Abstract
Background & objectives:
There is paucity of studies on the quality of anticoagulation in neurological patients from India. This study evaluates the quality of oral anticoagulation therapy in neurology patients.
Methods:
Consecutive patients attending a tertiary care neurology service in north India who were prescribed oral anticoagulant (OAC), were included. Their international normalized ratio (INR) values were prospectively monitored and the earlier INR values of the patients who were already on OAC were retrospectively analyzed. The patients with multi-organ dysfunction, pregnancy and those below 18 yr of age were excluded. The therapeutic INR range was defined as per standard recommendations. The level of anticoagulation, factors interfering with OAC and complications were noted.
Results:
The results were based on 77 patients with median age 40 yr. Fifty one patients received OAC for secondary stroke prevention, 23 for cerebral venous sinus thrombosis (CVST) and three for deep vein thrombosis (DVT). A total 167.9 person-years of follow up was done with a median of 1.2 (0.3-9.3) years. Of the 1287 INR reports, 505 (39.3%) reports were in the therapeutic range, 496 (38.5%) were below and 282 (21.91%) were above the therapeutic level. Stable INR was obtained in 33 (42.86%) patients only. INR level was improved by dose adjustment in 20 (26%), drug modification in two (2.6%), and dietary adjustment in six (7.8%) patients. Three patients were sensitive and five were resistant to OAC. Complications were noted in 28 instances; thromboembolic in 16 and haemorrhagic stroke in 12. The overall complication rate was 16.7 per 100 person-years.
Interpretation & conclusions:
It may be concluded that stable therapeutic INR is difficult to maintain in neurological patients. Optimal modification of diet, drug and dose of oral anticoagulant may help in stabilization of INR.
Keywords: Complications, INR, nicoumalone, oral anticoagulation, stroke, warfarin
Oral anticoagulation (OAC) is used in neurology practice for primary and secondary stroke prevention in atrial fibrillation (AF), prosthetic mechanical valve; dilated cardiomyopathy (DCMP) and cerebral venous sinus thrombosis (CVST)1,2,3. In many of these indications OACs are used lifelong. In CVST, OAC is used for six months or longer depending on the aetiology4. Oral anticoagulants have a narrow therapeutic range with risk of bleeding or thromboembolism. Effectiveness of OAC therapy is monitored by measurement of prothrombin time, expressed as international normalized ratio (INR). The frequency of monitoring depends on the stability of INR and may vary from daily to three monthly5. In North America, INR monitoring is indicated four weekly after achieving stabile INR6. In spite of regular monitoring, good INR control is difficult to achieve. In a meta-analysis including 47 studies on patients with AF on OAC, the median percentage of INR was in target range in 53 per cent (range 34-68%), below in 26 per cent (range10-51%) and above in 17 per cent (range 14-29%) in the retrospective studies7. In another meta-analysis including 95 studies done mostly prospectively or in anticoagulation clinic on patients with AF on OAC, median percentage of INR in therapeutic range was 56 per cent (range 34.2-70.7%), below in 26 per cent (range 8.7-51%) and above in 13 per cent (range 6-38.5%)8. Maintaining the INR in the therapeutic range may be difficult as the effect of OAC is influenced by food, drug, age, gender and genetic polymorphism5,9.
There are only a few studies evaluating the status of anticoagulation in neurology patients10,11,12. Neurology patients often are dependent on their relatives for their treatment and INR study especially in the developing countries. In the present study, the quality and stability of INR were evaluated in the neurology patients receiving OAC as also the influence of modification of correctable factors on these parameters.
Material & Methods
Consecutive patients on OAC attending to the neurology service of Sanjay Gandhi Post Graduate Institute of Medical Sciences, Lucknow, Uttar Pradesh, India, between March 2011 and February 2013 were included in the study. The patients who were prescribed OAC for the first time were prospectively followed up. The patients who were already on OAC were also prospectively followed up and their prior data were retrospectively evaluated. The study was approved by the Institute Ethics Committee. Informed written consent was obtained from all the patients or their first degree relatives.
