Abstract
Objective
To observe the effect on total liver volume (TLV) on and off therapy in selected symptomatic patients with autosomal dominant polycystic kidney disease (ADPKD) or autosomal dominant polycystic liver disease (PLD) who received octreotide long-acting release (OctLAR) for up to 4 years.
Patients and Methods
Twenty-eight of 42 participants in a prospective 2-year clinical trial of OctLAR (40 mg monthly) consisting of double-blind, randomized (year 1) and open-label treatment (year 2) phases reenrolled in a 2-year open-label extension (OLE) study after being off OctLAR a mean of 8.3 months (original study: July 1, 2007, through June 30, 2013). Participants underwent magnetic resonance imaging at baseline, years 1 and 2, reenrollment, and study completion. Primary end point: change in TLV; secondary end points: changes in total kidney volume, glomerular filtration rate, quality of life (QoL), safety, vital signs, and laboratory parameters.
Results
Twenty-five participants (59.5%) completed the OLE. Off therapy, TLVs increased a mean ± SD of 3.4%±8.2% per year; after resuming therapy, TLVs decreased a mean ± SD of −4.7%±6.1% per year. Despite regrowth off treatment, overall reductions were observed, with a median (interquartile range) TLV of 4047 mL (3107–7402 mL) at baseline and 3477 (2653–7131 mL) at study completion (−13.2%; P<.001) and with improved health-related QoL. Total kidney volumes increased, and glomerular filtration rates declined from 58.2 mL/min to 54.5 mL/min (n=16) in patients with ADPKD on therapy from baseline to study completion.
Conclusion
Therapy with OctLAR over 4 years in selected patients with symptomatic PLD arrested PLD progression, alleviating symptoms and improving health-related QoL. Discontinuation led to organ regrowth.
Trial Registration: clinicaltrials.gov Identifier: NCT00426153.
Autosomal dominant polycystic kidney disease (ADPKD) is the most common hereditary kidney disease and is characterized by the development of kidney cysts and progressive kidney function loss, often leading to end-stage renal disease. The liver is the most common extrarenal site of involvement.1 Mutations in 1 of 2 genes, PKD1 and PKD2, can lead to this disease. Another disease, autosomal dominant polycystic liver disease (PLD), leads to a similar liver cystic phenotype and no renal failure. There are currently no Food and Drug Administration–approved treatments for either disease capable of attenuating the rate of cyst formation. However, several clinical trials with somatostatin analogs have shown short-term benefit in the symptomatic PLD associated with these disorders.2–4 We previously completed a double-blind, randomized, placebo-controlled clinical trial using octreotide long-acting release depot (OctLAR) (Novartis Pharmaceuticals Corporation) over 2 years in 42 patients with severe PLD due to autosomal dominant PLD or ADPKD.5,6 A relatively small, prospective, randomized clinical trial with 3 years of follow-up in patients with ADPKD focused on the renal but not the hepatic manifestations of the disease.7 Two other prospective clinical trials have shown similar positive effects of somatostatin analogs in autosomal dominant PLD and ADPKD.2–4,8 One of these, a 12-month trial of somatostatin analog therapy, extended follow-up another 6 months in an open-label extension (OLE) study.9 A few other published reports relating to the use of somatostatin analogs further substantiate these observations.8,10,11
To evaluate whether long-term therapy retards liver growth beyond 2 years, the protocol design included a further 2-year OLE with OctLAR. Herein, we report the effects of OctLAR in patients treated for up to 4 years who were randomized in the original study and the effects of therapy discontinuation after the first 1 to 2 years of treatment.
PATIENTS AND METHODS
This is a 2-year OLE study of patients with severe PLD who had completed a clinical trial consisting of a 1-year, randomized, placebo-controlled, double-blind study of OctLAR with 2:1 randomization and a second-year open-label treatment study of all participants with OctLAR. The rationale, design, eligibility criteria, and implementation of the original trial have been described elsewhere.5,6 The Mayo Clinic Institutional Review Board approved this study, and it was conducted in adherence with the Declaration of Helsinki. All the authors had access to the study data and reviewed and approved the final manuscript.
Severe PLD was defined as a liver volume greater than 4000 mL or symptomatic disease due to mass effects from hepatic cysts.
