Abstract
Purpose
Tissue reservoirs of HIV may promote the persistent immunopathology responsible for non-AIDS morbidity and data support multifocal reactivation from tissues as the source of viral rebound during ART interruption. The heterogeneity of tissue reservoirs and incomplete knowledge about their composition are obstacles to an HIV cure.
Recent findings
In addition to the higher concentration of infected CD4+ T cells found in both central lymphoid tissues and gut, specific subsets of CD4+ T cells appear to play a disproportionate role in HIV persistence. Recently, a subset of central memory T cells enriched in lymphnode germinal centers called T-follicular helper cells have been identified that express more viral RNA and occupy an anatomic niche inaccessible to CTL killing. Additional observations suggest that ARV concentrations may be lower in some tissues raising the possibility for localized, low-level viral replication. Finally, some recent data implicate the persistence of infected, non-CD4+ T cell types in tissues during ART.
Summary
The retention of infected cells in a wide variety of tissues, often with distinct viral and cellular characteristics, underscores the importance of studying tissue reservoirs in the development and assessment of cure strategies. Both inhibitory ARVs and latency reversing drugs must reach these sites and novel strategies may be needed to attack virus in cells as variable as Tfh and macrophages.
Keywords: HIV, anatomic reservoirs, viral persistence
Introduction
Where and how much HIV persists in the body are central questions when developing strategies to eradicate HIV and for understanding the factors contributing to the persistent immune dysfunction thought to be responsible for non-AIDS clinical morbidities despite combination antiretroviral therapy (ART)[1]. While cells from the peripheral blood are easily accessed for study and have provided many basic insights into HIV pathogenesis with and without ART, it is important to consider that the circulating CD4+ T cells comprise <2% of total-body CD4+ T cell numbers[2,3]. Whether residual viral replication persists in the setting of combination ART that suppresses plasma virus levels to <20-50copies/ml remains controversial but if occurring is likely to involve tissue reservoirs[4,5]. Tissue sanctuaries of low ARV penetration might permit limited viral propagation at levels insufficient to allow the emergence of drug resistance or other detectable sequence change[6,7]. In addition, some tissues act as immune privileged sites that could theoretically prevent effective immune recognition, resulting in delayed immune clearance of infected cells[8,9]. Finally, although many if not all tissues harbor some CD4+ T cells, the distribution of T cell subsets and other infectable cell types including macrophages and possibly other cell types will vary among tissues[10-12]. Each of these factors may contribute to the complexity of HIV reservoirs across organs and tissues in HIV infected persons on ART.
We use the definition of HIV tissue reservoir as a tissue or organ containing cells that continue to harbor HIV in the setting of combination ART, regardless of the mechanism maintaining them (cell quiescence and stability, cell proliferation, low-level viral replication). Most studies of HIV persistence in tissues rely on the detection of viral nucleic acids and occasionally viral antigen but don't distinguish between replication competent and defective virus or viral remnants incapable of producing viral rebound. The presence of a large excess of genetically defective proviruses has been observed in cells in blood[13,14] and in brain[15] but likely applies to all tissues. Nevertheless, “replication incompetent” viral reservoirs might still contribute to pathogenesis if they were able to support abortive viral expression (either viral RNA or antigen) that could elicit inflammatory or immune responses as encountered with other viral infections[16,17]. We review observations concerning tissue reservoirs by organ system with particular attention to the nature of the infected cell types, evidence for viral compartmentalization if any, and evidence for differences in ARV concentrations. We conclude with a discussion of some implications and unanswered questions in the field.
Lymph Nodes and Spleen
Central lymphoid tissues are a primary site for viral replication and contain massive numbers of infected cells and free virions captured on the follicular dendritic cell network[18,19]. Although potent ART results in an exponential 3-log decrease in HIV-RNA in lymph node (LN), with a clearance half-life only slightly longer than the blood (6 vs. 1.9 days)[20,21], HIV-RNA and DNA can still be detected in the LN after years of ART[22-24].
One study also detected abundant amounts of HIV p24, p17, and gp120/gp41 in the germinal centers of LN after 5-13 months of suppressive ART, although HIV-RNA was not detected in this study[25]. Several studies of ART-treated macaques have demonstrated that viral DNA and/or RNA levels are highest in LN and spleen[26-28]. In one study, SIV-RNA levels in LN and spleen decreased much less than in plasma and gut, which was attributed to lower drug levels in these secondary lymphoid tissues[7]. Studies from humans also suggest that levels of some ARVs may be lower in LN[6,29].
There are conflicting data on whether virus found in LN is compartmentalized and genetically separate from that in blood. Some studies report differences between LN and blood in viral sequences and drug resistance mutational patterns[24,30], while other studies have shown that HIV sequences in lymphoid tissues are similar to blood[31,32]. Additional, unanswered questions concern whether regional differences exist among LN for infected cell content, infected cell viral expression or likelihood of compartmentalized virus with distinct genetic attributes.
Of note, while the subset of CD4+ T cells bearing markers of central and transitional memory maturation phenotypes comprise the largest proportion of the reservoir of infected CD4+ T cells in peripheral blood from patients on ART[10,33], some surveys suggest a greater contribution of CD4+ T cells with an effector memory phenotype in lymph nodes compared to blood[34]. Furthermore, several recent studies in SIV/macaques and patients on ART identify a memory subset, the CD4+ T-follicular helper cells (Tfh) in LN follicles bearing CXCR5 and PD-1 as highly enriched for replication competent virus and viral RNA[35,36]. The survival of infected Tfh has been attributed to the paucity of virus-specific CTL in LN follicles and to the expression of anti-apoptotic BCL2 by Tfh in the setting of chronic HIV/SIV infection [37-39]. Recently, preferential retention of HIV in cells in peripheral blood with Tfh markers has also been described[40].
Bone Marrow
HIV can infect various cell types in bone marrow, and HIV-DNA can be detected in bone marrow obtained from on-ART individuals. It remains unresolved whether hematopoietic progenitor cells constitute a reservoir in vivo[41]. In ART-suppressed individuals, two studies did not detect HIV-DNA in CD34+ progenitor cells[42,43], while a third found HIV-DNA in CD133+ bone marrow progenitor cells from 6/11 patients[44]. Bone marrow mast cell progenitors can be latently infected with HIV, and one study detected infected mast cells in some tissues of treated patients[45], although another study found no evidence of infected mast cells in multiple organs[46].
Thymus
A variety of cell types found in thymus of humans and non-human primates (NHP) support HIV and SIV infection and can be demonstrated in vivo in the absence of treatment. However, little evidence is available demonstrating persistence of HIV or SIV infection in the thymus of individuals on suppressive ART. In one study of ARV treated macaques, neither SIV-DNA nor replication-competent virus was detected in the thymus, while SIV-DNA was detected in spleen and LN, and infectious virus was isolated from LN[47]. In another study of SHIV-infected macaques on suppressive ART, Gag RNA was detected in the thymus in only 1 of 6 animals, and multiply-spliced HIV-RNA was not detected in the thymus[48].
Liver
Studies in untreated individuals have shown that HIV can infect Kupffer cells and T cells in the liver. In humans, one study detected HIV in the liver of some patients who died of AIDS[32], while a second detected HIV-RNA in 9/16 HIV+ participants[49]; the latter study also found evidence of compartmentalization between plasma and liver. In rhesus monkeys infected with SHIV or SIVmac251, SHIV-RNA can be detected in liver macrophages[50] and SIV protein as well as lentiviral particles can be detected in hepatic Kupffer cells and lymphocytes. A recent study of two patients on suppressive ART, observed ex vivo viral production from highly purified liver Kupffer cells that could be passaged to lymphoblasts[51].
Gastrointestinal Tract
The gut is among the earliest targets of HIV infection and one of the organs with highest numbers of infected cells, even in on-ART individuals. The gut contains a large proportion of the lymphoid tissue (up to 85%) and lymphocytes (up to 90%) in the body[52,53]. Primary gut mucosal CD4+T cells show increased susceptibility to in vitro infection with HIV[54,55] and support higher levels of viral replication[55,56] attributed to greater CCR5 expression[54,56] and T cell activation[54].
