Abstract
A new rearranged spongian diterpene, darwinolide, has been isolated from the Antarctic Dendroceratid sponge Dendrilla membranosa. Characterized on the basis of spectroscopic and crystallographic analysis, the central seven-membered ring is hypothesized to originate from a ring-expansion of a spongian precursor. Darwinolide displays 4-fold selectivity against the biofilm phase of methicillin-resistant Staphylococcus aureus compared to the planktonic phase and may provide a scaffold for the development of therapeutics for this difficult to treat infection.
Biofilms, formed by Staphylococcus aureus and many other pathogenic bacteria during infection, are a collection of cells coated in an extracellular matrix comprising polysaccharides, proteins, and DNA. Importantly, it is estimated that up to 80% of all infections are caused by bacterial biofilms, which are recalcitrant to therapeutic intervention.1 Indeed, at present our options for controlling and eradicating biofilms during infection are essentially nonexistent.2,3 As such, it is imperative that efforts are exerted to develop new and novel antibiofilm agents to treat drug resistant bacterial infections.
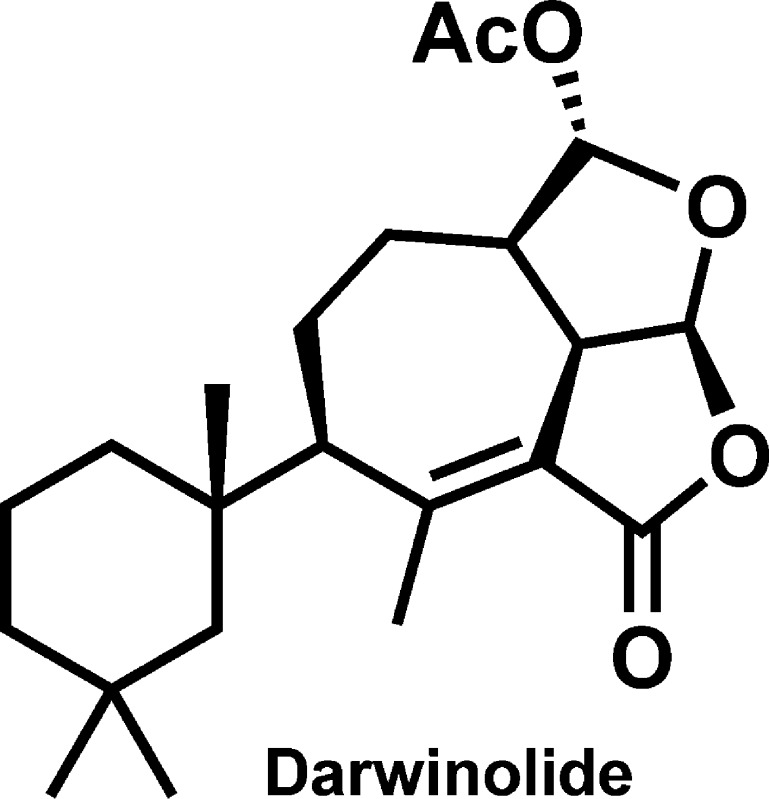
In the course of acquiring biodiversity to support our antibiotic screening program, we obtained the sponge Dendrilla membranosa from the vicinity of Palmer Station, Antarctica. The dichloromethane extract of the freeze-dried sponge was subjected to reversed-phase solid-phase extraction eluted with acetonitrile. The extract underwent high-performance liquid chromatography purification to yield four major natural products, including three previously reported spongian diterpenes, aplysulphurin, tetrahydroaplysulphurin, and membranolide,4−6 and a new rearranged spongian diterpene, darwinolide7 (Figure 1). The darwinolide skeleton is the newest of over a dozen structural motifs distinguishing the broad chemodiversity found in the Darwinellidae family of sponges.8
Figure 1.
Darwinolide.
The chemical formula of darwinolide, C22H32O5, was determined from the high-resolution electrospray ionization mass spectrum (HRESIMS) (m/z 377.2356 [M + H]+, calculated 377.2328), corroborating the 13C NMR spectrum, which displayed correlations in the hetereonuclear single quantum coherence (HSQC) spectrum indicative of six quaternary, five methine, six methylene, and five methyl carbons (Table 1). The methyl group signals observed in the 1H NMR spectrum were similar to those of the other spongian diterpenes found in the extract. A gem-dimethyl group (δ 0.86, 0.98) was evident, as was a singlet angular-type methyl group at δ 1.14, and vinyl and acetoxy methyl groups at δ 2.39 and 2.08, respectively. The lowfield shift of the vinyl methyl group, taken with its small (2.3 Hz) coupling, is reminiscent of tetrahydroaplysulphurin-3.4 Other notable 1H NMR signals include downfield singlet and doublet signals of the acetal methine groups (δ 5.93, 6.07), where the singlet observed at δ 5.93 is due to the roughly 90° dihedral angle between H-16 and H-13 resulting in a small JH13,H16.