All participants who completed the original 2-year clinical trial were offered participation in the 2-year OLE except those who had reached advanced stage 4 or 5 chronic kidney disease. After completion of the original clinical trial, participants were off therapy for a mean of 8.3 months (mean, 249 days; median, 306 days; range, 0–442 days) before starting the OLE. Original trial enrollment took place from January 1, 2007, through May 19, 2008, and patients reenrolled in the 2-year OLE from January 1 through July 31, 2010. All patients enrolling in the 2-year OLE signed an informed consent form and were evaluated by the principal investigator (M.C.H.) or a co-investigator (V.E.T.) every 6 months during the OLE. Evaluations included physical examination, vital signs, and laboratory parameters (aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, bilirubin, electrolytes, blood urea nitrogen, creatinine, fasting glucose, complete blood cell count, activated partial thromboplastin time, and prothrombin time). On reenrollment, all women of childbearing age had a pregnancy test, and all patients were required to use contraception or were postmenopausal. Magnetic resonance imaging (MRI) or computed tomography (CT) of the liver and kidneys was performed at reenrollment in the OLE (OLEbaseline) and at the end of year 4 (OLEend). Three monthly telephone follow-up monitoring visits were made by a study coordinator or as needed when there were adverse events. If drug adverse effects were identified during a telephone visit, a decision was made by the study team to reduce the next injection dose by 10 or 20 mg. The OctLAR was dispensed at 4-month intervals by the Mayo Research Pharmacy during the OLE.
The main goal of the OLE study was to analyze the change in total liver volume (TLV). Additional goals were to assess the effects of OctLAR on total kidney volume (TKV), glomerular filtration rate (GFR) as measured by iothalamate clearance, and quality of life (QoL), as well as its safety and toxicity. Liver volume was measured by MRI (or CT in 2 patients) at OLEbaseline and OLEend (eg, after up to 4 years of treatment) and was compared with the baseline, 12-month, and 24-month measurements of the original trial. The TKV, estimated GFR (eGFR), QoL as measured by the 36-item Short-Form Health Survey Version 2.0, safety as ascertained by reported adverse events, vital signs, and laboratory tests were measured at the same time points. Adverse effects were classified by Common Terminology Criteria for Adverse Events version 3. The study coordinator confirmed monthly drug and dose administration.
