Abstract
Objective
To investigate the association of morphine exposure in very preterm infants with cerebral volumes and neurodevelopmental outcome from birth through middle childhood.
Study design
Observational study of very preterm infants in the Victorian Infant Brain Study cohort. 230 infants born <30 weeks’ gestational age or <1,250 g were recruited from all admissions to the neonatal intensive care unit (NICU) of the Royal Women’s Hospital. 57 (25%) infants received morphine analgesia during their NICU stay at the attending physician’s discretion. Primary outcomes were regional brain volumes at term and 7 years; neurobehavioral performance at term; and cognitive, motor, emotional, behavioral, communication, and executive function scores at age 2 and 7 years. Linear regressions were used to compare outcomes between participants who did and did not receive morphine.
Results
At term, preterm infants who received morphine had similar rates of grey matter injury to no-morphine infants, but a trend towards smaller cortical volumes in the orbitofrontal (pleft=0.002, pright=0.01) and subgenual (pleft=0.01) regions. At seven years, cortical volumes did not differ between groups. At 2 years, morphine-exposed children were more likely to show behavioral dysregulation (p=0.007) than no-morphine children, but at seven years no detrimental impacts of morphine on neurobehavioral outcome were observed.
Conclusions
Low-dose morphine analgesia received during neonatal intensive care was associated with early alterations in cerebral structure and short-term neurobehavioral problems that did not persist into childhood.
Keywords: Preterm infants, Neurodevelopmental, Neonatal intensive care, Analgesics, Opioids
Preterm infants are highly susceptible to the harmful effects of pain and stress to which they are routinely exposed in the neonatal intensive care unit (NICU).1,2 NICU patients receive a daily average of 5 to 15 procedures classified as uncomfortable, painful, or stressful.3 Exposure to a greater number of stressors in the NICU has been reported to result in smaller frontal and parietal brain widths, altered connectivity in the temporal lobes, and abnormal neurobehavior at term equivalent.4 Consistent with these findings, higher neonatal exposure to procedural pain is associated with reduced white matter volume and subcortical gray matter maturation, as determined by diffusion magnetic resonance imaging (MRI) and MR spectroscopy, by 40 weeks.5 To ameliorate the consequences of painful neonatal procedures the administration of opioid analgesics is a common NICU practice.6
Morphine is one of the more common and well-studied opioids administered to preterm infants in the NICU, but concerns over the neurological consequences of morphine exposure remain2. In animal studies of opioid exposure, alterations in neuronal proliferation and survival have been detected. Chronic exposure to morphine produced neuronal degeneration7, and perinatal exposure reduced cortical neuron number and density8, reduced basilar dendritic growth9, and decreased metabolic activity in motor areas of the brain.10 These alterations were often accompanied by behavioral changes. Rats exposed prenatally to a long-acting opiate demonstrated more reference and working memory errors in the radial arm maze11, and postnatal morphine exposure impaired reward-mediated learning in adulthood.12
In human studies, reports on the neurological consequences of morphine exposure have been inconsistent. Neonatal exposure to continuous morphine infusions to reduce pain did not result in neurodevelopmental benefit,13, 14 and concerns have been raised for subtle neurobehavioral differences at term in those exposed to morphine.15 At age 5-7 years, children randomized to continuous morphine infusions during the NICU period had smaller head circumferences and body size, in addition to poorer performance on tests of short-term memory and a higher likelihood of social problems than those given a placebo infusion.16 However, in a separate trial, five year-olds who received morphine as preterm infants performed similarly on tests of movement, behavior, and intelligence compared with children who had received no morphine.17 In this study, we aimed to compare the short- and long-term outcomes of very preterm infants with and without exposure to low-dose morphine. Short-term outcomes include brain volumes and neurobehavior performance at term equivalent age, and longer term outcomes include cognitive and behavioral outcomes at 2 and 7 years and brain volumes at 7 years.
Methods
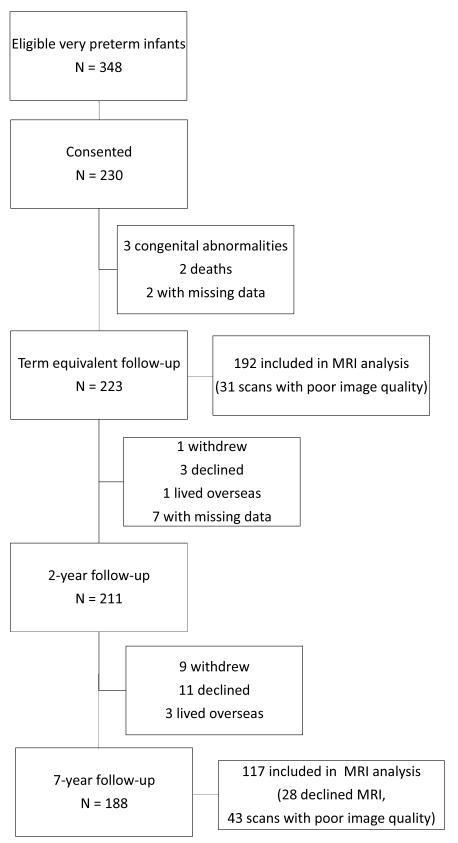
In this longitudinal cohort study, we enrolled 230 infants born at <30 weeks’ gestation or <1250 g at the Royal Women’s Hospital between July 2001 and December 2003. Patients were recruited consecutively from all eligible admissions to the hospital. Details of subject eligibility, recruitment, and follow-up at term, 2-years of age, and 7-years of age are available in the Figure (available at www.jpeds.com). Newborns received morphine analgesia at the attending physician’s discretion; no other form of pharmacologic sedation or analgesia, including benzodiazepines, were employed in the NICU, with the exception of intraoperative anesthesia. Ethical permission was granted for each stage of the study by the Human Research Ethics Committees of the Royal Women’s and Royal Children’s Hospitals. Parents of participants provided written informed consent.
Figure 1.
online only: Subject eligibility, recruitment, and follow-up
Term Equivalent MRI
Brain magnetic resonance imaging (MRI) was performed without sedation between 38 to 42 weeks’ postmenstrual age (PMA) using a 1.5 T General Electric Signa System (GE Medical Systems), with the following sequences: (i) a three-dimensional spoiled gradient recalled (SPGR) sequence (1.2 mm coronal slices, flip angle 45°, repetition time (TR) 35 ms, echo time (TE) 9 ms, field of view [FOV] 21 × 15 cm2, matrix 256 × 192); (ii) a double-echo (proton density and T2- weighted) spin-echo sequence (2 mm axial slices, TR 4000 ms, TE 60 and 160 ms, FOV 22 × 16 cm2, matrix 256 × 192, interpolated 512 × 512, interleaved acquisition); and (iii) a linescan sequence (4-6 mm axial slices with a 0.5-1 mm gap, TR 2139 ms, TE 78 ms, FOV 22 cm, matrix 128×128, 2 images at b=5 s/mm2, 6 images at b=700 s/mm2. The diffusion gradients for b=700 s/mm2 were oriented in six directions). Qualitative MR images were classified according to degree of intraventricular hemorrhage (IVH), cerebellar hemorrhage, white matter (WM) abnormality, and grey matter (GM) abnormality as has been previously reported.18
Quantitative volumetric MR analysis was undertaken by a single operator. The methods for total brain and hippocampal segmentation have been described previously.19,20 For total brain segmentation, brain tissue was separated into myelinated and unmyelinated WM, cortical and deep nuclear GM, and cerebrospinal fluid (CSF). Volumes were automatically parcellated into eight cortical regions per hemisphere, as described in Shah et al.21
7-year MRI
Data at age 7 years were obtained on a Siemens 3-T Trio imaging systems using a 32-channel, phased array RF head coil. The acquisition included a 3D MPRAGE T1-weighted sequence (TR 30 ms, TE 4.1 ms, flip angle 8°, 256 × 256 matrix; 0.9-mm isotropic voxels).
