Abstract
Background & objectives:
The changing spectrum of Candida species in causation of oropharyngeal candidiasis and their antifungal susceptibility pattern among the HIV infected individuals has made the identification to species level mandatory and detection of drug resistance necessary for patient care. The present study was carried out to determine the species distribution and antifungal susceptibility profile of oral Candida isolates colonizing or infecting both HIV seropositive and seronegative individuals.
Methods:
A case-control study was conducted including 141 consecutive, non-repeat HIV-seropositive individuals and an equal number of sex and age matched HIV-seronegative control. Speciation of the oropharyngeal Candida isolates was done using standard yeast identification protocol. Antifungal susceptibility testing was done by the disk-diffusion method as well as by Fungitest method.
Results:
From the 59 culture positive HIV seropositive cases, 61 Candida isolates were recovered; Candida albicans (n=47, 77.0%), C. dubliniensis (n=9, 14.7%), C. parapsilosis (n=2, 3.2%), C. glabrata (n=2, 3.2%), and C. famata (n=1, 1.6%). Candida colonization in HIV-seropositive individuals was significantly higher than that of HIV-seronegative (control) group. Antifungal susceptibility testing revealed (n=6, 9.3%) C. albicans isolates resistant to voriconazole and fluconazole by disk-diffusion method whereas no resistance was seen by Fungitest method.
Interpretation & conclusions:
C. albicans was the commonest Candida species infecting or colonizing HIV seropositive individuals. Oropharyngeal Candida isolates had high level susceptibility to all the major antifungals commonly in use. Increased level of immunosuppression in HIV-seropositives and drug resistance of non-albicans Candida species makes identification and susceptibility testing of Candida species necessary in different geographical areas of the country.
Keywords: Antifungal susceptibility testing, Candida colonization, geographical variation, non-albicans Candida species, oropharyngeal candidiasis
Oropharyngeal candidiasis (OPC) is the most frequent opportunistic infection encountered in human immunodeficiency virus (HIV)-infected individuals and is considered as an independent predictor of immunodeficiency in patients with AIDS1. Though Candida albicans is the most frequently isolated species as colonizer and pathogen of the oral mucosa, other Candida species, such as C. tropicalis, C. krusei, C. parapsilosis and C. glabrata have also been recovered increasingly1,2,3. The azoles, particularly fluconazole, remain among the most common antifungal drugs, but their intensive clinical use for both therapy and prophylaxis has favoured the emergence of resistant strains3.
Although many studies have been conducted on oropharyngeal candidiasis in HIV population across the country3,4,5,6, there is paucity of such studies in the north-east region of India. Thus the present study was carried out to determine the species distribution and antifungal susceptibility profile of oropharyngeal Candida isolates colonizing or infecting both HIV seropositive and seronegative individuals.
Material & Methods
This study was conducted over a period of one year from April, 2011 to March, 2012 in the department of Microbiology, Assam Medical College and Hospital, Dibrugarh, Assam, India. Clearance from the ethical committee of the institute was obtained for the study.
A total of 141 consecutive, non-repeat HIV-seropositive individuals, irrespective of oropharyngeal lesions attending Integrated Counselling and Testing Centre (ICTC) either for HIV serostatus detection or for CD4 cell counting were included in the study. The subjects already treated with highly active antiretroviral therapy (HAART) were excluded from the study. The participants were grouped on the basis of age as ≤ 20, 21-40, 41-60 and >60 yr. Equal number of HIV-negative individuals who came for detection of their HIV serostatus without oral mucosal lesions were included as control group. For each case, an individual of same sex and age falling within the range of that age group was selected as control. An informed written consent was obtained from all adult participants and parents/guardians in case of children.
A standardized data collection form was used to retrieve demographic and personal information and other relevant information like route of HIV transmission, diabetes status, history of tuberculosis, history of drug (antibacterial or antifungal) intake, etc. After thorough examination of oral cavity, the HIV-positive subjects were further divided into two groups: subjects with oral/oropharyngeal candidiasis (symptomatic seropositive) and subjects without oral/oropharyngeal candidiasis (asymptomatic seropositive). The clinical stages of disease were determined according to the World Health Organization (WHO) guidelines7.