Acquisition of MRIs and CT scan at OLEbaseline and OLEend was performed using the protocol as described in the previous clinical trial.5,6 The TLVs and TKVs were measured blindly (M.V.I.) in the Imaging Core of the Mayo PKD Translational Center as previously described.5,6 The comparability of volumetric measurements from MRI and CT and the low interobserver variability have been previously established.6 Absolute TLV and TKV at OLEbaseline and OLEend were compared with baseline, year 1, and year 2 measurements, and percentage changes were calculated on a per-patient basis.
The TLVs and TKVs are expressed as means, medians, and interquartile ranges (IQRs). X2 and 2-sample t tests were used for group comparisons. Because time spent off OctLAR between the end of the clinical trial and the beginning of the OLE was variable, annualized rate of change was calculated for TLV and TKV. Percentage changes were calculated on a per-patient basis, and then these values were analyzed using standard methods. The 1-sample t test was used to determine the significance of the annualized rate in each period, and the Wilcoxon signed rank test was used between on and off drug periods. A statistical software program (SAS version 9.2; SAS Institute Inc) was used for all analyses. A P<.05 was considered statistically significant.
RESULTS
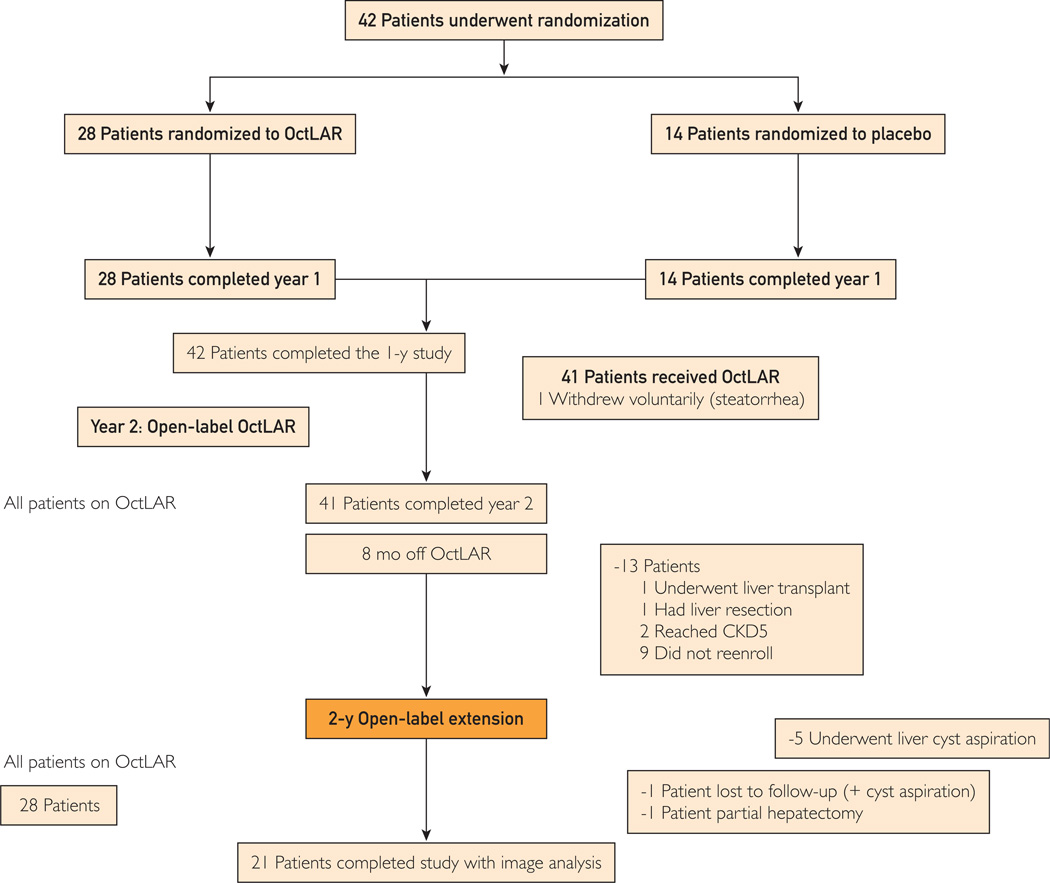
Twenty-eight of the 42 patients who completed the original trial opted to participate in the OLE (Figure 1). Fourteen patients decided not to continue: 1 with steatorrhea after the first year; 2 with continued liver growth who chose to undergo liver transplant or liver resection; 2 reached stage 5 chronic kidney disease, a contraindication to enrollment; 4 mainly owing to lack of effect on liver growth; and 5 owing to the expense of travel to the study site.
FIGURE 1.
Study flowchart. CKD5 = chronic kidney disease stage 5; OctLAR = octreotide long-acting release.
Of the 28 patients who enrolled in the study (Figure 1), 2 did not complete the OLE study protocol (1 decided to have a combined liver resection/fenestration and 1 was lost to follow-up). Five other patients who underwent liver cyst aspiration at the completion of the 2-year study were also excluded from the TLV analysis, leaving 21 patients for the final 4-year analysis at OLEend: 13 from the original OctLAR group and 8 from the original placebo group. The baseline characteristics, previous allocation to receive OctLAR or placebo, and absolute change in TLV during the first 2 years in the 21 enrolled patients vs the 20 patients who declined participation (or underwent procedures that precluded data analysis) were not different except for their responsiveness to OctLAR therapy in the first 2 years (reflected in mean ± SD percentage change in TLV: −2.5%±8.7% in those who declined OLE participation vs −9.6%±8% in OLE enrollees; P=.02, 2-sample t test).
Liver Volumes
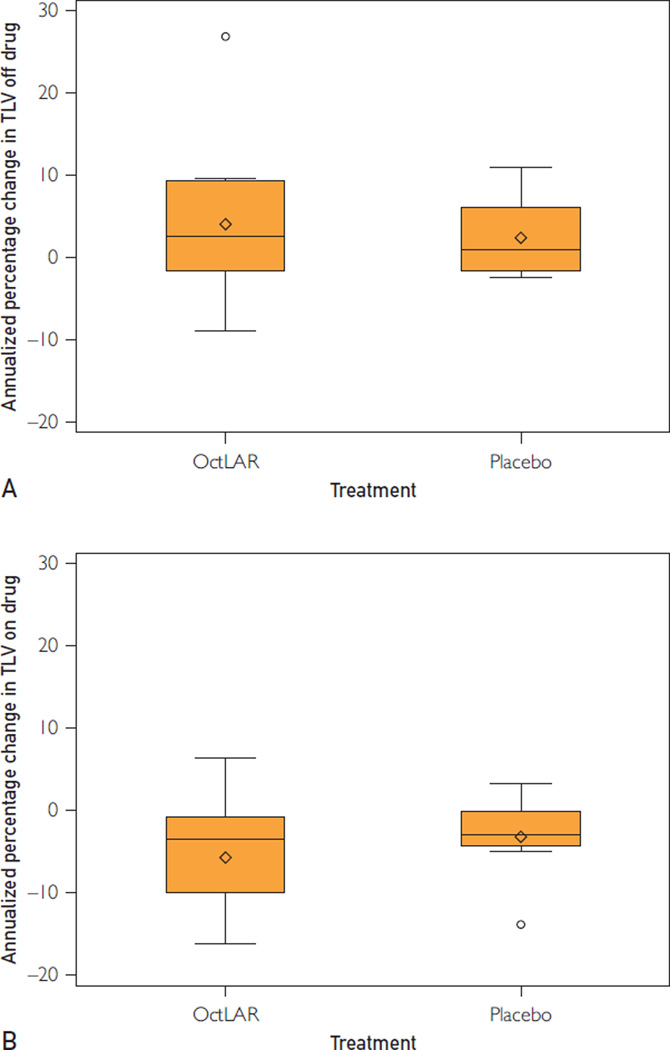
