Abstract
After many decades of improvements in mechanical circulatory assist devices (CADs), blood damage remains a serious problem during support contributing to variety of adverse events, and consequently affecting patient survival and quality of life. The mechanisms of cumulative cell damage in continuous-flow blood pumps are still not fully understood despite numerous in vitro, in vivo, and in silico studies of blood trauma. Previous investigations have almost exclusively focused on lethal blood damage, namely hemolysis, which is typically negligible during normal operation of current generation CADs. The measurement of plasma free hemoglobin (plfHb) concentration to characterize hemolysis is straightforward, however sublethal trauma is more difficult to detect and quantify since no simple direct test exists. Similarly, while multiple studies have focused on thrombosis within blood pumps and accessories, sublethal blood trauma and its sequelae have yet to be adequately documented or characterized. This review summarizes the current understanding of sublethal trauma to red blood cells (RBCs) produced by exposure of blood to flow parameters and conditions similar to those within CADs. It also suggests potential strategies to reduce and/or prevent RBC sublethal damage in a clinically-relevant context, and encourages new research into this relatively uncharted territory.
Keywords: Heart-assist devices, Erythrocyte aggregation, Erythrocyte deformability, Hemolysis, RBC mechanical fragility, Sublethal damage
Introduction
Whole blood is a complex, heterogeneous, multi-cellular suspension, the importance of which cannot be overstated. Blood is responsible for gas exchange, nutrient transport, waste removal, cell signaling, immunomodulation and immunoprotection, wound healing, and overall homeostasis. The cellular (~45%) and plasma (~55%) components of blood, while distinct, are interdependent and essential for maintaining proper functions. The phenomenon of mechanical blood trauma arises from the exposure of flowing blood to nonphysiological conditions found within circulatory assist devices (CADs), summarized concisely as “too many nonphysiological factors affecting too many physiological objects.” Although yet to be identified or discovered. These objects include blood cells (erythrocytes/RBCs, platelets, leukocytes) with various functions, cell volumes, rheological properties, life spans, and population sizes and other numerous components inside blood plasma, like proteins (albumin/globulins), blood coagulation factors, immunoglobulins, nutrients, minerals, salts, ions, and hundreds of other dissolved constituents. The most common nonphysiological factors are mechanical stresses, excessive temperatures, turbulence, cavitation, inappropriate flow rates, the presence (or absence) of flow pulsatility, incompatible foreign surfaces, etc. Inside a CAD, these factors may act independently, collectively, or synergistically. All cellular species of blood are sensitive to these factors and can be activated, deactivated, or irreversibly damaged. Furthermore, many plasma components may be denatured due to exposure to CADs.
Healthy, denucleated red blood cells (RBCs) have the longest life span in circulation (100–120 days), maintaining an intravascular population of about 5.1 ± 1.0 million/mm3 of blood or approximately 40% to 45% of blood volume. Due to their remarkable deformability, RBCs with size of ~8 µm are able to easily enter and pass through blood capillaries with diameters as small as 3 µm. Best known for gas transport, they are also essential to the dynamic, non-Newtonian rheological properties of blood which provide adaptive viscosity in various compartments of the vascular system. This is in turn responsible for the vessel wall shear stresses that affect endothelial function. Healthy platelets are 2 µm to 3 µm in size, with an in vivo life span of about 10 days and a concentration of 0.15 to 0.45 million/mm3 of blood. Their major function is to prevent and stop bleeding by creating plugs/clots involving the very complex coagulation cascade. As a key component in hemostasis, platelets are extremely sensitive to the factors listed above leading to their activation, aggregation, deposition, or dysfunction. Due to exposure to nonphysiological environments, activated platelets may create microthrombi in the CAD that can cause the device to fail and/or create emboli, potentially blocking blood flow to vital organs. The standard practice to temper the risk of CAD thrombogenicity is to administer anticoagulants directly into the blood (such as heparin) or indirectly by pills (such as warfarin, clopidogrel, etc.), an intervention that raises the risk of unintended bleeding. Consequently, coagulopathy related complications (including bleeding, thromboembolism, neurological dysfunction, and pump thrombosis) are the leading cause of adverse events in patients on mechanical circulatory support (1). Therefore, the reduction of blood damage remains a major challenge for developers of blood-contacting devices.
Over many decades, there have been an abundance of studies on blood damage to understand, reduce, and eliminate complications related to blood-contacting foreign surfaces and nonphysiological flow conditions. The best known, easily detectable, and reproducible marker of in vivo or in vitro blood damage is hemolysis, which is manifested by hemoglobin released from ruptured, overstretched, overheated, or prematurely aged RBCs, or some combination thereof. Numerous publications are dedicated to the in vitro, in vivo, and computational investigations of the fluid dynamic and microenvironmental conditions in CADs leading to device-induced hemolysis and the consequences of plfHb in blood. Despite considerable efforts by device developers to mitigate the risk of blood trauma, a significant increase in hemolysis takes place preceding the diagnosis of pump thrombosis. This phenomenon is associated with substantial morbidity and mortality of CAD recipients, which is now increasingly reported (2–4).
Sublethal trauma, which is difficult to detect and characterize compared to hemolysis, has yet to receive much attention. Even mild hemolysis in vivo, which is not an abrupt threat to renal function, can be a harbinger of potential ongoing damage to other blood components (including platelets, white blood cells, von Willebrand factor, etc.), which can cause or indicate serious complications like thrombosis. Therefore it is important to assess the susceptibility of blood to sublethal trauma or possibly the extent of sublethal damage incurred to serve as an early diagnostic marker. While there are many questions regarding what tests could predict complications, unfortunately no machine or single experiment exists that can measure both lethal and sublethal injury in a CAD patient’s blood. Acknowledging the breadth and depth of sublethal blood damage, this review will primarily focus on sublethal trauma to RBCs including the mechanisms of damage, the resulting physiological changes, and the consequences of pathological erythrocytes.
Hemolysis
Extensive experience with heart-assist devices has demonstrated that they have the propensity to damage blood to some extent. Therefore, special attention must be paid to the prediction and reduction of blood damage when designing, testing, and clinically operating CADs. The prolonged contact and collision between blood cells and foreign surfaces, cavitation, excessive exposure to high fluid stresses, and turbulence may cause the complete mechanical destruction of RBCs. These factors can also denature proteins, activate platelets and leukocytes, elevate inflammatory mediators, cause complement activation, and change the mechanical properties of red blood cells akin to accelerated aging (5–10). Hemolysis manifests clinically as anemia, fatigue, jaundice, hematuria, and kidney failure, among many other symptoms (11). In addition to renal damage, mechanical hemolysis can cause hypercoagulation, bleeding, thromboembolism, and neurologic dysfunction (stroke, altered mental status, etc.) (5, 7, 12). Even low levels of hemolysis have been shown to drastically increase RBC aggregation at low shear conditions (13). Additionally, the release of hemoglobin from the overstretched RBCs into the plasma may have a toxic effect on the cardiovascular system due to its ability to bind nitric oxide, an endothelium-derived relaxing factor, leading to vasoconstriction, hypertension, renal damage, and platelet activation (14). By the end of the 19th century, it was already recognized that hemolysis promotes intravascular thrombosis (15). Experiments using stroma-free RBC lysate revealed that a hemoglobin concentration as low as 30 mg/dL induced spontaneous platelet aggregation, which increased in direct proportion to the concentration of lysate (16).
