Abstract
Purpose
To report the first case of melanoma-associated retinopathy (MAR) and underlying occult melanoma diagnosed based on the presence of serum transient receptor potential melastatin 1 (TRPM1) autoantibodies.
Design
Interventional case report with basic science correlation.
Participants
One patient with melanoma-associated retinopathy (MAR).
Intervention
Testing for the presence of serum TRPM1 autoantibodies.
Main Outcome Measure
Diagnosis of an occult melanoma involving the axillary lymph nodes (unknown primary) and MAR based on the presence of TRPM1 autoantibodies in the patient’s serum.
Results
The patient's clinical exam was remarkable for mild intraocular inflammation in both eyes and retinal hemorrhages with an apparent choroidal neovascularization (CNV) in the left eye, which was confirmed by fluorescein angiogram (FA) and indocyanine green angiography (ICG) testing. Humphrey visual field (HVF) demonstrated diffuse depression in both eyes out of proportion to the clinical exam prompting electroretinogram (ERG) testing which revealed an electronegative response. Dark-adapted thresholds were markedly elevated and mediated by cones. Due to concern for MAR, a systemic work-up for melanoma was performed by the primary care physician which was unrevealing. Given our continued clinical suspicion for MAR, the patient's serum was sent for evaluation for TRPM1 autoantibodies. The patient’s serum applied to normal human retina exhibited positivity in the inner nuclear layer. Application of the patient’s serum to wild-type and TRPM1 knockout mouse retina revealed strongly labeled bipolar cells in the wild-type retina, but not in the TRPM1 knockout retina indicating TRPM1-dependent immunoreactivity. The antigen was confirmed as TRPM1 by labeling of TRPM1-transfected human embryonic kidney 293 (HEK293) cells. Additional systemic work-up prompted by this finding resulted in identification of an occult metastatic melanoma involving the axillary lymph nodes with an unknown primary. The patient underwent surgical excision of his occult melanoma without evidence of other sites of metastases. He also received intravenous immunoglobulin therapy and his vision has stabilized.
Conclusions
This is the first reported case of a melanoma-associated retinopathy diagnosed utilizing the application of innovative approach by testing for serum TRPM1 autoantibodies.
Introduction
Melanoma-associated retinopathy (MAR) is a rare paraneoplastic retinopathy that is most commonly associated with cutaneous melanoma, although ocular, visceral, and unknown primary melanomas have also been described.1–6 In most cases, it presents months to years after the diagnosis of the primary melanoma.1 Common presenting symptoms include decreased visual acuity, nyctalopia, photopsias, and visual field abnormalities. In many cases, the clinical examination is normal, especially in early stages. Other findings can include optic nerve pallor, retinal vessel attenuation, and vitreous cells.1 Selective reduction in the electroretinogram (ERG) b-wave, which represents an interruption in the ON-bipolar cell function, can be a distinguishing feature. The first case of MAR was described by Gass in 19847 and to date there have been 74 cases described in the literature.1–7 Diagnosis is most often made based on the patient's symptoms, an electronegative ERG in which there is selective loss of the b-wave, and a known history of melanoma. We present the first case of MAR where the underlying melanoma was diagnosed based on the presence of TRPM1 autoantibodies in the patient's serum.
Methods
The clinical record of the patient, which included clinical notes, laboratory testing, and psychophysical testing such as visual fields and electroretinogram, was reviewed. TRPM1 knockout and wild-type mice used in this study have been described previously.12 Human embryonic kidney 293 (HEK293) cells were transiently transfected with a plasmid encoding TRPM1 fused to the C-terminus of enhanced green fluorescent protein (EGFP), resulting in EGFP-TRPM1 expression in 10–20% of the cells. Immunofluorescent labeling was performed similarly for both mouse retina sections and transfected cells.12 Patient serum was diluted 1:1000 for retina sections and 1:250 for TRPM1-transfected HEK293 cells. Immunoreactivity was revealed with anti-human IgG coupled to Alexa 594 (1:1000; Invitrogen, Carlsbad, CA). Images were acquired with an Olympus FluoView FV1000 confocal microscope using a 60X/1.42 oil immersion objective. Images were adjusted for brightness and contrast using Pixelmator (Pixelmator Team LTD, London, UK). In addition, the available literature was surveyed by searching PubMed for transient receptor potential melastatin 1 (TRPM1), melanoma, melanoma-associated retinopathy (MAR), and paraneoplastic retinopathy.