Table 1. NMR Data for Darwinolide (CDCl3).
| position | δC, typea | δH (J in Hz)b | COSY | HMBC | ROESY |
|---|---|---|---|---|---|
| 1a | 38.6, CH2 | 1.08, m | 1b,2a,2b | 2,3,4,9,18,19,20 | |
| b | 1.54, m | 1a,2a,2b | 2,3,4,5,10,20 | ||
| 2a | 18.7, CH2 | 1.50, m | 1b,3a,3b | 1,4,10 | |
| b | 1.59, m | 1a,1b,3b | 4 | ||
| 3a | 39.2, CH2 | 1.11, m | 2a | 2,4,18,19 | |
| b | 1.37, m | 2a,2b | 4,10,18,19 | ||
| 4 | 30.8, C | ||||
| 5a | 50.5, CH2 | 1.08, d (14.1) | 5b | 3,4,9,18,19,20 | |
| b | 1.38, d (14.1) | 5a | 1,2,3,4,11,18,19,20 | ||
| 6 | 15.6, CH3 | 2.39, d (2.3) | 14 | 7,8,9,13,17 | 20 |
| 7 | 119.5, C | ||||
| 8 | 159.5, C | ||||
| 9 | 57.3, CH | 2.08, m | 11a,11b | 1,5,6,7,8,10,11,12,20 | 14 |
| 10 | 36.0, C | ||||
| 11a | 19.2, CH2 | 1.42, m | 9,12b | 8,9,10,12,13,16 | |
| b | 1.64, m | 9,12a,12b | 8,9,12,13 | ||
| 12a | 25.6, CH2 | 1.92, m | 11b,13 | 9,13,14,16 | |
| b | 1.19, m | 11a,11b | 13,14,16 | ||
| 13 | 43.2, CH | 2.24, m | 12a,14,16 | 12,16 | |
| 14 | 45.1, CH | 3.93, tt (7.0, 2.4) | 6,13,15 | 7 | 9,13,15 |
| 15 | 103.9, CH | 6.07, d (7.0) | 14 | 7,14,17 | 14 |
| 16 | 103.8, CH | 5.93, s | 13 | 12,13,14,15,21 | 12a |
| 17 | 167.7, C | ||||
| 18 | 33.9, CH3 | 0.86, s | 19 | 2,3,4,5,10,19 | 19 |
| 19 | 28.5, CH3 | 0.98, s | 18 | 3,4,5,10,18 | 18 |
| 20 | 22.1, CH3 | 1.14, s | 1,5,9,10 | 6 | |
| 21 | 169.7, C | ||||
| 22 | 21.2, CH3 | 2.08, s | 16,21 |
Recorded at 125 MHz.
Recorded at 500 MHz.