Sublethal RBC Damage
For many decades, it has been observed that the mechanical properties of RBCs could be altered during prolonged exposure to shear stresses well below the hemolytic threshold (6). The concept of sublethal RBC damage was first introduced by Dr. Galletti (a pioneering researcher in artificial organs and tissue engineering), who attributed the observed development of anemia and shortened RBC life spans in animals that underwent extracorporeal perfusion for 10 to 48 hours to a process of ongoing sublethal blood trauma (17). Additional studies by Bernstein et al and Indeglia et al confirmed this relationship between subhemolytic shear stresses during assisted circulation and the premature removal of damaged RBCs, eventually leading to postperfusion anemia (18–20). This sublethal trauma is much harder to detect and characterize than total lysis. The experiments of Sandza et al, where isolated rabbit spleens were perfused by a mixture of sheared and nonsheared autologous RBCs, proved that the spleen could “recognize” and selectively remove cells that had been exposed to shear stresses lower than 10 Pa for 2 hours, suggesting that some changes had occurred to the mechanical or chemical properties of the RBCs (21). Clinically observed, chronic anemia in patients supported with CADs is sometimes attributed to undetermined mechanisms (22). However, it might directly result from the sublethal RBC damage and lifespan shortening described by Galletti. This is corroborated by previously published clinical data on anemic patients with circulatory support devices and heart valves that showed alterations in patient blood rheology such as increased blood viscosity, RBC aggregation, and decreased RBC deformability (22–25). Moreover, an increasing occurrence of thrombosis and inflammatory events without measurable hemolysis may be unknowingly triggered by sublethal damage to blood, as it is not yet a part of clinical practice.
RBC Deformability
RBC deformability is critically important for the passage of these cells through the entire vascular system, including the smallest capillaries in microcirculation, to provide adequate transport of gases, sufficient supply of nutrients, and efficient removal of waste products. It has been found that naturally aged RBCs are removed from circulation by the spleen as they become less deformable (26). Each single RBC enters the spleen about twice per hour where it is “tested” for the ability to pass through tiny slits in the red pulp. The body applies this built-in “rheometer” to measure RBC deformability and remove those that fail this examination. Therefore, the deformability of RBCs appears to be the major determinant of their survival in the vascular system, and hence, their lifespan. RBCs exposed to extensive mechanical stress can therefore be prematurely removed from circulation because of an increase in their rigidity. In this context, sublethal trauma may be considered analogous to an accelerated RBC aging process. Healthy RBCs are able to enter and pass through much smaller capillaries, thus decreasing blood viscosity 5 to 6 times below that of blood containing rigid RBCs (27). Impairment of RBC deformability reduces the number of functioning capillaries, inducing tissue ischemia. The decrease in RBC deformability after exposure to mechanical stress has been reported in many studies (28–34). Baskurt et al illustrated this phenomenon by subjecting human RBCs to a uniform shear stress of 120 Pa for 15 to 120 seconds at 37°C. This level of stress significantly impaired RBC deformability as assessed by ektacytometry, with no visible hemolysis observed in the sheared RBC suspensions (31). Kameneva et al demonstrated that the reduction of RBC deformability induced by mechanical stress was worsened when combined with hypothermia and a decrease in plasma protein concentration due to hemodilution, especially in cases of moderate and deep hypothermia widely applied during cardiopulmonary bypass in infants (28). Dao et al found a reduction of human RBC filterability accompanied by significant changes in the RBC lipid bilayer due to exposure of RBC suspensions to sublethal shear stress of 100 Pa for 120 seconds (35).
There are various methods for the assessment of RBC deformability described in the literature including but not limited to micropipette aspiration, filtration, viscometry, and imaging-based modalities (36). Ektacytometry, a common imaging-based technique, uses laser diffraction analysis to assess cellular deformation in low hematocrit RBC suspensions under shear (37). These authors prefer to assess RBC deformability with bright-field illumination instead, enabling direct visualization of cell shape, using the Linkam Optical Shearing Stage (CSS-450; Linkam Scientific Instruments) (29). The apparatus consists of a parallel disc sample chamber with an adjustable height top plate (typically 10–20 µm) and rotating bottom stage. RBC samples are suspended in a concentrated polyvinyl pyrrolidone (PVP) solution (µ = 30 cP or mPa·s) and exposed to Couette flow at various shear rates while simultaneously observed using a synchronized CCD camera (QICAM Fast Color, QImaging). The resulting images are then analyzed to quantitatively determine cell deformability using ImageJ (NIH) or automated software to yield an Elongation Index calculated as (L-W)/(L+W), where L is the length and W the width of the RBC under shear. A representative image produced by the Linkam system is shown in Figure 1, where a mixture of normal and rigidified RBCs was sheared at (A) 0 and (B) 1000 s−1 (corresponding to a shear stress of 0 and 30 Pa, respectively).
Figure 1.
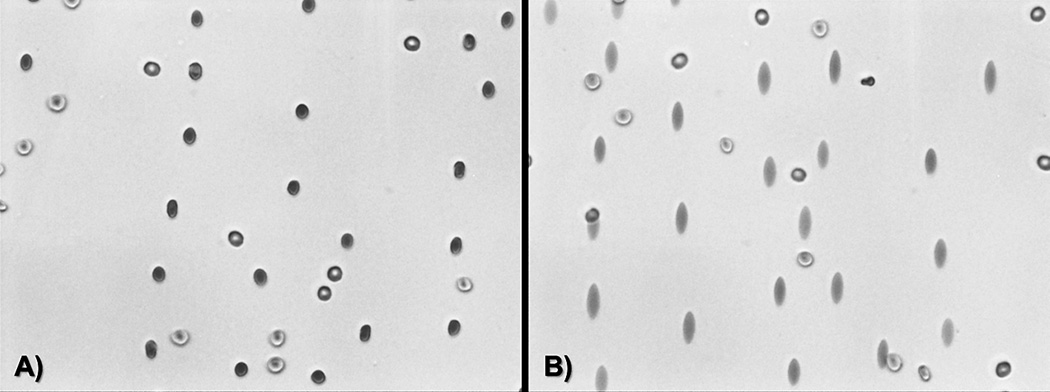
Bright-field illuminated normal and rigidified erythrocytes undergoing shear at (A) 0 and (B) 1000 s−1 for RBC deformability assessment using the Linkam Shearing Stage. Normal RBCs elongate becoming ellipsoidal under shear stress while the rigid cells remain circular.
RBC Aggregation
The RBCs of human and some other mammalian species, especially athletic versus sedentary ones such as a horse, feline and antelope, build aggregates that can be observed in blood in vitro and in vivo under no flow or low flow states. RBC aggregation is caused by fibrinogen and other large protein molecules in blood plasma. The physiological significance of RBC aggregation is not yet fully understood, but it has been shown to promote the development of a cell-depleted plasma layer near walls of small blood vessels, thereby reducing wall shear stress loading and diminishing the release of endothelium-dependent vasodilators (38). A drastic increase in RBC aggregation at low flow conditions has been seen even at low levels of hemolysis (39). Furthermore, it is known that an increase in RBC aggregation relative to normal physiological levels is associated with inflammation, infection, or some other pathological states; which is usually related to an increase in the concentrations of plasma fibrinogen, immunoglobulin, and other plasma macromolecules (40, 41). The majority of preclinical animal studies for CADs are performed in ruminants because of their acceptable size, docile behavior, long-term manageability, and cost effectiveness (42). Despite a relatively high physiological concentration of fibrinogen for bovines (up to 0.5–0.8 g/dL), ovines and bovines typically exhibit no RBC aggregation. Nevertheless, it was possible to observe aggregation of RBCs in bovine blood during CAD implantations as shown in Figure 2 (43). Figure 2A is a micrograph of blood from a CAD-implanted animal with a fibrinogen concentration of 1.0 g/dL showing a very strong aggregation of RBCs. Due to a typical postsurgical reaction after CAD implantation, these animals exhibited increased fibrinogen levels. Figure 2B shows blood of a control calf with no tendency for RBC aggregation. When RBCs of this normal calf (B) were resuspended in the plasma of the implanted calf (A), they still did not show any tendency to aggregate (C). However, RBCs of the CAD-supported calf (A) re-suspended in the plasma of the control calf (B) began aggregating (D) even at the much lower fibrinogen concentration of 0.2 g/dL in plasma. The same phenomenon was reported during testing of CAD-implanted ovines (44). These observations support the hypothesis that sublethal mechanical stress increases RBC aggregability, perhaps via decreased membrane sialic acid concentration and cell surface negative charge, which normally creates repulsing forces between RBCs (34, 45–47). While the difference in RBC aggregation behavior in these preclinical models should be noted, the abnormal aggregation of ovine or bovine erythrocytes may serve as an indicator for sublethal blood trauma during CAD testing. RBC aggregation is of basic scientific and clinical interest, with a number of reports associating a strong increase in this parameter to multiple pathologies, and a variety of experimental studies aimed at finding and testing drugs that are able to reduce RBC aggregation. Yet the advantages and disadvantages of having low or no RBC aggregation are not clear. A significant increase in RBC aggregation from CAD-induced sublethal mechanical blood damage could affect patient microcirculation by increasing the near- wall cell-free layer, allowing more platelets and leukocytes to concentrate near the vessel wall. This in turn, may promote inflammation (due to excess leukocytes) and thrombosis (due to excess platelets), which are major complications of mechanically assisted circulation.