Informed consent was obtained and all work was Health Insurance Portability and Accountability Act (HIPAA)-compliant. Research adhered to the tenets of the Declaration of Helsinki. Institutional review board (IRB) approval was obtained. All animal experiments were conducted in accordance with National Institutes of Health guidelines and approved by the Institutional Animal Care and Use Committee at Oregon Health & Science University.
Case Report and Results
A 60-year-old Caucasian male in good health without systemic complaints was referred for evaluation of chronic bilateral vitritis and slowly progressive vision loss in both eyes over the past year. He had been evaluated by multiple ophthalmologists without a definite diagnosis. His evaluation prior to presentation included a normal neurologic evaluation and magnetic resonance imaging (MRI) of the brain. Vitreous biopsy of the left eye was negative for malignancy. Additionally, he had undergone a trial of oral prednisone followed by mycophenolate mofetil for approximately 4 months without therapeutic response.
On initial evaluation at the National Eye Institute (NEI), his best-corrected visual acuity was 20/32 in the right eye and 20/63 in the left eye. Anterior segment exam was notable for trace cells without flare and minimal nuclear sclerotic cataracts in both eyes. Posterior segment exam revealed trace vitreous cells without haze and a mild epiretinal membrane with an otherwise normal fundus in the right eye. Posterior segment exam was significant for 1+ vitreous cell without haze and a deep hypopigmented lesion with surrounding fluid and intraretinal hemorrhage superior to the fovea suspicious for a choroidal neovascular membrane (CNVM) in the left eye. Color fundus photography, fundus autofluorescence, fluorescein angiography, indocyanine green angiography, and Spectralis optical coherence tomography (OCT) were performed and confirmed a CNMV in the left eye. Humphrey visual field 30-2 demonstrated severe generalized depression, greater in the right eye compared to the left eye, out of proportion to the clinical exam. An ERG was performed and demonstrated the absence of rod responses in the dark-adapted state, and a negative ERG with an almost complete absence of the b-wave in the presence of a near normal a-wave in both eyes (Figure 1). Dark adapted thresholds obtained on a Goldmann-Weekers dark adaptometer were elevated by over 3 log units indicating that these thresholds were being mediated by cones. Although the patient did not have a history of cancer including melanoma, our clinical suspicion at this time was concerning for a paraneoplastic retinopathy, specifically MAR, in the light of the negative ERG. A systemic work-up was recommended to the patient's local primary care physician; his evaluation included a complete blood count, chemistry, liver function testing, repeat neurologic exam, chest x-ray, MRI brain, colonoscopy, and prostate examination which were all normal. Computed tomography of the chest, abdomen, and pelvis had been recommended but not done. Given our continued clinical suspicion for MAR and the patient's precipitous decline in vision to 20/200 OD and 20/400 OS within several weeks his serum was tested for antiretinal antibody, specifically for TRPM1 autoantibody. Immunohistochemistry demonstrated staining at the inner nuclear layer on normal human frozen retina. Using the patient's serum as the primary antibody, immunofluorescent labeling of bipolar cells was detected in the outer plexiform layer and inner nuclear layer of the wild-type mouse retina while no immunoreactivity was detected when applied to the TRPM1 knockout mouse retina (Figure 2). The bipolar cell antigen was confirmed as TRPM1 by labeling HEK293 cells transiently transfected with an EGFP-TRPM1 fusion protein. The patient’s serum labeled transfected cells (identified by EGFP fluorescence), but not untransfected cells (Fig 3). Given his clinical and ERG findings and the antiretinal antibody results, the patient was diagnosed with MAR and referred to oncology and dermatology for evaluation for an underlying melanoma. CT of the chest, abdomen, and pelvis was performed and revealed two enlarged right axillary lymph nodes (2.7 × 2.0 cm and 2.1 × 1.6 cm) that were hypermetabolic on positron emission tomography (PET) scan. Excisional biopsy revealed melanoma in 1 of the 4 lymph nodes (Figure 4). There were no other detectable foci of disease or primary site for melanoma. The patient was diagnosed with metastatic melanoma with an unknown primary and has been negative for evidence of any melanoma on repeat imaging 12 months following diagnosis. Prior to completing his systemic evaluation, the patient received a subtenon's triamcinolone injection in the right eye, anti-vascular endothelial growth factor injection in the left eye, and one cycle of intravenous immunoglobulin due to concern for declining visual function. He has not required chemotherapy or radiation. His vision remains stable at 20/200 in the right eye and 20/400 in the left eye.