Those with pregnancy, hepatic and renal failure, multi-organ dysfunction and those below 18 yr of age or unwilling to participate in the study were excluded. A detailed history and clinical examination were done. The demographic details of the patients including age, gender, diet, ethnicity and religion were noted. Presence of anaemia, oedema, petechial haemorrhage, epistaxis, haematemesis and melena were enquired. Their pulse rate and rhythm were noted and abnormalities in cardiac examination were recorded. Neurological examination included level of consciousness, mental status, muscle power, tone, tendon reflexes, sensations and co-ordination. Electrocadiography, Doppler, echocardiography and MR venography were done as per clinical indication. The patients were categorized into rheumatic heart disease (RHD), prosthetic valve, dilated cardiomyopathy (DCMP), cerebral venous sinus thrombosis (CVST), deep vein thrombosis (DVT) and miscellaneous disorders. Adequacy of anticoagulation was defined by the INR value based on recommended therapeutic range for different conditions13,14.
Advice about dietary and drug interaction with OAC was given at the first visit. On follow up, adequacy of OAC was defined based on INR value. The starting dose of nicoumalone was 2 mg/day and the dose was adjusted based on INR value. The INR was measured every three days until two consecutive INR values were in the therapeutic range. Thereafter, INR was repeated after one week; if INR was in therapeutic range then repeated monthly. The patient with suboptimal INR was enquired about diet and OAC dose and compliance. Any of these factors if found abnormal, was corrected and INR was repeated after 1-2 wk. If INR was suboptimal even after correcting these factors, the dose of OAC was increased by 10-20 per cent. The INR values in the initial two weeks of OAC treatment and those after temporary discontinuation of drug due to surgical procedures or other conditions were excluded.
Nicoumalone was the preferred OAC but those already on OAC other than this drug were advised to continue the prescribed drug. Usually INR was done in our institute laboratory but patients from far off place were advised to get it done locally. The abnormal report was varified from our laboratory before modifying the OAC dose. The anticoagulation status was categorized into three groups based on the recommended INR value for the respective disease- optimal (in the therapeutic range), suboptimal (below the therapeutic range) and above the therapeutic range. Stable INR was defined by three consecutive INR values in therapeutic range at least one month apart without changing dose of oral anticoagulant. Polypills therapy was defined if the patient was taking ≥ 4 drugs.
During the follow up period, complications were noted and corresponding INR values were recorded. Total duration of follow up was calculated by adding follow up of all the patients and complication was calculated in per 100 person-years.
Statistical analysis: The relationship of anticoagulation status with demographic and clinical variables was evaluated using Chi square test for categorical and independent variables or Mann-Whitney U test for continuous variables. The factors influencing the stability of INR were evaluated by univariate followed by multivariate analysis. The variable was considered significant if two tailed P value was <0.05. The statistical tests were done using SPSS 16 version software (SPSS, Inc., Chicago, USA).
Results
During the study period, 89 patients on OAC were enrolled. Twelve patients were excluded because eight patients were lost to follow up, three had follow up for one month only and one patient stopped OAC within two months before reaching therapeutic INR level. The median age of the remaining 77 patients was 37 (range19-79) years and 46 were males (59.7%). Forty (51.9%) patients belonged to the rural area, 19 (24.7%) had ≤8 years of education and 40 (51.9%) were vegetarians. Fifty one patients were receiving OAC for secondary prophylaxis of stroke: 28 had RHD (21 had associated AF), 17 prosthetic valve, two antiphospholipid antibody (APLA) syndrome and four had DCMP. Three patients had DVT following ischaemic stroke (2) and Guillain-Barré syndrome (1) during hospital stay in the acute phase of illness. The remaining received OAC for CVST. Nicoumalone was prescribed in 68 (88.3%) patients and warfarin in the remaining. Three patients took alcohol occasionally, three smoked about 5-8 cigarettes/day and seven chewed tobacco. Two patients had associated diabetes mellitus, two had hypertension, two had both diabetes mellitus and hypertension, and one had chronic obstructive pulmonary disease (COPD).
Forty eight of the 77 patients were followed up prospectively, 28 had both retrospective and prospective data, and one had only retrospective data.
Quality of oral anticoagulation: Total 167.9 person-years follow up was done with a median of 1.17 (range 0.3-9.3 yr). During the follow up, 1287 INR reports were available, of which 505 (39.3%) were optimal, 496 (38.5%) suboptimal, 286 (22.2%) above the therapeutic range. Average time between two INR tests was 1.6 month.