Laboratory methods: HIV serostatus of the patients was determined by commercially available ELISA (SD HIV ELISA 3.0, Standard Diagnostic Pvt. Ltd.; Republic of Korea) and three rapid antibody tests using National AIDS Control Organization (NACO) recommended algorithm8. CD4 cell counts were measured by using Partec CyFlow counter (Partec GmbH, Munster, Germany).
Two oral swabs were collected from each patient by firmly swabbing the lesion site in case of symptomatic individuals and the dorsum of tongue and buccal mucosa in case of asymptomatic ones. One swab was used to prepare a smear for Gram staining. The second swab was immediately inoculated on two slopes of Sabouraud dextrose agar (SDA) supplemented with antibiotics (50 μg/ml of chloramphenicol and 5 μg/ml of gentamicin) and two slopes of SDA without antibiotics. Two sets of tubes were incubated at 37° C and room temperature respectively and examined every 48 h till growth was obtained. Growth of yeast-like organisms was confirmed by Gram staining. Species identification of the isolates was performed by standard methods, i.e. Germ tube test, corn meal agar morphology (Dalmau plate technique), carbohydrate fermentation and assimilation (auxanographic) technique, urea hydrolysis and growth at 45°C9,10, colony colour on Hicrome Candida differential agar (Hi-media, Mumbai)11, and Vitek 2 identification system (BioMerieux, France). After the final identification, isolates were stored at -80°C in 50 per cent glycerol until susceptibility tests were performed.
In vitro antifungal susceptibility testing: The in vitro activities of fluconazole (25 μg), voriconazole (1 μg) (procured from Hi-media, Mumbai) was determined by using disk-diffusion method according to the Clinical and Laboratory Standards Institute (CLSI) guidelines for antifungal disk diffusion susceptibility testing of yeasts (M44-A2)12 and results were expressed as susceptible, susceptible- dose dependent and resistant as per Zone Diameter Interpretive Standards-CLSI13. Commercially available modified microtitre broth breakpoint test from BIO-RAD, France (Fungitest TM)14 was also used for susceptibility testing for fluconazole (8-64 µg/ml), itraconazole (0.5-4 µg/ml), ketoconazole (0.5-4 µg/ml), miconazole (0.5-8 µg/ml), amphotericin-B (2-8 µg/ml) and 5-fluorocytosine (2-32 µg/ml) and interpretated according to the manufacturers’ instructions. Results were expressed as no growth or sensitive (isolate inhibited by antifungal agent in vitro), low growth (intermediate) and growth or resistant (not inhibited by the antifungal agent in vitro).
Quality control: Quality control procedures were performed as per CLSI guidelines12 using C. albicans ATCC 90028 and C. parapsilosis ATCC 22019 strains, as quality control strains for fluconazole and voriconazole disk diffusion testing.
Statistical analysis: Data entry, database management and analysis was done using SPSS version 16.0. Student's t test was performed to compare the continuous variable and chi-square test was performed to compare the categorical variables.
Results
The age of HIV-seropositive individuals (n=141) ranged from 2-65 yr with mean age of 35±11.95 yr with female preponderance (male: female ratio was 1:1.1). Ninety three (66.0%) individuals belonged to the age group of 21-40 yr. Among all, 78.7 per cent (n=111) were married and 58.2 per cent (n=82) were rural inhabitants. History of alcohol intake was positive in 31.9 per cent (n=45) cases and 24.8 per cent (n=35) were smoker. Most of the seropositive patients (70.9%, n=100) were categorized into WHO clinical stage 1 followed by 15.6 per cent (n=22), 8.5 (n=12) and 5 per cent (n=7) in clinical stages 3, 2 and 4, respectively. Heterosexual route (83.0%, n=117) was the most common mode of infection followed by blood transfusion (5.7%, n=8), vertical transmission (4.3%, n=6) and homosexual route (2.8%, n=4). One patient was intravenous drug user (needle sharing) while modes of transmission of 3.5 per cent (n=5) individuals were unknown. Among the HIV-seropositives 14 (9.9%) were co-infected with tuberculosis, 15 were hospitalized and 17 individuals had history of antifungal treatment within preceding 90 days. In control group, all individuals were asymptomatic. No obvious risk factors for colonization have been observed.
