Abstract
Photodynamic therapy (PDT) is an emerging treatment for malignant and inflammatory dermal disorders. Photoirradiation of the silicon phthalocyanine (Pc) 4 photosensitizer with red light generates singlet oxygen and other reactive oxygen species to induce cell death. We previously reported that Pc 4-PDT elicited cell death in lymphoid-derived (Jurkat) and epithelial-derived (A431) cell lines in vitro, and furthermore that Jurkat cells were more sensitive than A431 cells to treatment. In this study, we examined the effectiveness of Pc 4-PDT on primary human CD3+ T cells in vitro. Fluorometric analyses of lysed T cells confirmed the dose-dependent uptake of Pc 4 in non-stimulated and stimulated T cells. Flow cytometric analyses measuring annexin V and propidium iodide (PI) demonstrated a dose-dependent increase of T cell apoptosis (6.6–59.9%) at Pc 4 doses ranging from 0–300 nM. Following T cell stimulation through the T cell receptor using a combination of anti-CD3 and anti-CD28 antibodies, activated T cells exhibited increased susceptibility to Pc 4-PDT-induced apoptosis (10.6–81.2%) as determined by Pc 4 fluorescence in each cell, in both non-stimulated and stimulated T cells, Pc 4 uptake increased with Pc 4 dose up to 300 nM as assessed by flow cytometry. The mean fluorescence intensity (MFI) of Pc 4 uptake measured in stimulated T cells was significantly increased over the uptake of resting T cells at each dose of Pc 4 tested (50, 100, 150 and 300nM, p<0.001 between 50 and 150nM, n=8). Treg uptake was diminished relative to other T cells. Cutaneous T cell lymphoma (CTCL) T cells appeared to take up somewhat more Pc 4 than normal resting T cells at 100 and 150nm Pc 4. Confocal imaging revealed that Pc 4 localized in cytoplasmic organelles, with approximately half of the Pc 4 co-localized with mitochondria in T cells. Thus, Pc 4-PDT exerts an enhanced apoptotic effect on activated CD3+ T cells that may be exploited in targeting T cell-mediated skin diseases, such as cutaneous T cell lymphoma (CTCL) or psoriasis.
Keywords: Photodynamic therapy, T cells, apoptosis
Introduction
Photodynamic therapy (PDT) uses visible light to activate a photosensitizing compound to generate cytotoxic reactive oxygen species that damage target and surrounding cells through apoptosis as well as other cell death processes. This technique has been demonstrated to be effective in non-melanoma skin cancers (NMSCs) (1–5) as well as other non-oncogenic skin conditions such as acne (6–8) and warts (9–11). Silicon phthalocyanine (Pc) 4 is a second-generation PDT photosensitizer discovered and developed at Case Western Reserve University (5) that has been recently shown to be safe in Pc 4-PDT-treated patients with skin neoplasms and to produce clinical improvement in cutaneous T cell lymphoma lesions (12–14). Pc 4 offers several advantages for PDT: 1.) It can be synthesized in high purity; 2.) The far-red absorption peak of Pc 4 has a high extinction coefficient, allowing efficient absorption of light at depth; 3.) Favorable pharmacodynamics allow for rapid clearance from the skin, minimizing post-treatment cutaneous photosensitivity. The use of Pc 4-PDT in clinical trials for psoriasis and cutaneous T-cell lymphoma has demonstrated this modality to be safe (12), with a maximum tolerated dose identified in CTCL, but not reached in psoriasis.
Pc 4 has been shown to preferentially bind to mitochondrial, lysosomal and endoplasmic reticulum (ER) membranes in lymphoid-derived (Jurkat) and epithelial-derived (A431) cell lines in vitro (15, 16) as well as in numerous other cancer cell lines (reviewed in ref. 15). However, whether or not there is differential uptake and function as PDT on primary human, resting, activated, and malignant T cells is unknown. Therefore, we examined the effectiveness of Pc 4-PDT on such primary human CD4+ T cells in vitro. In this paper, we found that Pc 4 is preferentially taken up by activated and malignant T cells, which are rich in mitochondria, with a resultant associated increased Pc 4-PDT mediated apoptosis.
Materials and Methods
Human subjects
All studies of human subjects were approved by the Institutional Review Board of University Hospitals Case Medical Center (Cleveland, OH). Peripheral blood samples and/or punch biopsies were obtained from volunteer healthy controls and CTCL patients with Sezary Syndrome following informed consent.
Cell Isolation
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized peripheral blood by Histopaque (Sigma-Aldrich), according to the manufacturer’s instructions. CD3+ or CD4+ cells were separated from PBMCs by negative selection on MACS LS separation columns (CD3+/CD4+ T cell isolation kit; Miltenyi Biotec), according to the manufacturer’s instructions. CD4+ cells were used for regulatory T cell (Treg) uptake experiments (see Figure 3). For Treg specific experiments, after overnight incubation, CD4+ T cells were incubated with phycoerythrin (PE)-labeled anti-CD25 mAb (BD Biosciences). Based on isotype comparators and expression levels, cells were then sorted into CD25high, CD25mid and CD25− subsets using a BD FACSAria cell sorter (Case Comprehensive Cancer Center Imaging and Cytometry Core Facility).
Fig 3. Treg take up less Pc 4 than activated dividing T cells.