Cortical reconstruction and volumetric segmentation were performed with the Freesurfer v4.4 analysis suite according to the procedure reported in Desikan et al.22 The output was manually edited by three operators, with an inter-rater reliability of 0.98. Freesurfer generated 34 subregions for each hemisphere using the Desikan-Killian Atlas. These subregions were then grouped into 18 hemispheric regions (Table I; available at www.jpeds.com).
Table 1.
online only: Parcellation at seven years
| Lobe | Region | Grouped Freesurfer parcellation |
|---|---|---|
| Frontal | Postcentral | Postcentral |
| Precentral | Precentral | |
| Superior frontal | Superior frontal | |
| Middle frontal | Caudal middle frontal + rostral middle frontal + frontal pole | |
| Inferior frontal | Pars opercularis + pars triangularis | |
| Orbital frontal | Pars orbitalis + lateral orbital frontal + medial orbital frontal | |
|
| ||
| Temporal | Superior temporal | Superior temporal + transverse temporal |
| Lateral temporal | Middle temporal + banks of superior temporal | |
| Inferior temporal | Inferior temporal + fusiform | |
| Medial temporal | Temporal pole + entorhinal + parahippocampal | |
|
| ||
| Parietal | Lateral parietal | Inferior parietal + superior parietal |
| Supramarginal | Supramarginal | |
| Medial occipital parietal | Cuneus + precuneus + paracentral lobule | |
|
| ||
| Occipital | Lateral occipital | Lateral occipital |
| Medial occipital | Lingual + pericalcarine | |
|
| ||
| Other | Cingulate | Rostral anterior cingulate + caudal anterior cingulate + posterior cingulate |
| Isthmus | Isthmus cingulate | |
| Insula | Insula | |
Neurodevelopmental assessment
At term equivalent PMA, neurobehavior was evaluated with the Hammersmith Neonatal Neurologic Examination (HNNE).23 At 24 months’ corrected age, survivors were assessed by examiners blinded to the infant’s birth weight and gestational age. Clinical assessment included outcomes such as cerebral palsy and other neurological impairments. Cognitive and motor development were assessed with the Mental Development Index (MDI) and the Psychomotor Development Index (PDI) of the Bayley Scales of Infant Development – 2nd Edition.24 The prepublication version of the Infant-Toddler Social and Emotional Assessment (ITSEA) is a parent report questionnaire that was used to measure emotional and behavioral problems.25 Communication skills were evaluated using the Communication Symbolic Behavior Scale Developmental Profile (CSBS-DP) Infant-Toddler Checklist, also completed by parents.26 Executive function, specifically inhibition and working memory, was assessed with the Delayed Alternation task27,28 and the Behavior Rating Inventory of Executive Function, Preschool Version29.
At 7 years of age, participants underwent detailed cognitive, educational, and behavioral assessment by trained examiners blinded to gestational age and clinical outcomes. General intelligence was measured with the Wechsler Abbreviated Scale of Intelligence (WASI),30 motor functioning with the Movement Assessment Battery for Children – 2nd Edition (MABC-2)31, emotional and behavioral problems with the Strengths and Difficulties Questionnaire (SDQ),31,32 language skills with the Clinical Evaluation of Language Fundamentals – 4th Edition (CELF4)33 executive function with the parent report form of the Behavior Rating Inventory of Executive Function (BRIEF),29 and basic educational skills with the Wide Range Achievement Test (WRAT-4)34. The variables of interest are summarized in Table II (available at www.jpeds.com).
Table 2.
online only: Variables of interest from the neurodevelopmental assessments
| Domain | Term | 2 years | 7 years |
|---|---|---|---|
| Motor and reflexes | HNNE | Psychomotor Development Index (PDI) from the BSID-II |
Total score from the MABC2 |
| Cognitive development | Mental Development Index (MDI) from the BSID-II |
Full-scale IQ from the WASI | |
| Emotional & behavior development |
Externalizing, internalizing, dysregulation and competence scales from the ITSEA |
Scales from SDQ | |
| Language development & basic educational skills |
Speech and social scores from the CSBS-DP | Core language index from the CELF-4; Reading, spelling, math computation from the WRAT-4 |
|
| Executive function | Perseverative behavior on DA task; Scale scores from the BRIEF-P |
Scale scores from the BRIEF |
HNNE – Hammersmith Neonatal Neurologic Examination; BSID-II – Bayley Scales of Infant Development, 2nd Edition, MABC2 – Movement Assessment Battery for Children
Statistical Analyses
Statistical analyses were performed in SPSS v20 (Chicago, Illinois). Clinical outcomes and qualitative brain injury were compared between participants who received morphine as infants and no-morphine controls using independent sample t-test for continuous variables and chi-square for categorical variables. Brain volumes and neurobehavioral outcomes were compared using multivariate linear regression (multivariate analysis of covariance) to adjust for major confounders (gestational age, sex, Clinical Risk Index for Babies [CRIB] score, hours on ventilation, intrauterine growth restriction, incidences of sepsis, exposure to postnatal dexamethasone, white matter injury, days on total parenteral nutrition, neonatal surgery [patent ductus arteriosus ligation, bowel resection for spontaneous intestinal perforation or necrotizing enterocolitis, and/or inguinal hernia repair], and length of NICU stay). Dose-dependent effects and the effect of modality (bolus, infusion, or combination) were assessed using a partial correlation controlling for the listed confounders. Statistical significance was regarded at p<0.01 reflecting a Bonferroni correction to compensate for measures across five domains (brain volumes at term and 7 years, neurobehavior at term, and development at 2 and 7 years).
Results
Participants (n=223) were assessed at term equivalent age. Fifty-seven participants received morphine in the NICU (median dose 0.79 mg/kg, interquartile range 0.12-0.95 mg/kg, range 0.1-5.3 mg/kg). Thirty-two participants received only boluses; twenty-one received a mixture of boluses and infusion; four received an infusion only; no clinical factors differed between these three subgroups. One hundred and sixty-six infants received no sedative or analgesic medication while in the NICU.
Table III summarizes the clinical characteristics of the morphine and no-morphine groups. The morphine group had a lower gestational age at birth, lower birth weight, higher mean CRIB score, and longer mean NICU stay than the no-morphine group. Moreover, the morphine group required longer courses of respiratory support and were more likely to receive postnatal dexamethasone. Incidences of grade III or IV intraventricular hemorrhage (IVH), cerebellar hemorrhage, moderate or severe white matter injury, abnormal GM, ventricular dilation, cerebral palsy, and other neurological impairments were similar between groups. No dose-dependent effects of morphine on clinical neonatal outcomes were found. The clinical characteristics of the subgroup of patients with brain volumes analyzed at seven years of age are summarized in Table IV (available at www.jpeds.com). There were no significant differences in the clinical characteristics of patients included in analyses of brain volume at age seven and those who were not.
Table 3.