During the first year of the original trial, the mean TLV of these 21 patients decreased from 5863 mL (IQR, 3107–7402 mL) to 5617 mL (IQR, 2867–8743 mL) (Table 1). After the second year, the TLV of the 21 patients increased from 5304 mL (IQR, 2595–7698 mL) to 5368 mL (IQR, 2799–7318 mL; mean ± SD, 3.4%±8.2%; P=.11) (Figure 2, A and Table 2) during a mean of 8.3 months off therapy. The difference in the rate of change in TLV on drug year 1 to year 2 (−5.6%) vs that occurring in the off-drug period from year 2 to OLEbaseline (3.4%) was significant (P<.001) (Table 2). On resumption of drug during the OLE, the mean TLV dropped from 5368 mL (IQR, 2799–7318 mL) to 5138 mL (IQR, 2653–7131 mL) (P=.002), at a rate of −4.7%±6.1% per year (Figure 2, B and Table 2). Despite the increase in TLV while off OctLAR, the mean TLV decreased from 5863 mL (IQR, 3107–7402 mL) at (the original) baseline to 5138 mL (IQR, 2653–7131 mL) at OLEend (−11.75%; P=.006) over nearly 4 years of OctLAR therapy (Figure 3).
TABLE 1.
Changes in Total Liver Volume in Open-Label Extension Participants With Volumes Available for Analysis (Censored for Patients Who Had Liver Cyst Aspirations)a
| Liver volume (mL) |
Baseline (n=21) |
Year 1 (n=21) |
Year 2 (n=20b) |
OLEbaseline (n=21) |
OLEend (n=21) |
|---|---|---|---|---|---|
| Mean | 5863 | 5617 | 5304 | 5368 | 5138 |
| Median | 4047 | 3885 | 3454 | 3607 | 3477 |
| IQR | 3107–7402 | 2867–8743 | 2595–7698 | 2799–7318 | 2653–7131 |
IQR = interquartile range; OLEbaseline = reenrollment into a 2-year open-label extension study; OLEend = open-label extension study completion.
The year 2 scan of 1 person was not usable.
FIGURE 2.
A, The original octreotide long-acting release (OctLAR) and placebo groups experienced increases in total liver volume (TLV) while off OctLAR from year 2 to reenrollment in a 2-year open-label extension study (OLEbaseline). B, The original OctLAR and placebo groups showed reductions in TLV while on OctLAR during the 2 years from OLEbaseline to open-label extension study completion (OctLAR n=1, placebo n=8). The boxes extend from the 25th to the 75th percentile and are bisected by the median; the whiskers extend to the most extreme value within 1.5 of the interquartile range; values beyond that and the mean are denoted by symbols. These data are censored for patients who underwent cyst aspiration.
TABLE 2.
Annualized Rates of Change in Total Liver Volume by Study Period (Censored for Patients Who Had Liver Cyst Aspiration)a
| Original 12-mo trial Treatment |
Patients, No. | Annualized rate (%/y), mean ± SD | |||
|---|---|---|---|---|---|
| Baseline to year 1 | Year 1 to year 2 (on drug) |
Year 2 to OLEbaseline (off drug) |
OLEbaseline to OLEend (on drug) |
||
| Octreotide | 13 | −6.5±4.9 (on drug) | −3.9±5.9 | 4.0±10.2 | −5.7±6.7 |
| Placebo | 8 | −1.7±8.9 (off drug) | −8.3±12.3 | 2.4±4.7 | −3.2±5.1 |
| Total | 21 | NA | −5.6±9.0b | 3.4±8.2 | −4.7±6.1c |
| P value (total rate=0) | NA | .01 | .11 | .002 | |
NA = not available; OLEbaseline = reenrollment in a 2-year open-label extension study; OLEend = open-label extension study completion.