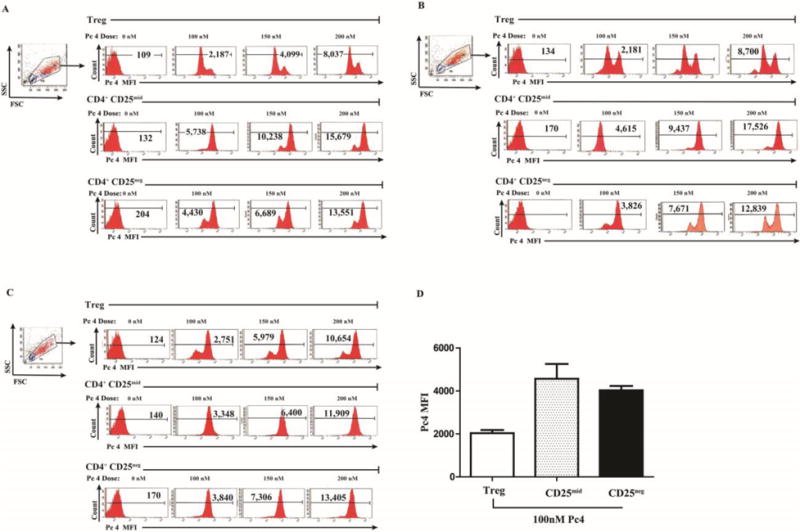
Treg cells from 3 subjects (defined as the highest 5% of CD4 cells expressing CD25) were sorted by flow cytometry and then incubated with Pc 4 (0–300 nM) in complete medium for 2 h. To measure monomeric Pc 4 levels, flow cytometric analysis was performed using a LSR II Flow Cytometer to obtain the mean fluorescence intensity (MFI) for each T cell subset. Pc 4 was excited by a broadband UV laser (335–365 nm) and fluorescence emission was collected with a 650-nm long-pass filter. Autofluorescence was subtracted from each sample. Three independent experiments are shown (A–C), indicating that CD4+ CD25high Treg cells incorporate less Pc 4 (lower Pc 4 MFI) than the proliferative populations of CD4+ CD25mid or CD4+ CD25neg T cells from the same subjects.
Cell Culture
For all experiments, cells were cultured in RPMI 1640 (HyClone) supplemented with 10% fetal bovine serum (Lonza), 1% L-glutamine (HyClone), 1% penicillin-streptomycin and 0.2 mM beta-mercaptoethanol (Sigma-Aldrich). To activate the sorted CD25high, CD25mid and CD25− cells, 24-well cell culture plates (Costar) were coated with 0.5–5 μg/mL anti-CD3 mAb (BD Pharmingen). Cells were cultured for 3–5 days in 500 μL complete medium in the presence or absence of 1 μg/mL soluble anti-CD28 mAb (BD Pharmingen).
Apoptosis Assay
CD25high, CD25mid and CD25− cells were cultured at 3–4 × 105 cells per well with or without CD3/CD28 stimulation for 48 h. After resting an additional 24 h, cells were incubated with varying doses of Pc 4 (50–300 nM) in RPMI complete medium for 2 h, followed by irradiation with red light using a light-emitting diode array (EFOS, Mississauga, Ontario, Canada) at a fluence of 200 mJ/cm2 (1 mW/cm2, λmax ~670–675 nm) at room temperature. After 4h, cells were harvested and stained with fluorescein isothiocyanate (FITC)-labeled anti-Annexin V mAb and PI), according to the manufacturer’s instructions (Annexin V-FITC Apoptosis Detection Kit I; BD Biosciences). Flow cytometric analysis was performed using the BD FACSAria Flow Cytometer.
Pc 4 uptake into T-cells
T cells were cultured at 3–4 × 105 cells per well with or without CD3/CD28 stimulation for 48 h. After resting an additional 24 h cells were incubated with the indicated concentration of Pc 4 (50–300 nM) in complete medium for 2 h. Cells were then harvested and washed with Hanks’ Balanced Salt Solution (1 mL) twice. Flow cytometric analysis was performed using a LSR II Flow Cytometer (Case Comprehensive Cancer Center Imaging and Cytometry Core Facility). Pc 4 was excited by a broadband UV laser (335–365 nm) and fluorescence emission was collected with a 650-nm long-pass filter. Autofluorescence of control cells was subtracted. In a subset of cells, cells were harvested, washed with Hanks’ solution (1 mL) twice and then lysed in sodium dodecyl sulfate (SDS; 2 mL of 0.5%). The SDS concentration was above the critical micelle concentration (SDS CMC = 0.24%). Cell lysates were collected, and fluorescence was measured (Vector X3 Spectrophotometer, Perkin Elmer). A standard curve was constructed from cells lysed without Pc 4 to which known concentrations of Pc 4 (0–300 nM) were added.
Confocal Microscopy
T cells were cultured at 2 × 106 cells/mL with stimulation for 72 h. After this period, cells were incubated with the indicated concentration of Pc 4 (0–300 nM) in complete medium for 2 h. Cells (100 μL) were incubated with 50 nM MitoTracker Green (Invitrogen) for 30 min at 37°C, then counterstained with 10 μg/mL Hoechst 33342 (Sigma-Aldrich) for an additional 15 min at 37°C. Five to 10 μL of stained cells were then placed on a slide with a glass coverslip and confocal images were acquired using an UltraVIEW VoX spinning disk confocal system (PerkinElmer) mounted onto a Leica DMI6000B microscope (Leica Microsystem, Inc.) equipped with a HCX PL APO 100X/1.4 oil immersion objective. Confocal images of Pc 4 fluorescence were collected using a solid state diode 640 nm laser and a 705(W90) band pass filter. Images of MitoTracker Green fluorescence and Hoechst 33342 were collected using a solid state diode 488 nm laser with a 527(W55) band pass filter and 405 nm laser with a 445(W60) band pass filter, respectively.
Results
Activated T cells incorporate higher levels of Pc 4 than resting T cells
CD3+ T cells were cultured with or without anti-CD3/CD28 stimulation and then exposed to various concentrations of Pc 4 in complete medium for 2 h. Cells were then harvested and washed to remove the medium and any associated non-internalized Pc 4. Internalized Pc 4 was quantified by flow cytometry as described in Methods. Activated T cells incorporated up to 4-fold higher levels of Pc 4 compared to resting T cells (Figs. 1A & B). Cumulative data for Pc 4 uptake are indicated in Fig. 1C. T cells incorporated Pc 4 in a dose-dependent manner; at each Pc 4 concentration, activated cells incorporated more Pc 4 than resting cells (p≤0.001 at each of 50, 100 and 150nM Pc 4, n=8). Note the double peak in the activated samples. There was variation in the degree of activation in this population such that, the larger peak correlated with more activated cells, while the smaller correlated with less activated cells.
Fig 1. Activated T cells incorporate higher levels of monomeric Pc 4 than resting T cells.