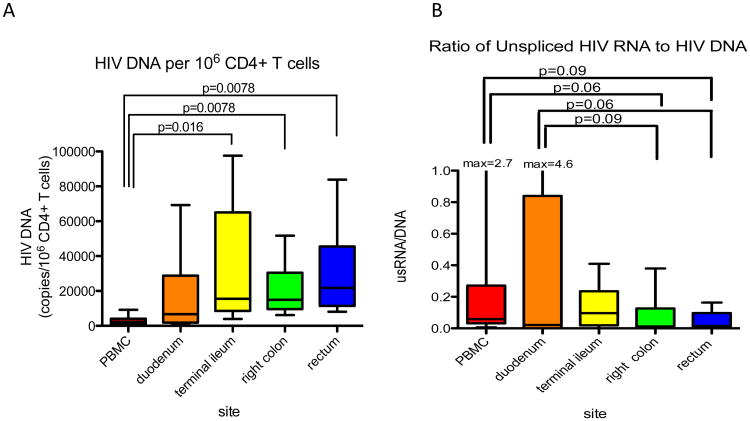
In ART-suppressed patients, HIV-DNA and RNA have been detected in gut CD4+T cells and non-CD4+T cells[10,57,58], including CD13+ myeloid cells[11]; HIV-DNA and p24 have also been detected in duodenal macrophages[59]. Another study found that early initiation of ART resulted in comparable reduction in HIV-RNA in blood and rectum at 6 months[60]. Poles et al showed that HIV-RNA and DNA levels in rectal biopsies appeared stable over one year of ART with gut HIV-DNA+ cells twice that in blood[61]. Chun et al reported that HIV-DNA levels per million CD4+T cells were on average 5-6 times higher in the ileum compared to blood in patients on up to 10 years of ART[62]. A subsequent study of four different regions of the gastrointestinal tract determined that levels of HIV-DNA and unspliced HIV-RNA per infected CD4+ T cell were higher (up to 12 fold) in all four gut sites compared to blood (Figure 1); differences in average transcription and the relation to immune activation suggested that different mechanisms control HIV persistence in blood and gut[63]. Based on the average level of HIV-DNA across the four gut sites, this study estimated that the gut harbors 1.2×109 infected CD4+ T cells reflecting 83-95% of all HIV-infected cells in the body. Another study of 1-2 drug ART intensification suggested that the ileum, but not blood or other gut sites, might be a site of ongoing replication in some patients on ART[4]. Finally, a recent study of RT-SHIV-infected, ART-suppressed macaques showed that gut tissues had the highest levels of multiply-spliced HIV-RNA and the ratio of multiply-spliced to Gag RNA, while Gag RNA tended to be highest in the mesenteric lymph nodes[48]. Some investigators have reported decreased penetration of certain antiretrovirals into some sites in gut[29], and both evidence for[64-66] and against[62,67,68] compartmentalization between virus in the gut and blood have been described.
Figure 1.
A) HIV DNA copies per million CD4 cells present in blood or different gut sites from 8 patients on ART with undetectable plasma virus. B) Ratio of HIV RNA (unspliced, genomic) to HIV DNA providing measures of HIV expression per average infected cell. Horizontal bars = medians; whiskers show standard deviations. Adapted from Yukl et al JID 2010[61]
Nervous System
Neurologic impairment is a long-recognized complication of HIV infection[69] and infectious HIV can be cultured from cerebrospinal fluid (CSF) and brains of HIV+persons[70,71]. HIV infects several cell types in the brain and nervous system, and multiple studies also suggest compartmentalized CNS virus[72-79]. In vitro infection of astrocytes[80,81] and fetal neural cells[82] and productive infection of fetal glial cells[83], human brain microglia[84], and human brain capillary endothelial cells[85] have each been demonstrated. Both nonproductive[86], and (transiently) productive infection of astrocytes and astroglial cells have been described including transient productive infection followed by viral latency that is reversible[81]. ARVs penetrate the CNS to different degrees and efforts have been made to categorize ARVs according to CNS activity[87].
HIV-DNA and RNA have also been detected in the brain of ART-treated individuals primarily localized to perivascular macrophages and microglial cells and occasionally to astrocytes. Several studies found that ART reduces CSF HIV-RNA to or near the limit of detection for most patients, although viral decay in CSF was slower than that in plasma in some cases[88,89]. However, HIV-RNA has been detected in the CSF of patients on suppressive ART[90-92], and both HIV-DNA[93] and HIV-RNA[91,94] have been detected in brain tissue obtained at autopsy from individuals on ART although whether these individuals remained on ARVs at the time of death cannot always be confirmed. In two of these studies, viruses from brain[93] and CSF[92] contained drug resistance mutations not found in the blood, further suggesting compartmentalization in the nervous system. Finally, HIV persistence in the brain is suggested by several small case series of patients presenting with new neurologic symptoms (including encephalitis) associated with rebound of CSF virus despite up to 8 years of suppressive ART[95,96].
Lung
HIV-DNA and RNA have been detected in lung cells from both untreated and on-ART individuals, and limited evidence suggests compartmentalization from blood. In untreated individuals, HIV has been detected in lung CD8+T cells[97] and macrophages obtained by bronchoalveolar lavage [98], and infection of alveolar macrophages is associated with impaired phagocytic function[12,99]. In one autopsy study of AIDS patients, HIV-DNA was detected in the lung tissues and Env sequences showed compartmentalization from blood[32]. HIV-DNA and RNA have also been detected in alveolar macrophages from some ART-treated patients[12].
Kidney and Urine
HIV-infected cells can be found in the kidney, and limited evidence suggests compartmentalization as well as persistence on ART. HIV-DNA Env sequences from infected renal epithelial cells showed clustering from viral sequences in the blood[100], and in one case, HIV-RNA was detected in tubular epithelial cells and glomerular podocytes after 3 months of suppressive ART[101]. In another study, HIV-DNA was detected in the urine of 23% of aviremic on-ART patients[102]. Finally, HIV nucleic acids and viral particles were detected in podocytes and tubular cells of renal biopsies of 19 post-renal transplant ARV-treated patients, and HIV-DNA and RNA were detected in the urine[103].
Male Reproductive Tract
HIV has been reported to infect a variety of cell-types in the male reproductive tract[104-112], and some but not all studies suggest viral compartmentalization. HIV antibody levels are lower in semen compared to blood[113], and testicular SIV-specific CD8+ T cells have reduced cytokine responses to mitogens[114], compatible with testis as an immune-privileged site.
In humans and primates on suppressive ART, several studies have demonstrated that HIV and/or SIV can occasionally be detected in genital tissues and secretions. HIV-RNA can be detected in the semen in 2-48% of on-ART, aviremic men[115-119], sometimes in association with drug resistance mutations[115]. Similarly, a subset of ART-treated, SIV-infected macaques continued to shed SIV in semen despite suppression in the plasma[120] with greater reduction of SIV-RNA in prostate and vas deferens, smaller decreases in epididymis and seminal vesicle but no change in urethra due to SIV+ macrophages. Multiple studies implicate a local source of seminal plasma virus showing compartmentalization from blood[32,121-123], suggesting a distinct reservoir. In contrast, one study in macaques showed that SIV-RNA sequences were evenly distributed among blood, LN, and genital tract[124].
Female Reproductive Tract
HIV can be detected in female cervical cells[125] and genital secretions[126] of most untreated patients. In addition, HIV can be detected in genital secretions of 8-87.5%women on suppressive ART[126-130], suggesting that some women may have a separate reservoir of HIV in the genital tract, perhaps due to reduced penetration of some antiretrovirals[131,132]. Compartmentalization of viral populations between blood and female genital organs has also been reported[133-137].
Skin and Adipose Tissue
In untreated HIV+ individuals, spliced HIV-RNA[138], HIV antigens, and viral particles[139] have been detected in epidermal Langerhans cells, though one study found equivocal evidence for HIV in these cells[140]. HIV-DNA has been detected in the epidermis[141] and dermis, and virus has been cultured from skin[139]. Recent studies also detected HIV/SIV-DNA/RNA in CD4+T cells from adipose tissue of ART-treated individuals and[142,143] with consequences for inflammation.
Conclusions
The persistence of virus or viral remnants in nearly all tissues studied (Table 1)[144] has important implications for viral pathogenesis and for cure. Given the diversity of cell types and anatomic environments where HIV reservoirs simultaneously persist, it is questionable whether single interventions and modalities will suffice to eliminate all possible viral reservoirs. Available data still support attempts to target latently-infected, resting memory CD4+ T cells but additional strategies may be needed to address specific cell types such as Tfh and macrophages found in tissues. Key too for the accurate assessment of curative interventions are better approaches to sample accessible tissue reservoirs complemented by NHP and other animal studies that permit more comprehensive analyses. The availability of biomarkers, for example measures of virus-specific antibody titers, as an indirect measure of whole body levels of residual virus [145,146] or novel in vivo imaging to assess anatomic reservoirs deserve continued development [2,147]. Consideration of both ARV and latency reversing drug delivery to different anatomic sites should be a priority. Finally, we remain ignorant about how specific tissue reservoirs in the body contribute to immunopathology and non-AIDS morbidity. Better ways to assess the activity (intermittent production of RNA, antigen or virus; low level residual replication) and the local immune and inflammatory responses to the presence of viral products are needed so that measures to reduce residual viral burden and virus activity [148,149] can be pursued in parallel with strategies aimed at eradication.