P<.001 for total group rate of change on drug from year 1 to year 2 (−5.6%) vs year 2 to OLEbaseline, off drug (3.4%).
P=.001 for total group rate of change on drug from OLEbaseline to OLEend (−4.7%) vs year 2 to OLEbaseline, off drug (3.4%).
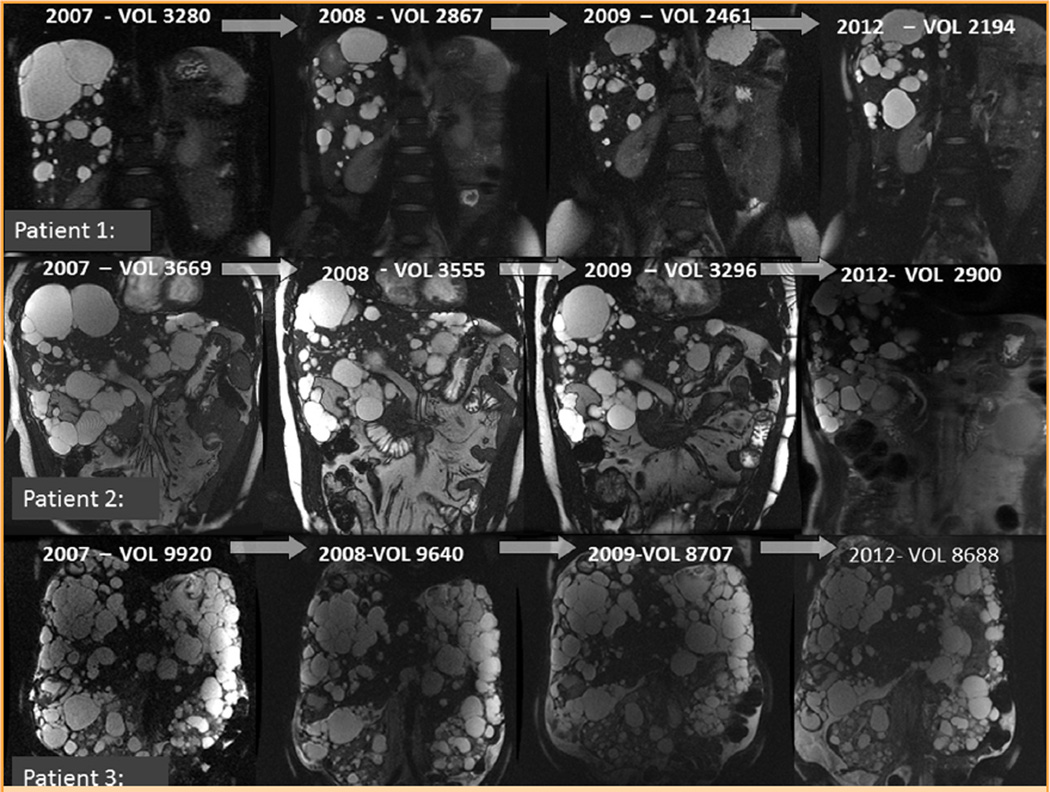
FIGURE 3.
Comparison of serial coronal liver magnetic resonance imaging studies in three participants, including additional liver volume data after their 2-year open-label extension period. Adapted from Nephrol Dial Transplant,6 with permission.
Kidney Volumes
Sixteen patients with ADPKD completed the OLE; 15 had complete TKV data for 4 years (Supplemental Table 1, available online at http://www.mayoclinicproceedings.org). During the original clinical trial, the mean TKV of these 15 patients increased from 777 mL (IQR, 443–1053 mL) to 792 mL (IQR, 437–1265 mL). During year 2, when all the patients received OctLAR, the mean ± SD annualized percentage change in TKV was 4.0%±6.8% (P=.05). After completion of the 2-year trial, mean TKVs increased from 819 mL (IQR, 469–1302 mL) to 862 mL (IQR, 471–1421 mL) (mean ± SD annualized rate: +5.5%±7.7%; P=.02) during a mean of 8.3 months off OctLAR. During the OLE, the mean annualized rate of growth increased from 862 mL (IQR, 471–1421 mL) to 879 mL (IQR, 474–1398 mL), or a mean ± SD of 0.7%±13.6% per annum (P=.85).