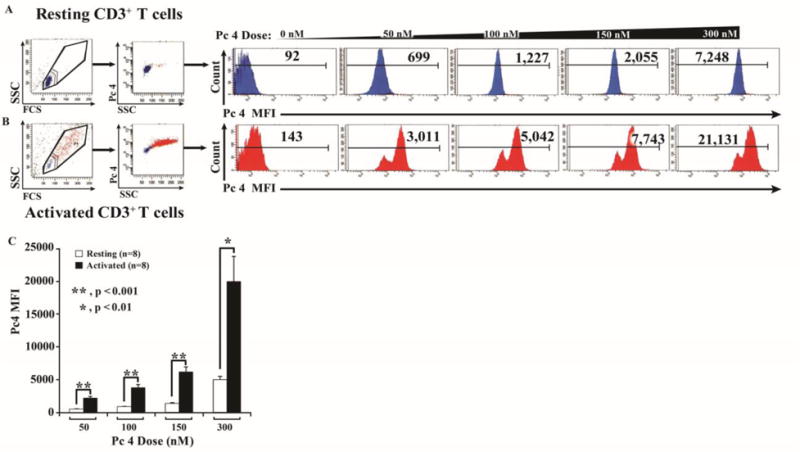
Activated or resting T cells were incubated with Pc 4 (0–300 nM) in complete medium for 2 h. To measure monomeric Pc 4 levels, flow cytometric analysis was performed using a LSR II Flow Cytometer to obtain the mean fluorescence intensity (MFI) for each sample. Pc 4 was excited by a broadband UV laser (335–365 nm) and fluorescence emission was collected with a 650-nm long-pass filter. Autofluorescence was subtracted from each sample. (A & B) Representative examples of the resting (A) and activated (B) populations are shown from 8 independent experiments (individuals). (C) Quantitative data demonstrating the average Pc 4 MFI ± SEM in resting and activated T cells.
Activated T cells exhibit greater susceptibility to Pc 4-PDT-induced cell death than resting T cells
We next sought to determine whether or not the increased uptake of Pc 4 translated into more effective cell killing following photoirradiation of the cells. CD3+ T cells were cultured with or without anti-CD3/CD28 stimulation for 48 h. Following stimulation, cells were incubated with Pc 4 (50–300 nM) in complete medium for 2 h, followed by irradiation with 200 mJ/cm2 of 675 nm red light. Four h after irradiation, T cells were harvested and stained with fluorescein isothiocyanate (FITC)-labeled anti-Annexin V monoclonal antibody and PI (Fig. 2A, quadrant 2). Activated T cells exhibited substantial apoptosis (Annexin V) and cell death (PI uptake) at or above 100nM Pc 4 PDT, whereas resting T cells were relatively resistant to Pc 4 PDT at the 50, 100 and 150 nM doses (p< 0.01, n=8). At 300nM Pc 4, the differential cytotoxicity was lost (Fig. 2B). To confirm that apoptosis was the preferential cell killing method induced by Pc 4, we measure Caspase-3 induction in activated versus regulatory T cells from healthy controls (Fig. 2C, n=2). We next determined whether activated T cells preferentially uptake Pc 4 relative to regulatory T cells, potentially targeting pathogenic T cells while sparing T regulatory cells. Indeed, as shown in Figure 3 representative Treg samples from healthy control individuals demonstrate that both CD4+ CD25neg and CD4+ CD25mid cell populations (recently activated cells), take up more Pc 4 than their corresponding Treg (CD4+ CD25+) population.
Fig 2. Activated T cells exhibit greater susceptibility to Pc 4-PDT induced cell death.
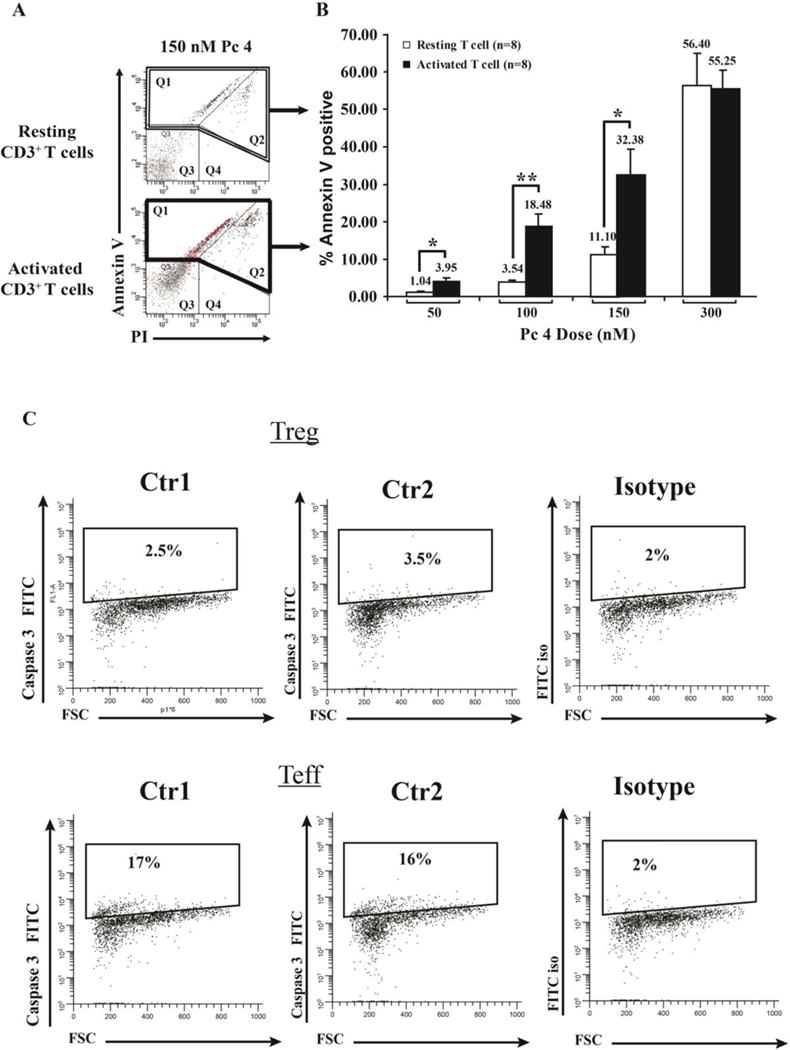
Activated or resting T cells were incubated with varying doses of Pc 4 (0–300 nM) in complete medium for 2 h, followed by irradiation with 200 mJ/cm2 of red light (λmax = 675 nm). After 4 h, cells were harvested and stained with fluorescein isothiocyanate (FITC)-labeled anti-Annexin V mAb and PI. Flow cytometric analysis was performed using the BD FACSAria flow cytometer. Baseline levels of cell death (~7% and ~15% in Q1 and Q2, respectively) were subtracted for each sample. (A) Representative histograms are shown for Annexin V and PI staining following 150 nM treatment of resting vs. activated T cells. (B) Data from 8 independent experiments were combined and analyzed for significance by paired Student’s t test.) Activated T cells exhibit a greater sensitivity than resting cells, a difference that was statistically significant at all Pc 4 concentrations except the highest (300 nM), where cell death was extensive in both cell populations. (C) Representative histograms (n=2) are shown for Caspase-3 staining following 100 nM treatment of activated T cells versus regulatory T cells.