Out of the 141 HIV seropositive individuals, 27 (19.1%) presented with oropharyngeal lesion (symptomatic seropositive); of whom, 66.7 per cent (n=18) experienced oropharyngeal lesion for the first time while the rest had recurrent episodes. Among them, 63 per cent (n=17) had pseudomembranous lesions (Figure) while the remaining (n=10) presented with erythematous type. The mean CD4 count in the group with oral/oropharyngeal candidiasis was significantly lower than the group without oral/oropharyngeal candidiasis (117 versus 360 cells/µl) (P<0.001).
Figure.
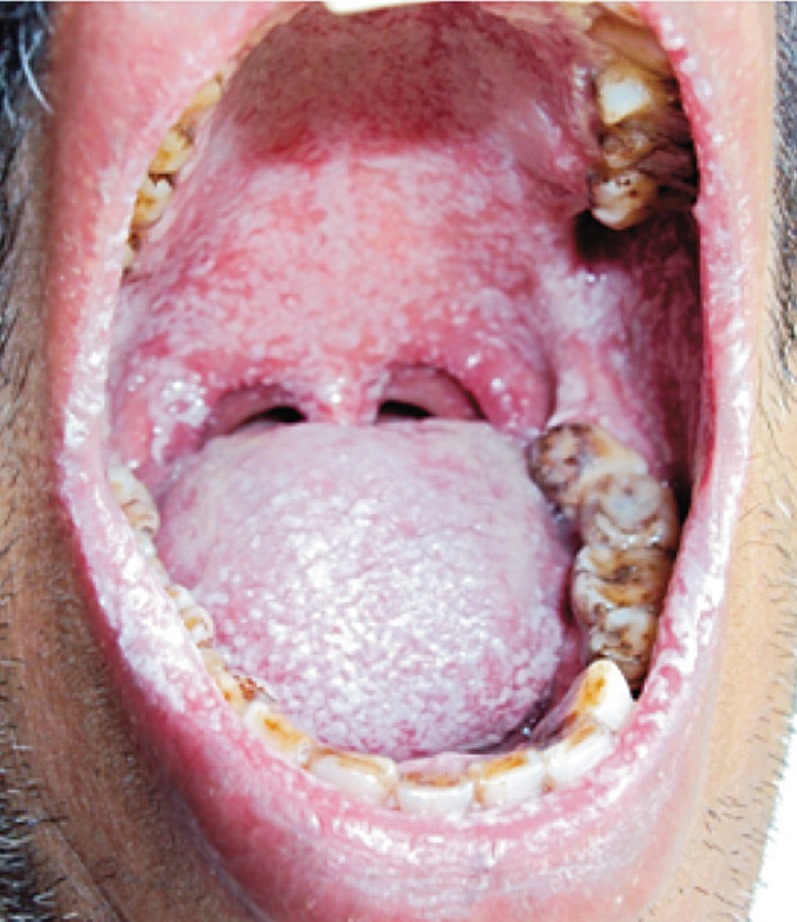
Oropharyngeal candidiasis-diffuse lesions.
Of the HIV-seropositive group, a significantly higher number of (41.8%, n=59) individuals were culture positive yielding 61 Candida isolates (2 cases yielded mixture of two isolates each) in comparison to the 22.7 per cent (32 isolates) culture positive individuals from control group (P<0.001). Isolates were further identified as C. albicans (n=47), C. dubliniensis (n=9), C. parapsilosis (n=2), C. glabrata (n=2) and C. famata (n=1). C. dubliniensis (n=9) was the most common non-albicans Candida species isolated from HIV-seropositive group while HIV seronegative (control) group revealed highest of 11 C. dubliniensis isolates. Other species isolated from control group were C. ablicans (n=17), C. parapsilosis (n=3) and C. famata (n=1). The combinations of mixed isolates were C. albicans + C. glabrata and C. albicans + C. parapsilosis. These two cases were excluded from statistical analysis. C. dubliniensis isolates were identified by bunch of chlamydospore formation in corn meal agar, dark green colony on HiChrome Candida Differential agar, no xylose assimilation and sparse or no growth at 45°C to differentiate between these two species. The isolation rate of Candida species from the HIV-seropositive symptomatic group was 81.5 per cent (n=22) with 72.7 per cent (n=16) C. albicans and 27.3 per cent (n=6) non albicans Candida isolates. The distribution of C. albicans and non-albicans Candida isolates between HIV-seropositive and seronegative (control) groups was found to be significant (P<0.001) (Table I).
Table I.