We had 101.2 person-years retrospective follow up and 666 INR values were obtained from 29 patients and 239 (35.9%) of these values were optimal. In 66.6 person-years prospective follow up period, 621 INR values were available; of these, 266 (42.8%) were optimal. In retrospective follow up, the average interval between two INR tests was 1.8 month and in the prospective follow up it was 1.3 month.
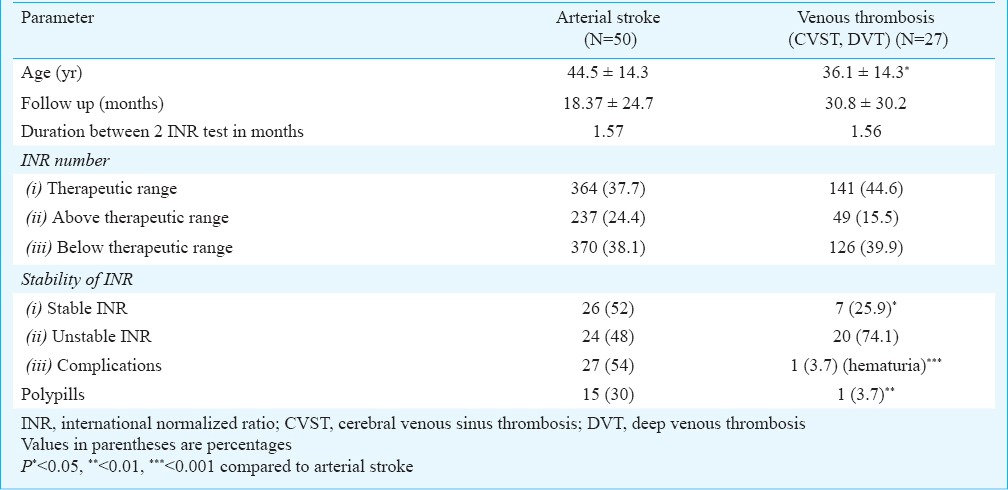
Stability of INR: Stable INR was noted in 33 of 77 (42.6%) patients only. Three patients had stable INR with dietary modification, two required lesser dose and one patient needed higher dose of OAC after dietary advice. The dose of OAC was adjusted to get stable INR in 20 patients (increased in 15 and decreased in 5). The dose of OAC was reduced in one patient after changing antiepileptic drug from carbamazepine to sodium valporate and in another after stopping atorvastatin. There was variable response of oral anticoagulants requiring variable doses to maintain INR in therapeutic range. Three patients were sensitive to oral anticoagulant and needed 1 mg or less nicoumalone while five were resistant needing more than 5 mg of nicoumalone for achieving optimal INR value. The stability of INR was related to the cadrioembolic stroke (P<0.05), duration of follow up (P<0.001), serum protein (P<0.001), albumin (P<0.001) and polypills (P<0.05). The details are summarized in Table I. The patients with stable INR had significantly fewer comorbidities [diabetes, hypertension, COPD: 1 versus 10; P<0.05] and number of drugs (<4 drugs: 3 versus 13; P<0.05) compared to those with unstable INR. Presence of diabetes mellitus, hypertension and COPD (r=0.28; P=0.01) and polypills (r=0.249; P=0.03) was significantly correlated with unstable INR. The patients with arterial stroke although had higher stability but had more complications (Table II).
Table I.
Predictors of stable anticoagulation in the patients receiving oral anticoagulant (n=77)
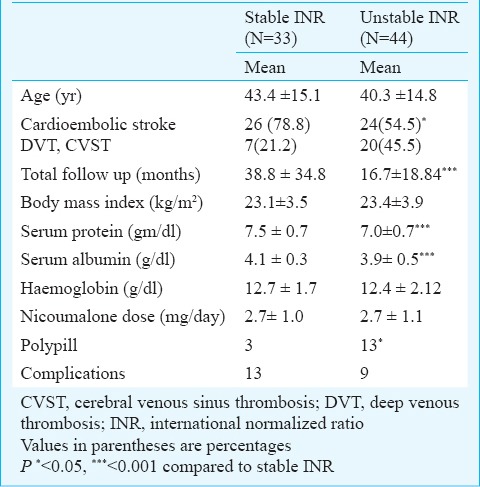
Table II.