Activated T cells incorporate more total Pc 4 than resting T cells
Only single Pc 4 monomers have significant fluorescence, while aggregated Pc 4 molecules are essentially non-fluorescent. Thus, the measurement of cellular Pc 4 by fluorescence may not account for all of the intracellular photosensitizer. Therefore, the total amount of Pc 4 in cells (Fig. 4A) was determined in the same cell populations described above by lysing an aliquot of cells in SDS, which solubilizes and monomerizes all Pc 4, and measuring the fluorescence emission after excitation of Pc 4 at 610 nm. The level of total Pc 4 increased in a dose dependent manner (Fig. 4A, B), as observed in the intact cell flow cytometry based assays (Fig. 1C); this observation rules out the possibility that differential Pc 4 fluorescence by flow cytometry is due to differences in aggregated monomeric Pc 4 when it is present and distributed in living T cells.
Fig 4. Activated T cells incorporate more total Pc 4 than do resting T cells.
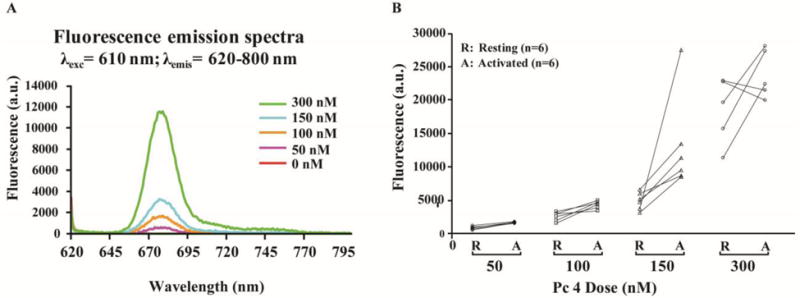
Activated or resting T cells were incubated with Pc 4 (0–300 nM) in complete medium for 2 h. To analyze total Pc 4 levels, cells were lysed in SDS. The SDS concentration was above the critical micelle concentration (SDS CMC = 0.24%). Cell lysates were collected, and fluorescence was measured. (A) A representative example of one experiment with resting T cells shows the level of total Pc 4 increased in a dose-dependent manner. (B) Cumulative data indicated that activated T cells incorporate more total Pc 4 than resting cells at each concentration, although this became less distinct at 300 nM Pc 4.
Activated T cells are larger and contain proportionally increased levels of Pc 4 in mitochondria
Activated or resting cells were treated with 150 nM Pc 4 in complete medium for 2 h. Prior to imaging, 50 nM MitoTracker Green and 10 μg/mL Hoechst 33342 were loaded into the cells for 15 min at 37°C. As indicated in Fig. 5, activated T cells were larger and appeared to have internalized more Pc 4 compared to unstimulated cells. Intracellular Pc 4 demonstrated striking co-localization with mitochondria at a higher rate in activated compared to non-activated T cells, suggesting that once internalized, Pc 4 is associated primarily with the mitochondrial membrane of T cells (Fig. 5). It has been reported that oncogenes and tumor suppressors can modulate signaling pathways that regulate mitochondrial dynamics and that mitochondrial mass and function vary between tumors and individuals (17–19). Therefore, the efficacy of Pc 4 PDT in CTCL patients may be related to enhanced uptake of Pc 4. At 50 and 100nM Pc 4, resting CTCL T cells appeared to take up more Pc 4 than resting T cells isolated from healthy control individuals (Supplementary Figure 1A, B), with an average of 953 MFI in CTCL versus 529 MFI in controls (50nM) and 1280 MFI in CTCL versus 893 MFI in controls (100nM), in 2 paired patient and control samples. Upon activation, the difference in Pc 4 uptake between CTCL T cells and T cells from healthy control individuals is lost.
Fig. 5. Activated T cells are larger and contain proportionally increased levels of Pc 4 in mitochondria.
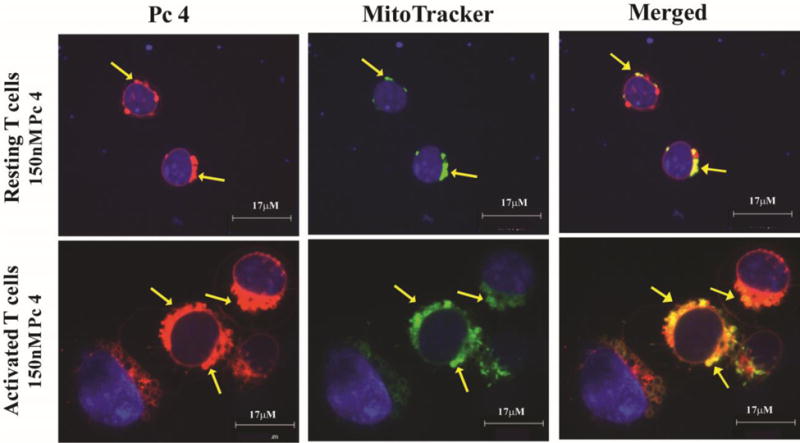
Activated or resting T cells were treated with Pc 4 (0–300 nM) in complete medium for 2 h. Prior to imaging, 50 nM MitoTracker Green and 10 μg/mL Hoechst 33342 were added to the cell culture for 15 min at 37°C. Stained cells were placed onto a slide with a glass coverslip and all images were acquired using the UltraVIEW VoX spinning disk confocal system mounted on a Leica DMI6000B microscope equipped with a HCX PL APO 100×/1.4 oil immersion objective. Confocal images were collected using solid-state diode lasers, with 640 nm, 488 nm and 405 nm excitation light for Pc 4, MitoTracker Green and Hoechst 33342, respectively, and with appropriate emission filters. Pc 4 (pseudo red) preferentially binds to mitochondrial membranes; mitochondria are marked using MitoTracker Green (pseudo green) and cell nuclei are marked with Hoechst 33342 (pseudo blue). The merged images in the right panel demonstrate that activated T cells, which are larger in size, as indicated by the scale bars, contain more mitochondria and proportionally increased levels of Pc 4. The yellow arrows indicate areas of co-localization between Pc 4 and MitoTracker Green.