Distribution of Candida isolates in HIV seropositive and seronegative groups
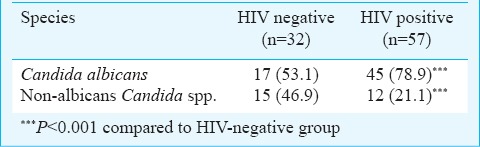
The HIV seropositive asymptomatic group (n=114) revealed Candida colonization of 30.7 per cent (n=35) which includes 82.8 per cent (n=29) C. albicans and 17.1 per cent (n=6) non albicans Candida isolates. HIV seronegative individuals (control group) revealed 22.7 per cent (n=32) colonization of Candida species, of which 53.1 per cent (n=17) were C. albicans and 46.9 per cent (n=15) non-albicans Candida species. Candida colonization in HIV-seropositive individuals was significantly (P<0.05) higher than that of the HIV seronegative control group.
C. albicans isolates (n=45) were associated with significantly higher mean CD4 cell count of 242 ± 207.8 cells/µl than 144 ±119.6 cells/µl of non-albicans Candida (n=12) isolates (P< 0.05). Candida isolation rate was similar (88.9%) in recurrent cases as well as the cases with first episode of candidiasis.
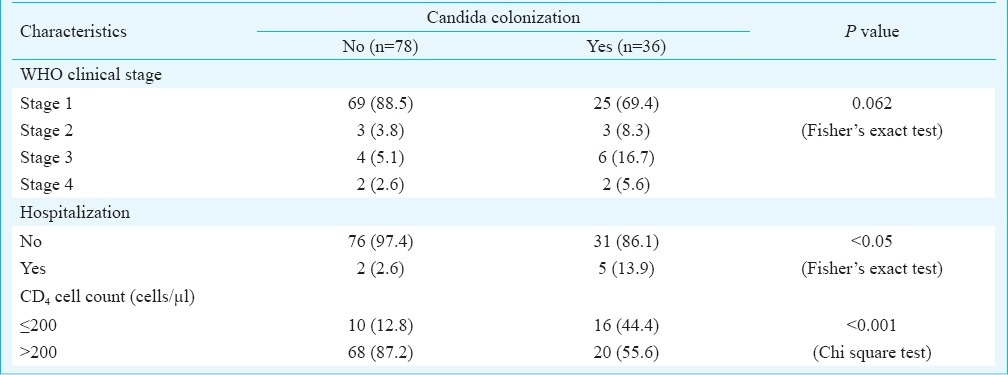
Factors associated with Candida colonization of asymptomatic HIV-seropositive individuals: Factors found to be significantly associated with candida colonization in HIV seropositive asymptomatic individuals were hospitalization (P<0.05), and CD4 cell count of ≤200 cells/µl) (P<0.001) (Table II). Factors not associated with increased rate of colonization were male respondents, literacy, employed status, marital status, urban habitation, alcohol consumption, heterosexual mode of HIV transmission and co-infection with tuberculosis.
Table II.
Characteristics among asymptomatic HIV seropositive individuals (n=114) with Candida colonization
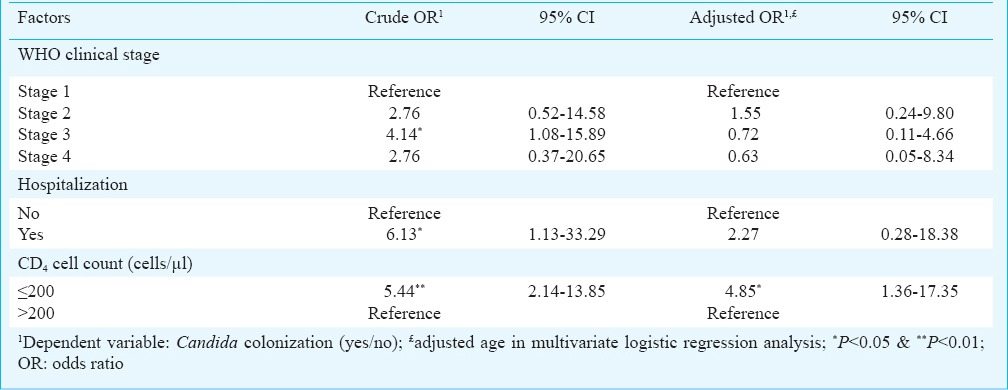
In univariate logistic regression analysis, factors found to be significantly associated with Candida colonization were WHO clinical stage 3, hospitalization and CD4 cell count ≤200 cells/µl while in multivariate analysis by adjusting the age, only CD4 cell count ≤200 cells/µl was found to be significant (Table III). Both univariate odds ratio (OR: 4.15, 95% CI, 1.98-8.69; P<0.01) and adjusted odds ratio (OR; 5.17, CI 95%, 2.31-11.55; P<0.01) of species C. albicans were found to be significantly associated with increased risk of HIV seropositivity in conditional logistic regression analysis. Though not significant, the factor non-albicans Candida species showed increased risk of HIV seropositivity in both univariate and adjusted regression analysis. Additional analysis among control group showed that the presence of tuberculosis was significantly associated with candida colonization (data not shown).