Figure 2.
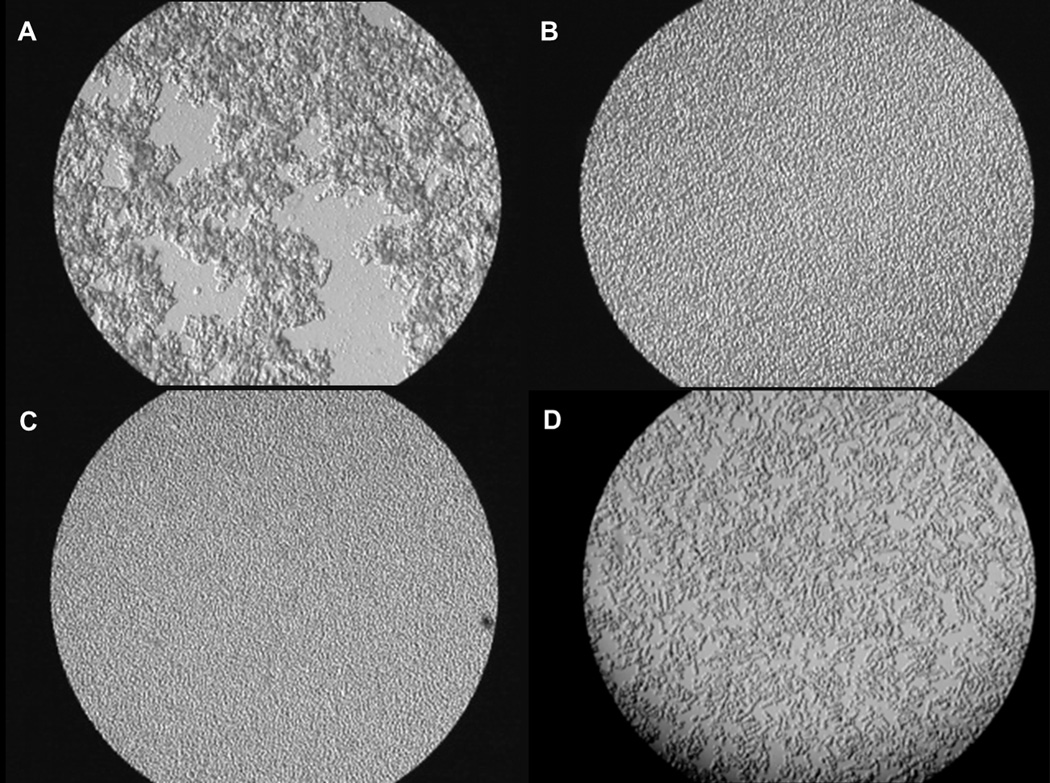
(A) Blood of a calf implanted with a CAD (high RBC aggregation and high plasma fibrinogen concentration); (B) Blood of a control calf (no RBC aggregation and low fibrinogen concentration); (C) Control RBCs placed in the CAD animal’s plasma (no RBC aggregation at high fibrinogen concentration); and (D) CAD calf RBCs placed in the control calf plasma (high RBC aggregation despite low fibrinogen concentration) (43). The aggregation present in frames (A) and, especially, (D) indicate that there are RBC-specific changes directly induced by mechanical stress related to CAD support. Reprinted with permission from IOS Press (43).
There are several methods used for evaluating RBC aggregation, namely, photometric techniques based on alterations of light reflectance or transmittance of RBC suspensions during the process of cell aggregation using special aggregometers (e.g., Myrenne Aggregometer or RheoScan) (48). In addition, RBC aggregation can be assessed using low-shear blood viscosity and erythrocyte sedimentation rate (ESR) parameters (49). Since the concentration of RBCs strongly affects the results of any aggregation measurement, the hematocrit of the blood sample should be always adjusted to a “standard” value (traditionally, 40% for most methods).
Mechanical Fragility of Red Blood Cells
The sensitivity of RBCs to mechanical stress is one indicator toward the extent of sublethal damage to blood. A sensitivemethod to assess the mechanical fragility (MF) of RBCs is the Rocker-bead MF test (50), which could be used during CAD support to warn of blood damage preceding measurable hemolysis (51). A corresponding Mechanical Fragility Index (MFI) is calculated by measuring the amount of free hemoglobin generated after blood vials containing stainless steel ball bearings are subjected to a defined amount of mechanical stress by rocking at specific parameters for a controlled period of time (50, 52, 53). The Rocker-bead MF test was found to be a practical, straightforward, and reproducible way to determine changes in RBC susceptibility to mechanical stress related to aging, excessive exposure to mechanical stresses, or disease states. It is worth noting that RBC mechanical fragility in human neonates was found to be higher than that in adults (54). In all tested species, younger RBCs were found to be less fragile than senescent RBCs (34, 55). This test has demonstrated that RBCs from blood obtained from premenopausal women were significantly less susceptible to shear stress-induced hemolysis compared to those obtained from age-matched males (56). The Rocker-bead MF test has also been applied to compare mechanical blood damage produced by cell salvage suction devices and more recently was used for evaluating sublethal RBC injury induced by Blood Bank storage (57, 58). While there was no significant difference in MF observed between ABO groups, there was a strong increase in RBC fragility proportional to storage time. In the same study of the Blood Bank-stored donor RBCs, the cells were tested for potential changes in their rheological properties using a viscoelasticity analyzer (Vilastic-3; Vilastic Instruments). All stored RBC suspensions demonstrated a progressive increase in viscosity and cell rigidity at equivalent shear rates over 7 weeks of storage, indicating a significant decrease in RBC deformability over that period of time (58, 59). The clinical effects of storage time on donor RBC units, including cell membrane changes, decreased oxygen deliverability, and increased oxidative species – termed RBC storage lesion – is well established (60, 61).
Because of the dependence of the Rocker-bead MF test results on RBC concentration, any comparison between groups or time points requires the adjustment of the sample hematocrit to a “standard” value before testing as performed in the studies presented above (58, 61). Further work to improve the test, including eliminating the additional time for hematocrit normalization and significantly reducing the blood volume requirement, would expand the utility of the Rocker-bead MF-test as an important scientific and possibly clinical hematological parameter.
Protection of Red Blood Cells from Mechanical Trauma
Several studies have demonstrated potential ways to reduce mechanical blood trauma caused by CAD using biocompatible and rheologically active additives to blood (31, 62–65). Kamada et al used albumin additives to the priming solution during extracorporeal circulation in patients undergoing coronary bypass surgery to prevent erythrocyte crenation, thus improving RBC deformability. This modification of the procedure also improved microcirculatory flow in patients undergoing open heart surgery (62). It is important to note that current circuit priming strategies, especially in pediatrics, remain unsettled over the use of albumin, synthetic colloid suspensions, packed RBC units, fresh frozen plasma (FFP), or some combination therein, due to clinical concerns of hemodilution, inflammation, bleeding, and cost effectiveness (66–70). Though as indicated in this review, sublethal mechanical blood trauma likely plays a role in some of these complications. Armstrong et al demonstrated that the nonionic surfactant poloxamer-188 (RheothRx) protected RBCs from damage caused by mechanical stress and reduced leakage of ADP from RBCs, thus potentially inhibiting ADP-induced platelet aggregation. The authors proposed that nonspecific adsorption of copolymer to the RBC surface via the hydrophobic polyoxypropylene moiety was responsible for shielding them from mechanical damage. RheothRx injection has subsequently been shown to effectively treat acute ischemic disorders such as myocardial infarction (63).