Discussion
Pc 4 has been found to be highly effective against numerous human cancer cells in vitro and model tumor systems (20–25). PDT with Pc 4 is currently in clinical trials for psoriasis and CTCL (14, 26, 27), and other applications are being developed. Pc 4 has been shown to bind preferentially to mitochondrial, lysosomal and ER membranes (28, 27, 16). Interestingly, recent publications have suggested that PDT leads to an antigen-specific immune response (29), which, taken together with our data indicating less Pc 4 uptake by Treg cells than by activated T cells, suggests a potential for rebalancing the ratio of Treg to Teff. For example, if Pc 4-PDT were applied during an ongoing effector T cell-mediated immune response, it is likely that more Tregs would be found at the response site due to less apoptosis associated with phototherapy due to less uptake of Pc 4. Therefore, in addition to having less effector T cells due to increased Pc 4 uptake and PDT-dependent apoptosis, there would be a higher ratio of Treg:Teff cells and a net suppressive environment which would result in a more effective suppression of a responding effector T cell response. Whether this will be demonstrable in autoinflammatory disorders and cancer remains to be seen, as a decrease in Treg in situ in esophageal cancer following PDT with Photofrin was not observed (30). Extensive investigations using various PDT photosensitizers have previously revealed preferential uptake of photosensitizers by 1.) leukemic cells (31–35); and 2.) activated lymphocytes compared to resting cells (36–39). The use of PDT for treatment of skin cancers has been slowly increasing with advances in light delivery systems and improved photosensitizers (40). The use of PDT in non-oncogenic skin disorders, such as the treatment of psoriatic plaques, has been evaluated > 25 years ago, and recent meta analyses have concluded that PDT may not be effective for psoriasis (41). One possible explanation for this lack of efficacy may be the uptake of the photosensitizer aminolevulinic acid (ALA) predominantly by keratinocytes of the skin (42), in essence acting as a sink for the photosensitizer following oral delivery. However, these same authors also demonstrated that oral delivery of the ALA also induced T lymphocyte apoptosis in psoriasis patients (43). Given new information regarding the potential target cells and immune response in psoriasis (e.g., Th17) differential uptake by activated lymphocytes compared to regulatory T cells, and the potential to one day specifically target Th17 cells, may present a viable attack strategy for PDT on psoriasis.
The mechanism of action of Pc 4 involves absorption of a photon by Pc 4, resulting in Pc 4 emerging as a triplet-state photosensitizer that transfers energy to ground state oxygen (Type II photochemistry), generating singlet oxygen, a highly reactive form of oxygen that reacts with many biological molecules, including lipids, proteins, and nucleic acids, producing an oxidative stress and eventually leading to cell death (44). Cell types and states of activation vary significantly in their susceptibility to oxidative stress, which may account for our previous observation that a human acute T-cell leukemia cell line (Jurkat) was far more susceptible to Pc 4-PDT-induced cell death than was a human epidermoid carcinoma cell line (A431) (15). Interestingly, within populations of normal human T cells, we observed a hierarchy in which the highest uptake of Pc 4 occurred in activated T cells with large numbers of mitochondria, the next highest uptake was in resting T cells, with the lowest Pc 4 uptake occurring in Treg. The high uptake of Pc 4 by activated T cells suggests that reactive T cells in chronic inflammatory conditions or neoplastic T cells in T cell lymphomas/leukemias may be highly susceptible to Pc 4-PDT-induced cell death, with a potential therapeutic window where resting naïve and memory T cells are left alive, along with sparing of Treg cells that may be able to restore quiescence once the auto-reactive inflammatory population numbers are reduced.
To quantify the susceptibility of primary resting versus activated T cells to Pc 4-PDT, we negatively selected CD3+ T cells from normal peripheral blood samples, rested half of the T cells for 24 h and stimulated the other half with CD3/CD28. Resting or activated T cells were then treated with various doses of Pc 4 for 2 h followed by photoirradiation. In response to PDT, we observed a Pc 4 dose-dependent increase in the percentage of cell death in both resting and activated T cells. Interestingly, activated T cells exhibited greater sensitivity than resting cells, a difference that was statistically significant at all except the highest dose of Pc 4.
Because activated T cells appear to be more susceptible to Pc 4-PDT than their resting counterparts, confirming observations by other investigators (34), we then determined whether there was any difference in the ability of activated and resting T cells to incorporate Pc 4 (45). Flow cytometric analysis of Pc 4 demonstrated that T cells incorporate Pc 4 in a dose-dependent manner and that activated cells incorporate more Pc 4 compared to resting cells. Using light scatter characteristics of T cells, it was apparent that larger, more granular cells (likely more activated) exhibited the most significant Pc 4 uptake (Fig. 1B).
This suggested that Pc 4 might localize in different sites in resting and activated T cells. Since Pc 4 preferentially binds to mitochondrial membranes, we used a mitochondrial marker (MitoTracker Green) to simultaneously visualize the mitochondria of the targeted T cells (28). Upon analysis of the confocal images, it was apparent that activated T cells are larger. Additionally, mitochondria were more numerous in activated T cells and contained proportionally increased levels of Pc 4. The increased numbers of mitochondria associated with activated T cells suggests that a mitochondrion-induced apoptosis may occur in T cells as observed for other cell types. Specifically, Pc 4-PDT has been demonstrated to damage the anti-apoptotic proteins Bcl-2 and Bcl-XL when they are bound to the mitochondrial or ER membranes, resulting in Bax translocation from the cytosol to mitochondria, and the release of cytochrome c and induction of the intrinsic apoptosis pathway that may be enhanced by lysosomal disruption (46, 15, 47–51).