Clinical Outcomes
| Morphine | No Morphine | |
|---|---|---|
| Number of Infants | 57 | 166 |
| Male sex (%) | 30 (53%) | 83 (50%) |
| Gestational Age (weeks)* | 26.8 ± 1.6 | 27.7 ± 2.0 |
| Birth weight (g)* | 887.9 ± 212.0 | 979.1 ± 226.6 |
| Small for Gestational Age | 4 (7%) | 17 (10%) |
| Multiparity | 27 (47%) | 68 (41%) |
| Antenatal betamethasone | 51 (90%) | 147 (89%) |
| Sepsis | 28 (49%) | 71 (43%) |
| Total Hours of invasive ventilation* | 473 ± 554 | 195 ± 359 |
| 25% | 80 | 0 |
| 50% | 258 | 33 |
| 75% | 810 | 192 |
| Total Hours of CPAP | 475 ± 368 | 381 ± 381 |
| 25% | 143 | 72 |
| 50% | 432 | 249 |
| 75% | 725 | 636 |
| Total Hours of Oxygen* | 2108 ± 2637 | 1038 ± 2224 |
| 25% | 336 | 41 |
| 50% | 1052 | 199 |
| 75% | 2635 | 1289 |
| Postnatal dexamethasone* | 12 (21%) | 9 (5%) |
| Neonatal surgery | 9 (16%) | 27 (16%) |
| CRIB score* | 4.8 ± 3.2 | 3.4 ± 2.9 |
| Days on parenteral nutrition | 14 ± 11 | 11 ± 10 |
| Length of hospital stay (days)* | 106 ± 45 | 85 ± 43 |
| Brain Injury at Term | ||
| High Grade IVH | 1 (2%) | 8 (5%) |
| Cerebellar Hemorrhage | 3 (5%) | 7 (4%) |
| Abnormal Gray Matter | 14 (25%) | 41 (25%) |
|
Moderate/Severe White Matter
Injury |
11 (19%) | 24 (14%) |
| Lateral Ventricle Size | ||
| Normal | 19 (33%) | 47 (28%) |
| Mild/Moderate Dilation | 36 (63%) | 110 (66%) |
| Marked Dilation | 4 (7%) | 9 (5%) |
| Cerebral palsy | 3 (5%) | 11 (7%) |
| Non-CP neurologic impairment | 2 (4%) | 15 (9%) |
Indicates statistically significant difference, p<0.01
Table 4.
online only: Clinical outcomes in subgroup with 7 year scans
| Morphine | No Morphine | |
|---|---|---|
| Number of Infants | 30 | 87 |
| Male sex (%) | 15 (50%) | 39 (44.8%) |
| Gestational Age (weeks) | 26.9 ± 1.6 | 27.6 ± 1.9 |
| Birth weight (g) | 913.6 ± 217.4 | 978.6 ± 207.5 |
| Small for Gestational Age | 1 (3.3%) | 9 (10.3%) |
| Multiparity | 16 (53.3%) | 42 (48.3%) |
| Antenatal betamethasone | 28 (93.3%) | 74 (85.1%) |
| Sepsis | 12 (40%) | 39 (44.8%) |
| Total Hours of invasive ventilation* | 404 ± 529 | 163 ± 282 |
| 25% | 74.25 | 0 |
| 50% | 216.5 | 32 |
| 75% | 599.25 | 179 |
| Total Hours of CPAP | 465 ± 338 | 407 ± 368 |
| 25% | 185 | 71 |
| 50% | 432 | 305 |
| 75% | 706.25 | 663 |
| Total Hours of Oxygen* | 1807 ± 2275 | 920 ± 1642 |
| 25% | 330.75 | 33 |
| 50% | 923.5 | 177 |
| 75% | 2776.5 | 1381 |
| Postnatal dexamethasone | 4 (13.3%) | 2 (2.3%) |
| Neonatal surgery | 5 (17%) | 12 (14%) |
| CRIB score | 4.4 ± 3.0 | 3.2 ± 2.9 |
| Days on parenteral nutrition | 14.3 ± 11.3 | 11.4 ± 9.8 |
| Length of hospital stay (days)* | 99.3 ± 33.9 | 79.5 ± 24.8 |
| Brain Injury at Term | ||
| High Grade IVH | 1 (3.3%) | 2 (2.2%) |
| Cerebellar Hemorrhage | 2 (7%) | 4 (5%) |
| Abnormal Gray Matter | 7 (23.3%) | 20 (23.0%) |
|
Moderate/Severe White Matter
Injury |
5 (16.7%) | 10 (11.4%) |
| Lateral Ventricle Size | ||
| Normal | 10 (33.3%) | 28 (32.2%) |
| Mild/Moderate Dilation | 19 (63.3%) | 55 (63.2%) |
| Marked Dilation | 1 (3.3%) | 3 (3.4%) |
| Cerebral palsy | 1 (3.3%) | 4 (4.5%) |
| Non-CP neurologic impairment | 2 (6.7%) | 5 (5.7%) |
Indicates statistically significant difference, p<0.01
Brain Volumes
Age, weight, and head circumference at the time of the term-equivalent and 7-year MRI brain scans were similar for the morphine and no-morphine groups. Thirty-one scans were excluded from the term-equivalent volume analysis due to poor image quality, leaving 192 scans (56 morphine, 136 no-morphine). At term-equivalent PMA, there were no significant differences in regional brain volumes between the morphine and no-morphine groups (Table V). Region-specific assessment of cortical gray matter demonstrated a trend towards smaller orbitofrontal (pleft=0.002, pright=0.01) and subgenual (pleft=0.01, pright=0.02) volumes bilaterally in the morphine group compared with the no-morphine group.
Table 5.
Term-equivalent brain volumes
| Morphine | No Morphine | Mean difference (95% CI) |
||||
|---|---|---|---|---|---|---|
| N | 56 | 136 | ||||
| Whole Brain | 383.3 (62.9) | 400.2 (63.3) | −16.9 (−36.7, 2.9) | |||
| Cortical Gray Matter |
151.3 (40.3) | 161.7 (41.6) | −10.4 (−23.3, 2.5) | |||
| White Matter | 208.3 (32.5) | 215.1 (32.5) | −6.8 (−16.9, 3.4) | |||
| Basal Ganglia | 14.3 (4.1) | 13.4 (3.8) | 0.9 (−0.3, 2.1) | |||
| Cerebellum | 20.6 (4.5) | 21.7 (4.5) | −1.1 (−2.4, 0.3) | |||
|
Regional cortical
gray matter |
Left Hemisphere | Right Hemisphere | ||||
| Morphine | No Morphine |
Mean difference
(95% CI) |
Morphine | No Morphine |
Mean difference (95% CI) |
|
| Dorsal Prefrontal | 5.9 (2.4) | 5.9 (2.3) | 0 (−0.7, 0.7) | 5.3 (2.6) | 5.3 (2.5) | 0 (−0.8, 0.8) |
| Orbitofrontal | 1.1 (0.9) | 1.6 (0.8) | −0.5 (−0.7, −0.2)* | 1.2 (0.9) | 1.6 (0.8) | −0.4 (−0.7, −0.2) |
| Premotor | 7.3 (2.6) | 7.4 (2.5) | −0.1 (−0.8, 0.7) | 7.3 (2.6) | 7.3 (2.8) | 0 (−0.9, 0.8) |
| Subgenual | 3.1 (1.2) | 3.6 (1.4) | −0.5 (−1.0, −0.2) | 3.1 (1.2) | 3.6 (1.4) | −0.5 (−1.0, −0.1) |
| Sensorimotor | 11.2 (3.3) | 11.6 (2.8) | −0.4 (−1.3, 0.5) | 12.3 (3.4) | 12.5 (3.2) | −0.2 (1.3, −0.8) |
| Midtemporal | 5.0 (1.8) | 5.5 (1.7) | −0.5 (−1.0, 0.1) | 5.2 (1.9) | 5.7 (1.7) | −0.5 (−1.0, 0.1) |
| Parieto-occipital | 22.0 (6.8) | 24.0 (8.3) | −2.0 (−4.4, 0.5) | 26.3 (7.7) | 27.4 (8.3) | −1.1 (−3.6, 1.5) |
| Inferior occipital | 16.1 (5.9) | 18.0 (7.3) | −1.9 (−4.1, 0.3) | 18.8 (6.6) | 20.4 (7.6) | −1.6 (−3.9, 0.7) |
Mean cc (SD)
Covariates: gestational age, sex, CRIB score, hours of ventilation, intrauterine growth restriction, sepsis, dexamethasone, white matter injury, days of parenteral nutrition, neonatal surgery, length of NICU stay
Indicates statistically significant difference, p<0.01
One hundred sixty participants were successfully rescanned at 7 years of age; 43 scans were excluded from the analysis of volumes at seven years due to poor image quality, leaving 117 analyzed scans (30 morphine, 87 no-morphine). At 7 years of age, no differences in regional volumes were found between morphine and no-morphine groups (Table VI). No dose-dependent effect of morphine on regional volumes at term or seven years was present. There were also no differences in regional volumes at term equivalent or seven years based upon the dosing modality. Ninety-nine patients (99/117 eligible scans) who were included in the analysis of volume at seven years also had high quality term imaging at term (30 morphine, 69 no-morphine). Within this subgroup, differences did not persist for any region (Table VII; available at www.jpeds.com).