Various plasma proteins have likewise demonstrated protective effects on RBC mechanical damage (64). In these studies, bovine cells suspended in various solutions were simultaneously exposed to the same mechanical stress using the standard RBC mechanical fragility test (as described above) in experiments performed at room temperature with controlled osmolality and viscosity of the suspension media. The lowest hemolysis was obtained for RBCs suspended in serum, plasma, and albumin solutions; while hemolysis in phosphate buffered saline (PBS) or in dextran (Dextran-40) suspensions was over 3 times greater than in plasma (p<0.001). The presence of relatively small amounts of plasma (30%) in PBS media significantly (p<0.001) decreased mechanical hemolysis compared to RBCs suspended in PBS only. The clinical significance of these results is that a decrease in the concentration of plasma proteins due to hemodilution may elevate blood damage during extracorporeal circulation (64). Protective effects of a 4%-modified fluid gelatin solution and a 4% albumin solution on blood bank-stored RBC exposed to mechanical stress has been reported by Sumpelmann et al (71). A similar protective effect was demonstrated by replacement of 20% of blood plasma with a perfluorochemical-based blood substitute in in vitro experiments, promising improvement in blood oxygenation as well (65). An additional benefit of this additive was a decrease in low shear viscosity and erythrocyte sedimentation rate, both related to the ability of the perfluorochemical emulsion to reduce RBC aggregation. Finally, a significant reduction of mechanical hemolysis was observed in suspensions of bovine RBCs in autologous bovine plasma and/or polyethylene glycol (PEG ~20,000 MW; Sigma) compared to Dextran-40 (40,000 MW; Sigma) and PBS as shown in Figure 3 (72).
Figure 3.
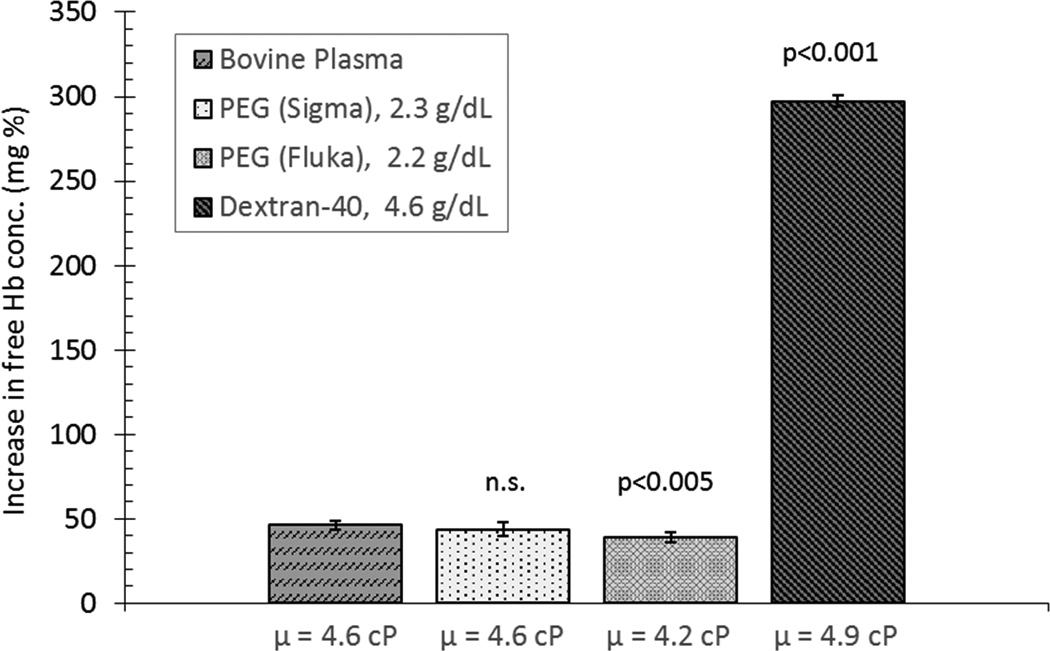
Effect of suspension media with matched viscosities on bovine RBC mechanical fragility, highlighting the protective effects of plasma proteins and PEG versus Dextran-40 solution, adapted from Kameneva et al (72).
Effects of Sublethal RBC Damage on Platelets
Exposure of platelets to supraphysiological mechanical stress can cause their activation and eventual dysfunction with far-reaching implications (73–77). However, in addition to hemolysis-induced platelet activation, there is growing evidence that sublethal erythrocyte damage promotes platelet activation as well. The potential loss of smaller molecules such as ATP and 2–3diphosphoglycerate (2–3 DPG) through 10 Å to 20 Å micropores in stretched RBCs was described by Sutera (6). Alkhamis et al followed with a study that found a 2% release of ADP from RBCs under shear induced platelet aggregation in vitro (78). The leakage of these platelet activating factors was also found after short exposure times of blood in extracorporeal systems (6, 79).
In addition to the agonistic effects of intracellular RBC contents, there is a rheological aberration that contributes to platelet behavior (80). For example, alterations in RBC deformability may modify their effect on platelet transport to boundary walls (81–83). Using chemically-modified RBCs to change deformability, Aarts et al showed an increase in platelet adhesions with increasing RBC rigidity within isolated arteries in vitro (84). This was corroborated in a follow-up, double-blind, clinical, in vivo study using isoxsuprine, a vasodilator found to increase erythrocyte deformability, which supported the previous findings that increased RBC deformability reduces platelet-wall interaction and adhesion (85). The results of computational simulations demonstrated that at a wide range of shear rates and hematocrit values, the rate of platelet adhesion is mainly limited by the frequency of their near-wall rebounding collisions with RBCs (86). These findings suggest that to examine and address thrombotic complications without the consideration of the rheological and biochemical influence of erythrocytes may be a gross oversimplification and should not be overlooked when investigating thrombosis in CADs.
Final Notes
First-generation, pulsatile-flow ventricular assist devices and now continuous-flow, rotary blood pumps has broadened the availability of CADs for patients with heart disease. While mechanical circulatory support has been gaining acceptance, there are growing reports of CAD-associated complications that require consideration including device thrombosis and nonsurgical bleeding (1, 3, 4, 87–92). Due to “first-in, first-out” policies to minimize waste, packed RBC units given to patients within the United States are typically close to their maximum storage duration limit (average 42 days) as dictated by the Food and Drug Administration (FDA). Since collected erythrocytes already have an age distribution before subsequently spending one-third of their lifespan deteriorating ex vivo, it is unsurprising that a 25% loss of donor RBCs within 24 hours of transfusion, while physiologically taxing, is still considered clinically acceptable (93, 94). The consequences of administering blood products (e.g., inflammation, allosensitization, pulmonary hypertension, right heart failure, hepatic congestion, coagulopathy, and mortality) are established, but acute and chronic postoperative transfusions remain prevalent for CAD-implanted patients (88, 95, 96). Although bleeding is unavoidable from surgical insult, one center reported an average packed RBC transfusion rate after implantation of 5.7 units per patient overall with an upper limit of 120 units (97).
With the acknowledgment that there is a significant increase in the mechanical fragility of stored Blood Bank RBCs and the results of sublethal mechanical stress from CADs, it appears paradoxical to transfuse patients on mechanical circulatory support only for these cells to lyse, be prematurely removed by the spleen, and unable to improve microcirculation. In the absence of active bleeding and clinically intolerable anemia, the use of bone marrow-stimulating therapies, such as erythropoietin, should be considered to encourage native production of healthy and well deformable erythrocytes that will remain in circulation longer. For CAD patients who require immediate transfusion, a new policy to infuse only recently donated blood products (for example: less than 7-days-old) and targeting a lower acceptable hemoglobin concentration goal, may maximize the clinical utility of each administered unit (93, 94). Indeed, a study published in the New England Journal of Medicine (NEJM) showed no difference in outcomes for critical condition patients who were transfused when blood hemoglobin dropped below a threshold of 7 g/dL versus 10 g/dL (98). Even more promising, a community teaching hospital saw a 28.6% reduction in associated complications after implementing an interventional monitoring program requiring physician adherence to restrictive transfusion criteria and blood product delivery protocols (99). Furthermore, despite critical care studies that suggest no difference between crystalloid and colloid solutions during general fluid resuscitation (100), for postoperative volume replacement in CAD patients one should consider the use of albumin and/or fresh frozen plasma (FFP) to improve microcirculation by reducing RBC aggregation and to protect RBCs from the increased mechanical stresses associated with blood pumps.