Table 1. Tissues with detectable HIV or SIV during ART with suppression of plasma virus.
| Tissue | HIV-DNA | HIV-RNA | Protein/Antigen | Virus (EM) | Infectious Virus | Compartmentalization from blood |
|---|---|---|---|---|---|---|
| Lymph nodes | X | X | X | X | Some but not all studies | |
| Spleen | X (SHIV, SIV) | X (SHIV, SIV) | No | |||
| Bone marrow | X | |||||
| Thymus | +/- (1 of 6 SHIV-infected macaques) | Some evidence | ||||
| Liver | X (macrophages) | X | Yes (one study) | |||
| Gut | X | X | X (macrophages) | Some but not other studies | ||
| Nervous system | X | X (brain, CSF) | Yes (multiple reports) | |||
| Lung | X | X | Yes (one study) | |||
| Heart | ||||||
| Kidney | X | X (3mo ART) | X | Yes (one study) | ||
| Male reproductive tract | X (SIV) | X (SIV; human genital secretions) | Most but not all studies | |||
| Female reproductive tract | X (genital secretions) | X (genital secretions) | Most but not all studies | |||
| Placenta | X (mast cells) | X (mast cells) | ||||
| Breast and breast milk | X | X | X | No | ||
| Skin | ||||||
| Adipose tissue | X | X |
Adapted from Yukl S and Wong J, Anatomic compartments as a barrier to HIV cure, 2015 [143]
Key Points.
In patients on ART without detectable plasma viremia, HIV infected cells can still be found in nearly all tissues but particularly in central and mucosal lymphoid tissues.
CD4+ T cell subsets that comprise HIV reservoirs in persons on ART differ in their distribution at different anatomic sites with particularly high contributions of central and transitional memory cells in blood, effector memory in GALT
While each major maturation subset of CD4+ T cells contributes to the reservoir of HIV DNA+ cells in lymph nodes, a subset of central memory cells, the T follicular helper cell expresses much higher levels of HIV RNA and may have particular importance in viral persistence
Some tissues, including some gut sites and lymph node regions where infected cell frequencies seem to be highest, have recently been noted to be associated with limited ARV penetration
Non-CD4+ T cell types that harbor HIV DNA including macrophages, microglial cells and astrocytes, though far fewer in number, persist in a variety of organs and tissues and are important because they may require different approaches to purge and for their potential contribution to local immunopathology.
Acknowledgments
We would like to thank our colleagues Harry Lampiris, Hiroyu Hatano, Peilin Li, Philipp Kaiser, Katsuya Fujimoto, Satish Pillai, Davey Smith, Matthew Strain, Teri Liegler, Kersten Koelsch, Satya Dandekar, Steve Deeks, Marek Fischer, Diane Havlir, Douglas Richman, Huldrych Guenthard and especially the many participants in the research studies that have advanced HIV treatment and our understanding of HIV pathogenesis.
Financial support and sponsorship: This work was supported by the Department of Veterans Affairs (JKW 5101 BX001048, SAY IK2 CX000520); NIH (JKW R56AI091573, R21AI116218; SY, JW U19AI096109); GIVI/UCSF Center for AIDS Research Virology Core P30AI027763 (JW) and American Foundation for AIDS Research (JW, SY)
Footnotes
Conflicts of interest: None
References
- 1.Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet. 2013;382:1525–1533. doi: 10.1016/S0140-6736(13)61809-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Mascio M, Paik CH, Carrasquillo JA, Maeng JS, Jang BS, Shin IS, Srinivasula S, Byrum R, Neria A, Kopp W, et al. Noninvasive in vivo imaging of CD4 cells in simian-human immunodeficiency virus (SHIV)-infected nonhuman primates. Blood. 2009;114:328–337. doi: 10.1182/blood-2008-12-192203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Westermann J, Pabst R. Distribution of lymphocyte subsets and natural killer cells in the human body. Clin Investig. 1992;70:539–544. doi: 10.1007/BF00184787. [DOI] [PubMed] [Google Scholar]
- 4.Yukl SA, Shergill AK, McQuaid K, Gianella S, Lampiris H, Hare CB, Pandori M, Sinclair E, Gunthard HF, Fischer M, et al. Effect of raltegravir-containing intensification on HIV burden and T-cell activation in multiple gut sites of HIV-positive adults on suppressive antiretroviral therapy. Aids. 2010;24:2451–2460. doi: 10.1097/QAD.0b013e32833ef7bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5*.Lorenzo-Redondo R, Fryer HR, Bedford T, Kim EY, Archer J, Kosakovsky Pond SL, Chung YS, Penugonda S, Chipman JG, Fletcher CV, et al. Persistent HIV-1 replication maintains the tissue reservoir during therapy. Nature. 2016;530:51–56. doi: 10.1038/nature16933. Study using phylodynamic modeling of genetic sequences reveals evidence for ongoing viral replication localized to lymphoid tissues in patients receiving potent antiretroviral therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6**.Fletcher CV, Staskus K, Wietgrefe SW, Rothenberger M, Reilly C, Chipman JG, Beilman GJ, Khoruts A, Thorkelson A, Schmidt TE, et al. Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proc Natl Acad Sci U S A. 2014;111:2307–2312. doi: 10.1073/pnas.1318249111. ARV levels are appreciably lower in lymph node and appear to correlate with increased expression/persistence of HIV RNA that is compatible with ongoing replication in pharmacologic sanctuaries. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bourry O, Mannioui A, Sellier P, Roucairol C, Durand-Gasselin L, Dereuddre-Bosquet N, Benech H, Roques P, Le Grand R. Effect of a short-term HAART on SIV load in macaque tissues is dependent on time of initiation and antiviral diffusion. Retrovirology. 2010;7:78. doi: 10.1186/1742-4690-7-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Louveau A, Harris TH, Kipnis J. Revisiting the Mechanisms of CNS Immune Privilege. Trends Immunol. 2015;36:569–577. doi: 10.1016/j.it.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao S, Zhu W, Xue S, Han D. Testicular defense systems: immune privilege and innate immunity. Cell Mol Immunol. 2014;11:428–437. doi: 10.1038/cmi.2014.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yukl SA, Shergill AK, Ho T, Killian M, Girling V, Epling L, Li P, Wong LK, Crouch P, Deeks SG, et al. The distribution of HIV DNA and RNA in cell subsets differs in gut and blood of HIV-positive patients on ART: implications for viral persistence. J Infect Dis. 2013;208:1212–1220. doi: 10.1093/infdis/jit308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yukl SA, Sinclair E, Somsouk M, Hunt PW, Epling L, Killian M, Girling V, Li P, Havlir DV, Deeks SG, et al. A comparison of methods for measuring rectal HIV levels suggests that HIV DNA resides in cells other than CD4+ T cells, including myeloid cells. AIDS. 2014;28:439–442. doi: 10.1097/QAD.0000000000000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cribbs SK, Lennox J, Caliendo AM, Brown LA, Guidot DM. Healthy HIV-1-infected individuals on highly active antiretroviral therapy harbor HIV-1 in their alveolar macrophages. AIDS Research and Human Retroviruses. 2015;31:64–70. doi: 10.1089/aid.2014.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanchez G, Xu X, Chermann JC, Hirsch I. Accumulation of defective viral genomes in peripheral blood mononuclear cells fo HIV infected individuals. Journal of Virology. 1997;71:2233–2240. doi: 10.1128/jvi.71.3.2233-2240.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho YC, Shan L, Hosmane NN, Wang J, Laskey SB, Rosenbloom DI, Lai J, Blankson JN, Siliciano JD, Siliciano RF. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell. 2013;155:540–551. doi: 10.1016/j.cell.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y, Kappes JC, Conway JA, Price RW, Shaw GM, Hahn BH. Molecular characterization of human immunodeficiency virus type 1 cloned directly from uncultured human brain tissue: identification of replication-competent and -defective viral genomes. Journal of Virology. 1991;65:3973–3985. doi: 10.1128/jvi.65.8.3973-3985.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mercado-Lopez X, Cotter CR, Kim WK, Sun Y, Munoz L, Tapia K, Lopez CB. Highly immunostimulatory RNA derived from a Sendai virus defective viral genome. Vaccine. 2013;31:5713–5721. doi: 10.1016/j.vaccine.2013.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17*.Lopez CB. Defective viral genomes: critical danger signals of viral infections. J Virol. 2014;88:8720–8723. doi: 10.1128/JVI.00707-14. Review of data showing that in the case of viral RNA detection by the pathogen pattern recognition receptor, RIG-I, defective viral forms may be more immunostimulatory than wild type virus. This has implications for how we interpret the presence of non-replication competent, defective HIV genomes and their contribution to immunopathology during ART. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Embretson J, Zupancic M, Ribas JL, Burke A, Racz P, Tenner-Racz K, Haase AT. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature. 1993;363:359–362. doi: 10.1038/362359a0. [DOI] [PubMed] [Google Scholar]