Table 6.
online only: Term equivalent volumes in the subgroup with 7 year scans
| Morphine | No Morphine |
Mean difference (95% CI) | ||||
|---|---|---|---|---|---|---|
| N | 30 | 69 | ||||
| Whole Brain | 388.3 (57.6) | 401.3 (64.7) | −13.0 (−40.1, 14.2) | |||
| Cortical Gray Matter | 151.3 (41.7) | 162.7 (43.8) | −11.4 (−30.1, 7.3) | |||
| White Matter | 212.9 (26.7) | 214.9 (28.6) | −2.0 (−14.2, 10.1) | |||
| Basal Ganglia | 13.7 (3.6) | 13.5 (3.6) | 0.2 (−1.3, 1.8) | |||
| Cerebellum | 20.9 (4.4) | 21.7 (4.0) | −0.8 (−2.5, 1.0) | |||
|
Regional cortical gray
matter |
Left Hemisphere | Right Hemisphere | ||||
| Morphine |
No
Morphine |
Mean difference (95% CI) | Morphine |
No
Morphine |
Mean difference
(95% CI) |
|
| Dorsal Prefrontal | 5.5 (2.2) | 6.1 (2.3) | −0.6 (−1.6, 0.4) | 4.7 (2.1) | 5.6 (2.6) | −0.9 (−1.9, 0.2) |
| Orbitofrontal | 1.1 (1.1) | 1.6 (0.8) | −0.5 (−0.9, 0.1) | 1.2 (1.1) | 1.6 (0.7) | −0.4 (−0.8, 0) |
| Premotor | 7.3 (2.7) | 7.7 (2.8) | −0.4 (−1.6, 0.8) | 7.2 (2.9) | 7.7 (3.0) | −0.5 (−1.7, 0.8) |
| Subgenual | 3.2 (1.5) | 3.6 (1.5) | −0.4 (−1.1, 0.2) | 3.2 (1.4) | 3.6 (1.5) | −0.4 (−1.1, 0.2) |
| Sensorimotor | 11.1 (2.9) | 12.1 (2.8) | −1.0 (−2.2, 0.2) | 12.4 (3.5) | 13.1 (3.2) | −0.7 (−2.1, 0.7) |
| Midtemporal | 5.1 (2.0) | 5.5 (1.7) | −0.4 (−1.1, 0.4) | 5.4 (2.1) | 5.7 (1.7) | −0.4 (−1.1, 0.5) |
| Parieto-occipital | 21.5 (6.2) | 24.1 (8.1) | −2.6 (−5.9, 0.7) | 26.4 (7.8) | 27.6 (8.6) | −1.2 (−4.8, 2.5) |
| Inferior occipital | 16.1 (6.1) | 17.3 (7.1) | −1.2 (−4.1, 1.8) | 19.5 (7.1) | 19.6 (6.7) | −0.1 (−3.2, 3.1) |
Mean cc (SD)
Covariates: gestational age, sex, CRIB score, hours of ventilation, intrauterine growth restriction, sepsis, dexamethasone, white matter injury, days of parenteral nutrition, neonatal surgery, length of NICU stay
Table 7.
7-year volumes
| Left Hemisphere | Right Hemisphere | |||||
|---|---|---|---|---|---|---|
| Region | Morphine | No Morphine |
Mean difference
(95% CI) |
Morphine | No Morphine |
Mean difference
(95% CI) |
| N | 30 | 87 | 30 | 87 | ||
| Total Brain Volume | 557.5 (55.0) | 561.8 (50.7) | −4.3 (−25.3, 16.7) | 557.7 (56.1) | 562.3 (49.2) | −4.6 (−25.2, 16.1) |
| Cortical Gray Matter | 285.3 (27.1) | 284.2 (24.6) | 1.1 (−9.3, 11.5) | 284.4 (28.1) | 283.9 (23.7) | 0.5 (−9.7, 10.7) |
| White Matter | 201.7 (25.0) | 201.1 (22.6) | 0.4 (−9.0, 10.0) | 202.5 (24.6) | 202.5 (22.5) | 0 (−9.4, 9.6) |
| Cerebellar Cortex | 59.9 (5.4) | 61.9 (5.9) | −2.0 (−4.5, 0.3) | 60.1 (6.4) | 61.5 (5.6) | −1.4 (−3.8, 1.0) |
| Cerebellar WM | 12.9 (2.2) | 12.9 (1.8) | 0 (−0.8, 0.8) | 12.9 (1.8) | 12.9 (1.7) | 0 (−0.7, 0.7) |
|
Regional cortical gray
matter | ||||||
| Superior Frontal | 28.0 (3.4) | 28.6 (3.3) | −0.6 (−2.0, 0.8) | 26.4 (3.0) | 27.1 (3.1) | −0.7 (−2.0, 0.6) |
| Middle Frontal | 29.7 (3.9) | 29.8 (3.0) | −0.1 (−1.3, 1.4) | 29.2 (4.0) | 29.4 (3.1) | −0.2 (−1.7, 1.2) |
| Inferior Frontal | 10.3 (1.6) | 10.2 (1.3) | 0.1 (−0.6, 0.6) | 9.9 (1.6) | 9.6 (1.1) | 0.3 (−0.1, 0.9) |
| Orbitofrontal | 16.9 (1.8) | 17.1 (1.8) | −0.2 (−1.0, 0.6) | 17.9 (1.9) | 18.1 (1.9) | −0.2 (−1.1, 0.6) |
| Precentral | 15.9 (1.8) | 15.5 (2.0) | 0.4 (−0.4, 1.2) | 15.4 (2.2) | 15.4 (1.6) | 0 (−0.8, 0.7) |
| Postcental | 12.4 (2.0) | 12.1 (1.7) | 0.3 (−0.5, 1.1) | 11.6 (2.2) | 11.4 (1.7) | 0.2 (−0.6, 1.0) |
| Superior Temporal | 15.8 (2.1) | 16.6 (2.4) | −0.8 (−1.9, 0.1) | 14.9 (2.0) | 15.2 (1.8) | −0.3 (−1.1, 0.5) |
| Inferior Temporal | 26.1 (3.5) | 24.9 (3.1) | 1.2 (−0.1, 2.5) | 25.9 (4.0) | 24.8 (3.4) | 1.1 (−0.5, 2.5) |
| Medial Temporal | 7.1 (1.0) | 6.8 (1.0) | 0.3 (−0.1, 0.7) | 6.7 (1.1) | 6.5 (0.8) | 0.2 (−0.2, 0.6) |
| Lateral Temporal | 15.7 (2.1) | 16.3 (2.4) | −0.5 (−1.4, 0.5) | 16.9 (2.2) | 17.6 (2.3) | −0.7 (−1.7, 0.2) |
| Medial Parietal | 20.9 (2.2) | 21.2 (2.2) | −0.3 (−1.2, 0.6) | 22.3 (2.2) | 22.5 (2.4) | −0.2 (−1.2, 0.8) |
| Lateral Parietal | 32.9 (3.8) | 32.8 (3.3) | 0.1 (−1.3, 1.6) | 36.4 (4.1) | 36.2 (4.0) | 0.2 (−1.5, 1.9) |
| Supramarginal | 13.8 (2.8) | 14.4 (2.4) | −0.6 (−1.6, 0.5) | 12.6 (1.8) | 13.2 (2.1) | −0.6 (−1.5, 0.2) |
| Medial Occipital | 10.7 (1.8) | 10.6 (1.6) | 0.1 (−0.6, 0.7) | 11.2 (1.5) | 11.0 (1.5) | 0.2 (−0.4, 0.8) |
| Lateral Occipital | 15.3 (2.6) | 15.6 (2.5) | −0.3 (−1.3, 0.8) | 15.0 (2.3) | 15.4 (2.4) | −0.4 (−1.5, 0.5) |