Figure 1. Full-field electroretinogram (ERG).
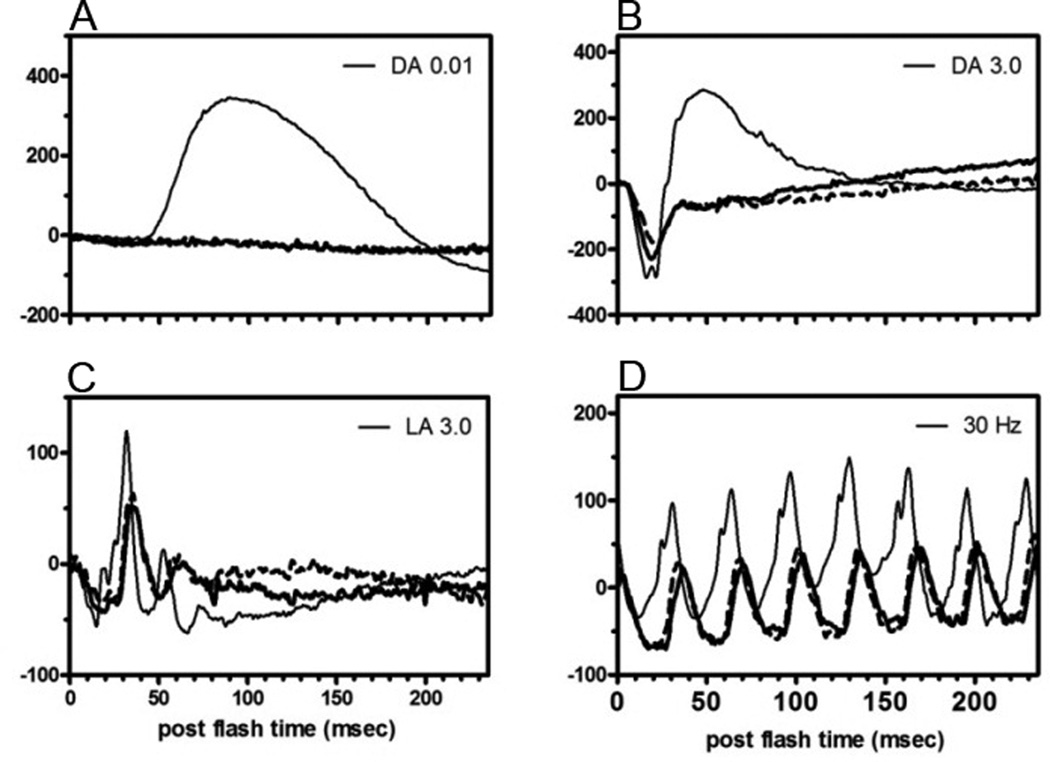
Thin solid line is from a normal subject and the thick solid and dashed lines are from the patient’s right eye and left, respectively. (A) Absence of a rod response. (B) Negative ERG with almost complete absence of the b-wave (bipolar response) in the presence of a near normal a-wave (photoreceptor response). (C) Prototypical loss of ON-bipolar cell responses. (D) Similar to photopic response, typical of loss of ON-bipolar cell responses. Abbreviations: DA, dark-adapted; LA, light-adapted; Hz, hertz.