Additional research is needed to further elucidate the factors, thresholds, and boundaries that define the realm of sublethal RBC trauma. We hypothesize a profile can be developed that describes sublethal RBC damage as a function of shear stress, exposure time, and cumulative history. This would be similar to how the “Leverett curve” has been used to suggest the hemolytic threshold between shear stress and exposure time (29, 101–103).
It seems that there is no way to completely avoid mechanical damage to blood cells produced by CADs. However, we should try to curb or diminish the negative impact and complications produced by mechanically damaged blood cells on organ and tissue functionality. While hemolysis is typically considered a symptom that will resolve after addressing the underlying condition, the consequences of free hemoglobin even at subclinical thresholds support exploring the simultaneous use of treatments to minimize its deleterious effects until complete resolution. Another example of such action is the potential modulation of blood traffic in microvessels that can be provided by nanomolar concentrations of blood soluble polymeric molecules, the so called drag-reducing polymers or DRPs (104). It is well known that inside microvessels with diameters below ~300 micron, well-deformable RBCs tend to move toward the vessel center, leaving a cell free layer near the vessel wall (Fåhraeus effect) (105). Due to this natural phenomenon, other blood cells (platelets and leukocytes) and less deformable RBCs flow closer to the vessel wall and at each microvessel bifurcation, a larger fraction of these cells are skimmed to side branches. RBC aggregation additionally stimulates the relocation of normal deformable RBCs toward the vessel center. Therefore an increase in RBC aggregation (e.g., due to mechanical damage and/or inflammation) promotes the margination of less deformable RBCs. Due to this segregation at bifurcations, mechanically damaged RBCs, which are concentrated near the vessel wall, enter into the capillary beds while healthy RBCs are shunted past, resulting in low tissue perfusion. Blood-soluble macromolecules such as polyethylene oxide (PEO) prevent shifting of the well-deformable RBCs toward the center of the vessel, providing equal distribution of blood cells across the vessel lumen (106). Increased near-wall traffic of normal RBCs in microvessels will improve gas exchange, reduce the accumulation and attachment of leukocytes and platelets to the endothelial cells, and stimulate the release of nitric oxide – a well-known vasodilator.
In summary, the rheological and biochemical impact of sublethal damage to blood should not be overlooked when investigating thrombosis, hemolysis, or anemia in CAD patients. Though mechanical trauma in blood pumps is commonly categorized as either erythrocyte destruction or coagulopathy centered, it is important to keep in mind the interdependence of all blood and blood flow components during the design, development, and use of CADs.
Acknowledgments
Financial support: Support was provided in part by the National Institutes of Health (NIH Grant R01 HL089456-01 Multi-scale Model of Thrombosis in Artificial Circulation) and the Cardiovascular Bioengineering Training Program (NIH Training Grant T32-HL076124 for Salim E. Olia).
Abbreviations
- ABO group
most common blood type antigen system relevant to compatibility in transfusion medicine and organ transplantation
- ADP
adenosine diphosphate
- ATP
adenosine triphosphate
- CAD
circulatory assist device
- DRP
drag reducing polymer
- FDA
Food and Drug Administration
- FFP
fresh frozen plasma
- MFI
Mechanical Fragility Index
- PBS
phosphate buffered saline (pH buffered)
- PEG
polyethylene glycol
- PEO
polyethylene oxide
- plfHb
plasma-free hemoglobin
- PVP
polyvinyl pyrrolidone
- RBC
red blood cell
Footnotes
Disclosures
Conflict of interest: None to disclose.
References
- 1.Kirklin JK, Naftel DC, Pagani FD, et al. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant. 2015;34(12):1495–1504. doi: 10.1016/j.healun.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 2.L’Acqua C, Hod E. New perspectives on the thrombotic complications of haemolysis. Br J Haematol. 2015;168(2):175–185. doi: 10.1111/bjh.13183. [DOI] [PubMed] [Google Scholar]
- 3.Whitson BA, Eckman P, Kamdar F, et al. Hemolysis, pump thrombus, and neurologic events in continuous-flow left ventricular assist device recipients. Ann Thorac Surg. 2014;97(6):2097–2103. doi: 10.1016/j.athoracsur.2014.02.041. [DOI] [PubMed] [Google Scholar]
- 4.Starling RC, Moazami N, Silvestry SC, et al. Unexpected abrupt increase in left ventricular assist device thrombosis. N Engl J Med. 2014;370(1):33–40. doi: 10.1056/NEJMoa1313385. [DOI] [PubMed] [Google Scholar]
- 5.Berger S, Salzman EW. Thromboembolic complication of prosthetic devices. Prog Hemost Thromb. 1974;2(0):273–309. [PubMed] [Google Scholar]
- 6.Sutera SP. Flow-induced trauma to blood cells. Circ Res. 1977;41(1):2–8. doi: 10.1161/01.res.41.1.2. [DOI] [PubMed] [Google Scholar]
- 7.Wurzinger L, Opitz R. Hematological principles of hemolysis and thrombosis with special reference to rotary blood pumps. In: Schima H, Thoma H, Weiselthaler G, Wolner E, editors. International Workshop on Rotary Blood Pumps. Vienna: University of Vienna; 1991. pp. 19–25. [Google Scholar]
- 8.Mecozzi G, Milano AD, De Carlo M, et al. Intravascular hemolysis in patients with new-generation prosthetic heart valves: a prospective study. J Thorac Cardiovasc Surg. 2002;123(3):550–556. doi: 10.1067/mtc.2002.120337. [DOI] [PubMed] [Google Scholar]
- 9.Maraj R, Jacobs LE, Ioli A, Kotler MN. Evaluation of hemolysis in patients with prosthetic heart valves. Clin Cardiol. 1998;21(6):387–392. doi: 10.1002/clc.4960210604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ismeno G, Renzulli A, Carozza A, et al. Intravascular hemolysis after mitral and aortic valve replacement with different types of mechanical prostheses. Int J Cardiol. 1999;69(2):179–183. doi: 10.1016/s0167-5273(99)00024-8. [DOI] [PubMed] [Google Scholar]
- 11.Shapira Y, Bairey O, Vatury M, Magen-Nativ H, Prokocimer M, Sagie A. Erythropoietin can obviate the need for repeated heart valve replacement in high-risk patients with severe mechanical hemolytic anemia: case reports and literature review. J Heart Valve Dis. 2001;10(4):431–435. [PubMed] [Google Scholar]
- 12.Blackshear P. Mechanical hemolysis in flowing blood. Englewood Cliffs, NJ: Prentice-Hall; 1972. pp. 501–528. [Google Scholar]
- 13.Seiyama A, Suzuki Y, Tateshi N, Maeda N. Viscous properties of partially hemolyzed erythrocyte suspension. Biorheology. 1991;28:452. [Google Scholar]
- 14.Minneci PC, Deans KJ, Zhi H, et al. Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin. J Clin Invest. 2005;115(12):3409–3417. doi: 10.1172/JCI25040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt A. Zur blutlehre: FCW Vogel. 1892 [Google Scholar]