Table III.
Factor associated with positive Candida colonization among asymptomatic HIV seropositive individuals in univariate (crude OR) and multivariate logistic (adjusted OR) regression analysis
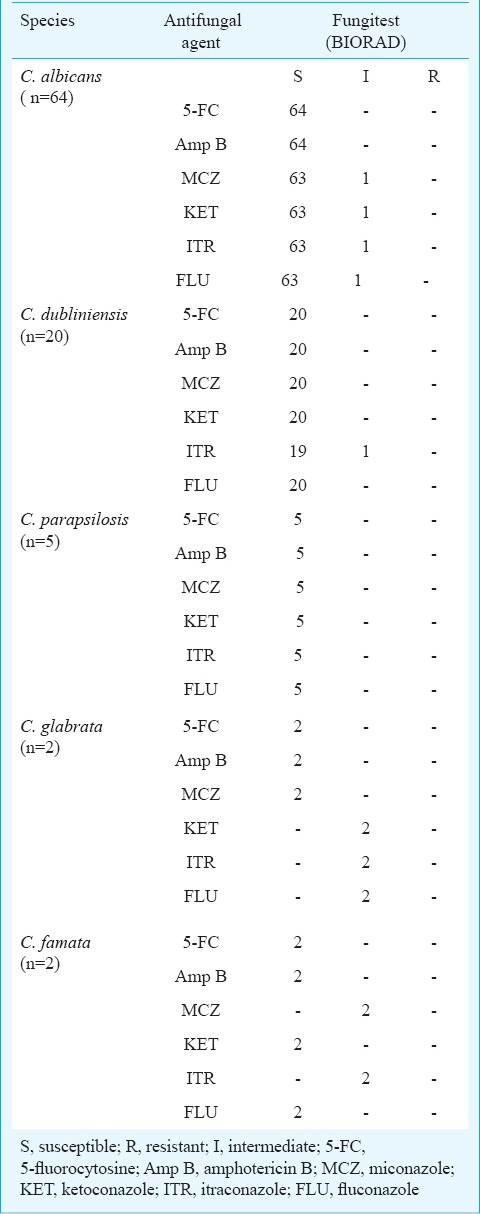
Antifungal susceptibility profile: Antifungal susceptibility testing by disk diffusion method revealed 9.3 per cent (n=6) C. albicans isolates to be resistant to each of fluconazole and voriconazole whereas Fungitest method revealed no fluconazole resistance among these isolates except one intermediate to all the azoles (Tables IV, V). Susceptibility to voriconazole could not be compared as Fungitest method lacks this antifungal agent. None of the non-albicans Candida isolates showed resistance to fluconazole and voriconazole by disk-diffusion method but some of these isolates were found intermediate to azoles by Fungitest method. All the isolates were found to be susceptible to amphotericin B and 5-fluorocytosine. None of the Candida isolates from the HIV-seropositive individuals with previous exposure to antifungal drugs showed resistance to any antifungal agents tested.
Table IV.
Antifungal susceptibility profile of Candida isolates by disk diffusion technique
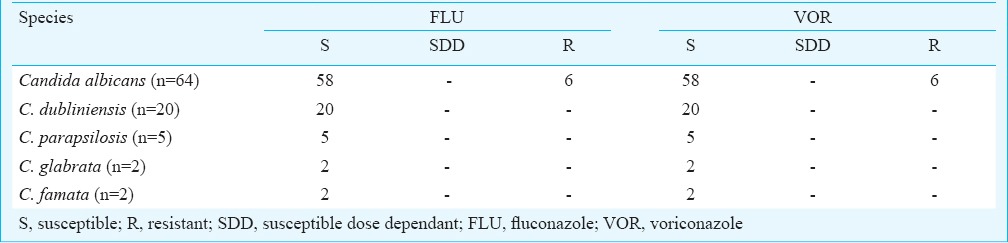
Table V.