Figure 2. Immunofluorescent labeling of retina sections from wildtype and transient receptor melastatin potential 1 (TRPM1) knockout mice.
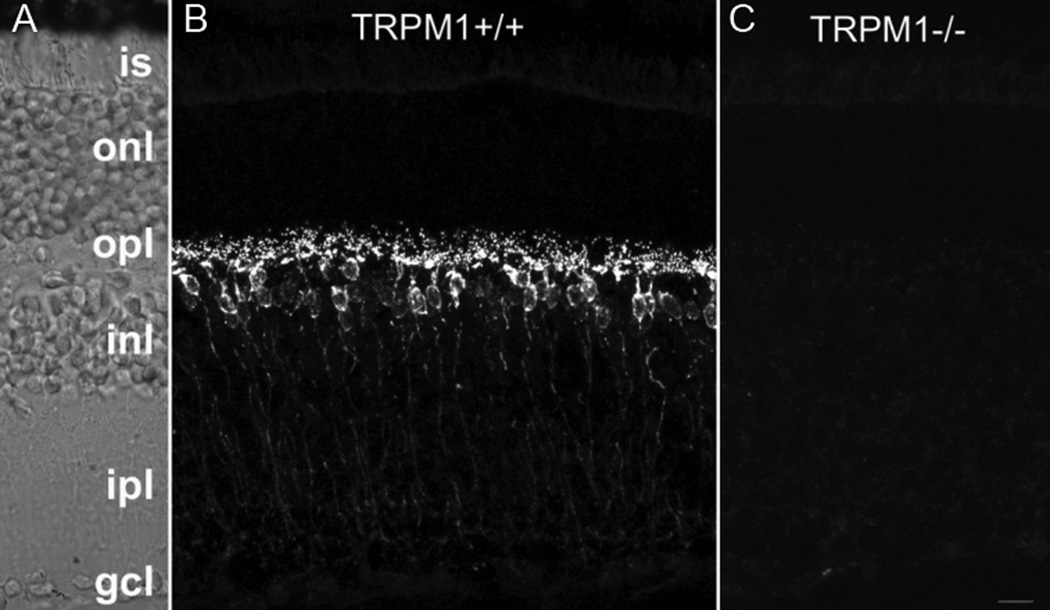
(A) Nomarski image of the retina section shown in panel B. (B) Antibodies in the patient’s serum react with bipolar cells in retina sections from wild-type mice, (C) but not TRPM1 knockout mice. Abbreviations: is, inner segments; onl, outer nuclear layer; opl, outer plexiform layer; inl, inner nuclear layer; ipl, inner plexiform layer; gcl, ganglion cell layer. Scale bar = 10um.
Figure 3. Positive staining of human embryonic kidney 293 (HEK293) cells transfected with enhanced green fluorescent protein (EGFP)-TRPM1.
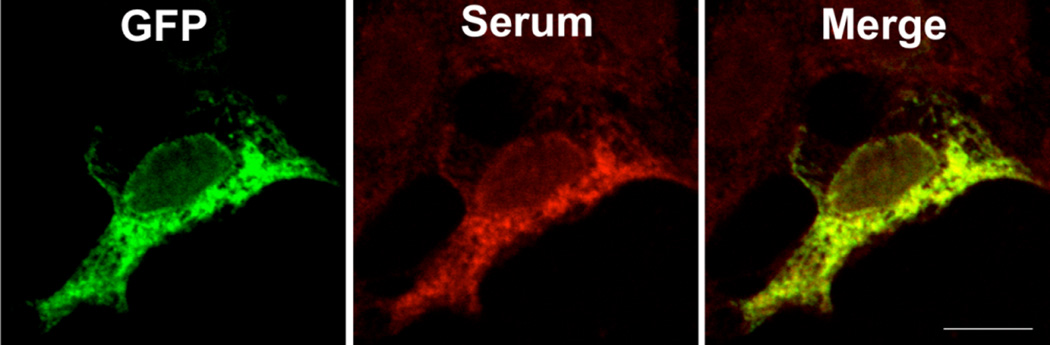
Green fluorescent protein (GFP) is shown in green and the patient serum immunoreactivity in red (aHu-594). Overlap between the red and green channels appears yellow (merge). Scale bar = 10um.