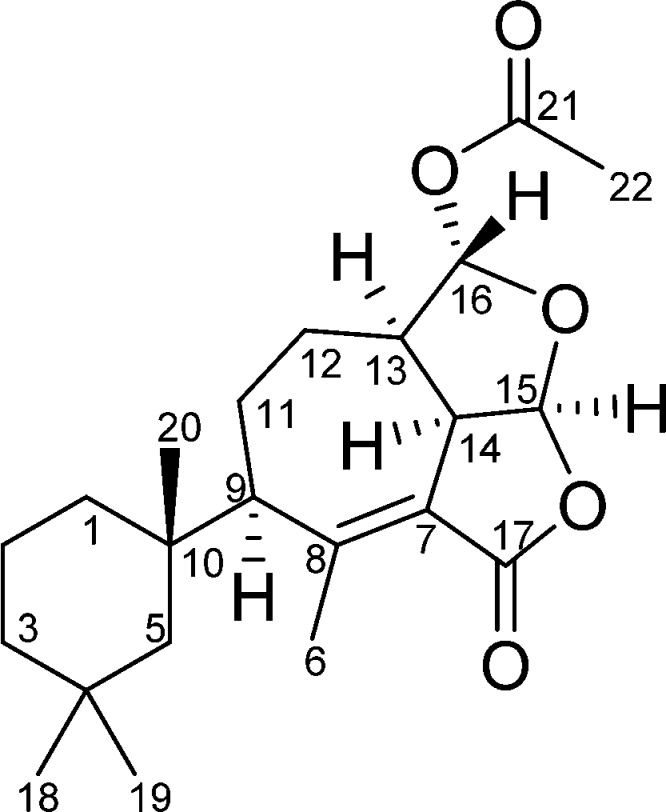
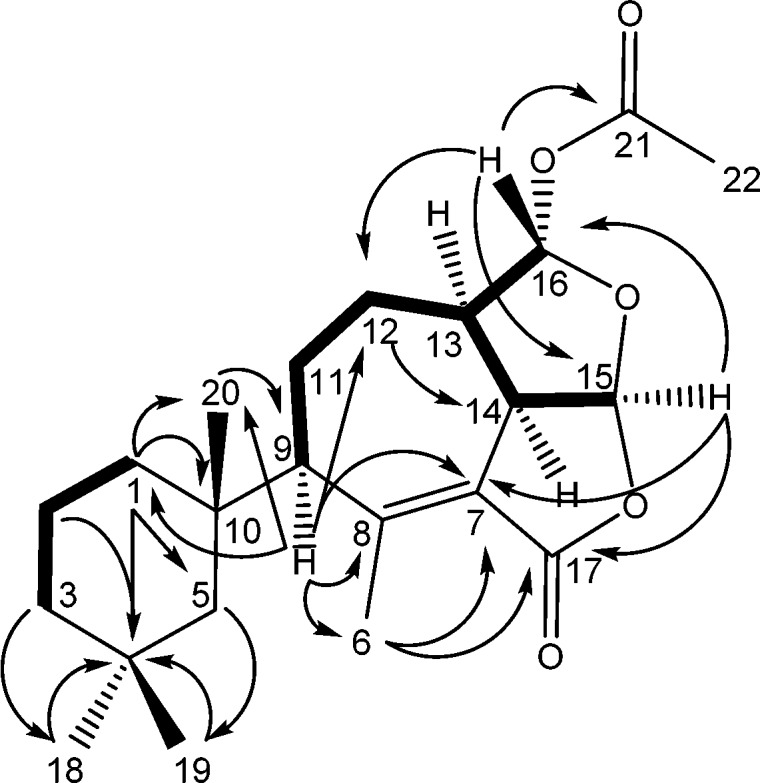
The 1H–1H correlation spectroscopy (COSY) spectrum showed coupling among most of the protons comprising the core tricyclic ring system (Figure 2). Coupling of H2-1/H2-2 and H2-2/H2-3 observed in the COSY spectrum, in addition to heteronuclear multiple bond correlations (HMBC) of Hb-1 (δ 1.54) to C-3, C-4, C-5, C-10, and C-20 and H3-20 (δ 1.14) to C-1, C-5, C-9, and C-10 helped to establish the trimethyl cyclohexane ring found among gracilin- and aplysulphurin-type seco-spongian metabolites. The protons of the gem-dimethyl group on that substructure, H3-18 and H3-19, correlated in the HMBC to C-3, C-4, and C-5. A proton at δ 2.08 displayed HMBC correlations spanning the tricyclic core and the trimethylcyclohexyl groups, including C-1, C-5, C-6, C-7, C-8, C-10, C-11, C-12, and C-20, securing its assignment as H-9. Completion of the carbon skeleton could be accomplished by observation of HMBC correlations of H-14 (δ 3.93) to C-7, C-8, C-13, and C-15. An acetate substituent was located at C-16 based on HMBC correlations between H-16 (δ 5.93) and carbonyl C-21, and the furan and furanone ring systems were required by HMBC correlation of H-15 (δ 6.08) to acetal carbon C-16 and lactone carbon C-17.
Figure 2.
Key COSY (bold) and HMBC (arrows) correlations observed for darwinolide.
The relative stereochemistry of the six chiral centers could be assigned with rotating frame Overhauser effect spectroscopy (ROESY) (Table 1), which demonstrated that H-9, H-13, H-14, and H-15 occupied the same face of the tricyclic core, as did Ha-12 and H-16. These observations were confirmed by X-ray analysis (Figure 3), which also established the absolute configuration of darwinolide based on Bijvoet-Pair Analysis and Bayesian Statistics (see SI).9,10
Figure 3.