- 19.Pantaleo G, Graziosi C, Demarest JF, Butini L, Montroni M, Fox CH, Orenstein JM, Kotler DP, Fauci AS. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 1993;362:355–358. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- 20.Lafeuillade A, Poggi C, Profizi N, Tamalet C, Costes O. Human immunodeficiency virus type 1 kinetics in lymph nodes compared with plasma. The Journal of infectious diseases. 1996;174:404–407. doi: 10.1093/infdis/174.2.404. [DOI] [PubMed] [Google Scholar]
- 21.Cavert W, Notermans DW, Staskus K, Wietgrefe S, Zupancic M, Gebhard K, Henry K, Zhang ZQ, Mills R, McDade H, et al. Kinetics of Response in lymphoid tissues to antiretroviral therapy of HIV-1 infection. Science. 1997;276:960–964. doi: 10.1126/science.276.5314.960. [DOI] [PubMed] [Google Scholar]
- 22.Wong JK, Gunthard HF, Havlir DV, Zhang ZQ, Haase AT, Ignacio CC, Kwok S, Emini E, Richman DD. Reduction of HIV-1 in blood and lymph nodes following potent antiretroviral therapy and the virologic correlates of treatment failure. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:12574–12579. doi: 10.1073/pnas.94.23.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lafeuillade A, Poggi C, Chadapaud S, Hittinger G, Chouraqui M, Delbeke E. HIV-1 induction-maintenance at the lymph node level: the “Apollo-97” Study. Journal of Acquired Immune Deficiency Syndromes. 2001;28:154–157. doi: 10.1097/00042560-200110010-00007. [DOI] [PubMed] [Google Scholar]
- 24.Gunthard HF, Havlir DV, Fiscus S, Zhang ZQ, Eron J, Mellors J, Gulick R, Frost SD, Brown AJ, Schleif W, et al. Residual human immunodeficiency virus (HIV) Type 1 RNA and DNA in lymph nodes and HIV RNA in genital secretions and in cerebrospinal fluid after suppression of viremia for 2 years. The Journal of infectious diseases. 2001;183:1318–1327. doi: 10.1086/319864. [DOI] [PubMed] [Google Scholar]
- 25.Popovic M, Tenner-Racz K, Pelser C, Stellbrink HJ, van Lunzen J, Lewis G, Kalyanaraman VS, Gallo RC, Racz P. Persistence of HIV-1 structural proteins and glycoproteins in lymph nodes of patients under highly active antiretroviral therapy. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:14807–14812. doi: 10.1073/pnas.0506857102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.North TW, Higgins J, Deere JD, Hayes TL, Villalobos A, Adamson L, Shacklett BL, Schinazi RF, Luciw PA. Viral sanctuaries during highly active antiretroviral therapy in a nonhuman primate model for AIDS. Journal of Virology. 2010;84:2913–2922. doi: 10.1128/JVI.02356-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kline C, Ndjomou J, Franks T, Kiser R, Coalter V, Smedley J, Piatak M, Jr, Mellors JW, Lifson JD, Ambrose Z. Persistence of viral reservoirs in multiple tissues after antiretroviral therapy suppression in a macaque RT-SHIV model. PLoS One. 2013;8:e84275. doi: 10.1371/journal.pone.0084275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horiike M, Iwami S, Kodama M, Sato A, Watanabe Y, Yasui M, Ishida Y, Kobayashi T, Miura T, Igarashi T. Lymph nodes harbor viral reservoirs that cause rebound of plasma viremia in SIV-infected macaques upon cessation of combined antiretroviral therapy. Virology. 2012;423:107–118. doi: 10.1016/j.virol.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 29.Cohen J. HIV/AIDS research. Tissue says blood is misleading, confusing HIV cure efforts. Science. 2011;334:1614. doi: 10.1126/science.334.6063.1614. [DOI] [PubMed] [Google Scholar]
- 30.Haddad DN, Birch C, Middleton T, Dwyer DE, Cunningham AL, Saksena NK. Evidence for late stage compartmentalization of HIV-1 resistance mutations between lymph node and peripheral blood mononuclear cells. AIDS. 2000;14:2273–2281. doi: 10.1097/00002030-200010200-00008. [DOI] [PubMed] [Google Scholar]
- 31.Ball JK, Holmes EC, Whitwell H, Desselberger U. Genomic variation of human immunodeficiency virus type 1 (HIV-1): molecular analyses of HIV-1 in sequential blood samples and various organs obtained at autopsy. The Journal of general virology. 1994;75(Pt 4):67–79. doi: 10.1099/0022-1317-75-4-867. [DOI] [PubMed] [Google Scholar]
- 32.van't Wout AB, Ran LJ, Kuiken CL, Kootstra NA, Pals ST, Schuitemaker H. Analysis of the temporal relationship between human immunodeficiency virus type 1 quasispecies in sequential blood samples and various organs obtained at autopsy. Journal of Virology. 1998;72:488–496. doi: 10.1128/jvi.72.1.488-496.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chomont N, El-Far M, Ancuta P, Trautmann L, Procopio FA, Yassine-Diab B, Boucher G, Boulassel MR, Ghattas G, Brenchley JM, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. 2009;15:893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong JK. Characteristics of Tissues Reservoirs of HIV in vivo. Keystone Conference: Mechanisms of HIV Persistence:Implications for a cure; April 26-30, 2015; Boston, MA. 2015. [Google Scholar]
- 35*.Fukazawa Y, Lum R, Okoye AA, Park H, Matsuda K, Bae JY, Hagen SI, Shoemaker R, Deleage C, Lucero C, et al. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat Med. 2015;21:132–139. doi: 10.1038/nm.3781. Even in the face of strong CTL responses as present in NHP elite controllers, viral replication takes place in LN germinal centers involving Tfh cells due to exclusion of CTL from the B cell follicle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36**.Banga R. PD-1+ and Tfh cells represent the major source of HIV-1 replication competent virus. Conference on Retroviruses and Opportunistic Infections; Feb 23-25, 2016; Boston, MA. 2016. PD-1+ Tfh cells in LN are highly enriched for replication competent virus and represent a large majority of all cells with infectious virus in this study of 11 patients on suppressive ART. [Google Scholar]
- 37.Haas MK, Levy DN, Folkvord JM, Connick E. Distinct patterns of Bcl-2 expression occur in R5- and X4-tropic HIV-1-producing lymphoid tissue cells infected ex vivo. AIDS Res Hum Retroviruses. 2015;31:298–304. doi: 10.1089/aid.2014.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Connick E, Mattila T, Folkvord JM, Schlichtemeier R, Meditz AL, Ray MG, McCarter MD, Mawhinney S, Hage A, White C, et al. CTL fail to accumulate at sites of HIV-1 replication in lymphoid tissue. J Immunol. 2007;178:6975–6983. doi: 10.4049/jimmunol.178.11.6975. [DOI] [PubMed] [Google Scholar]
- 39.Connick E, Folkvord JM, Lind KT, Rakasz EG, Miles B, Wilson NA, Santiago ML, Schmitt K, Stephens EB, Kim HO, et al. Compartmentalization of simian immunodeficiency virus replication within secondary lymphoid tissues of rhesus macaques is linked to disease stage and inversely related to localization of virus-specific CTL. J Immunol. 2014;193:5613–5625. doi: 10.4049/jimmunol.1401161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pallikkuth S, Sharkey M, Babic DZ, Gupta S, Stone GW, Fischl MA, Stevenson M, Pahwa S. Peripheral T Follicular Helper Cells Are the Major HIV Reservoir within Central Memory CD4 T Cells in Peripheral Blood from Chronically HIV-Infected Individuals on Combination Antiretroviral Therapy. J Virol. 2015;90:2718–2728. doi: 10.1128/JVI.02883-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carter CC, Onafuwa-Nuga A, McNamara LA, Riddell Jt, Bixby D, Savona MR, Collins KL. HIV-1 infects multipotent progenitor cells causing cell death and establishing latent cellular reservoirs. Nat Med. 2010;16:446–451. doi: 10.1038/nm.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Durand CM, Ghiaur G, Siliciano JD, Rabi SA, Eisele EE, Salgado M, Shan L, Lai JF, Zhang H, Margolick J, et al. HIV-1 DNA is detected in bone marrow populations containing CD4+ T cells but is not found in purified CD34+ hematopoietic progenitor cells in most patients on antiretroviral therapy. The Journal of infectious diseases. 