In conclusion, these results suggest that a window exists in which activated normal primary T cells are more susceptible to Pc 4-PDT-induced cell death compared to their resting counterparts. Additionally, our flow cytometry and fluorometry experiments demonstrate that activated cells incorporate more Pc 4 proportionally to size and granularity. The presence of activated T cells in T cell-mediated skin diseases, such as CTCL, may provide a favorable therapeutic window in which Pc 4-PDT may act. Lastly, confocal imaging appears to indicate an increase of mitochondria and thus a proportional increase in Pc 4 levels. Our results indicate that activation of T cells provides an increased target area (mitochondria) upon which Pc 4 may act, and that apoptosis induction by Pc 4 may be useful in skin diseases where T cell proliferation is ongoing. These observations may account for the increased sensitivity of activated T cells to Pc 4-PDT.
Supplementary Material
Acknowledgments
Funding
This study has been supported in part by the National Institutes of Health (P30AR039750, P50AR055508; R01AR051498 KDC, P30CA043703 NO,), Ohio Department of Development, Center for Innovative Immunosuppressive Therapeutics (TECH 09-023 KDC and TSM), Murdough Family Center for Psoriasis and the Flow Cytometry Center of the Case Comprehensive Cancer Center (P30 CA43703).
References
- 1.Bahner JD, Bordeaux JS. Non-melanoma skin cancers: photodynamic therapy, cryotherapy, 5-fluorouracil, imiquimod, diclofenac, or what? Facts and controversies. Clin Dermatol. 2013;31:792–798. doi: 10.1016/j.clindermatol.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 2.De Vijlder HC, Middelburg T, De Bruijn HS, Martino Neumann HA, Sterenborg HC, Robinson DJ, De Haas ER. Optimizing ALA-PDT in the management of non-melanoma skin cancer by fractionated illumination. G Ital Dermatol Venereol. 2009;144:433–439. [PubMed] [Google Scholar]
- 3.Fien SM, Oseroff AR. Photodynamic therapy for non-melanoma skin cancer. Journal of the National Comprehensive Cancer Network: JNCCN. 2007;5:531–540. doi: 10.6004/jnccn.2007.0046. [DOI] [PubMed] [Google Scholar]
- 4.Ibbotson SH, Moseley H, Brancaleon L, Padgett M, O’Dwyer M, Woods JA, Lesar A, Goodman C, Ferguson J. Photodynamic therapy in dermatology: Dundee clinical and research experience. Photodiagnosis and photodynamic therapy. 2004;1:211–223. doi: 10.1016/S1572-1000(04)00045-6. [DOI] [PubMed] [Google Scholar]
- 5.Segura S, Puig S, Carrera C, Lecha M, Borges V, Malvehy J. Non-invasive management of non-melanoma skin cancer in patients with cancer predisposition genodermatosis: a role for confocal microscopy and photodynamic therapy. J Eur Acad Dermatol Venereol. 2011;25:819–827. doi: 10.1111/j.1468-3083.2010.03871.x. [DOI] [PubMed] [Google Scholar]
- 6.Bissonnette R, Maari C, Nigen S, Provost N, Bolduc C. Photodynamic therapy with methylaminolevulinate 80 mg/g without occlusion improves acne vulgaris. J Drugs Dermatol. 2010;9:1347–1352. [PubMed] [Google Scholar]
- 7.Jeon YM, Lee HS, Jeong D, Oh HK, Ra KH, Lee MY. Antimicrobial photodynamic therapy using chlorin e6 with halogen light for acne bacteria-induced inflammation. Life sciences. 2015;124:56–63. doi: 10.1016/j.lfs.2014.12.029. [DOI] [PubMed] [Google Scholar]
- 8.Pinto C, Schafer F, Orellana JJ, Gonzalez S, Hasson A. Efficacy of red light alone and methyl-aminolaevulinate-photodynamic therapy for the treatment of mild and moderate facial acne. Indian journal of dermatology, venereology and leprology. 2013;79:77–82. doi: 10.4103/0378-6323.104673. [DOI] [PubMed] [Google Scholar]
- 9.Chen K, Chang BZ, Ju M, Zhang XH, Gu H. Comparative study of photodynamic therapy vs CO2 laser vaporization in treatment of condylomata acuminata: a randomized clinical trial. Br J Dermatol. 2007;156:516–520. doi: 10.1111/j.1365-2133.2006.07648.x. [DOI] [PubMed] [Google Scholar]
- 10.Giomi B, Pagnini F, Cappuccini A, Bianchi B, Tiradritti L, Zuccati G. Immunological activity of photodynamic therapy for genital warts. Br J Dermatol. 2011;164:448–451. doi: 10.1111/j.1365-2133.2010.10089.x. [DOI] [PubMed] [Google Scholar]
- 11.Helsing P, Togsverd-Bo K, Veierod MB, Mork G, Haedersdal M. Intensified fractional CO2 laser-assisted photodynamic therapy vs. laser alone for organ transplant recipients with multiple actinic keratoses and wart-like lesions: a randomized half-side comparative trial on dorsal hands. Br J Dermatol. 2013;169:1087–1092. doi: 10.1111/bjd.12507. [DOI] [PubMed] [Google Scholar]
- 12.Baron ED, Malbasa CL, Santo-Domingo D, Fu P, Miller JD, Hanneman KK, Hsia AH, Oleinick NL, Colussi VC, Cooper KD. Silicon phthalocyanine (Pc 4) photodynamic therapy is a safe modality for cutaneous neoplasms: results of a phase 1 clinical trial. Lasers Surg Med. 2010;42:728–735. doi: 10.1002/lsm.20984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lam M, Hsia AH, Liu Y, Guo M, Swick AR, Berlin JC, McCormick TS, Kenney ME, Oleinick NL, Cooper KD, Baron ED. Successful cutaneous delivery of the photosensitizer silicon phthalocyanine 4 for photodynamic therapy. Clin Exp Dermatol. 2011;36:645–651. doi: 10.1111/j.1365-2230.2010.03989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lam M, Lee Y, Deng M, Hsia AH, Morrissey KA, Yan C, Azzizudin K, Oleinick NL, McCormick TS, Cooper KD, Baron ED. Photodynamic therapy with the silicon phthalocyanine pc 4 induces apoptosis in mycosis fungoides and sezary syndrome. Adv Hematol. 2010;2010:896161. doi: 10.1155/2010/896161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ke MS, Xue LY, Feyes DK, Azizuddin K, Baron ED, McCormick TS, Mukhtar H, Panneerselvam A, Schluchter MD, Cooper KD, Oleinick NL, Stevens SR. Apoptosis mechanisms related to the increased sensitivity of Jurkat T-cells vs A431 epidermoid cells to photodynamic therapy with the phthalocyanine Pc 4. Photochem Photobiol. 2008;84:407–414. doi: 10.1111/j.1751-1097.2007.00278.x. [DOI] [PubMed] [Google Scholar]