Quality of anticoagulation in the patients with arterial stroke and venous thrombosis receiving oral anticoagulant
Complications: During the study period, 22(28.6.%) patients developed 28 complications. During a total of 167.9 person-years follow up period,16 thromboembolic episodes and 12 (10 patients) intracerebral haemorrhagic occurred. These complications were at a rate of 9.5 and 7.1 per 100 person-years, respectively with an overall complication rate 16.7 per 100 person-years. In prospective follow up of 66.8 person-years, two episodes of thromboembolic stroke and 10 episodes of intracerebral haemorrhage occurred; five of which were major. The average complication rate in prospective follow up was 19.5 per 100 person-years. During the retrospective 101.1 person-years follow up, there were 14 episodes of thromboembolic stroke and two intracerebral haemorrhages with an average complication rate of 12.9 per 100 person-years.
Discussion
In the present study, 39.3 per cent INR reports were in the therapeutic range. This was lower than the reported frequency of 43-72 per cent6,15. Retrospective studies reported 51 per cent INR in therapeutic range in US, 58 per cent in Canadian15 and 17.8 per cent in Indian patients12. In Stroke Prevention in Atrial Fibrillation II (SPAF II) study, 72 per cent INR values were in the therapeutic range16. The poorer quality of OAC in our study compared to that reported in prospective studies may be due to less frequent INR testing. Several studies suggest that adverse events can be reduced and optimal INR may be maximized by more frequent INR testing17-20. The optimal INR can be achieved in 90 per cent on alternate day monitoring compared to 50 per cent when monitored monthly20. In our study, the retrospective data showed inferior results compared to the prospective. In the literature, the retrospective studies have shown inferior results compared to prospective studies and randomized controlled trials, and this was attributed to regular and frequent follow up6,15. Therapeutic INR is difficult to achieve in the first six months21. The type of OAC and co-medications may also affect the quality of anticoagulation. The patients receiving warfarin had 72 per cent INR in the therapeutic range compared to 67 per cent on acenocoumarol22. Majority of our patients were on nicoumalone and they were followed up for a variable period of time which might have affected the results.
Stable INR was obtained in 42.9 per cent patients which was higher than a previous study23. In our study, use of multiple drugs and associated co-morbidities were related to unstable INR which was consistent with earlier reports23,24. The level of anticoagulation is affected not only by comorbid conditions but also by other innate characteristics5. In our study, dietary modification resulted in stable INR in six and drug modification in two patients. Diet plays an important role in maintaining stability of anticoagulation. There is great variation in Indian diet with respect to consumption of non-vegetarian diet and green vegetables in vegetarian diet. The first generation antiepileptic and other hepatic enzyme inducing drugs which have interaction with oral anticoagulants should be avoided25. In two of our patients, INR values were stabilized after change in drug.
The overall complication rate in our study was 16.7 per 100 patient-years. In an earlier study, median rate of major haemorrhage was 2.2 per 100 patients years and that of thromboembolism was 2.5 per 100 patient years1 which were much lower compared to our results. There are studies showing complications rate similar and/or higher than ours26,27,28,29. In a randomized controlled trial 33 per cent INR was in therapeutic range, 12 per cent patient years had major bleeding and 13 per cent had thromboembolic complications27. The high incidence of complications may be due to low quality of oral anticoagulation. In our study, 39.3 per cent INR was in therapeutic range and only 42.7 per cent patients had three consecutive INR in therapeutic range. A study showed that at centres achieving median time in therapeutic range (TTR) less than 65 per cent, there was no benefit of oral anticoagulation over dual antiplatelet therapy in preventing vascular complications28. We also had a proportion of patients with prosthetic valve (22.1%). These patients require higher level of anticoagulation (INR 2.5-3.5) which is more difficult to maintain29. Most of the cardiac patients on anticoagulants when developed complications were referred to neurology and inclusion of these complications as OAC related could be responsible for higher complication rate in the present study. Drug response was also variable in our patients to maintain INR in therapeutic range. The variability in drug response may have genetic basis which needs further studies.
Our study was a single centre hospital-based study on a small sample with heterogeneous follow up. A large well plan study is needed on stability of INR in Indian patients and the factors responsible for variability.
In neurological patients achievement and maintenance of optimal INR is often difficult. Various modifiable factors including diet, choice of drugs and their dosage are important predictors of stability hence should be carefully adjusted.
Acknowledgment
Authors thank Dr S. Kumar for review of literature in the final stage of preparation of the manuscript.