2012;205:1014–1018. doi: 10.1093/infdis/jir884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Josefsson L, Eriksson S, Sinclair E, Ho T, Killian M, Epling L, Shao W, Lewis B, Bacchetti P, Loeb L, et al. Hematopoietic precursor cells isolated from patients on long-term suppressive HIV therapy did not contain HIV-1 DNA. The Journal of infectious diseases. 2012;206:28–34. doi: 10.1093/infdis/jis301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McNamara LA, Onafuwa-Nuga A, Sebastian NT, Riddell Jt, Bixby D, Collins KL. CD133+ hematopoietic progenitor cells harbor HIV genomes in a subset of optimally treated people with long-term viral suppression. The Journal of infectious diseases. 2013;207:1807–1816. doi: 10.1093/infdis/jit118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sundstrom JB, Ellis JE, Hair GA, Kirshenbaum AS, Metcalfe DD, Yi H, Cardona AC, Lindsay MK, Ansari AA. Human tissue mast cells are an inducible reservoir of persistent HIV infection. Blood. 2007;109:5293–5300. doi: 10.1182/blood-2006-11-058438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nelson AM, Auerbach A, Man YG. Failure to detect active virus replication in mast cells at various tissue sites of HIV patients by immunohistochemistry. International journal of biological sciences. 2009;5:603–610. doi: 10.7150/ijbs.5.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shen A, Zink MC, Mankowski JL, Chadwick K, Margolick JB, Carruth LM, Li M, Clements JE, Siliciano RF. Resting CD4+ T lymphocytes but not thymocytes provide a latent viral reservoir in a simian immunodeficiency virus-Macaca nemestrina model of human immunodeficiency virus type 1-infected patients on highly active antiretroviral therapy. Journal of Virology. 2003;77:4938–4949. doi: 10.1128/JVI.77.8.4938-4949.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deere JD, Kauffman RC, Cannavo E, Higgins J, Villalobos A, Adamson L, Schinazi RF, Luciw PA, North TW. Analysis of multiply spliced transcripts in lymphoid tissue reservoirs of rhesus macaques infected with RT-SHIV during HAART. PloS one. 2014;9:e87914. doi: 10.1371/journal.pone.0087914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blackard JT, Ma G, Martin CM, Rouster SD, Shata MT, Sherman KE. HIV variability in the liver and evidence of possible compartmentalization. AIDS Research and Human Retroviruses. 2011;27:1117–1126. doi: 10.1089/aid.2010.0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Igarashi T, Brown CR, Endo Y, Buckler-White A, Plishka R, Bischofberger N, Hirsch V, Martin MA. Macrophage are the principal reservoir and sustain high virus loads in rhesus macaques after the depletion of CD4+ T cells by a highly pathogenic simian immunodeficiency virus/HIV type 1 chimera (SHIV): Implications for HIV-1 infections of humans. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:658–663. doi: 10.1073/pnas.021551798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kandathil AJ, Durand CM, Quinn J, Cameron AM, Thomas DL, Balagopal A. Liver macrophages and HIV persistence. Conference on retroviruses and opportunistic infections; Feb 23-26, 2015; Seattle, WA. 2015. Abstract 380. [Google Scholar]
- 52.Cerf-Bensussan N, Guy-Grand D. Intestinal intraepithelial lymphocytes. Gastroenterology Clinics of North America. 1991;20:549–576. [PubMed] [Google Scholar]
- 53.Mowat AM, Viney JL. The anatomical basis of intestinal immunity. Immunological Reviews. 1997;156:145–166. doi: 10.1111/j.1600-065x.1997.tb00966.x. [DOI] [PubMed] [Google Scholar]
- 54.Lapenta C, Boirivant M, Marini M, Santini SM, Logozzi M, Viora M, Belardelli F, Fais S. Human intestinal lamina propria lymphocytes are naturally permissive to HIV-1 infection. European Journal of Immunology. 1999;29:1202–1208. doi: 10.1002/(SICI)1521-4141(199904)29:04<1202::AID-IMMU1202>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 55.Poles MA, Elliott J, Taing P, Anton PA, Chen IS. A preponderance of CCR5(+) CXCR4(+) mononuclear cells enhances gastrointestinal mucosal susceptibility to human immunodeficiency virus type 1 infection. Journal of Virology. 2001;75:8390–8399. doi: 10.1128/JVI.75.18.8390-8399.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anton PA, Elliott J, Poles MA, McGowan IM, Matud J, Hultin LE, Grovit-Ferbas K, Mackay CR, Chen ISY, Giorgi JV. Enhanced levels of functional HIV-1 co-receptors on human mucosal T cells demonstrated using intestinal biopsy tissue. AIDS. 2000;14:1761–1765. doi: 10.1097/00002030-200008180-00011. [DOI] [PubMed] [Google Scholar]
- 57.Belmonte L, Olmos M, Fanin A, Parodi C, Bare P, Concetti H, Perez H, de Bracco MM, Cahn P. The intestinal mucosa as a reservoir of HIV-1 infection after successful HAART. AIDS. 2007;21:2106–2108. doi: 10.1097/QAD.0b013e3282efb74b. [DOI] [PubMed] [Google Scholar]
- 58.Lafeuillade A, Cheret A, Hittinger G, Bernardini D, Cuquemelle C, Jullian E, Poggi C. Rectal cell-associated HIV-1 RNA: a new marker ready for the clinic. HIV Clin Trials. 2009;10:324–327. doi: 10.1310/hct1005-324. [DOI] [PubMed] [Google Scholar]
- 59.Zalar A, Figueroa MI, Ruibal-Ares B, Bare P, Cahn P, de Bracco MM, Belmonte L. Macrophage HIV-1 infection in duodenal tissue of patients on long term HAART. Antiviral Research. 2010;87:269–271. doi: 10.1016/j.antiviral.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 60.Tincati C, Biasin M, Bandera A, Violin M, Marchetti G, Piacentini L, Vago GL, Balotta C, Moroni M, Franzetti F, et al. Early initiation of highly active antiretroviral therapy fails to reverse immunovirological abnormalities in gut-associated lymphoid tissue induced by acute HIV infection. Antiviral therapy. 2009;14:321–330. [PubMed] [Google Scholar]
- 61.Poles MA, Boscardin WJ, Elliott J, Taing P, Fuerst MM, McGowan I, Brown S, Anton PA. Lack of decay of HIV-1 in gut-associated lymphoid tissue reservoirs in maximally suppressed individuals. Journal of Acquired Immune Deficiency Syndromes. 2006;43:65–68. doi: 10.1097/01.qai.0000230524.71717.14. [DOI] [PubMed] [Google Scholar]
- 62.Chun TW, Nickle DC, Justement JS, Meyers JH, Roby G, Hallahan CW, Kottilil S, Moir S, Mican JM, Mullins JI, et al. Persistence of HIV in gut-associated lymphoid tissue despite long-term antiretroviral therapy. J Infect Dis. 2008;197:714–720. doi: 10.1086/527324. [DOI] [PubMed] [Google Scholar]
- 63.Yukl SA, Gianella S, Sinclair E, Epling L, Li Q, Duan L, Choi AL, Girling V, Ho T, Li P, et al. Differences in HIV burden and immune activation within the gut of HIV-positive patients receiving suppressive antiretroviral therapy. J Infect Dis. 2010;202:1553–1561. doi: 10.1086/656722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Poles MA, Elliott J, Vingerhoets J, Michiels L, Scholliers A, Bloor S, Larder B, Hertogs K, Anton PA. Despite high concordance, distinct mutational and phenotypic drug resistance profiles in human immunodeficiency virus type 1 RNA are observed in gastrointestinal mucosal biopsy specimens and peripheral blood mononuclear cells compared with plasma. The Journal of infectious diseases. 2001;183:143–148. doi: 10.1086/317640. [DOI] [PubMed] [Google Scholar]
- 65.Katzenstein TL, Petersen AB, Storgaard M, Obel N, Jensen-Fangel S, Nielsen C, Jorgensen LB. Phylogeny and resistance profiles of HIV-1 POL sequences from rectal biopsies and blood. Journal of Medical Virology. 2010;82:1103–1109. doi: 10.1002/jmv.21796. [DOI] [PubMed] [Google Scholar]
- 66.Lewis MJ, Frohnen P, Ibarrondo FJ, Reed D, Iyer V, Ng HL, Elliott J, Yang OO, Anton P. HIV-1 Nef sequence and functional compartmentalization in the gut is not due to differential cytotoxic T lymphocyte selective pressure. PloS one. 2013;8:e75620. doi: 10.1371/journal.pone.0075620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Avettand-Fenoel V, Hocqueloux L, Muller-Trutwin M, Prazuck T, Melard A, Chaix ML, Agoute E, Michau C, Rouzioux C. Greater diversity of HIV DNA variants in the rectum compared to variants in the blood in patients without HAART. Journal of Medical Virology. 2011;83:1499–1507. doi: 10.1002/jmv.22132. [DOI] [PubMed] [Google Scholar]