Antifungal susceptibility profile of Candida isolates by Fungitest
Discussion
The present study showed that oral/oropharyngeal candidiasis was a common manifestation of declining immune system in HIV-seropositive individuals. Mean CD4 cell count of patients with oral/oropharyngeal candidiasis in the present study was 117 cells/μl that correlated well with previous studies from other parts of India15,16,17 However, quantitative estimation of yeast carriage and phenotypic changes over time will be a better predictor of level of immunosuppression in HIV seropositive individuals. Finding of pseudomembranous candidiasis as the commonest type of lesion in the present study was consistent with earlier findings4,15.
The colonization was significantly higher in HIV-seropositive group than that of control group as has been reported earlier3,16,17. Our study revealed C. albicans as the predominant species in both the seropositive as well as seronegative groups as reported in previous studies5,6. There were some differences in the spectrum of non-albicans Candida species and the percentage of recovered isolates observed in studies from different parts of India. A higher number of C. dubliniensis isolates in our study indicates a need of further study in this geographical region. Some of the previous studies reported C. tropicalis or C. krusei as the predominant non-albicans Candida species5,6 though Nadagir et al18 reported C. dubliniensis as the predominant non-albicans species in their study. As C. dubliniensis is phenotypically related to C. albicans, there may be the possibility of missing this non-albicans species in most of the laboratories. Emergence of C. dubliniensis in HIV-seropositive individual is a cause of concern because of its ability to develop drug resistance to commonly used antifungal, fluconazole that may lead to increased morbidity and mortality in HIV-infected individuals with oro-pharyngeal candidiasis19,20. However, none of the C. dubliniensis isolates in our study was found to be resistant to fluconazole. Also no azole resistance was found in C. glabrata and C. famata isolates in the present study which was in contrast to the findings from Mane et al3 (50% resistant to azoles).
Our study had some limitations. Molecular methods would have been useful for confirmation of the species identified phenotypically. Further, lesser number of non-albicans Candida species isolates limited the scope of this study in predicting their susceptibility pattern in this geographical area.
In conclusion, C. albicans was the commonest Candida species infecting or colonizing HIV- seropositive individuals and C. dubliniensis, the most common non-albicans Candida species isolate. In view of increased level of immunosuppression due to HIV seropositivity and drug resistance being more commonly associated with non-albicans Candida species; it becomes important to identify the Candida up to species level in different geographical areas of the country with their antifungal susceptibility pattern.
Acknowledgment
The authors acknowledge Department of Biotechnology Nodal cell, Tezpur University, Napaam, Tezpur, Assam, for financial support. The authors thank Dr Vicky Lahkar, Shri Abidur Rahman, Counsellor ICTC and Shri Rajesh Sonar, laboratory technician for their assistance.
Footnotes
Conflicts of Interest: None.
References
- 1.Hamza OJ, Matee MI, Moshi MJ, Simon EN, Mugusi F, Mikx FH. Species distribution and in vitro antifungal susceptibility of oral yeast isolates from Tanzanian HIV-infected patients with primary and recurrent oropharyngeal candidiasis. BMC Microbiol. 2008;8:1. doi: 10.1186/1471-2180-8-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gugnani HC, Becker K, Fegeler W, Basu S, Chattopadhya D, Baveja U, et al. Oropharyngeal carriage of Candida species in HIV-infected patients in India. Mycoses. 2003;46:299–306. doi: 10.1046/j.1439-0507.2003.00896.x. [DOI] [PubMed] [Google Scholar]
- 3.Mane A, Panchvalli S, Bembalkar S, Risbud A. Species distribution & antifungal susceptibility of oral Candida colonising or infecting HIV infected individuals. Indian J Med Res. 2010;131:836–8. [PubMed] [Google Scholar]
- 4.Ranganathan K, Narasimhan P, Gunaseelan R, Solomon S, Samaranayake LP. Oral Candida spp. In healthy and HIV infected subjects in Chennai. Trop Med Health. 2008;3:101. [Google Scholar]
- 5.Jagder M, Arora U. Isolation, characterization and antifungal susceptibility pattern of Candida species causing oropharyngeal candidiasis in HIV positive patients. J Commun Dis. 2008;40:177–81. [PubMed] [Google Scholar]
- 6.Baradkar VP, Kumar S. Species identification of Candida isolates obtained from oral lesions of HIV infected patients. Indian J Dermatol. 2009;54:385–6. doi: 10.4103/0019-5154.57622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children. [accessed on February 22, 2011]. Available from: http://www.who.int/hiv/pub/guidelines/hivstaging150307.pdf .