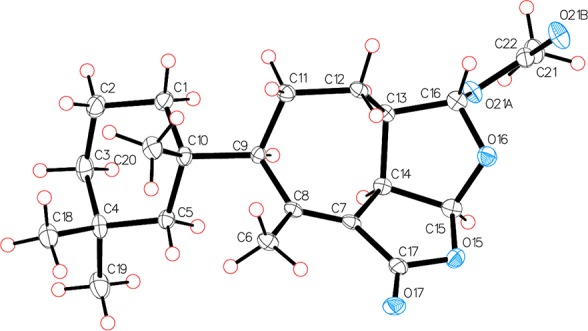
Asymmetric unit of darwinolide showing absolute stereostructure. Thermal ellipsoids have been drawn at 50% probability level.
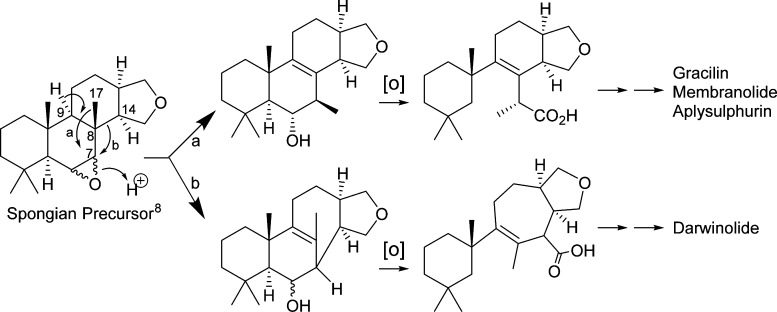
Darwinolide likely derives biosynthetically from the same gracilane pathway as other known aplysulphurides,8 but with a ring-expansion rearrangement not previously observed (Figure 4). All previously reported D. membranosa metabolites, including 9,11-dihydrogracilin, membranolide, and tetrahydroaplysulphurin, are conjectured8 to originate with a C-8 to C-7 shift of Me-17 in a suitable spongian precursor (Figure 4, path a). Darwinolide, however, results from the C-8/C-14 bond migration to C-7 (Figure 4, path b), a ring expansion that forms the new seven-membered carbocyclic ring. Further oxidation steps common in the spongian family (e.g., both paths a and b) results in cleavage of the C-5/C-6 bond and leads to the acid moiety at C-7, manifested in some D. membranosa compounds as the acid11 or methyl ester6 and in others as lactones.12
Figure 4.
Proposed biosynthetic pathway to Dendrilla membranosa diterpene metabolites. Path a leads to all known D. membranosa spongian diterpenes, presumably through a concerted cascade starting with removal of H-9 and ending with stereospecific ring opening of an α-epoxide.8 Path b may occur in a similar concerted fashion with the β-epoxide or may involve one or more carbocation intermediates to accommodate the stereochemical requirements of orbital orientations.
Spongian diterpenes are well-known as bioactive natural products. We screened darwinolide for activity against a clinical strain of a highly methicillin-resistant Staphylococcus aureus (MRSA). A broth dilution assay determined the MIC for darwinolide as 132.9 μM. The remaining colony was subjected to a cell recovery experiment overnight after washout of darwinolide. This study revealed that only 1.6% of the treated bacteria were able to recover and grow, therefore indicating darwinolide was cytotoxic, rather than cytostatic, toward S. aureus. A biofilm was established in vitro with the same MRSA strain and the experiment revealed an IC50 value of 33.2 μM against the biofilm. Cytotoxicity against a J774 macrophage cell line found darwinolide lacks mammalian cytotoxicity (IC50 = 73.4 μM). While there are currently no treatments for MRSA biofilms for use as positive controls in this assay, a comparison with contemporary work in the field13 found more potent biofilm inhibitors, but in all cases planktonic cells were considerably more sensitive than those within a biofilm. Based on the 4-fold selectivity of darwinolide for MRSA biofilms over planktonic cells and its low mammalian cytotoxicity, we suggest that darwinolide may present a highly suitable scaffold for the development of urgently needed, novel antibiofilm-specific antibiotics.14
Acknowledgments
This work was funded by the National Science Foundation awards ANT-0838773 and PLR-1341333 (to C.D.A., J.B.M.) and ANT-08328776 and PLR-1341339 (to B.J.B.) from the Antarctic Organisms and Ecosystems program, by the National Institutes of Health grants AI103715 (to L.N.S. and B.J.B.) and AI80626 (to L.N.S.), and by a Center of Excellence award from the State of Florida to support the Center for Drug Discovery and Innovation, whose facilities made much of the chemical analysis possible. J.B.M. acknowledges the support of an Endowed Professorship from the University of Alabama at Birmingham. We thank Ashley Souza from the Kyle lab at USF for the mammalian cytotoxicity data, the staff and technical support services of Antarctic Support Contract for logistical support in the field, and the USF X-ray crystallography facility and its director, Dr. Lukasz Wojtas.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.orglett.6b00979.