| Cingulate Lobule | 8.6 (1.5) | 8.8 (1.4) | −0.2 (−0.9, 0.4) | 8.0 (1.8) | 8.2 (1.3) | −0.2 (−0.8, 0.4) |
| Isthmus | 3.2 (0.4) | 3.3 (0.6) | −0.1 (−0.3, 0.2) | 3.0 (0.7) | 2.9 (0.6) | 0.1 (−0.1, 0.4) |
| Insula | 6.8 (0.7) | 6.7 (0.7) | 0.1 (−0.2, 0.3) | 6.8 (0.8) | 6.7 (0.7) | 0.1 (−0.2, 0.4) |
Mean cc (SD)
Covariates: gestational age, sex, CRIB score, hours of ventilation, intrauterine growth restriction, sepsis, dexamethasone, white matter injury, days of parenteral nutrition, neonatal surgery, length of NICU stay
Neurobehavioral outcomes
The group means for the neurobehavioral assessments performed at term equivalent, two, and seven years of age, controlled for major confounders, are presented in Table VIII. At term equivalent PMA the morphine group had a trend toward lower tone (p=0.01) on the HNNE than the no-morphine group.
Table 8.
Neurobehavioral Assessment
| Test (N morphine, N no morphine) | Morphine Mean (SD) |
No Morphine Mean (SD) |
Mean difference (95% CI) |
|---|---|---|---|
|
| |||
| Term | |||
| HNNE (37, 122) | |||
| Tone | 6.6 (2.0) | 7.6 (1.9) | −1.0 (−1.8, −0.3) |
| Tone patterns | 3.9 (0.9) | 4.2 (0.8) | −0.3 (−0.6, 0.04) |
| Reflexes | 5.2 (0.9) | 5.5 (0.6) | −0.3 (−0.6, 0.01) |
| Spontaneous movements | 1.9 (1.0) | 2.0 (0.9) | −0.1 (−0.5, 0.2) |
| Abnormal signs | 2.4 (0.6) | 2.3 (0.6) | 0.1 (−0.1, 0.4) |
| Behavior | 5.5 (1.6) | 5.3 (1.4) | 0.2 (−0.4, 0.7) |
| Total | 25.5 (4.9) | 27.0 (3.8) | −1.5 (−3.1, 0.07) |
|
| |||
| 2 years | |||
| BSID-II (51, 160) | |||
| MDI index score | 84.2 (19.2) | 83.5 (19.4) | 0.7 (−5.2, 6.6) |
| PDI index score | 87.6 (19.0) | 88.4 (16.3) | −0.8 (−6.0, 4.3) |
| ITSEA (47, 137) | |||
| Externalizing | 49.5 (8.1) | 49.3 (9.7) | 0.2 (−3.3, 3.6) |
| Internalizing | 50.1 (12.1) | 48.7 (11.9) | 1.4 (−3.0, 5.8) |
| Dysregulation* | 58.0 (11.2) | 52.0 (11.8) | 6.0 (1.8, 10.2) |
| Competence | 45.6 (9.5) | 46.4 (10.6) | −0.8 (−4.5, 2.9) |
| CSBS (47, 138) | |||
| Social composite | 21.5 (3.9) | 21.1 (3.6) | 0.4 (−0.8, 1.7) |
| Social N of concern (%) | 9 (19%) | 23 (17%) | |
| Speech composite | 11.1 (3.7) | 11.4 (2.8) | 0.3 (−1.4, 0.6) |
| Speech N of concern | 13 (28°%) | 15 (11%) | |
| Delayed alternation, N failed (39, 142)* | 19 (49%) | 36 (25%) | |
| BRIEF-P GEC (46, 139) | 56.0 (9.9) | 52.4 (10.7) | 3.6 (−0.2, 7.4) |
|
| |||
| 7 years | |||
| WASI Full Scale IQ (46, 142) | 98.0 (13.2) | 96.6 (13.7) | 1.4 (−3.1, 5.8) |
| MABC2 total motor (41, 126) | 8.6 (3.1) | 8.6 (3.6) | 0 (−1.3, 1.2) |
| SDQ (44, 134) | |||
| Total | 10.1 (5.1) | 10.7 (6.8) | −0.6 (−3.0, 1.8) |
| Emotional problems | 2.5 (2.2) | 2.5 (2.3) | 0 (−0.9, 0.8) |
| Conduct problems | 1.3 (1.3) | 1.9 (1.8) | −0.6 (−1.1, 0.1) |
| Hyperactivity | 4.5 (2.4) | 4.5 (3.0) | 0 (−1.0, 1.1) |
| Peer problems | 1.8 (1.9) | 1.9 (1.7) | −0.1 (−0.7, 0.6) |
| Prosocial scale | 8.3 (2.1) | 8.5 (1.8) | −0.2 (−0.8, 0.4) |
| CELF4 core language (44, 138) | 97.7 (14.8) | 90.6 (18.3) | 7.1 (1.1, 13.1) |
| WRAT (46, 138)* | |||
| Reading* | 106.4 (18.7) | 96.6 (18.6) | 9.8 (3.6, 16.0) |
| Spelling* | 107.1 (17.6) | 96.4 (18.2) | 10.7 (4.6, 16.7) |
| Computation | 95.2 (16.1) | 87.9 (18.3) | 7.3 (1.2, 13.4) |
| BRIEF GEC (46, 135) | 55.4 (10.2) | 56.4 (13.6) | −1.0 (−5.6, 3.7) |
Covariates: gestational age, sex, CRIB score, hours of ventilation, intrauterine growth restriction, sepsis, dexamethasone, white matter injury, days of parenteral nutrition, neonatal surgery, length of NICU stay
Indicates statistically significant difference, p<0.01
At two years of age, the morphine and no-morphine groups scored similarly in terms of cognitive (MDI) and motor (PDI) development, as well as the speech and social components of the CSBS-DP. Although there were no between-group differences on the ITSEA externalizing, internalizing, and competence scales, the morphine group children were reported by parents to exhibit more dysregulation (p=0.007).
At seven years of age, children in the morphine and no-morphine groups performed similarly on measures of IQ (WASI), motor function (MABC-2), executive function (BRIEF), and behavior (SDQ). However, the morphine group achieved higher scores in basic educational skills assessed by the WRAT 4 (p=0.008) after controlling for covariates. Basic educational superiority persisted across subgroup tests of reading (p=0.002) and spelling (p=0.001).