- 68.Imamichi H, Degray G, Dewar RL, Mannon P, Yao M, Chairez C, Sereti I, Kovacs JA. Lack of compartmentalization of HIV-1 quasispecies between the gut and peripheral blood compartments. The Journal of infectious diseases. 2011;204:309–314. doi: 10.1093/infdis/jir259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Navia BA, Jordan BD, Price RW. The AIDS dementia complex: I. Clinical features. Ann Neurol. 1986;19:517–524. doi: 10.1002/ana.410190602. [DOI] [PubMed] [Google Scholar]
- 70.Levy JA, Shimabukuro J, Hollander H, Mills J, Kaminsky L. Isolation of AIDS-associated retroviruses from cerebrospinal fluid and brain of patients with neurological symptoms. Lancet. 1985;2:586–588. [PubMed] [Google Scholar]
- 71.Ho DD, Rota TR, Schooley RT, Kaplan JC, Allan JD, Groopman JE, Resnick L, Felsenstein D, Andrews CA, Hirsch MS. Isolation of HTLV-III from cerebrospinal fluid and neural tissues of patients with neurologic syndromes related to the acquired immunodeficiency syndrome. The New England journal of medicine. 1985;313:1493–1497. doi: 10.1056/NEJM198512123132401. [DOI] [PubMed] [Google Scholar]
- 72.Koyanagi Y, Miles S, Mitsuyasu RT, Merrill JE, Vinters HV, Chen IS. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science. 1987;236:819–822. doi: 10.1126/science.3646751. [DOI] [PubMed] [Google Scholar]
- 73.Cheng-Mayer C, Levy JA. Distinct biological and serological properties of human immunodeficiency viruses from the brain. Annals of Neurology. 1988;23 Suppl:S58–61. doi: 10.1002/ana.410230716. [DOI] [PubMed] [Google Scholar]
- 74.Chiodi F, Valentin A, Keys B, Schwartz S, Asjo B, Gartner S, Popovic M, Albert J, Sundqvist VA, Fenyo EM. Biological characterization of paired human immunodeficiency virus type 1 isolates from blood and cerebrospinal fluid. Virology. 1989;173:178–187. doi: 10.1016/0042-6822(89)90233-x. [DOI] [PubMed] [Google Scholar]
- 75.Korber BT, Kunstman KJ, Patterson BK, Furtado M, McEvilly MM, Levy R, Wolinsky SM. Genetic differences between blood- and brain-derived viral sequences from human immunodeficiency virus type 1-infected patients: evidence of conserved elements in the V3 region of the envelope protein of brain-derived sequences. Journal of Virology. 1994;68:7467–7481. doi: 10.1128/jvi.68.11.7467-7481.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wong JK, Ignacio CC, Torriani F, Havlir D, Fitch NJ, Richman DD. In vivo compartmentalization of human immunodeficiency virus: evidence from the examination of pol sequences from autopsy tissues. Journal of Virology. 1997;71:2059–2071. doi: 10.1128/jvi.71.3.2059-2071.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Strain MC, Letendre S, Pillai SK, Russell T, Ignacio CC, Gunthard HF, Good B, Smith DM, Wolinsky SM, Furtado M, et al. Genetic composition of human immunodeficiency virus type 1 in cerebrospinal fluid and blood without treatment and during failing antiretroviral therapy. Journal of Virology. 2005;79:1772–1788. doi: 10.1128/JVI.79.3.1772-1788.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Harrington PR, Schnell G, Letendre SL, Ritola K, Robertson K, Hall C, Burch CL, Jabara CB, Moore DT, Ellis RJ, et al. Cross-sectional characterization of HIV-1 env compartmentalization in cerebrospinal fluid over the full disease course. AIDS. 2009;23:907–915. doi: 10.1097/QAD.0b013e3283299129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schnell G, Spudich S, Harrington P, Price RW, Swanstrom R. Compartmentalized human immunodeficiency virus type 1 originates from long-lived cells in some subjects with HIV-1-associated dementia. PLoS pathogens. 2009;5:e1000395. doi: 10.1371/journal.ppat.1000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rytik PG, Eremin VF, Kvacheva ZB, Poleschuk NN, Popov SA, Schroder HC, Bachmann M, Weiler BE, Muller WE. Susceptibility of primary human glial fibrillary acidic protein-positive brain cells to human immunodeficiency virus infection in vitro: anti-HIV activity of memantine. AIDS Research and Human Retroviruses. 1991;7:89–95. doi: 10.1089/aid.1991.7.89. [DOI] [PubMed] [Google Scholar]
- 81.Tornatore C, Nath A, Amemiya K, Major EO. Persistent human immunodeficiency virus type 1 infection in human fetal glial cells reactivated by T-cell factor(s) or by the cytokines tumor necrosis factor alpha and interleukin-1 beta. Journal of Virology. 1991;65:6094–6100. doi: 10.1128/jvi.65.11.6094-6100.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Harouse JM, Kunsch C, Hartle HT, Laughlin MA, Hoxie JA, Wigdahl B, Gonzalez-Scarano F. CD4-independent infection of human neural cells by human immunodeficiency virus type 1. Journal of Virology. 1989;63:2527–2533. doi: 10.1128/jvi.63.6.2527-2533.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Christofinis G, Papadaki L, Sattentau Q, Ferns RB, Tedder R. HIV replicates in cultured human brain cells. AIDS. 1987;1:229–234. [PubMed] [Google Scholar]
- 84.Watkins BA, Dorn HH, Kelly WB, Armstrong RC, Potts BJ, Michaels F, Kufta CV, Dubois-Dalcq M. Specific tropism of HIV-1 for microglial cells in primary human brain cultures. Science. 1990;249:549–553. doi: 10.1126/science.2200125. [DOI] [PubMed] [Google Scholar]
- 85.Moses AV, Bloom FE, Pauza CD, Nelson JA. Human immunodeficiency virus infection of human brain capillary endothelial cells occurs via a CD4/galactosylceramide-independent mechanism. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:10474–10478. doi: 10.1073/pnas.90.22.10474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sharpless N, Gilbert D, Vandercam B, Zhou JM, Verdin E, Ronnett G, Friedman E, Dubois-Dalcq M. The restricted nature of HIV-1 tropism for cultured neural cells. Virology. 1992;191:813–825. doi: 10.1016/0042-6822(92)90257-p. [DOI] [PubMed] [Google Scholar]
- 87.Letendre S, Marquie-Beck J, Capparelli E, Best B, Clifford D, Collier AC, Gelman BB, McArthur JC, McCutchan JA, Morgello S, et al. Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol. 2008;65:65–70. doi: 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Spudich SS, Nilsson AC, Lollo ND, Liegler TJ, Petropoulos CJ, Deeks SG, Paxinos EE, Price RW. Cerebrospinal fluid HIV infection and pleocytosis: relation to systemic infection and antiretroviral treatment. BMC infectious diseases. 2005;5:98. doi: 10.1186/1471-2334-5-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ellis RJ, Gamst AC, Capparelli E, Spector SA, Hsia K, Wolfson T, Abramson I, Grant I, McCutchan JA. Cerebrospinal fluid HIV RNA originates from both local CNS and systemic sources. Neurology. 2000;54:927–936. doi: 10.1212/wnl.54.4.927. [DOI] [PubMed] [Google Scholar]
- 90.Spudich S, Lollo N, Liegler T, Deeks SG, Price RW. Treatment benefit on cerebrospinal fluid HIV-1 levels in the setting of systemic virological suppression and failure. The Journal of infectious diseases. 2006;194:1686–1696. doi: 10.1086/508750. [DOI] [PubMed] [Google Scholar]
- 91.Kumar AM, Borodowsky I, Fernandez B, Gonzalez L, Kumar M. Human immunodeficiency virus type 1 RNA Levels in different regions of human brain: quantification using real-time reverse transcriptase-polymerase chain reaction. Journal of Neurovirology. 2007;13:210–224. doi: 10.1080/13550280701327038. [DOI] [PubMed] [Google Scholar]
- 92.Canestri A, Lescure FX, Jaureguiberry S, Moulignier A, Amiel C, Marcelin AG, Peytavin G, Tubiana R, Pialoux G, Katlama C. Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2010;50:773–778. doi: 10.1086/650538. [DOI] [PubMed] [Google Scholar]
- 93.Smit TK, Brew BJ, Tourtellotte W, Morgello S, Gelman BB, Saksena NK. Independent evolution of human immunodeficiency virus (HIV) drug resistance mutations in diverse areas of the brain in HIV-infected patients, with and without dementia, on antiretroviral treatment. Journal of Virology. 2004;78:10133–10148. doi: 10.1128/JVI.78.18.10133-10148.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Langford D, Marquie-Beck J, de Almeida S, Lazzaretto D, Letendre S, Grant I, McCutchan JA, Masliah E, Ellis RJ. Relationship of antiretroviral treatment to postmortem brain tissue viral load in human immunodeficiency virus-infected patients. Journal of Neurovirology. 2006;12:100–107. doi: 10.1080/13550280600713932. [DOI] [PubMed] [Google Scholar]
- 95.Bingham R, Ahmed N, Rangi P, Johnson M, Tyrer M, Green J. HIV encephalitis despite suppressed viraemia: a case of compartmentalized viral escape. International Journal of STD and AIDS. 2011;22:608–609. doi: 10.1258/ijsa.2011.010507. [DOI] [PubMed] [Google Scholar]