Footnotes
Conflicts of Interest: None.
References
- 1.Hylek EM, Go AS, Chang Y, Jensvold NG, Henault LE, Selby JV, et al. Effect of intensity of oral anticoagulation on stroke severity and mortality in atrial fibrillation. N Engl J Med. 2003;49:1019–26. doi: 10.1056/NEJMoa022913. [DOI] [PubMed] [Google Scholar]
- 2.Einhäupl K, Stam J, Bousser MG, De Bruijn SF, Ferro JM, Martinelli I, et al. EFNS guideline on the treatment of cerebral venous and sinus thrombosis in adult patients. Eur J Neurol. 2010;17:1229–35. doi: 10.1111/j.1468-1331.2010.03011.x. [DOI] [PubMed] [Google Scholar]
- 3.Prandoni P, Lensing AW, Cogo A, Cuppini S, Villalta S, Carta M, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125:1–7. doi: 10.7326/0003-4819-125-1-199607010-00001. [DOI] [PubMed] [Google Scholar]
- 4.Einhäupl K, Stam J, Bousser MG, De Bruijn SF, Ferro JM, Martinelli I, et al. European Federation of Neurological Societies. EFNS guideline on the treatment of cerebral venous and sinus thrombosis in adult patients. Eur J Neurol. 2010;17:1229. doi: 10.1111/j.1468-1331.2010.03011.x. [DOI] [PubMed] [Google Scholar]
- 5.Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuünemann HJ. Antithrombotic therapy and prevention of thrombosis panel, 9th ed. Evidence-based clinical practice guidelines. Chest. 2012;141:7S–47S. doi: 10.1378/chest.1412S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G. Pharmacology and management of the vitamin K antagonists: Evidence-based clinical practice guidelines, 8th ed. Chest. 2008;133:160S–98S. doi: 10.1378/chest.08-0670. [DOI] [PubMed] [Google Scholar]
- 7.Wan Y, Heneghan C, Perera R, Roberts N, Hollowell J, Glasziou P, et al. Anticoagulation control and prediction of adverse events in patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2008;1:84–91. doi: 10.1161/CIRCOUTCOMES.108.796185. [DOI] [PubMed] [Google Scholar]
- 8.Mearns ES, White CM, Kohn CG, Hawthorne J, Song JS, Meng J, et al. Quality of vitamin K antagonist control and outcomes in atrial fibrillation patients: a meta-analysis and meta-regression. Thromb J. 2014;12:14. doi: 10.1186/1477-9560-12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Apostolakis S, Sullivan RM, Olshansky B, Lip GY. Factors affecting quality of anticoagulation control among patients with atrial fibrillation on warfarin: the SAMe-TT2R2 score. Chest. 2013;144:1555–63. doi: 10.1378/chest.13-0054. [DOI] [PubMed] [Google Scholar]
- 10.Benavente L, Calleja s, de la Vega V, Lahoz CH. Oral anticoagulation in elderly patients as secondary prevention of cardioembolic strokes. Int Arch Med. 2010;3:8. doi: 10.1186/1755-7682-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhanya PS, Nidheesh C, Kuriakose KM, Puthiyaveetil N. Pattern of oral anticoagulant use following prosthetic heart Valve replacement. Indian J Thorac Cardiovasc Surg. 2011;27:119–24. [Google Scholar]
- 12.Kakkar N, Kaur R, John M. Outpatient oral anticoagulant management: audit of 82 patients. J Assoc Phyicians India. 2005;53:847–52. [PubMed] [Google Scholar]
- 13.Keeling D, Baglin T, Tait C, Watson H, Perry D, Baglin C, et al. Guidelines on oral anticoagulation with warfarin - fourth edition. Br J Haematol. 2011;154:311–24. doi: 10.1111/j.1365-2141.2011.08753.x. [DOI] [PubMed] [Google Scholar]
- 14.Saposnik G, Barinagarrementeria F, Brown RD, Jr, Bushnell CD, Cucchiara B, Cushman M, et al. Diagnosis and management of cerebral venous thrombosis. Stroke. 2011;42:1158–92. doi: 10.1161/STR.0b013e31820a8364. [DOI] [PubMed] [Google Scholar]