- 16.Trivedi NS, Wang HW, Nieminen AL, Oleinick NL, Izatt JA. Quantitative analysis of Pc 4 localization in mouse lymphoma (LY-R) cells via double-label confocal fluorescence microscopy. Photochem Photobiol. 2000;71:634–639. doi: 10.1562/0031-8655(2000)071<0634:qaopli>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 17.Boland ML, Chourasia AH, Macleod KF. Mitochondrial dysfunction in cancer. Front Oncol. 2013;3:292. doi: 10.3389/fonc.2013.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chourasia AH, Boland ML, Macleod KF. Mitophagy and cancer. Cancer Metab. 2015;3:4. doi: 10.1186/s40170-015-0130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12:9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.George JE, 3rd, Ahmad Y, Varghai D, Li X, Berlin J, Jackowe D, Jungermann M, Wolfe MS, Lilge L, Totonchi A, Morris RL, Peterson A, Lust WD, Kenney ME, Hoppel CL, Sun J, Oleinick NL, Dean D. Pc 4 photodynamic therapy of U87-derived human glioma in the nude rat. Lasers Surg Med. 2005;36:383–389. doi: 10.1002/lsm.20185. [DOI] [PubMed] [Google Scholar]
- 21.Master AM, Livingston M, Oleinick NL, Sen Gupta A. Optimization of a nanomedicine-based silicon phthalocyanine 4 photodynamic therapy (Pc 4-PDT) strategy for targeted treatment of EGFR-overexpressing cancers. Molecular pharmaceutics. 2012;9:2331–2338. doi: 10.1021/mp300256e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Master AM, Rodriguez ME, Kenney ME, Oleinick NL, Gupta AS. Delivery of the photosensitizer Pc 4 in PEG-PCL micelles for in vitro PDT studies. Journal of pharmaceutical sciences. 2010;99:2386–2398. doi: 10.1002/jps.22007. [DOI] [PubMed] [Google Scholar]
- 23.Separovic D, Breen P, Boppana NB, Van Buren E, Joseph N, Kraveka JM, Rahmaniyan M, Li L, Gudz TI, Bielawska A, Bai A, Bielawski J, Pierce JS, Korbelik M. Increased killing of SCCVII squamous cell carcinoma cells after the combination of Pc 4 photodynamic therapy and dasatinib is associated with enhanced caspase-3 activity and ceramide synthase 1 upregulation. International journal of oncology. 2013;43:2064–2072. doi: 10.3892/ijo.2013.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whitacre CM, Feyes DK, Satoh T, Grossmann J, Mulvihill JW, Mukhtar H, Oleinick NL. Photodynamic therapy with the phthalocyanine photosensitizer Pc 4 of SW480 human colon cancer xenografts in athymic mice. Clin Cancer Res. 2000;6:2021–2027. [PubMed] [Google Scholar]
- 25.Whitacre CM, Satoh TH, Xue L, Gordon NH, Oleinick NL. Photodynamic therapy of human breast cancer xenografts lacking caspase-3. Cancer Lett. 2002;179:43–49. doi: 10.1016/s0304-3835(01)00853-9. [DOI] [PubMed] [Google Scholar]
- 26.Lee TK, Baron ED, Foster TH. Monitoring Pc 4 photodynamic therapy in clinical trials of cutaneous T-cell lymphoma using noninvasive spectroscopy. Journal of biomedical optics. 2008;13:030507. doi: 10.1117/1.2939068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller JD, Baron ED, Scull H, Hsia A, Berlin JC, McCormick T, Colussi V, Kenney ME, Cooper KD, Oleinick NL. Photodynamic therapy with the phthalocyanine photosensitizer Pc 4: the case experience with preclinical mechanistic and early clinical-translational studies. Toxicol Appl Pharmacol. 2007;224:290–299. doi: 10.1016/j.taap.2007.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lam M, Oleinick NL, Nieminen AL. Photodynamic therapy-induced apoptosis in epidermoid carcinoma cells. Reactive oxygen species and mitochondrial inner membrane permeabilization. J Biol Chem. 2001;276:47379–47386. doi: 10.1074/jbc.M107678200. [DOI] [PubMed] [Google Scholar]
- 29.Reginato E, Mroz P, Chung H, Kawakubo M, Wolf P, Hamblin MR. Photodynamic therapy plus regulatory T-cell depletion produces immunity against a mouse tumour that expresses a self-antigen. British journal of cancer. 2013;109:2167–2174. doi: 10.1038/bjc.2013.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reginato E, Lindenmann J, Langner C, Schweintzger N, Bambach I, Smolle-Juttner F, Wolf P. Photodynamic therapy downregulates the function of regulatory T cells in patients with esophageal squamous cell carcinoma. Photochem Photobiol Sci. 2014;13:1281–1289. doi: 10.1039/c4pp00186a. [DOI] [PubMed] [Google Scholar]
- 31.Fiedorowicz M, Galindo JR, Julliard M, Mannoni P, Chanon M. Efficient photodynamic action of Victoria blue BO against the human leukemic cell lines K-562 and TF-1. Photochem Photobiol. 1993;58:356–361. doi: 10.1111/j.1751-1097.1993.tb09574.x. [DOI] [PubMed] [Google Scholar]
- 32.Jamieson CH, McDonald WN, Levy JG. Preferential uptake of benzoporphyrin derivative by leukemic versus normal cells. Leuk Res. 1990;14:209–219. doi: 10.1016/0145-2126(90)90128-v. [DOI] [PubMed] [Google Scholar]