- 96.Peluso MJ, Ferretti F, Peterson J, Lee E, Fuchs D, Boschini A, Gisslen M, Angoff N, Price RW, Cinque P, et al. Cerebrospinal fluid HIV escape associated with progressive neurologic dysfunction in patients on antiretroviral therapy with well controlled plasma viral load. AIDS. 2012;26:1765–1774. doi: 10.1097/QAD.0b013e328355e6b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Semenzato G, Agostini C, Ometto L, Zambello R, Trentin L, Chieco-Bianchi L, De Rossi A. CD8+ T lymphocytes in the lung of acquired immunodeficiency syndrome patients harbor human immunodeficiency virus type 1. Blood. 1995;85:2308–2314. [PubMed] [Google Scholar]
- 98.Sierra-Madero JG, Toossi Z, Hom DL, Finegan CK, Hoenig E, Rich EA. Relationship between load of virus in alveolar macrophages from human immunodeficiency virus type 1-infected persons, production of cytokines, and clinical status. The Journal of infectious diseases. 1994;169:18–27. doi: 10.1093/infdis/169.1.18. [DOI] [PubMed] [Google Scholar]
- 99.Jambo KC, Banda DH, Kankwatira AM, Sukumar N, Allain TJ, Heyderman RS, Russell DG, Mwandumba HC. Small alveolar macrophages are infected preferentially by HIV and exhibit impaired phagocytic function. Mucosal immunology. 2014;7:1116–1126. doi: 10.1038/mi.2013.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Marras D, Bruggeman LA, Gao F, Tanji N, Mansukhani MM, Cara A, Ross MD, Gusella GL, Benson G, D'Agati VD, et al. Replication and compartmentalization of HIV-1 in kidney epithelium of patients with HIV-associated nephropathy. Nature Medicine. 2002;8:522–526. doi: 10.1038/nm0502-522. [DOI] [PubMed] [Google Scholar]
- 101.Winston JA, Bruggeman LA, Ross MD, Jacobson J, Ross L, D'Agati VD, Klotman PE, Klotman ME. Nephropathy and establishment of a renal reservoir of HIV type 1 during primary infection. The New England journal of medicine. 2001;344:1979–1984. doi: 10.1056/NEJM200106283442604. [DOI] [PubMed] [Google Scholar]
- 102.Chakrabarti AK, Caruso L, Ding M, Shen C, Buchanan W, Gupta P, Rinaldo CR, Chen Y. Detection of HIV-1 RNA/DNA and CD4 mRNA in feces and urine from chronic HIV-1 infected subjects with and without anti-retroviral therapy. AIDS research and therapy. 2009;6:20. doi: 10.1186/1742-6405-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Canaud G, Dejucq-Rainsford N, Avettand-Fenoel V, Viard JP, Anglicheau D, Bienaime F, Muorah M, Galmiche L, Gribouval O, Noel LH, et al. The kidney as a reservoir for HIV-1 after renal transplantation. Journal of the American Society of Nephrology : JASN. 2014;25:407–419. doi: 10.1681/ASN.2013050564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Borzy MS, Connell RS, Kiessling AA. Detection of human immunodeficiency virus in cell-free seminal fluid. Journal of Acquired Immune Deficiency Syndromes. 1988;1:419–424. [PubMed] [Google Scholar]
- 105.Lecatsas G, Houff S, Macher A, Gelman E, Steis R, Reichert C, Masur H, Sever JL. Retrovirus-like particles in salivary glands, prostate and testes of AIDS patients. Proceedings of the Society for Experimental Biology and Medicine Society for Experimental Biology and Medicine. 1985;178:653–655. doi: 10.3181/00379727-178-4-rc3. [DOI] [PubMed] [Google Scholar]
- 106.Baccetti B, Benedetto A, Burrini AG, Collodel G, Elia C, Piomboni P, Renieri T, Sensini C, Zaccarelli M. HIV particles detected in spermatozoa of patients with AIDS. Journal of Submicroscopic Cytology and Pathology. 1991;23:339–345. [PubMed] [Google Scholar]
- 107.Pudney J, Anderson D. Orchitis and human immunodeficiency virus type 1 infected cells in reproductive tissues from men with the acquired immune deficiency syndrome. The American journal of pathology. 1991;139:149–160. [PMC free article] [PubMed] [Google Scholar]
- 108.da Silva M, Shevchuk MM, Cronin WJ, Armenakas NA, Tannenbaum M, Fracchia JA, Ioachim HL. Detection of HIV-related protein in testes and prostates of patients with AIDS. American Journal of Clinical Pathology. 1990;93:196–201. doi: 10.1093/ajcp/93.2.196. [DOI] [PubMed] [Google Scholar]
- 109.Muciaccia B, Uccini S, Filippini A, Ziparo E, Paraire F, Baroni CD, Stefanini M. Presence and cellular distribution of HIV in the testes of seropositive subjects: an evaluation by in situ PCR hybridization. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1998;12:151–163. doi: 10.1096/fasebj.12.2.151. [DOI] [PubMed] [Google Scholar]
- 110.Muciaccia B, Filippini A, Ziparo E, Colelli F, Baroni CD, Stefanini M. Testicular germ cells of HIV-seropositive asymptomatic men are infected by the virus. Journal of Reproductive Immunology. 1998;41:81–93. doi: 10.1016/s0165-0378(98)00050-3. [DOI] [PubMed] [Google Scholar]
- 111.Shevchuk MM, Nuovo GJ, Khalife G. HIV in testis: quantitative histology and HIV localization in germ cells. Journal of Reproductive Immunology. 1998;41:69–79. doi: 10.1016/s0165-0378(98)00049-7. [DOI] [PubMed] [Google Scholar]
- 112.Bagasra O, Farzadegan H, Seshamma T, Oakes JW, Saah A, Pomerantz RJ. Detection of HIV-1 proviral DNA in sperm from HIV-1-infected men. AIDS. 1994;8:1669–1674. doi: 10.1097/00002030-199412000-00005. [DOI] [PubMed] [Google Scholar]
- 113.Wolff H, Mayer K, Seage G, Politch J, Horsburgh CR, Anderson D. A comparison of HIV-1 antibody classes, titers, and specificities in paired semen and blood samples from HIV-1 seropositive men. Journal of Acquired Immune Deficiency Syndromes. 1992;5:65–69. [PubMed] [Google Scholar]
- 114.Winnall WR, Lloyd SB, De Rose R, Alcantara S, Amarasena TH, Hedger MP, Girling JE, Kent SJ. Simian immunodeficiency virus infection and immune responses in the pig-tailed macaque testis. Journal of Leukocyte Biology. 2015;97:599–609. doi: 10.1189/jlb.4A0914-438R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lafeuillade A, Solas C, Halfon P, Chadapaud S, Hittinger G, Lacarelle B. Differences in the detection of three HIV-1 protease inhibitors in non-blood compartments: clinical correlations. HIV clinical trials. 2002;3:27–35. doi: 10.1310/WMWL-6W9Y-PXV2-X148. [DOI] [PubMed] [Google Scholar]
- 116.Marcelin AG, Tubiana R, Lambert-Niclot S, Lefebvre G, Dominguez S, Bonmarchand M, Vauthier-Brouzes D, Marguet F, Mousset-Simeon N, Peytavin G, et al. Detection of HIV-1 RNA in seminal plasma samples from treated patients with undetectable HIV-1 RNA in blood plasma. AIDS. 2008;22:1677–1679. doi: 10.1097/QAD.0b013e32830abdc8. [DOI] [PubMed] [Google Scholar]
- 117.Sheth PM, Kovacs C, Kemal KS, Jones RB, Raboud JM, Pilon R, la Porte C, Ostrowski M, Loutfy M, Burger H, et al. Persistent HIV RNA shedding in semen despite effective antiretroviral therapy. AIDS. 2009;23:2050–2054. doi: 10.1097/QAD.0b013e3283303e04. [DOI] [PubMed] [Google Scholar]
- 118.Halfon P, Giorgetti C, Khiri H, Penaranda G, Terriou P, Porcu-Buisson G, Chabert-Orsini V. Semen may harbor HIV despite effective HAART: another piece in the puzzle. PloS one. 2010;5:e10569. doi: 10.1371/journal.pone.0010569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Politch JA, Mayer KH, Welles SL, O'Brien WX, Xu C, Bowman FP, Anderson DJ. Highly active antiretroviral therapy does not completely suppress HIV in semen of sexually active HIV-infected men who have sex with men. AIDS. 2012;26:1535–1543. doi: 10.1097/QAD.0b013e328353b11b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Matusali G, Dereuddre-Bosquet N, Le Tortorec A, Moreau M, Satie AP, Mahe D, Roumaud P, Bourry O, Sylla N, Bernard-Stoecklin S, et al. Detection of Simian Immunodeficiency Virus in Semen, Urethra, and Male Reproductive Organs during Efficient Highly Active Antiretroviral Therapy. Journal of Virology. 2015;89:5772–5787. doi: 10.1128/JVI.03628-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Paranjpe S, Craigo J, Patterson B, Ding M, Barroso P, Harrison L, Montelaro R, Gupta P. Subcompartmentalization of HIV-1 quasispecies between seminal cells and seminal plasma indicates their origin in distinct genital tissues. AIDS Research and Human Retroviruses. 2002;18:1271–1280. doi: 10.1089/088922202320886316. [DOI] [PubMed] [Google Scholar]
- 122.Smith DM, Kingery JD, Wong JK, Ignacio CC, Richman DD, Little SJ. The prostate as a reservoir for HIV-1. Aids. 2004;18:1600–1602. doi: 10.1097/01.aids.0000131364.60081.01. [DOI] [PubMed] [Google Scholar]