Figure 4. Photomicrograph of immunostaining of melanoma cells in the axillary lymph node.
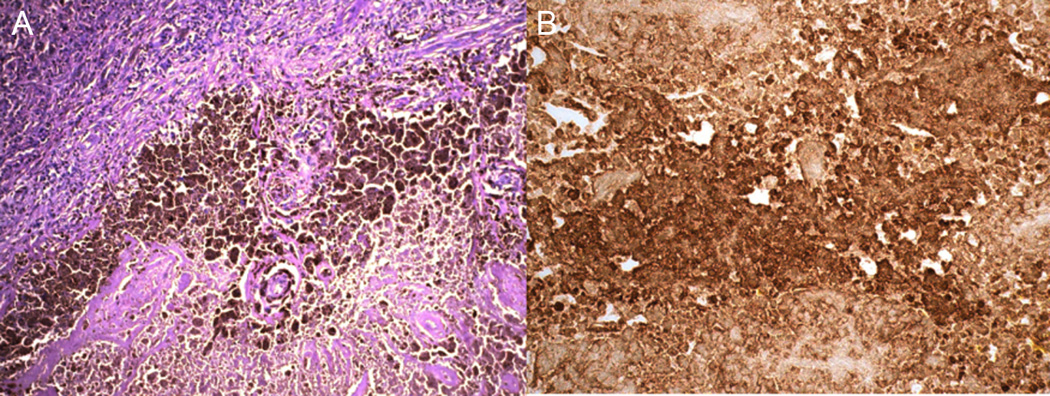
(A) Infiltration of melanoma cells in lymph node tissue (hematoxylin and eosin). (B) Positive human melanoma black-45 (HMB-45) staining of melanoma cells.
Discussion
Recently, the presence of retinal autoantibodies in MAR has become t he subject of much interest. Milam et al.8 first proposed that MAR resulted from autoantibodies that circulate in the patient's serum based on the observation that the serum of MAR patients react with rod bipolar cells. In support of this, Lei et al.9 demonstrated injection of purified IgG from MAR patients into monkey eyes caused a reduction in the ERG b-wave. Based on ERG and immunohistochemistry studies, the results suggested the main target in MAR is the retinal ON-bipolar cell; however the exact antigen was unknown until recently. Dhingra et al.10 and Kondo et al.11 both demonstrated the primary target of autoantibodies in MAR is the TRPM1 cation channel. TRPM1 is required for the depolarizing response of ON-bipolar cells to light.12–13 While other autoantibodies such as carbonic anhydrase II, enolase, arrestin and aldolase have been demonstrated in the sera of MAR patients, TRPM1 appears to be important in those that demonstrate selective b-wave reductions but near normal a-waves, since bipolar cells are the primary targets in these cases.12–13 Recently, TRPM1 channels on the ON bipolar dendritic tips in the outer plexiform layer have also been illustrated in the retina of a patient with paraneoplastic vitelliform retinopathy, and atypical form of MAR.14 TRPM1 is expressed in normal melanocytes, and is downregulated in metastatic melanoma.15 This downregulation may be an immunologic defense against metastatic melanocytes, and the generation of TRPM1 autoantibodies may contribute to host defense.10 TRPM1, is a non-selective cation channel that is required for light responses in all types of ON-bipolar cells.12–13 As suggested by Dhingra et al. (2011), it is likely that circulating antibodies bind the TRPM1 channel on the ON-bipolar cells and either directly block the channel or indirectly interfere with the cell's signal transduction. Night vision is mediated by rod bipolar cells. Importantly, rods are only connected to ON-type bipolar cells, whereas cones signal through both ON- and OFF-type which explains why nyctalopia is a common complaint in MAR patients. However, some impairment of daylight vision can occur as well reflecting the ON class of cone bipolar cells, which also use TRPM1.12 Preservation of cone-mediated vision is achieved through unaffected OFF-bipolar cells which do not utilize TRPM1 channels.10
While there are no proven treatments for MAR, previous therapies have focused on reducing tumor-burden, which incites autoantibody formation, through metastasectomy, chemotherapy, and radiation, and removal of autoantibodies present in the serum via intravenous immunoglobulin (IVIG), systemic corticosteroids, and plasmapheresis.16 Subhadra et al.17 demonstrated improvement in visual field testing after IVIG and Jacobzone et al.18 showed some recovery in ERG after systemic corticosteroids, although this is not typical as treatment of visual loss in MAR has been largely ineffective. Additionally, there is some concern that immunomodulatory therapy such as IVIG, although decreasing the titer of circulating autoantibodies, may increase cancer mortality because MAR patients may have antibodies that are protective against tumor spread.1 However, there is no clear evidence to date that treatment has a negative effect. In our patient, tumor excision and intravenous immunoglobulin treatment have appeared to stabilize his ocular disease at 12 months follow-up. At present he is considered tumor-free, however he will require close continued monitoring for a primary etiology and potentially new metastases.