Renal Function
In the 21 patients in the OLE, the eGFR was estimated at baseline, year 2, and OLEend for the 16 patients with ADPKD with native kidneys. Overall, the mean ± SD eGFR dropped from 58.2±18.8 mL/min at baseline to 54.5±20.6 mL/min by OLEend. The total 4-year mean ± SD change was −3.7±11.2 mL/min, and the mean ± SD percentage change was −7.2%±20% (P=.12). When we compared the absolute change in the eGFR in those receiving placebo in year 1 (a drop of −10.6 mL/min per annum) compared with the group receiving OctLAR in year 1 (a drop of 0.5 mL/min per annum), there were no significant differences between the 2 groups (P=.10). When we examined percentage change in GFR, again no differences between the 2 groups were observed (−2% vs −18.8% per annum; P=.12).
Safety
A few serious adverse events occurred during the OLE but were probably not related to OctLAR. Three patients were hospitalized for abdominal hernia repair surgery. One patient was hospitalized for what was thought to be an infected liver cyst, and another with chest pain. Liver cyst infection was considered when there was right upper quadrant pain, fever (temperature >38°C) for 3 days, increased C-reactive protein levels (>4.7 nmol/L and the absence of CT evidence of recent intracystic bleeding.12 One patient developed radiologic evidence of gallstones but did not require surgical intervention.
Tolerability
The most common adverse effects were injection site pain, bruising, and granuloma formation (9 patients). Occasional needle-clogging events were reported by 1 patient. Most participants tolerated 40-mg monthly dosing, but because of frequent loose stools or flatulence, 2 participants required dose reduction from 40 to 30 mg, and a third was reduced to 20 mg, permitting continued gastrointestinal tolerability. A fourth patient was reduced to 30 mg for 6 months, then to 20 mg for 6 months, and then completed the study on 30 mg.
Laboratory Parameters
Prothrombin time (mean ± SD: 13.1±3.9 seconds) was prolonged at the end of year 4 but without any clinically significant hemorrhagic consequences. Glucose levels increased from year 1 to year 2 by a mean ± SD of 2.6%±10.2% per annum compared with a mean ± SD decrease of 4.5%±12.3% per annum while off OctLAR. Glucose levels increased again by a mean ± SD of 4.7%±8.0% while on OctLAR by OLEend; however, no patient developed diabetes.
Quality of Life
From baseline to the end of year 4 (OLEend), mean 36-item Short-Form Health Survey sub-domain scores increased significantly for social functioning (from a mean of 77 to 85; P=.04), mental health (from a mean of 76 to 81; P=.03), and standardized mental health (from a mean of 50 to 53; P=.01) (Supplemental Table 2, available online at http://www.mayoclinicproceedings.org). Although the bodily pain index improved from a mean of 65 to 71, this change was not significant (P=.13).
DISCUSSION
This study suggests that long-acting somatostatin analogs—specifically OctLAR—are effective in controlling PLD over 4 years in a selected cohort of symptomatic patients. We previously reported positive effects of OctLAR in a 2-year, randomized, crossover clinical trial in reducing the rate of increase in TLV and possibly the rate of increase in TKV.5,6 After interruption of therapy, TLVs increased a mean ± SD of 3.4%±8.2% per year; observation on OctLAR for another 2 years in an OLE saw further reduction in TLV growth (mean ± SD: −4.7%±6.1% per annum) in individuals who chose to be treated for up to 4 years. Mean ± SD liver regrowth (3.4%±8.2% per year) seen after the discontinuation of OctLAR in this study was similar to that observed after discontinuation of lanreotide (4%) in another study.9
During the OLE, these patients experienced lower mean ± SD rates of kidney growth (0.7%±13.6%; P=.46) vs 5.5%±7.7% (n=15) while off therapy, but the small number of patients limits interpretation. These results compare favorably with mean ± SD observed growth rates of 5.27%±3.92% in the Consortium for Radiologic Imaging Studies in Polycystic Kidney Disease (CRISP) (n=241) and 5.5% per year in the Tolvaptan Phase 3 Efficacy and Safety Study in ADPKD (TEMPO) (placebo group n=464) studies and correspond with the findings from the Somatostatin in Polycystic Kidney: a Long-term Three Year Follow up Study (ALADIN) with OctLAR.7,13,14 The GFR declined over the 4 years; however, this decline was small (only a −2% total change), and interpretation is limited because of the small sample size (n=11), although lower than the GFR declines seen in the CRISP and the Study of Heart and Renal Protection (SHARP) (−3.8%±2.5%) cohort studies.13,15 These rates of GFR decline were similar to those reported in the ALADIN trial, where percentage GFR reductions compared with baseline values were numerically smaller in the OctLAR group than in the placebo group from 1 year onward.7
Also note that individuals who chose to continue participation in this OLE seemed to self-select based on therapy responsiveness as reflected by the higher mean percentage change in TLV from the original study, where the group that decided to enroll in the OLE had a mean ± SD response of −9.6%±8.0% vs −2.5%±8.7% in the group that decided not to enroll.