- 123.Coombs RW, Lockhart D, Ross SO, Deutsch L, Dragavon J, Diem K, Hooton TM, Collier AC, Corey L, Krieger JN. Lower genitourinary tract sources of seminal HIV. Journal of Acquired Immune Deficiency Syndromes. 2006;41:430–438. doi: 10.1097/01.qai.0000209895.82255.08. [DOI] [PubMed] [Google Scholar]
- 124.Fieni F, Stone M, Ma ZM, Dutra J, Fritts L, Miller CJ. Viral RNA levels and env variants in semen and tissues of mature male rhesus macaques infected with SIV by penile inoculation. PloS one. 2013;8:e76367. doi: 10.1371/journal.pone.0076367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Iversen AK, Attermann J, Gerstoft J, Fugger L, Mullins JI, Skinhoj P. Longitudinal and cross-sectional studies of HIV-1 RNA and DNA loads in blood and the female genital tract. European Journal of Obstetrics, Gynecology, and Reproductive Biology. 2004;117:227–235. doi: 10.1016/j.ejogrb.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 126.Kovacs A, Wasserman SS, Burns D, Wright DJ, Cohn J, Landay A, Weber K, Cohen M, Levine A, Minkoff H, et al. Determinants of HIV-1 shedding in the genital tract of women. Lancet. 2001;358:1593–1601. doi: 10.1016/S0140-6736(01)06653-3. [DOI] [PubMed] [Google Scholar]
- 127.Nunnari G, Sullivan J, Xu Y, Nyirjesy P, Kulkosky J, Cavert W, Frank I, Pomerantz RJ. HIV type 1 cervicovaginal reservoirs in the era of HAART. AIDS Research and Human Retroviruses. 2005;21:714–718. doi: 10.1089/aid.2005.21.714. [DOI] [PubMed] [Google Scholar]
- 128.Wahl SM, Redford M, Christensen S, Mack W, Cohn J, Janoff EN, Mestecky J, Jenson HB, Navazesh M, Cohen M, et al. Systemic and mucosal differences in HIV burden, immune, and therapeutic responses. Journal of Acquired Immune Deficiency Syndromes. 2011;56:401–411. doi: 10.1097/QAI.0b013e31820cdfdb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Launay O, Tod M, Tschope I, Si-Mohamed A, Belarbi L, Charpentier C, Goujard C, Taburet AM, Lortholary O, Leroy V, et al. Residual HIV-1 RNA and HIV-1 DNA production in the genital tract reservoir of women treated with HAART: the prospective ANRS EP24 GYNODYN study. Antiviral therapy. 2011;16:843–852. doi: 10.3851/IMP1856. [DOI] [PubMed] [Google Scholar]
- 130.Fiscus SA, Cu-Uvin S, Eshete AT, Hughes MD, Bao Y, Hosseinipour M, Grinsztejn B, Badal-Faesen S, Dragavon J, Coombs RW, et al. Changes in HIV-1 subtypes B and C genital tract RNA in women and men after initiation of antiretroviral therapy. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013;57:290–297. doi: 10.1093/cid/cit195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Taylor S, Davies S. Antiretroviral drug concentrations in the male and female genital tract: implications for the sexual transmission of HIV. Current opinion in HIV and AIDS. 2010;5:335–343. doi: 10.1097/COH.0b013e32833a0b69. [DOI] [PubMed] [Google Scholar]
- 132.Else LJ, Taylor S, Back DJ, Khoo SH. Pharmacokinetics of antiretroviral drugs in anatomical sanctuary sites: the male and female genital tract. Antiviral therapy. 2011;16:1149–1167. doi: 10.3851/IMP1919. [DOI] [PubMed] [Google Scholar]
- 133.Overbaugh J, Anderson RJ, Ndinya-Achola JO, Kreiss JK. Distinct but related human immunodeficiency virus type 1 variant populations in genital secretions and blood. AIDS Research and Human Retroviruses. 1996;12:107–115. doi: 10.1089/aid.1996.12.107. [DOI] [PubMed] [Google Scholar]
- 134.Tirado G, Jove G, Kumar R, Noel RJ, Reyes E, Sepulveda G, Yamamura Y, Kumar A. Differential virus evolution in blood and genital tract of HIV-infected females: evidence for the involvement of drug and non-drug resistance-associated mutations. Virology. 2004;324:577–586. doi: 10.1016/j.virol.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 135.Chomont N, Hocini H, Gresenguet G, Brochier C, Bouhlal H, Andreoletti L, Becquart P, Charpentier C, de Dieu Longo J, Si-Mohamed A, et al. Early archives of genetically-restricted proviral DNA in the female genital tract after heterosexual transmission of HIV-1. AIDS. 2007;21:153–162. doi: 10.1097/QAD.0b013e328011f94b. [DOI] [PubMed] [Google Scholar]
- 136.Bull ME, Learn GH, McElhone S, Hitti J, Lockhart D, Holte S, Dragavon J, Coombs RW, Mullins JI, Frenkel LM. Monotypic human immunodeficiency virus type 1 genotypes across the uterine cervix and in blood suggest proliferation of cells with provirus. Journal of Virology. 2009;83:6020–6028. doi: 10.1128/JVI.02664-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kelley CF, Sullivan ST, Lennox JL, Evans-Strickfaden T, Hart CE. Lack of effect of compartmentalized drug resistance mutations on HIV-1 pol divergence in antiretroviral-experienced women. AIDS. 2010;24:1361–1366. doi: 10.1097/QAD.0b013e3283394f3f. [DOI] [PubMed] [Google Scholar]
- 138.Giannetti A, Zambruno G, Cimarelli A, Marconi A, Negroni M, Girolomoni G, Bertazzoni U. Direct detection of HIV-1 RNA in epidermal Langerhans cells of HIV-infected patients. Journal of Acquired Immune Deficiency Syndromes. 1993;6:329–333. [PubMed] [Google Scholar]
- 139.Rappersberger K, Gartner S, Schenk P, Stingl G, Groh V, Tschachler E, Mann DL, Wolff K, Konrad K, Popovic M. Langerhans' cells are an actual site of HIV-1 replication. Intervirology. 1988;29:185–194. doi: 10.1159/000150045. [DOI] [PubMed] [Google Scholar]
- 140.Kalter DC, Greenhouse JJ, Orenstein JM, Schnittman SM, Gendelman HE, Meltzer MS. Epidermal Langerhans cells are not principal reservoirs of virus in HIV disease. Journal of Immunology. 1991;146:3396–3404. [PubMed] [Google Scholar]
- 141.Dusserre N, Dezutter-Dambuyant C, Mallet F, Delorme P, Philit F, Ebersold A, Desgranges C, Thivolet J, Schmitt D. In vitro HIV-1 entry and replication in Langerhans cells may clarify the HIV-1 genome detection by PCR in epidermis of seropositive patients. The Journal of investigative dermatology. 1992;99:99S–102S. doi: 10.1111/1523-1747.ep12669977. [DOI] [PubMed] [Google Scholar]
- 142.Couturier J, Suliburk JW, Brown JM, Luke DJ, Agarwal N, Yu X, Nguyen C, Iyer D, Kozinetz CA, Overbeek PA, et al. Human adipose tissue as a reservoir for memory CD4+ T cells and HIV. AIDS. 2015;29:667–674. doi: 10.1097/QAD.0000000000000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Damouche A, Lazure T, Avettand-Fenoel V, Huot N, Dejucq-Rainsford N, Satie AP, Melard A, David L, Gommet C, Ghosn J, et al. Adipose Tissue Is a Neglected Viral Reservoir and an Inflammatory Site during Chronic HIV and SIV Infection. PLoS Pathog. 2015;11:e1005153. doi: 10.1371/journal.ppat.1005153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yukl SA, Wong JK. Anatomic Compartments as a Barrier to HIV Cure. In: Hope T, Stevenson M, Richman D, editors. Encyclopedia of AIDS. New York: Springer; 2015. pp. 1–29. [Google Scholar]
- 145.Henrich TJ, Hu Z, Li JZ, Sciaranghella G, Busch MP, Keating SM, Gallien S, Lin NH, Giguel FF, Lavoie L, et al. Long-term reduction in peripheral blood HIV type 1 reservoirs following reduced-intensity conditioning allogeneic stem cell transplantation. J Infect Dis. 2013;207:1694–1702. doi: 10.1093/infdis/jit086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yukl SA, Boritz E, Busch M, Bentsen C, Chun TW, Douek D, Eisele E, Haase A, Ho YC, Hutter G, et al. Challenges in detecting HIV persistence during potentially curative interventions: a study of the Berlin patient. PLoS Pathog. 2013;9:e1003347. doi: 10.1371/journal.ppat.1003347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sharaf R, Mempel TR, Murooka TT. Visualizing the Behavior of HIV-Infected T Cells In Vivo Using Multiphoton Intravital Microscopy. Methods Mol Biol. 2016;1354:189–201. doi: 10.1007/978-1-4939-3046-3_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mousseau G, Clementz MA, Bakeman WN, Nagarsheth N, Cameron M, Shi J, Baran P, Fromentin R, Chomont N, Valente ST. An analog of the natural steroidal alkaloid cortistatin A potently suppresses Tat-dependent HIV transcription. Cell Host Microbe. 2012;12:97–108. doi: 10.1016/j.chom.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mediouni S, Jablonski J, Paris JJ, Clementz MA, Thenin-Houssier S, McLaughlin JP, Valente ST. Didehydro-cortistatin A inhibits HIV-1 Tat mediated neuroinflammation and prevents potentiation of cocaine reward in Tat transgenic mice. Curr HIV Res. 2015;13:64–79. doi: 10.2174/1570162x13666150121111548. [DOI] [PMC free article] [PubMed] [Google Scholar]