Full NMR and mass spectra, details of the crystal analysis, and experimental protocols describing the isolation and bioassay of darwinolide (PDF)
Author Present Address
⊥ Department of Chemistry, Simon Fraser University, 8888 University Drive, Burnaby, British Columbia V5A 1S6, Canada.
Author Present Address
# Alternative Medical Enterprises, 1451 Global Court, Sarasota, Florida 34240, United States.
The authors declare no competing financial interest.
Supplementary Material
References
- Akers K. S.; Mende K.; Cheatle K. A.; Zera W. C.; Yu X.; Beckius M. L.; Aggarwal D.; Li P.; Sanchez C. J.; Wenke J. C.; Weintrob A. C.; Tribble D. R.; Murray C. K. BMC Infect. Dis. 2014, 14, 190. 10.1186/1471-2334-14-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römling U.; Balsalobre C. J. Intern. Med. 2012, 272, 541–561. 10.1111/joim.12004. [DOI] [PubMed] [Google Scholar]
- Scali C.; Kunimoto B. J. Cutaneous Med. Surg. 2013, 17, 371–376. [DOI] [PubMed] [Google Scholar]
- Karuso P.; Skelton B. W.; Taylor W. C.; White A. H. Aust. J. Chem. 1984, 37, 1081–1093. 10.1071/CH9841081. [DOI] [Google Scholar]
- Karuso P.; Bergquist P. R.; Cambie R. C.; Buckleton J. S.; Clark G. R.; Rickard C. E. F. Aust. J. Chem. 1986, 39, 1643–1653. [Google Scholar]
- Molinski T. F.; Faulkner D. J. J. Org. Chem. 1987, 52, 296–298. 10.1021/jo00378a031. [DOI] [Google Scholar]
- Darwinolide: White crystals; [α]23D + 53 (c 1.0, CHCl3). UV–vis (CH3CN) λmax (log ε): 240 (4.73), 233 (4.73), 215 (4.77) nm. IR (thin film): 3049, 2921, 1756, 1636, 1372, 1234 cm–1; for 1H and 13C NMR please refer to Table 1. HRESIMS m/z 377.2356 [M + H]+ (calculated for 377.2328 C22H33O5); 317.2134 [M – HOAC + H]+ (calcd 317.2117); 335.2246 [M – OAc + H2O]+ (calcd 335.2222); 394.2588 [M + H2O]+ (calcd 394.2355); 633.4160 [2(M-HOAc) + H]+ (calcd 633.4155); 775.4423 [2 M + Na]+ (calcd 775.4397). Cambridge Crystallographic Data Center deposition number: CCDC 1477648.
- Keyzers R. A.; Northcote P. T.; Davies-Coleman M. T. Nat. Prod. Rep. 2006, 23, 321–334. 10.1039/b503531g. [DOI] [PubMed] [Google Scholar]
- Spek A. L. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2009, D65, 148–155. 10.1107/S090744490804362X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooft R. W. W.; Straver L. H.; Spek A. L. J. Appl. Crystallogr. 2008, 41, 96–103. 10.1107/S0021889807059870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Marrero A. R.; Dorta E.; Cueto M.; San-Martin A.; Darias J. Tetrahedron 2004, 60, 1073–1078. 10.1016/j.tet.2003.11.077. [DOI] [Google Scholar]
- Ankisetty S.; Amsler C. D.; McClintock J. B.; Baker B. J. J. Nat. Prod. 2004, 67, 1172–1174. 10.1021/np0340551. [DOI] [PubMed] [Google Scholar]
- Fleeman R. M.; LaVoi T.; Santos R. G.; Morales A.; Nefzi A.; Welmaker G. S.; Medina-Franco J.; Houghten R. A.; Giulianotti M. A.; Shaw L. N. J. Med. Chem. 2015, 58, 3340–3355. 10.1021/jm501628s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthington R. J.; Richards J. J.; Melander C. Anti-Infect. Agents 2014, 12, 120–138. 10.2174/22113525113119990107. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.