The presence of TRPM1 autoantibodies in the serum of this patient helped establish the diagnosis of MAR and discovery of metastatic melanoma without a known primary. MAR is generally believed to occur only after patients have developed metastatic melanoma.19 In the case series by Keltner et al. (2001) they disclosed two patients who presented with MAR before the diagnosis of a primary melanoma, similar to our patient. To date, only 4 cases of established MAR were demonstrated to have positive TRPM1 antibody, however these cases had known melanoma.10–11 To our knowledge, this is the first case of MAR where TRPM1 testing lead to the diagnosis of occult melanoma. While positive serum TRPM1 autoantibody testing alone in not sufficient to diagnose MAR, and its presence alone does not indicate MAR, TRPM1 testing can be a useful objective adjunct when there is a clinical suspicion for MAR, especially in the setting of an electronegative ERG. While our case was rare in that the patient did not have a known melanoma, TRPM1 autoantibody testing may also be valuable for earlier detection of ocular involvement in those with known melanoma.
Acknowledgments
Financial Support: Grant support was provided by NEI Intramural Research Program (Bethesda, MD). The sponsor or funding organization had no role in the design or conduct of this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: CWM and RMD have filed an invention disclosure with Oregon Health & Sciences University (OHSU) that may have commercial applications relating to the results of this research. This potential institutional and individual conflict of interest has been reviewed and managed by OHSU. The other authors have no conflict of interest.
References
- 1.Keltner JL, Thirkill CE, Yip PT. Clinical and immunologic characteristics of melanoma-associated retinopathy syndrome: eleven new cases and review of 51 previously published cases. J Neuroophthalmol. 2001;21:173–187. doi: 10.1097/00041327-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Lu Y, Jia L, He S, et al. Melanoma-associated retinopathy: a paraneoplastic autoimmune complication. Arch Ophthalmol. 2009;127:1572–1580. doi: 10.1001/archophthalmol.2009.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diamant PM, Prause JU, Rosenberg T, Fledelius HC. Melanomaassociated retinopathy in a patient without a primary tumour [in Danish] Ugeskr Laeger. 2004;166:2812–2813. [PubMed] [Google Scholar]
- 4.Murayama K, Takita H, Kiyohara Y, et al. Melanoma-associated retinopathy with unknown primary site in a Japanese woman [in Japanese] Nihon Ganka Gakkai Zasshi. 2006;110:211–217. [PubMed] [Google Scholar]
- 5.Kiratli H, Thirkill CE, Bilgic S, et al. Paraneoplastic retinopathy associated with metastatic cutaneous melanoma of unknown primary site. Eye (Lond) 1997;11:889–892. doi: 10.1038/eye.1997.227. [DOI] [PubMed] [Google Scholar]
- 6.Rappoport D, Leiba H. Presumed melanoma-associated retinopathy (MAR): a presenting sign of primary small intestinal melanoma? Int Ophthalmol. 2012;32:387–391. doi: 10.1007/s10792-012-9564-y. [DOI] [PubMed] [Google Scholar]
- 7.Gass JD. Acute Vogt-Koyanagi-Harada-like syndrome occurring in a patient with metastatic cutaneous melanoma. In: Saari KM, editor. Uveitis Update: Proceedings of the First International Symposium on Uveitis; Elsevier Science; Amsterdam. 1984. pp. 407–408. [Google Scholar]