- 16.Wurzinger LJ, Blasberg P, Schmid-Schönbein H. Towards a concept of thrombosis in accelerated flow: rheology, fluid dynamics, and biochemistry. Biorheology. 1985;22(5):437–450. doi: 10.3233/bir-1985-22507. [DOI] [PubMed] [Google Scholar]
- 17.Brinsfield DE, Hopf MA, Geering RB, Galletti PM. Hematological changes in long-term perfusion. J Appl Physiol. 1962;17:531–534. doi: 10.1152/jappl.1962.17.3.531. [DOI] [PubMed] [Google Scholar]
- 18.Bernstein EF, Castaneda AR, Varco RL. Some biologic limitations to prolonged blood pumping. Trans Am Soc Artif Intern Organs. 1965;11:118–121. doi: 10.1097/00002480-196504000-00023. [DOI] [PubMed] [Google Scholar]
- 19.Bernstein EF, Indeglia RA, Shea MA, Varco RL. Sublethal damage to the red blood cell from pumping. Circulation. 1967;35(4)(Suppl):I226–I233. doi: 10.1161/01.cir.35.4s1.i-226. [DOI] [PubMed] [Google Scholar]
- 20.Indeglia RA, Shea MA, Forstrom R, Bernstein EF. Influence of mechanical factors on erythrocyte sublethal damage. Trans Am Soc Artif Intern Organs. 1968;14:264–272. [PubMed] [Google Scholar]
- 21.Sandza JG, Jr, Clark RE, Weldon CS, Sutera SP. Subhemolytic trauma of erythrocytes: recognition and sequestration by the spleen as a function of shear. Trans Am Soc Artif Intern Organs. 1974;20B:457–462. [PubMed] [Google Scholar]
- 22.Pierce CN, Larson DF, Arabia FA, Copeland JG. Inflammatory mediated chronic anemia in patients supported with a mechanical circulatory assist device. J Extra Corpor Technol. 2004;36(1):10–15. [PubMed] [Google Scholar]
- 23.Pierce CN, Larson DF. Inflammatory cytokine inhibition of erythropoiesis in patients implanted with a mechanical circulatory assist device. Perfusion. 2005;20(2):83–90. doi: 10.1191/0267659105pf793oa. [DOI] [PubMed] [Google Scholar]
- 24.Hung T-C, Butter DB, Yie C, et al. Effects of long-term novacor artificial heart support on blood rheology. ASAIO Trans. 1990;37:M312–M313. [PubMed] [Google Scholar]
- 25.Hung T-C, Butter DB, Kormos RL, et al. Characteristics of blood rheology in patients during Novacor left ventricular assist system support. ASAIO Trans. 1989;35(3):611–613. doi: 10.1097/00002480-198907000-00144. [DOI] [PubMed] [Google Scholar]
- 26.Ganong WF. Review of medical physiology. 15. Norwalk, CT: Appleton & Lange; 1991. [Google Scholar]
- 27.Chien S. Shear dependence of effective cell volume as a determinant of blood viscosity. Science. 1970;168(3934):977–979. doi: 10.1126/science.168.3934.977. [DOI] [PubMed] [Google Scholar]
- 28.Kameneva MV, Ündar A, Antaki JF, Watach MJ, Calhoon JH, Borovetz HS. Decrease in red blood cell deformability caused by hypothermia, hemodilution, and mechanical stress: factors related to cardiopulmonary bypass. ASAIO J. 1999;45(4):307–310. doi: 10.1097/00002480-199907000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Lee SS, Antaki JF, Kameneva MV, et al. Strain hardening of red blood cells by accumulated cyclic supraphysiological stress. Artif Organs. 2007;31(1):80–86. doi: 10.1111/j.1525-1594.2007.00344.x. [DOI] [PubMed] [Google Scholar]
- 30.Marascalco PJ, Ritchie SP, Snyder TA, Kameneva MV. Development of standard tests to examine viscoelastic properties of blood of experimental animals for pediatric mechanical support device evaluation. ASAIO J. 2006;52(5):567–574. doi: 10.1097/01.mat.0000242248.66083.48. [DOI] [PubMed] [Google Scholar]
- 31.Baskurt OK, Uyuklu M, Meiselman HJ. Protection of erythrocytes from sub-hemolytic mechanical damage by nitric oxide mediated inhibition of potassium leakage. Biorheology. 2004;41(2):79–89. [PubMed] [Google Scholar]
- 32.Lee SS, Ahn KH, Lee SJ, Sun K, Goedhart PT, Hardeman MR. Shear induced damage of red blood cells monitored by the decrease of their deformability. Korea-Australia Rheology Journal. 2004;16(3):141–146. [Google Scholar]
- 33.Mizuno T, Tsukiya T, Taenaka Y, et al. Ultrastructural alterations in red blood cell membranes exposed to shear stress. ASAIO J. 2002;48(6):668–670. doi: 10.1097/00002480-200211000-00017. [DOI] [PubMed] [Google Scholar]
- 34.Kameneva MV, Antaki JF, Borovetz HS, et al. Mechanisms of red blood cell trauma in assisted circulation. Rheologic similarities of red blood cell transformations due to natural aging and mechanical stress. ASAIO J. 1995;41(3):M457–M460. [PubMed] [Google Scholar]
- 35.Dao KM, O’Rear EA, Johnson AE, Peitersen SE. Sensitivity of the erythrocyte membrane bilayer to subhemolytic mechanical trauma as detected by fluorescence anisotropy. Biorheology. 1994;31(1):69–76. doi: 10.3233/bir-1994-31106. [DOI] [PubMed] [Google Scholar]
- 36.Chien S. Red cell deformability and its relevance to blood flow. Annu Rev Physiol. 1987;49:177–192. doi: 10.1146/annurev.ph.49.030187.001141. [DOI] [PubMed] [Google Scholar]
- 37.Baskurt OK, Hardeman MR, Uyuklu M, et al. Comparison of three commercially available ektacytometers with different shearing geometries. Biorheology. 2009;46(3):251–264. doi: 10.3233/BIR-2009-0536. [DOI] [PubMed] [Google Scholar]
- 38.Fåhræus R, Lindqvist T. The viscosity of the blood in narrow capillary tubes. Am J Physiol. 1931;96:562–568. [Google Scholar]
- 39.Seiyama A, Suzuki Y, Maeda N. Increased viscosity of erythrocyte suspension upon hemolysis. Colloid Polym Sci. 1993;271:63–69. [Google Scholar]
- 40.Ami RB, Barshtein G, Zeltser D, et al. Parameters of red blood cell aggregation as correlates of the inflammatory state. Am J Physiol Heart Circ Physiol. 2001;280(5):H1982–H1988. doi: 10.1152/ajpheart.2001.280.5.H1982. [DOI] [PubMed] [Google Scholar]
- 41.Gu YJ, Graaff R, de Hoog E, et al. Influence of hemodilution of plasma proteins on erythrocyte aggregability: an in vivo study in patients undergoing cardiopulmonary bypass. Clin Hemorheol Microcirc. 2005;33(2):95–107. [PubMed] [Google Scholar]
- 42.Carney E, Litwak K, Weiss W Group AMW; Animal Models Working Group. Animal models for pediatric circulatory support device pre-clinical testing: National heart, lung, and blood institute pediatric assist device contractor’s meeting animal models working group. ASAIO J. 2009;55(1):6–9. doi: 10.1097/MAT.0b013e318198e11c. [DOI] [PubMed] [Google Scholar]
- 43.Kameneva MV, Antaki JF. Mechanical trauma to blood. In: Baskurt OK, Harderman MR, Rampling MW, Meiselman HJ, editors. Handbook of hemorheology and hemodynamics. Amsterdam: IOS Press; 2007. pp. 206–227. [Google Scholar]
- 44.Kameneva MV, Antaki JF, Butler KC, et al. A sheep model for the study of hemorheology with assisted circulation. Effect of an axial flow blood pump. ASAIO J. 1994;40(4):959–963. [PubMed] [Google Scholar]
- 45.Maeda N, Imaizumi K, Sekiya M, Shiga T. Rheological characteristics of desialylated erythrocytes in relation to fibrinogen-induced aggregation. Biochimica et Biophysica Acta (BBA) Biomembranes. 1984;776:151–158. doi: 10.1016/0005-2736(84)90261-x. [DOI] [PubMed] [Google Scholar]
- 46.Hadengue A, Razavian SM, Del-Pino M, Simon A, Levenson J. Influence of sialic acid on erythrocyte aggregation in hypercholesterolemia. Thromb Haemost. 1996;76(6):944–949. [PubMed] [Google Scholar]