Overall, the safety profile of OctLAR in this study was similar to that seen where the therapy is used for other indications and as reported in the prescribing information. We monitored glucose levels, coagulation studies, and for gallstones (2 patients developed gallstones during the study period; 1 required cholecystectomy at the end of the 2-year study and decided not to reenroll in the OLE).5,6 Patients receiving long-term OctLAR should be monitored for cholelithiasis symptoms or signs because this is a known complication.16–18 We discontinued 1 patient from therapy after 1 year owing to steatorrhea and weight loss.5 Another concern with intramuscular OctLAR injections is the potential for increased risk of liver cyst infections, but we observed only 3 cases (1 definite, 2 possible) over the 4 years of OctLAR therapy.5,6,12,19–21 No patients had diagnostic hepatic cyst aspiration confirming neutrophils and bacteria.
CONCLUSION
In summary, the results of the present study suggest that long-term administration of a somatostatin analog provides a substantial benefit to a selected group of patients with severe PLD, achieving a sustained and continued reduction in TLV for up to 4 years with relative safety. Whether somatostatin analogs have a similar beneficial effect on the progression of PKD is less clear. Longer and larger studies are required to confirm their beneficial effect on the progression of PLD and to better ascertain their effect on the progression of PKD.
Supplementary Material
Acknowledgments
Grant Support: This study was partially funded by Novartis USA (M.C.H.). Novartis USA also supplied the OctLAR.
Potential Competing Interests: Drs Masyuk and LaRusso are named as inventors on patents filed by Mayo Clinic claiming methods for using somatostatin analogs to treat PLD: EP1885384A1, EP1885384A4, US20090170754, US20120277153, and WO2006127214A1.
Abbreviations and Acronyms
- ADPKD
autosomal dominant polycystic kidney disease
- ALADIN
Somatostatin in Polycystic Kidney: a Long-term Three Year Follow up Study
- CRISP
Consortium for Radiologic Imaging Studies in Polycystic Kidney Disease
- CT
computed tomography
- eGFR
estimated glomerular filtration rate
- GFR
glomerular filtration rate
- IQR
interquartile range
- MRI
magnetic resonance imaging
- OctLAR
octreotide long-acting release
- OLE
open-label extension
- OLEbaseline
reenrollment in open-label extension
- OLEend
open-label extension study completion
- PLD
polycystic liver disease
- QoL
quality of life
- SHARP
Study of Heart and Renal Protection
- TEMPO
Tolvaptan Phase 3 Efficacy and Safety Study in ADPKD
- TKV
total kidney volume
- TLV
total liver volume
Footnotes
SUPPLEMENTAL ONLINE MATERIAL
Supplemental material can be found online at http://www.mayoclinicproceedings.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
REFERENCES
- 1.Bae KT, Zhu F, Chapman AB, et al. Magnetic resonance imaging evaluation of hepatic cysts in early autosomal-dominant polycystic kidney disease: the Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease cohort. Clin J Am Soc Nephrol. 2006;1(1):64–69. doi: 10.2215/CJN.00080605. [DOI] [PubMed] [Google Scholar]
- 2.Ruggenenti P, Remuzzi A, Ondei P, et al. Safety and efficacy of long-acting somatostatin treatment in autosomal-dominant polycystic kidney disease. Kidney Int. 2005;68(1):206–216. doi: 10.1111/j.1523-1755.2005.00395.x. [DOI] [PubMed] [Google Scholar]
- 3.Caroli A, Antiga L, Cafaro M, et al. Reducing polycystic liver volume in ADPKD: effects of somatostatin analogue octreotide. Clin J Am Soc Nephrol. 2010;5(5):783–789. doi: 10.2215/CJN.05380709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Keimpema L, Nevens F, Vanslembrouck R, et al. Lanreotide reduces the volume of polycystic liver: a randomized, double-blind, placebo-controlled trial. Gastroenterology. 2009;137(5):1661–1668. doi: 10.1053/j.gastro.2009.07.052. [DOI] [PubMed] [Google Scholar]