- 8.Milam AH, Saari JC, Jacobson SG, et al. Autoantibodies against retinal bipolar cells in cutaneous melanoma-associated retinopathy. Invest Ophthalmol Vis Sci. 1993;34:91–100. [PubMed] [Google Scholar]
- 9.Lei B, Bush RA, Milam AH, Sieving PA. Human melanoma-associated retinopathy (MAR) antibodies alter the retinal ON-response of the monkey ERG in vivo. Invest Ophthalmol Vis Sci. 2000;41:262–266. [PubMed] [Google Scholar]
- 10.Dhingra A, Fina ME, Neinstein A, et al. Autoantibodies in melanoma-associated retinopathy target TRPM1 cation channels of retinal ON bipolar cells. J Neurosci. 2011;31:3962–3967. doi: 10.1523/JNEUROSCI.6007-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kondo M, Sanuki R, Ueno S, et al. Identification of autoantibodies against TRPM1 in patients with paraneoplastic retinopathy associated with ON bipolar cell dysfunction. [Accessed July 21, 2013];PLoS ONE [serial online] 2011 6:e19911. doi: 10.1371/journal.pone.0019911. Available at: http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0019911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morgans CW, Zhang J, Jeffrey BG, et al. TRPM1 is required for the depolarizing light response in retinal ON-bipolar cells. Proc Natl Acad Sci U S A. 2009;106:19174–19178. doi: 10.1073/pnas.0908711106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koike C, Obara T, Uriu Y, et al. TRPM1 is a component of the retinal ON bipolar cell transduction channel in the mGluR6 cascade. Proc Natl Acad Sci U S A. 2010;107:332–337. doi: 10.1073/pnas.0912730107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Abu-Asab MS, Li W, et al. Autoantibody against transient receptor potential M1 cation channels of retinal ON bipolar cells in paraneoplastic vitelliform retinopathy. [Accessed July 21, 2013];BMC Ophthalmol [serial online] 2012 12:56. doi: 10.1186/1471-2415-12-56. Available at: http://www.biomedcentral.com/1471-2415/12/56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duncan LM, Deeds J, Cronin FE, et al. Melastatin expression and prognosis in cutaneous malignant melanoma. J Clin Oncol. 2001;19:568–576. doi: 10.1200/JCO.2001.19.2.568. [DOI] [PubMed] [Google Scholar]
- 16.Powell SF, Dudek AZ. Treatment of melanoma-associated retinopathy. Curr Treat Options Neurol. 2010;12:54–63. doi: 10.1007/s11940-009-0057-x. [DOI] [PubMed] [Google Scholar]
- 17.Subhadra C, Dudek AZ, Rath PP, Lee MS. Improvement in visual fields in a patient with melanoma-associated retinopathy treated with intravenous immunoglobulin. J Neuroophthalmol. 2008;28:23–26. doi: 10.1097/WNO.0b013e31816754c4. [DOI] [PubMed] [Google Scholar]
- 18.Jacobzone C, Cochard-Marianowski C, Kupfer I, et al. Corticosteroid treatment for melanoma-associated retinopathy: effect on visual acuity and electrophysiologic findings. Arch Dermatol. 2004;140:1258–1261. doi: 10.1001/archderm.140.10.1258. [DOI] [PubMed] [Google Scholar]
- 19.Milam AH. Clinical aspects: paraneoplastic retinopathy. In: Djamgoz MB, Archer SN, Vellerga S, editors. Neurobiology and Clinical Aspects of the Outer Retina. London: Chapman and Hall; 1995. pp. 461–471. [Google Scholar]