- 47.Hadengue AL, Del-Pino M, Simon A, Levenson J. Erythrocyte disaggregation shear stress, sialic acid, and cell aging in humans. Hypertension. 1998;32(2):324–330. doi: 10.1161/01.hyp.32.2.324. [DOI] [PubMed] [Google Scholar]
- 48.Baskurt OK, Uyuklu M, Ulker P, et al. Comparison of three instruments for measuring red blood cell aggregation. Clin Hemorheol Microcirc. 2009;43(4):283–298. doi: 10.3233/CH-2009-1240. [DOI] [PubMed] [Google Scholar]
- 49.Hardeman MR, Goedhart PT, Shin S. Methods in hemorheology. In: Baskurt OK, Hardeman MR, Rampling MW, Meiselman HJ, editors. Handbook of hemorheology and hemodynamics. Amsterdam: IOS Press; 2007. pp. 242–266. [Google Scholar]
- 50.Kameneva MV, Antaki JF, Konishi H, et al. Effect of perfluorochemical emulsion on blood trauma and hemorheology. ASAIO J. 1994;40(3):M576–M579. doi: 10.1097/00002480-199407000-00064. [DOI] [PubMed] [Google Scholar]
- 51.Kameneva MV, Watach MJ, Litwak P, et al. Chronic animal health assessment during axial ventricular assistance: importance of hemorheologic parameters. ASAIO J. 1999;45(3):183–188. doi: 10.1097/00002480-199905000-00015. [DOI] [PubMed] [Google Scholar]
- 52.Gu L, Smith WA, Chatzimavroudis GP. Mechanical fragility calibration of red blood cells. ASAIO J. 2005;51(3):194–201. doi: 10.1097/01.mat.0000161940.30190.6d. [DOI] [PubMed] [Google Scholar]
- 53.Herbertson LH, Olia SE, Daly AR, et al. Multi-laboratory study of flow-induced hemolysis using the fda benchmark nozzle model. Artif Organs. 2014 doi: 10.1111/aor.12368. (accepted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Böhler T, Leo A, Stadler A, Linderkamp O. Mechanical fragility of erythrocyte membrane in neonates and adults. Pediatr Res. 1992;32(1):92–96. doi: 10.1203/00006450-199207000-00018. [DOI] [PubMed] [Google Scholar]
- 55.Kameneva MV, Garrett KO, Watach MJ, Borovetz HS. Red blood cell aging and risk of cardiovascular diseases. Clin Hemorheol Microcirc. 1998;18(1):67–74. [PubMed] [Google Scholar]
- 56.Kameneva MV, Watach MJ, Borovetz HS. Gender difference in rheologic properties of blood and risk of cardiovascular diseases. Clin Hemorheol Microcirc. 1999;21(3–4):357–363. [PubMed] [Google Scholar]
- 57.Yazer MH, Waters JH, Elkin KR, Rohrbaugh ME, Kameneva MV. A comparison of hemolysis and red cell mechanical fragility in blood collected with different cell salvage suction devices. Transfusion. 2008;48(6):1188–1191. doi: 10.1111/j.1537-2995.2008.01670.x. [DOI] [PubMed] [Google Scholar]
- 58.Raval JS, Waters JH, Seltsam A, et al. The use of the mechanical fragility test in evaluating sublethal RBC injury during storage. Vox Sang. 2010;99(4):325–331. doi: 10.1111/j.1423-0410.2010.01365.x. [DOI] [PubMed] [Google Scholar]
- 59.Daly A, Raval JS, Waters JH, Yazer MH, Kameneva MV. Effect of blood bank storage on the rheological properties of male and female donor red blood cells. Clin Hemorheol Microcirc. 2014;56(4):337–345. doi: 10.3233/CH-131754. [DOI] [PubMed] [Google Scholar]
- 60.Vandromme MJ, McGwin G, Jr, Weinberg JA. Blood transfusion in the critically ill: does storage age matter? Scand J Trauma Resusc Emerg Med. 2009;17:35. doi: 10.1186/1757-7241-17-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sparrow RL. Red blood cell storage duration and trauma. Transfus Med Rev. 2015;29(2):120–126. doi: 10.1016/j.tmrv.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 62.Kamada T, McMillan DE, Sternlieb JJ, Björk VO, Otsuji S. Albumin prevents erythrocyte crenation in patients undergoing extracorporeal circulation. Scand J Thorac Cardiovasc Surg. 1988;22(2):155–158. doi: 10.3109/14017438809105949. [DOI] [PubMed] [Google Scholar]
- 63.Armstrong JK, Meiselman HJ, Fisher TC. Inhibition of red blood cell-induced platelet aggregation in whole blood by a nonionic surfactant, poloxamer 188 (RheothRx injection) Thromb Res. 1995;79(5–6):437–450. doi: 10.1016/0049-3848(95)00134-d. [DOI] [PubMed] [Google Scholar]
- 64.Kameneva MV, Antaki JF, Yeleswarapu KK, Watach MJ, Griffith BP, Borovetz HS. Plasma protective effect on red blood cells exposed to mechanical stress. ASAIO J. 1997;43(5):M571–M575. [PubMed] [Google Scholar]
- 65.Kameneva MV, Borovetz HS, Antaki JF, et al. Effect of perfluorochemical emulsion on hemorheology and shear induced blood trauma. In: Nemoto E, Lamanna J, Cooper C, et al., editors. Oxygen transport to tissue XVIII. New York: Springer; 1997. pp. 383–390. [DOI] [PubMed] [Google Scholar]
- 66.Faraoni D, Willems A, Savan V, Demanet H, De Ville A, Van der Linden P. Plasma fibrinogen concentration is correlated with postoperative blood loss in children undergoing cardiac surgery. A retrospective review. Eur J Anaesthesiol. 2014;31(6):317–326. doi: 10.1097/EJA.0000000000000043. [DOI] [PubMed] [Google Scholar]
- 67.Miao X, Liu J, Zhao M, et al. Evidence-based use of FFP: the influence of a priming strategy without FFP during CPB on postoperative coagulation and recovery in pediatric patients. Perfusion. 2015;30(2):140–147. doi: 10.1177/0267659114537328. [DOI] [PubMed] [Google Scholar]
- 68.Agarwal HS, Barrett SS, Barry K, et al. Association of blood products administration during cardiopulmonary bypass and excessive post-operative bleeding in pediatric cardiac surgery. Pediatr Cardiol. 2015;36(3):459–467. doi: 10.1007/s00246-014-1034-z. [DOI] [PubMed] [Google Scholar]
- 69.Moret E, Jacob MW, Ranucci M, Schramko AA. Albumin— beyond fluid replacement in cardiopulmonary bypass surgery: Why, how, and when? Semin Cardiothorac Vasc Anesth. 2014;18(3):252–259. doi: 10.1177/1089253214535667. [DOI] [PubMed] [Google Scholar]
- 70.Cho JE, Shim JK, Song JW, Lee HW, Kim DH, Kwak YL. Effect of 6% hydroxyethyl starch 130/0.4 as a priming solution on coagulation and inflammation following complex heart surgery. Yonsei Med J. 2014;55(3):625–634. doi: 10.3349/ymj.2014.55.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sümpelmann R, Schürholz T, Marx G, Zander R. Protective effects of plasma replacement fluids on erythrocytes exposed to mechanical stress. Anaesthesia. 2000;55(10):976–979. doi: 10.1046/j.1365-2044.2000.01531.x. [DOI] [PubMed] [Google Scholar]
- 72.Kameneva MV, Repko BM, Krasik EF, Perricelli BC, Borovetz HS. Polyethylene glycol additives reduce hemolysis in red blood cell suspensions exposed to mechanical stress. ASAIO J. 2003;49(5):537–542. doi: 10.1097/01.mat.0000084176.30221.cf. [DOI] [PubMed] [Google Scholar]
- 73.Hellums JD. 1993 Whitaker Lecture: biorheology in thrombosis research. Ann Biomed Eng. 1994;22(5):445–455. doi: 10.1007/BF02367081. [DOI] [PubMed] [Google Scholar]
- 74.Rubenstein DA, Yin W. Quantifying the effects of shear stress and shear exposure duration regulation on flow induced platelet activation and aggregation. J Thromb Thrombolysis. 2010;30(1):36–45. doi: 10.1007/s11239-009-0397-0. [DOI] [PubMed] [Google Scholar]
- 75.Kawahito K, Mohara J, Misawa Y, Fuse K. Platelet damage caused by the centrifugal pump: in vitro evaluation by measuring the release of alpha-granule packing proteins. Artif Organs. 1997;21(10):1105–1109. doi: 10.1111/j.1525-1594.1997.tb00450.x. [DOI] [PubMed] [Google Scholar]