- 5.Hogan MC, Masyuk TV, Page LJ, et al. Randomized clinical trial of long-acting somatostatin for autosomal dominant polycystic kidney and liver disease. J Am Soc Nephrol. 2010;21(6):1052–1061. doi: 10.1681/ASN.2009121291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hogan MC, Masyuk TV, Page L, et al. Somatostatin analog therapy for severe polycystic liver disease: results after 2 years. Nephrol Dial Transplant. 2012;27(9):3532–3539. doi: 10.1093/ndt/gfs152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caroli A, Perico N, Perna A, et al. Effect of longacting somatostatin analogue on kidney and cyst growth in autosomal dominant polycystic kidney disease (ALADIN): a randomised, placebo-controlled, multicentre trial. Lancet. 2013;382(9903):1485–1495. doi: 10.1016/S0140-6736(13)61407-5. [DOI] [PubMed] [Google Scholar]
- 8.van Keimpema L, de Man RA, Drenth JP. Somatostatin analogues reduce liver volume in polycystic liver disease. Gut. 2008;57(9):1338–1339. doi: 10.1136/gut.2008.155721. [DOI] [PubMed] [Google Scholar]
- 9.Chrispijn M, Nevens F, Gevers TJ, et al. The long-term outcome of patients with polycystic liver disease treated with lanreotide. Aliment Pharmacol Ther. 2012;35(2):266–274. doi: 10.1111/j.1365-2036.2011.04923.x. [DOI] [PubMed] [Google Scholar]
- 10.Peces R, Cuesta-Lopez E, Peces C, Perez-Duenas V, Vega-Cabrera C, Selgas R. Octreotide reduces hepatic, renal and breast cystic volume in autosomal-dominant polycystic kidney disease. Int Urol Nephrol. 2011;43(2):565–569. doi: 10.1007/s11255-010-9748-1. [DOI] [PubMed] [Google Scholar]
- 11.Treille S, Bailly JM, Van Cauter J, Dehout F, Guillaume B. The use of lanreotide in polycystic kidney disease: a single-centre experience. Case Rep Nephrol Urol. 2014;4(1):18–24. doi: 10.1159/000358268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sallee M, Rafat C, Zahar JR, et al. Cyst infections in patients with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2009;4(7):1183–1189. doi: 10.2215/CJN.01870309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grantham JJ, Torres VE, Chapman AB, et al. Volume progression in polycystic kidney disease. N Engl J Med. 2006;354(20):2122–2130. doi: 10.1056/NEJMoa054341. [DOI] [PubMed] [Google Scholar]
- 14.Torres VE, Chapman AB, Devuyst O, et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367(25):2407–2418. doi: 10.1056/NEJMoa1205511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haynes R, Staplin N, Emberson J, et al. Evaluating the contribution of the cause of kidney disease to prognosis in CKD: results from the Study of Heart and Renal Protection (SHARP) Am J Kidney Dis. 2014;64(1):40–48. doi: 10.1053/j.ajkd.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bigg-Wither GW, Ho KK, Grunstein RR, Sullivan CE, Doust BD. Effects of long term octreotide on gall stone formation and gall bladder function. BMJ. 1992;304(6842):1611–1612. doi: 10.1136/bmj.304.6842.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davies PH, Stewart SE, Lancranjan L, Sheppard MC, Stewart PM. Long-term therapy with long-acting octreotide (Sandostatin-LAR) for the management of acromegaly. Clin Endocrinol (Oxf) 1998;48(3):311–316. doi: 10.1046/j.1365-2265.1998.00389.x. [DOI] [PubMed] [Google Scholar]
- 18.Ho KY, Weissberger AJ, Marbach P, Lazarus L. Therapeutic efficacy of the somatostatin analog SMS 201–995 (octreotide) in acromegaly: effects of dose and frequency and long-term safety. Ann Intern Med. 1990;112(3):173–181. doi: 10.7326/0003-4819-112-3-173. [DOI] [PubMed] [Google Scholar]
- 19.Telenti A, Torres VE, Gross JB, Jr, Van Scoy RE, Brown ML, Hattery RR. Hepatic cyst infection in autosomal dominant polycystic kidney disease. Mayo Clin Proc. 1990;65(7):933–942. doi: 10.1016/s0025-6196(12)65154-4. [DOI] [PubMed] [Google Scholar]
- 20.Jouret F, Lhommel R, Devuyst O, et al. Diagnosis of cyst infection in patients with autosomal dominant polycystic kidney disease: attributes and limitations of the current modalities. Nephrol Dial Transplant. 2012;27(10):3746–3751. doi: 10.1093/ndt/gfs352. [DOI] [PubMed] [Google Scholar]
- 21.Lantinga MA, Drenth JP, Gevers TJ. Diagnostic criteria in renal and hepatic cyst infection. Nephrol Dial Transplant. 2015;30(5):744–751. doi: 10.1093/ndt/gfu227. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.