- 33.Kapsokalyvas D, Dimitriou H, Skalkos D, Konstantoudakis G, Filippidis G, Stiakaki E, Papazoglou T, Kalmanti M. Does Hypericum perforatum L. extract show any specificity as photosensitizer for HL-60 leukemic cells and cord blood hemopoietic progenitors during photodynamic therapy? J Photochem Photobiol B. 2005;80:208–216. doi: 10.1016/j.jphotobiol.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 34.Rittenhouse-Diakun K, Van Leengoed H, Morgan J, Hryhorenko E, Paszkiewicz G, Whitaker JE, Oseroff AR. The role of transferrin receptor (CD71) in photodynamic therapy of activated and malignant lymphocytes using the heme precursor delta-aminolevulinic acid (ALA) Photochem Photobiol. 1995;61:523–528. doi: 10.1111/j.1751-1097.1995.tb02356.x. [DOI] [PubMed] [Google Scholar]
- 35.Traitcheva N, Berg H. Electroporation and alternating current cause membrane permeation of photodynamic cytotoxins yielding necrosis and apoptosis of cancer cells. Bioelectrochemistry. 2010;79:257–260. doi: 10.1016/j.bioelechem.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 36.Canti G, Marelli O, Ricci L, Nicolin A. Haematoporphyrin-treated murine lymphocytes: in vitro inhibition of DNA synthesis and light-mediated inactivation of cells responsible for GVHR. Photochem Photobiol. 1981;34:589–594. doi: 10.1111/j.1751-1097.1981.tb09047.x. [DOI] [PubMed] [Google Scholar]
- 37.Hunt DW, Jiang H, Granville DJ, Chan AH, Leong S, Levy JG. Consequences of the photodynamic treatment of resting and activated peripheral T lymphocytes. Immunopharmacol. 1999;41:31–44. doi: 10.1016/s0162-3109(98)00051-4. [DOI] [PubMed] [Google Scholar]
- 38.Jiang H, Granville DJ, North JR, Richter AM, Hunt DW. Selective action of the photosensitizer QLT0074 on activated human T lymphocytes. Photochem Photobiol. 2002;76:224–231. doi: 10.1562/0031-8655(2002)076<0224:saotpq>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 39.Marelli O, Franco P, Canti G, Ricci L, Prandoni N, Nicolin A, Festenstein H. DTIC xenogenized lines obtained from an L1210 clone: clonal analysis of cytotoxic T lymphocyte reactivity. British journal of cancer. 1988;58:171–175. doi: 10.1038/bjc.1988.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown SB, Brown EA, Walker I. The present and future role of photodynamic therapy in cancer treatment. Lancet Oncol. 2004;5:497–508. doi: 10.1016/S1470-2045(04)01529-3. [DOI] [PubMed] [Google Scholar]
- 41.Almutawa F, Thalib L, Hekman D, Sun Q, Hamzavi I, Lim HW. Efficacy of localized phototherapy and photodynamic therapy for psoriasis: a systematic review and meta-analysis. Photodermatol Photoimmunol Photomed. 2015;31:5–14. doi: 10.1111/phpp.12092. [DOI] [PubMed] [Google Scholar]
- 42.Bissonnette R, Zeng H, McLean DI, Korbelik M, Lui H. Oral aminolevulinic acid induces protoporphyrin IX fluorescence in psoriatic plaques and peripheral blood cells. Photochem Photobiol. 2001;74:339–345. doi: 10.1562/0031-8655(2001)074<0339:oaaipi>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 43.Bissonnette R, Tremblay JF, Juzenas P, Boushira M, Lui H. Systemic photodynamic therapy with aminolevulinic acid induces apoptosis in lesional T lymphocytes of psoriatic plaques. J Invest Dermatol. 2002;119:77–83. doi: 10.1046/j.1523-1747.2002.01827.x. [DOI] [PubMed] [Google Scholar]
- 44.Panzarini E, Tenuzzo B, Dini L. Photodynamic therapy-induced apoptosis of HeLa cells. Ann N Y Acad Sci. 2009;1171:617–626. doi: 10.1111/j.1749-6632.2009.04908.x. [DOI] [PubMed] [Google Scholar]
- 45.Yang Y, Samas B, Kennedy VO, Macikenas D, Chaloux BL, Miller JA, Speer RL, Jr, Protasiewicz J, Pinkerton AA, Kenney ME. Long, directional interactions in cofacial silicon phthalocyanine oligomers. The journal of physical chemistry A. 2011;115:12474–12485. doi: 10.1021/jp2019445. [DOI] [PubMed] [Google Scholar]
- 46.Chiu SM, Xue LY, Usuda J, Azizuddin K, Oleinick NL. Bax is essential for mitochondrion-mediated apoptosis but not for cell death caused by photodynamic therapy. British journal of cancer. 2003;89:1590–1597. doi: 10.1038/sj.bjc.6601298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morris RL, Azizuddin K, Lam M, Berlin J, Nieminen AL, Kenney ME, Samia AC, Burda C, Oleinick NL. Fluorescence resonance energy transfer reveals a binding site of a photosensitizer for photodynamic therapy. Cancer Res. 2003;63:5194–5197. [PubMed] [Google Scholar]
- 48.Quiogue G, Saggu S, Hung HI, Kenney ME, Oleinick NL, Lemasters JJ, Nieminen AL. Signaling From Lysosomes Enhances Mitochondria-Mediated Photodynamic Therapy In Cancer Cells. Proc SPIE Int Soc Opt Eng. 2009;7380:1–8. doi: 10.1117/12.823752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Usuda J, Azizuddin K, Chiu SM, Oleinick NL. Association between the photodynamic loss of Bcl-2 and the sensitivity to apoptosis caused by phthalocyanine photodynamic therapy. Photochem Photobiol. 2003;78:1–8. doi: 10.1562/0031-8655(2003)078<0001:abtplo>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 50.Xue LY, Chiu SM, Azizuddin K, Joseph S, Oleinick NL. The death of human cancer cells following photodynamic therapy: apoptosis competence is necessary for Bcl-2 protection but not for induction of autophagy. Photochem Photobiol. 2007;83:1016–1023. doi: 10.1111/j.1751-1097.2007.00159.x. [DOI] [PubMed] [Google Scholar]
- 51.Xue LY, Chiu SM, Fiebig A, Andrews DW, Oleinick NL. Photodamage to multiple Bcl-xL isoforms by photodynamic therapy with the phthalocyanine photosensitizer Pc 4. Oncogene. 2003;22:9197–9204. doi: 10.1038/sj.onc.1207019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