Within the subgroup of children included in brain volume analyses at seven years of age, differences in neurobehavior at term and neurodevelopment at two and seven years did not persist (Table IX; available at www.jpeds.com).
Table 9.
online only: Neurobehavioral assessment in the subgroup with 7 year scans
| Test (N morphine, N no morphine) | Morphine Mean (SD) |
No Morphine Mean (SD) |
Mean difference (95% CI) |
|---|---|---|---|
|
| |||
| Term | |||
| HNNE (21, 64) | |||
| Tone | 6.6 (1.2) | 7.6 (1.0) | −1.0 (−2.0, −0.02) |
| Tone patterns | 3.7 (0.7) | 4.2 (0.6) | −0.5 (−0.9, −0.08) |
| Reflexes | 5.2 (0.6) | 5.5 (0.5) | −0.3 (−0.7, 0.03) |
| Spontaneous movements | 1.9 (0.6) | 2.1 (0.5) | −0.2 (−0.7, 0.2) |
| Abnormal signs | 2.5 (0.4) | 2.4 (0.4) | 0.1 (−0.3, 0.3) |
| Behavior | 5.3 (1.2) | 5.4 (1.0) | −0.1 (−0.8, 0.7) |
| Total | 25.2 (2.8) | 27.3 (2.4) | −2.0 (−4.0, −0.07) |
|
| |||
| 2 years | |||
| BSID-II (29, 86) | |||
| MDI index score | 87.8 (12.9) | 89.0 (10.9) | −1.2 (−8.6, 6.3) |
| PDI index score | 88.6 (12.9) | 92.3 (10.9) | −3.7 (−10.2, 2.8) |
| ITSEA (26, 74) | |||
| Externalizing | 48.9 (6.3) | 48.4 (5.4) | 0.5 (−3.4, 4.4) |
| Internalizing | 47.6 (10.0) | 48.4 (8.5) | −0.8 (−6.4, 4.8) |
| Dysregulation | 55.2 (9.1) | 51.6 (7.7) | 3.6 (−1.9, 9.2) |
| Competence | 47.7 (8.4) | 47.8 (7.1) | −0.1 (−4.9, 4.8) |
| CSBS (26, 74) | |||
| Social composite | 22.2 (2.9) | 21.8 (2.5) | 0.4 (−1.1, 1.8) |
| Social N of concern (%) | |||
| Speech composite | 11.1 (2.2) | 12.0 (1.9) | −0.9 (−2.2, 0.3) |
| Speech N of concern | |||
| Delayed alternation, N failed (25, 82) | 10 (33.3%) | 21 (24.1%) | |
| BRIEF-P GEC (26, 74) | 54.9 (6.7) | 51.5 (5.7) | 3.4 (−0.8, 7.5) |
|
| |||
| 7 years | |||
| WASI Full Scale IQ (30, 87) | 98.1 (10.0) | 99.1 (8.5) | −1.0 (−6.5, 4.5) |
| MABC2 total motor (28, 80) | 8.9 (2.3) | 9.6 (1.9) | −0.7 (−2.0, 0.6) |
| SDQ (29, 83) | |||
| Total | 10.1 (3.9) | 9.6 (3.3) | 0.5 (−2.2, 3.1) |
| Emotional problems | 2.6 (1.7) | 2.4 (1.4) | 0.2 (−0.8, 1.3) |
| Conduct problems | 1.2 (0.9) | 1.6 (0.7) | −0.3 (−0.9, 0.3) |
| Hyperactivity | 4.7 (1.7) | 4.0 (1.4) | 0.7 (−0.5, 0.9) |
| Peer problems | 1.6 (1.3) | 1.7 (1.1) | −0.1 (−0.9, 0.5) |
| Prosocial scale | 8.7 (1.2) | 8.8 (1.0) | −0.1 (−0.7, 0.7) |
| CELF4 core language (28, 87) | 97.1 (14.6) | 93.4 (12.4) | 3.7 (−3.8, 11.3) |
| WRAT (29, 86) | |||
| Reading | 105.2 (13.7) | 99.3 (11.6) | 5.9 (−1.7, 13.4) |
| Spelling | 105.6 (13.2) | 100.1 (11.2) | 5.5 (−2.0, 13.0) |
| Computation | 95.9 (13.1) | 91.2 (11.1) | 4.7 (−2.9, 12.3) |
| BRIEF GEC (29, 83) | 53.6 (8.4) | 54.3 (7.1) | −0.6 (−6.3, 5.1) |
Covariates: gestational age, sex, CRIB score, hours of ventilation, intrauterine growth restriction, sepsis, dexamethasone, white matter injury, days of parenteral nutrition, neonatal surgery, length of NICU stay
Discussion
In this study, very preterm infants exposed to low-dose morphine analgesia did not demonstrate long-term detriments in cortical development or neurobehavioral outcomes at age seven years compared with very preterm infants without morphine exposure. It is of interest that morphine-exposed infants displayed a trend toward smaller orbitofrontal and subgenual volumes and lower scores on tests of tone at term equivalent PMA than no-morphine infants. Furthermore, at 2 years of age, the morphine exposed infants were reported by parents to demonstrate more dysregulated behavior than their no-morphine exposed peers. Despite these early findings, morphine exposed children who were able to be followed at 7 years of age did not show any detriments on a wide range of neuropsychological domains compared with their peers exposed to no morphine.
The observational nature of this longitudinal cohort study is a limitation in evaluating the outcomes associated with morphine exposure. Infants received morphine based upon the attending physicians’ clinical judgment, and this is reflected in the different clinical characteristics of the two groups. Infants in our morphine group had a lower gestational age at birth, lower birth weight, higher CRIB score, and were more likely to require postnatal dexamethasone and prolonged ventilation. We attempted to isolate the effects of morphine from those of a higher initial clinical burden by adjusting for numerous confounders in our analyses. A second limitation is that clinicians did not perform any quantifications of neonatal pain, so the potential analgesic effect of morphine could not be examined. Additionally, not all patients returned for the MRI and neurodevelopmental follow-up at seven years, and some scans were not of sufficient quality to use in the comparison of regional volumes. Those included in the 7-year analyses did not differ from those in the cohort without data in terms of gestational age, sex, WM injury, intrauterine growth restriction, hours on invasive ventilation, incidences of sepsis, receipt of postnatal dexamethasone, exposure to morphine, number of days on morphine, and total dose of morphine received. Analyses of only those children with brain volumes available at age seven revealed loss of significance on both volume and neurodevelopmental differences observed at the earlier time points, which may reflect either a loss of power or selection bias within the cohort followed longitudinally. Further, numerous outcome variables were tested in this longitudinal cohort study which may have increased the risk of type I error (ie, erroneous attribution of an effect to morphine). Despite liberal criteria for statistical significance, few significant findings were generated, supporting the key conclusion of no detrimental impact of low-dose morphine on neurologic outcomes of preterm infants assessed in early childhood.
Previous studies on the impact of receiving morphine in the NICU have examined acute brain injury and behavioral outcomes. The design of the NEOPAIN trial involved pre-emptive morphine infusions to a randomized sample of preterm neonates on mechanical ventilation. Morphine infusion was found to decrease the clinical indications of pain, but did not alter the incidence of adverse neurological outcomes, including IVH, periventricular leukomalacia, and death.13 In another randomized trial, morphine infusion was found to reduce the incidence of IVH, but did not mitigate poor neurological outcome, or result in a clinically significant analgesic effect.14 In our study, we also found equal incidences of IVH and other forms of brain injury in morphine and no-morphine groups, although morphine exposure was associated with trends toward reductions in the orbitofrontal and subgenual cortical gray matter volumes at term equivalent PMA. Though we attempted to control for disparities in clinical burden between groups, the initial trends observed in these regional volumes and neurobehavior outcomes at term and 24 months may reflect the greater severity of illness in the newborns who received morphine, rather than the impact of the opiate itself, as alterations in the temporal and frontal regions have been shown in association with pain35 and stress4 in preterm infants at term, rather than with exposure to morphine alone The similarity in brain structure and function at age seven years, regardless of morphine exposure, may reflect recovery from the impact of stressful events during the neonatal period. The paucity of longitudinal studies on the adaptability and recovery of volume and function in the preterm infant has limited our ability to determine the additional impact of factors such as neonatal analgesia. Further data on the long-term effects of stress, pain, and opioid exposure in the neonatal period alongside early childhood environments may provide greater insight.