- 8.National AIDS Control Organization. Operational guidelines for integrated counselling and Testing centres; Annexure II: testing algorithm. [accessed on February 21, 2011]. Available from: http://naco.gov.in/upload/Policies%20&%20Guidelines/20,%20Operational%20Guidelines%20for%20Integrated%20Counseling%20and%20Testing%20Centres.pdf .
- 9.Chander J. Candidiasis. In: Chander J, editor. Text book of medical mycology. 3rd ed. New Delhi: Mehta Offset Pvt Ltd; 2009. pp. 266–90. [Google Scholar]
- 10.Chakrabarty A, Shivprakash MR. Medical mycology laboratory procedure. Chandigarh: Postgraduate Institute of Medical Education & Research; 2005. (modified in 2008) [Google Scholar]
- 11.Baradkar VP, Mathur M, Kumar S. HiCrome Candida agar for identification of Candida species. Indian J Pathol Microbiol. 2010;53:93–5. doi: 10.4103/0377-4929.59192. [DOI] [PubMed] [Google Scholar]
- 12.CLSI document M44-A2. 2nd ed. Wayne, PA: CLSI; 2009. Clinical and Laboratory Standards Institute (CLSI). Method for antifungal disk diffusion susceptibility testing of yeasts; Approved guideline. [Google Scholar]
- 13.Wayne, PA: CLSI; 2009. Clinical and Laboratory Standards Institute (CLSI). Zone diameter interpretive standards, corresponding minimal inhibitory concentration (MIC) interpretive breakpoints, and quality control limits for antifungal disk diffusion susceptibility testing of yeasts, Third International Supplement CLSI document - M44-S3. [Google Scholar]
- 14.Determination of the sensitivity of yeasts to antifungal agents. [accessed on February 20, 2011]. Available from: www.bio-rad.com/60780_fungitest_product_insert/pdf .
- 15.Ranganathan K, Umadevi M, Saraswathi TR, Kumarasamy N, Solomon S, Johnson N. Oral lesions and conditions associated with human immunodeficiency virus infection in 1000 South Indian patients. Ann Acad Med Singapore. 2004;33(Suppl):37S–42S. [PubMed] [Google Scholar]
- 16.Ananthalakshmi R, Murali S, Sekar B. Association of asymptomatic oral candidal carriage, oral candidiasis with CD4 lymphocyte count in HIV/AIDS patients. J Indian Acad Dent Spec. 2011;2:6–10. [Google Scholar]
- 17.Jain PA, Kulkarni RD, Ajantha GS, Shubhada C. A comparative evaluation of oral candida carriage in HIV-infected individuals and HIV seronegative healthy individuals in North Karnataka. J Biosci Tech. 2011;2:232–7. [Google Scholar]
- 18.Nadagir SD, Chunchanur SK, Halesh LH, Yasmeen K, Chandrasekhar MR, Patil BS. Significance of isolation and drug susceptibility testing of non- Candida albicans spp causing oropharyngeal candidiasis in HIV patients. Southeast Asian J Trop Med Public Health. 2008;39:492–5. [PubMed] [Google Scholar]
- 19.Hazen KC, Baron EJ, Colombo AL, Girmenia C, Sanchez-Sousa A, del Palacio A, et al. Global Antifungal Surveillance Group. Comparison of the susceptibilities of Candida spp. To fluconazole and voriconazole in a 4-year global evaluation using disk diffusion. J Clin Microbiol. 2003;41:5623–32. doi: 10.1128/JCM.41.12.5623-5632.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quindós G, Carrillo-Muñoz AJ, Arévalo MP, Salgado J, Alonso-Vargas RI, Rodrigo JE, et al. In vitro susceptibility of Candida dubliniensis to current and new antifungal agents. Chemotherapy. 2000;46:395–401. doi: 10.1159/000007320. [DOI] [PubMed] [Google Scholar]