- 76.Koster A, Loebe M, Hansen R, et al. Alterations in coagulation after implantation of a pulsatile Novacor LVAD and the axial flow MicroMed DeBakey LVAD. Ann Thorac Surg. 2000;70(2):533–537. doi: 10.1016/s0003-4975(00)01404-1. [DOI] [PubMed] [Google Scholar]
- 77.Brown CH, III, Leverett LB, Lewis CW, Alfrey CP, Jr, Hellums JD. Morphological, biochemical, and functional changes in human platelets subjected to shear stress. J Lab Clin Med. 1975;86(3):462–471. [PubMed] [Google Scholar]
- 78.Alkhamis TM, Beissinger RL, Chediak JR. Red blood cell effect on platelet adhesion and aggregation in low-stress shear flow. Myth or fact? ASAIO Trans. 1988;34(3):868–873. [PubMed] [Google Scholar]
- 79.Nevaril CG, Lynch EC, Alfrey CP, Jr, Hellums JD. Erythrocyte damage and destruction induced by shearing stress. J Lab Clin Med. 1968;71(5):784–790. [PubMed] [Google Scholar]
- 80.Turitto VT, Weiss HJ. Red blood cells: their dual role in thrombus formation. Science. 1980;207(4430):541–543. doi: 10.1126/science.7352265. [DOI] [PubMed] [Google Scholar]
- 81.Keller KH. Effect of fluid shear on mass transport in flowing blood. Fed Proc. 1970;30(5):1591–1599. [PubMed] [Google Scholar]
- 82.Wang NH, Keller KH. Solute transport induced by erythrocyte motions in shear flow. Trans Am Soc Artif Intern Organs. 1979;25:14–18. doi: 10.1097/00002480-197902500-00003. [DOI] [PubMed] [Google Scholar]
- 83.Aarts PA, Bolhuis PA, Sakariassen KS, Heethaar RM, Sixma JJ. Red blood cell size is important for adherence of blood platelets to artery subendothelium. Blood. 1983;62(1):214–217. [PubMed] [Google Scholar]
- 84.Aarts PA, Heethaar RM, Sixma JJ. Red blood cell deformability influences platelets—vessel wall interaction in flowing blood. Blood. 1984;64(6):1228–1233. [PubMed] [Google Scholar]
- 85.Aarts PA, Banga JD, van Houwelingen HC, Heethaar RM, Sixma JJ. Increased red blood cell deformability due to isoxsuprine administration decreases platelet adherence in a perfusion chamber: a double-blind cross-over study in patients with intermittent claudication. Blood. 1986;67(5):1474–1481. [PubMed] [Google Scholar]
- 86.Tokarev AA, Butylin AA, Ataullakhanov FI. Platelet adhesion from shear blood flow is controlled by near-wall rebounding collisions with erythrocytes. Biophys J. 2011;100(4):799–808. doi: 10.1016/j.bpj.2010.12.3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rosenthal JL, Starling RC. Coagulopathy in mechanical circulatory support: A fine balance. Curr Cardiol Rep. 2015;17(12):114. doi: 10.1007/s11886-015-0670-0. [DOI] [PubMed] [Google Scholar]
- 88.Schaffer JM, Arnaoutakis GJ, Allen JG, et al. Bleeding complications and blood product utilization with left ventricular assist device implantation. Ann Thorac Surg. 2011;91(3):740–747. doi: 10.1016/j.athoracsur.2010.11.007. discussion 747–749. [DOI] [PubMed] [Google Scholar]
- 89.John R, Lee S. The biological basis of thrombosis and bleeding in patients with ventricular assist devices. J Cardiovasc Transl Res. 2009;2(1):63–70. doi: 10.1007/s12265-008-9072-7. [DOI] [PubMed] [Google Scholar]
- 90.Stern DR, Kazam J, Edwards P, et al. Increased incidence of gastrointestinal bleeding following implantation of the HeartMate II LVAD. J Card Surg. 2010;25(3):352–356. doi: 10.1111/j.1540-8191.2010.01025.x. [DOI] [PubMed] [Google Scholar]
- 91.Amer S, Shah P, Hassan S. Gastrointestinal bleeding with continuous-flow left ventricular assist devices. Clin J Gastroenterol. 2015;8(2):63–67. doi: 10.1007/s12328-015-0551-5. [DOI] [PubMed] [Google Scholar]
- 92.Stulak JM, Lee D, Haft JW, et al. Gastrointestinal bleeding and subsequent risk of thromboembolic events during support with a left ventricular assist device. J Heart Lung Transplant. 2014;33(1):60–64. doi: 10.1016/j.healun.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 93.Hod EA, Brittenham GM, Billote GB, et al. Transfusion of human volunteers with older, stored red blood cells produces extravascular hemolysis and circulating non-transferrin-bound iron. Blood. 2011;118(25):6675–6682. doi: 10.1182/blood-2011-08-371849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Koch CG, Figueroa PI, Li L, Sabik JF, III, Mihaljevic T, Blackstone EH. Red blood cell storage: how long is too long? Ann Thorac Surg. 2013;96(5):1894–1899. doi: 10.1016/j.athoracsur.2013.05.116. [DOI] [PubMed] [Google Scholar]
- 95.Goldstein DJ, Seldomridge JA, Chen JM, et al. Use of aprotinin in LVAD recipients reduces blood loss, blood use, and perioperative mortality. Ann Thorac Surg. 1995;59(5):1063–1067. doi: 10.1016/0003-4975(95)00086-z. discussion 1068. [DOI] [PubMed] [Google Scholar]
- 96.Goldstein DJ, Beauford RB. Left ventricular assist devices and bleeding: adding insult to injury. Ann Thorac Surg. 2003;75(6)(Suppl):S42–S47. doi: 10.1016/s0003-4975(03)00478-8. [DOI] [PubMed] [Google Scholar]
- 97.Uriel N, Pak S-W, Jorde UP, et al. Acquired von Willebrand syndrome after continuous-flow mechanical device support contributes to a high prevalence of bleeding during long-term support and at the time of transplantation. J Am Coll Cardiol. 2010;56(15):1207–1213. doi: 10.1016/j.jacc.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 98.Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340(6):409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 99.Politsmakher A, Doddapaneni V, Seeratan R, Dosik H. Effective reduction of blood product use in a community teaching hospital: when less is more. Am J Med. 2013;126(10):894–902. doi: 10.1016/j.amjmed.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 100.Finfer S, Bellomo R, Boyce N, French J, Myburgh J, Norton R SAFEStudy Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350(22):2247–2256. doi: 10.1056/NEJMoa040232. [DOI] [PubMed] [Google Scholar]
- 101.Yeleswarapu KK, Antaki JF, Kameneva MV, Rajagopal KR. A mathematical model for shear-induced hemolysis. Artif Organs. 1995;19(7):576–582. doi: 10.1111/j.1525-1594.1995.tb02384.x. [DOI] [PubMed] [Google Scholar]
- 102.Dobbe JG, Streekstra GJ, Hardeman MR, Ince C, Grimbergen CA. Measurement of the distribution of red blood cell deformability using an automated rheoscope. Cytometry. 2002;50(6):313–325. doi: 10.1002/cyto.10171. [DOI] [PubMed] [Google Scholar]
- 103.Leverett LB, Hellums JD, Alfrey CP, Lynch EC. Red blood cell damage by shear stress. Biophys J. 1972;12(3):257–273. doi: 10.1016/S0006-3495(72)86085-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kameneva MV. Microrheological effects of drag-reducing polymers in vitro and in vivo. Int J Eng Sci. 2012;59:168–183. [Google Scholar]
- 105.Fåhraeus R. The suspension stability of the blood. [Accessed February 19, 2016];Physiol Rev. 1929 9(2):241–274. http://physrev.physiology.org/content/9/2/241. [Google Scholar]
- 106.Marhefka JN, Zhao R, Wu ZJ, Velankar SS, Antaki JF, Kameneva MV. Drag reducing polymers improve tissue perfusion via modification of the RBC traffic in microvessels. Biorheology. 2009;46(4):281–292. doi: 10.3233/BIR-2009-0543. [DOI] [PMC free article] [PubMed] [Google Scholar]