Our results are consistent with previous studies examining the long-term neurodevelopmental impact of early morphine exposure on preterm infants. Abnormal tone at 36 weeks’ postmenstrual age was observed in morphine-treated infants in the NEOPAIN trial.36 Poorer motor development at eight months was associated with intravenous morphine exposure in an observational cohort by Grunau et al; however, this finding did not persist at 18 months of age.35 Similarly, children randomized to early morphine infusion in the European morphine trial performed poorly on the visual analysis domain of intelligence quotient at five years compared with control children.37 However, these children had better executive function reported by parents at eight years of age.38 Conversely, a 5-year follow-up study of participants in the NEOPAIN trial demonstrated a negative impact of neonatal morphine exposure on head circumference and body weight, short-term memory, and complex social skills.16 One possible explanation for the discrepancy is the difference in dosing protocols used in the studies. Infants in the NEOPAIN trial were exposed to higher levels of morphine than patients in other trials of morphine infusion.37 Patients in our study received lower doses of morphine, and fewer than half of the 57 newborns received continuous morphine infusions.
The suggestion of a positive effect of morphine at 7 years of age with regard to educational skills surprised the investigators. It is essential to note that this finding occurred after multivariate analyses adjusting for potential confounders which were more heavily present in the morphine treated infants. However, it is interesting to note that although the morphine group had a lower gestational age, birth weight, and higher CRIB score than the no-morphine group, children exposed to morphine performed similarly on all tests of cognition and behavior by age seven years when the outcomes were compared with t-tests that did not control for any confounders. This is despite the fact that both birth weight and gestational age correlate positively with tests of cognition in preterm children.39 This finding is consistent with a study showing that either neonatal stress or morphine exposure alone impaired place-preference conditioning in adulthood in a rat model, but when experienced together, they had no effect on adult learning.12 Further, opioid analgesia has been shown to reduce harmful metabolite changes in the basal ganglia, thalami, and occipital GM following tissue-damaging procedures.40 Taken together, these results and our data suggest that targeted morphine exposure in infants with high neonatal stress may ameliorate the negative effect of the high stress, with the caveat that the multiplicity of interwoven factors affecting the development of a preterm infant, ranging from early clinical burden to the innate plasticity of the immature brain, complicates the prediction of clinical outcomes based upon one variable. Future studies exploring the interaction between analgesia and severity of illness are needed to determine if newborns experiencing greater distress in the NICU derive more benefit from opioid analgesia than those experiencing less distress. Certainly, our findings of a potential benefit from neonatal morphine in sick preterm infants require replication.
Low-dose morphine analgesia exposure in the NICU was not associated with sustained alterations in brain development or neurodevelopmental performance at seven years of age. Our findings highlight the need for further prospective, randomized studies into the safety and efficacy of different analgesics and dosing regimens in preterm infants, not only in regard to short term clinical outcomes, but also with respect to brain development and later childhood cognitive skills.
Acknowledgments
We would like to acknowledge the imaging analysis work that was undertaken by Yuning Zhang, MD, Dimitrios Alexopoulos, MS, and Deanne Thompson, along with the input of the clinical research team for VIBeS, particularly the lead research nurse Merilyn Bear, and all the families who participated in this study.
Funded by the National Institute of Child Health and Development (R01 HD057098 and 1P30 HD062171), Doris Duke Charitable Foundation, and the National Health Medical Research Council (Project Grants 23117 and 491209 and Senior Research Fellowship 628371 [to P.A.]).
Abbreviations
- CRIB
Clinical Risk Index for Babies
- IVH
intraventricular hemorrhage
- MRI
magnetic resonance imaging
- NICU
neonatal intensive care unit
- PMA
postmenstrual age
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- [1].Grunau RE, Holsti L, Peters JW. Long-term consequences of pain in human neonates. Seminars in fetal & neonatal medicine. 2006;11:268–75. doi: 10.1016/j.siny.2006.02.007. [DOI] [PubMed] [Google Scholar]
- [2].Anand KJ. Pharmacological approaches to the management of pain in the neonatal intensive care unit. Journal of perinatology: official journal of the California Perinatal Association. 2007;27(Suppl 1):S4–S11. doi: 10.1038/sj.jp.7211712. [DOI] [PubMed] [Google Scholar]
- [3].Carbajal R, Rousset A, Danan C, Coquery S, Nolent P, Ducrocq S, et al. Epidemiology and treatment of painful procedures in neonates in intensive care units. JAMA. 2008;300:60–70. doi: 10.1001/jama.300.1.60. [DOI] [PubMed] [Google Scholar]
- [4].Smith GC, Gutovich J, Smyser C, Pineda R, Newnham C, Tjoeng TH, et al. Neonatal intensive care unit stress is associated with brain development in preterm infants. Annals of neurology. 2011;70:541–9. doi: 10.1002/ana.22545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Brummelte S, Grunau RE, Chau V, Poskitt KJ, Brant R, Vinall J, et al. Procedural pain and brain development in premature newborns. Annals of neurology. 2012;71:385–96. doi: 10.1002/ana.22267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kahn DJ, Richardson DK, Gray JE, Bednarek F, Rubin LP, Shah B, et al. Variation among neonatal intensive care units in narcotic administration. Arch Pediatr Adolesc Med. 1998;152:844–51. doi: 10.1001/archpedi.152.9.844. [DOI] [PubMed] [Google Scholar]
- [7].Atici S, Cinel L, Cinel I, Doruk N, Aktekin M, Akca A, et al. Opioid Neurotoxicity: Comparison of Morphine and Tramadol in an Experimental Rat Model. International Journal of Neuroscience. 2004;114:1001–11. doi: 10.1080/00207450490461314. [DOI] [PubMed] [Google Scholar]
- [8].Seatriz JV, Hammer RP., Jr. Effects of opiates on neuronal development in the rat cerebral cortex. Brain Research Bulletin. 1991;30:523–7. doi: 10.1016/0361-9230(93)90078-p. [DOI] [PubMed] [Google Scholar]
- [9].Ricalde AA, Hammer RP., Jr. Perinatal opiate treatment delays growth of cortical dendrites. Neuroscience Letters. 1990;115:137–43. doi: 10.1016/0304-3940(90)90444-e. [DOI] [PubMed] [Google Scholar]
- [10].Handelmann GE, Dow-Edwards D. Modulation of brain development by morphine: Effects on central motor systems and behavior. Peptides. 1985;6:29–34. doi: 10.1016/0196-9781(85)90131-7. [DOI] [PubMed] [Google Scholar]