- 15.Ansell J, Hollowell J, Pengo V, Martinez-Brotons F, Caro J, Drouet L. The international study of anticoagulation management (ISAM) J Thromb Thrombolysis. 2007;23:83–91. doi: 10.1007/s11239-006-9022-7. [DOI] [PubMed] [Google Scholar]
- 16.Ezekowitz MD, James KE. Warfarin versus aspirin for prevention of thromboembolism in atrial fibrillation. Stroke Prevention in Atrial Fibrillation II Study. Lancet. 1994;343:687–91. [PubMed] [Google Scholar]
- 17.Cannegieter SC, Rosendaal FR, Wintzen AR, van der Meer FJ, Vandenbroucke JP, Briët E. Optimal oral anticoagulant therapy in patients with mechanical heart valves. N Engl J Med. 1995;333:11–7. doi: 10.1056/NEJM199507063330103. [DOI] [PubMed] [Google Scholar]
- 18.Palareti G, Leali N, Coccheri S, Poggi M, Manotti C, D’Angelo A, et al. Study on complications of oral anticoagulant therapy (ISCOAT) Lancet. 1996;348:423–8. doi: 10.1016/s0140-6736(96)01109-9. [DOI] [PubMed] [Google Scholar]
- 19.Horstkotte D, Piper C, Wiemer M. Optimal frequency of patient monitoring and intensity of oral anticoagulation therapy in valvular heart disease. J Thromb Thrombolysis. 1998;1:19–24. doi: 10.1023/a:1013228718768. [DOI] [PubMed] [Google Scholar]
- 20.Sawicki PT. A structured teaching and self-management program for patients receiving oral anticoagulation. JAMA. 1999;281:145–50. doi: 10.1001/jama.281.2.145. [DOI] [PubMed] [Google Scholar]
- 21.Azar AJ, Deckers JW, Rosendaal FR, van Bergen PF, van der Meer FJ, Jonker JJ, et al. Assessment of therapeutic quality control in a long-term anticoagulant trial in post-myocardial infarction patients. Thromb Haemost. 1994;72:347–51. [PubMed] [Google Scholar]
- 22.Pattacini C, Manotti C, Pini M, Quintavalla R, Dettori AG. A comparative study on the quality of oral anticoagulant therapy (warfarin versus acenocoumarol) Thromb Haemost. 1994;71:188–91. [PubMed] [Google Scholar]
- 23.Witt DM, Delate T, Clark NP, Martell C, Tran T, Crowther MA, et al. Outcomes and predictors of very stable INR control during chronic anticoagulation therapy (WARPED) Blood. 2009;114:952–6. doi: 10.1182/blood-2009-02-207928. [DOI] [PubMed] [Google Scholar]
- 24.Witt DM, Delate T, Clark NP, Martell C, Tran T, Crowther MA, et al. Twelve-month outcomes and predictors of very stable INR control in prevalent warfarin users. J Thromb Haemost. 2010;8:744–9. doi: 10.1111/j.1538-7836.2010.03756.x. [DOI] [PubMed] [Google Scholar]
- 25.Holbrook AM, Pereira JA, Labiris R, McDonald H, Douketis JD, Crowther M, et al. Systematic overview of warfarin and its drug and food interactions. Arch Intern Med. 2005;165:1095–106. doi: 10.1001/archinte.165.10.1095. [DOI] [PubMed] [Google Scholar]
- 26.Horstkotte D, Piper C. Improvement of oral anticoagulation therapy by INR self-management. J Heart Valve Dis. 2004;13:335–8. [PubMed] [Google Scholar]
- 27.Beyth RJ, Quinn L, Landefeld CS. A multicomponent intervention to prevent major bleeding complications in older patients receiving warfarin. A randomized, controlled trial. Ann Intern Med. 2000;133:687–95. doi: 10.7326/0003-4819-133-9-200011070-00010. [DOI] [PubMed] [Google Scholar]
- 28.Connolly SJ, Pogue J, Eikelboom J, Flaker G, Commerford P, Franzosi MG, et al. Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of INR control achieved by centres and countries as measured by time in therapeutic range. Circulation. 2008;118:2029–37. doi: 10.1161/CIRCULATIONAHA.107.750000. [DOI] [PubMed] [Google Scholar]
- 29.Rose PE. Audit of anticoagulant therapy. J Clin Pathol. 1996;49:5–9. doi: 10.1136/jcp.49.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]