- [11].Schrott LM, Franklin L, Serrano PA. Prenatal opiate exposure impairs radial arm maze performance and reduces levels of BDNF precursor following training. Brain research. 2008;1198:132–40. doi: 10.1016/j.brainres.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Boasen JF, McPherson RJ, Hays SL, Juul SE, Gleason CA. Neonatal stress or morphine treatment alters adult mouse conditioned place preference. Neonatology. 2009;95:230–9. doi: 10.1159/000165379. [DOI] [PubMed] [Google Scholar]
- [13].Anand KJS, Hall RW, Desai N, Shephard B, Bergqvist LL, Young TE, et al. Effects of morphine analgesia in ventilated preterm neonates: primary outcomes from the NEOPAIN randomised trial. The Lancet. 2004;363:1673–82. doi: 10.1016/S0140-6736(04)16251-X. [DOI] [PubMed] [Google Scholar]
- [14].Simons SHP, van Dijk M, van Lingen RA, Roofthooft DW, Duivenvoorden HJ, Jongeneel N, et al. Routine morphine infusion in preterm newborns who received ventilatory support. JAMA. 2003;290:2419–27. doi: 10.1001/jama.290.18.2419. [DOI] [PubMed] [Google Scholar]
- [15].Rao R, Sampers JS, Kronsberg SS, Brown JV, Desai NS, Anand KJ. Neurobehavior of preterm infants at 36 weeks postconception as a function of morphine analgesia. American journal of perinatology. 2007;24:511–7. doi: 10.1055/s-2007-986675. [DOI] [PubMed] [Google Scholar]
- [16].Ferguson SA, Ward WL, Paule MG, Hall RW, Anand KJ. A pilot study of preemptive morphine analgesia in preterm neonates: effects on head circumference, social behavior, and response latencies in early childhood. Neurotoxicology and teratology. 2012;34:47–55. doi: 10.1016/j.ntt.2011.10.008. [DOI] [PubMed] [Google Scholar]
- [17].MacGregor R, Evans D, Sugden D, Gaussen T, Levene M. Outcome at 5-6 years of prematurely born children who received morphine as neonates. Arch Dis Child Fetal Neonatal Ed. 1998;79:F40–F3. doi: 10.1136/fn.79.1.f40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. New England Journal of Medicine. 2006;355:685–94. doi: 10.1056/NEJMoa053792. [DOI] [PubMed] [Google Scholar]
- [19].Thompson DK, Warfield SK, Carlin JB, Pavlovic M, Wang HX, Bear M, et al. Perinatal risk factors altering regional brain structure in the preterm infant. Brain: a journal of neurology. 2007;130:667–77. doi: 10.1093/brain/awl277. [DOI] [PubMed] [Google Scholar]
- [20].Thompson DKWS, Doyle LW, Warfield SK, Lodygensky GA, Anderson PJ, Egan GF, Inder TE. Neonate hippocampal volumes: prematurity, perinatal predictors, and 2-year outcome. Annals of neurology. 2008;63:642–51. doi: 10.1002/ana.21367. [DOI] [PubMed] [Google Scholar]
- [21].Shah DK, Anderson PJ, Carlin JB, Pavlovic M, Howard K, Thompson DK, et al. Reduction in cerebellar volumes in preterm infants: relationship to white matter injury and neurodevelopment at two years of age. Pediatr Res. 2006;60:97–102. doi: 10.1203/01.pdr.0000220324.27597.f0. [DOI] [PubMed] [Google Scholar]
- [22].Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31:968–80. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- [23].Dubowitz L, Mercuri E. An optimality score for the neurologic examination of the term newborn. The Journal of pediatrics. 1998;133:406–16. doi: 10.1016/s0022-3476(98)70279-3. V D. [DOI] [PubMed] [Google Scholar]
- [24].Corporation TP, editor. Bayley Scales of Infant Development. Second Edition. San Antonio, TX1993: N B. [Google Scholar]
- [25].Briggs-Gowan M. Infant Toddler Social and Emotional Assessment Manual. 2001 A C. unpublished. [Google Scholar]
- [26].Wetherby A. Communication and Symbolic Behavior Scales Developmental Profile Manual. Paul H Brookes Publishing Co; Sydney, Australia: 2002. B P. [Google Scholar]
- [27].Beauchamp MTD, Howard K, Doyle LW, Egan GF, Inder TE, Anderson PJ. Preterm infant hippocampal volumes correlate with later working memory deficits. Brain: a journal of neurology. 2008;131:2986–94. doi: 10.1093/brain/awn227. [DOI] [PubMed] [Google Scholar]
- [28].Espy KA, Stalets MM, McDiarmid MM, Senn TE, Cwik MF. Executive functions in preschool children born preterm: application of cognitive neuroscience paradigms. Child Neuropsychol. 2002;8:83–92. doi: 10.1076/chin.8.2.83.8723. A H. [DOI] [PubMed] [Google Scholar]
- [29].Gioia, Espy Isquith. Behavior Rating Inventory of Executive Function, Preschool Version. Psychological Assessment Resources Inc; Odessa, FL: 2003. [Google Scholar]
- [30].WPPSI-III (Australian): Administration and scoring manual. The Psychological Corporation; Marrickville, NSW: 2004. D W. [Google Scholar]
- [31].Schoemaker MM, Smits-Engelsman BC, MJ J. Psychometric properties of the movement assessment battery for children-checklist as a screening instrument for children with a developmental co-ordination disorder. Br J Educ Psychol. 2003;73:425–41. doi: 10.1348/000709903322275911. [DOI] [PubMed] [Google Scholar]
- [32].The Strengths and Difficulties Questionnaire: A research note. J Child Psych Psychiatry. 1997;38:581–6. doi: 10.1111/j.1469-7610.1997.tb01545.x. R G. [DOI] [PubMed] [Google Scholar]
- [33].Semel E,M, Wiig EH, Secord W. Clinical Evaluation of Language Fundamentals (CELF-4) The Psychological Corporation; San Antonio: 2003. [Google Scholar]
- [34].Wilkinson G. Wide Range Achievement Test. 4th edition. Wilmington, Delaware: 2005. G R. [Google Scholar]
- [35].Grunau RE, Whitfield MF, Petrie-Thomas J, Synnes AR, Cepeda IL, Keidar A, et al. Neonatal pain, parenting stress and interaction, in relation to cognitive and motor development at 8 and 18 months in preterm infants. Pain. 2009;143:138–46. doi: 10.1016/j.pain.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rao R, Sampers JS, Kronsberg SS, Brown JV, Desai NS, Anand KJ. Neurobehavior of preterm infants at 36 weeks postconception as a function of morphine analgesia. American journal of perinatology. 2007;24:511–7. doi: 10.1055/s-2007-986675. [DOI] [PubMed] [Google Scholar]
- [37].de Graaf J, van Lingen RA, Simons SH, Anand KJ, Duivenvoorden HJ, Weisglas-Kuperus N, et al. Long-term effects of routine morphine infusion in mechanically ventilated neonates on children’s functioning: five-year follow-up of a randomized controlled trial. Pain. 2011;152:1391–7. doi: 10.1016/j.pain.2011.02.017. [DOI] [PubMed] [Google Scholar]
- [38].de Graaf J, van Lingen RA, Valkenburg AJ, Weisglas-Kuperus N, Groot Jebbink L, Wijnberg-Williams B, et al. Does neonatal morphine use affect neuropsychological outcomes at 8 to 9 years of age? Pain. 2013;154:449–58. doi: 10.1016/j.pain.2012.12.006. [DOI] [PubMed] [Google Scholar]
- [39].Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJS. Cognitive and behavioral outcomes of school-aged children who were born preterm. JAMA. 2002;288:728–37. doi: 10.1001/jama.288.6.728. [DOI] [PubMed] [Google Scholar]
- [40].Angeles DM, Ashwal S, Wycliffe ND, Ebner C, Fayard E, Sowers L, et al. Relationship between opioid therapy, tissue-damaging procedures, and brain metabolites as measured by proton MRS in asphyxiated term neonates. Pediatric research. 2007;61:614–21. doi: 10.1203/pdr.0b013e318045bde9. [DOI] [PubMed] [Google Scholar]