Abstract
Purpose
To investigate local control, survival outcomes, and complication rates of patients treated with aggressive surgery and radiation therapy (RT) for retroperitoneal sarcomas (RPS).
Methods
We reviewed the medical records of 121 consecutive patients treated for RPS with surgery and RT between 1965 and 2012. The most common histology was liposarcomas (n=42, 35%). The median follow-up was 100 months (range, 20–467 months). Eighty six (71%) patients were treated for initial presentation of RPS, and 35 patients (29%) presented with and were treated for RPS recurrence. RT was preoperative in 88 patients (73%) (median dose, 50.4 Gy) and post-operative in 33 (27%) (median dose, 55 Gy).
Results
The 5-year LC and OS rates were 56% and 57%, respectively. Two factors were associated with higher risk of any intra-abdominal recurrence at 5 years: positive or uncertain margins (58% vs. 30% for negative margins, P<0.001, HR 2.7 95% CI 1.6–4.8) and presenting with recurrent disease after previous resection (76% vs. 31% for de novo RPS, P<0.001, HR 4.4 95% CI 2.5–7.5). The 10-year complication rate was 5% and RT-related complications were associated with postoperative RT (P<0.001) and a RT dose ≥ 60 Gy (P<0.001).
Conclusions
Intra-abdominal RPS recurrences continue to be a significant challenge despite the use of aggressive surgery and radiation therapy. Given the complications associated with postoperative radiation therapy, we recommend that preoperative radiation therapy is the preferred strategy when combined modality therapy is recommended.
Keywords: retroperitoneal sarcoma, radiation, multimodality therapy, combined modality therapy, intra-abdominal recurrence, local recurrence
INTRODUCTION
Retroperitoneal sarcomas (RPS) are rare, heterogenous tumors that represent approximately 15% of all soft tissue sarcomas with an incidence of 0.5 to 1 new case per 100,000 people per year.1,2 Different histologic subtypes of RPS can display varying biologic behaviors, responses to treatment, and clinical outcomes.3 However, surgery remains the standard potentially curative treatment for RPS. Wide resection margins are crucial for durable local control but are often unattainable in the retroperitoneal location. In fact, the completeness of surgical resection has been a prominent factor in several recent nomograms for predicting outcome, and an incomplete resection has the same expected outcome as unresectable disease.4,5
Given the documented high rates of local recurrence with surgery alone, radiation therapy (RT) has historically also been included in combination with surgery for local management of RPS. When delivered preoperatively, RT may possibly improve the probability of achieving a negative resection margin, either by sterilizing peripheral microscopic disease or by affecting cytoreduction of the gross tumor.6 Additional advantages for preoperative RT include clear delineation of the target, the displacement of normal tissues, and the radiobiologic benefit of better tumor oxygenation, which facilitates radiation-induced damage to the tumor cells.7 No randomized data are currently available proving the benefit of preoperative RT versus resection alone. However, non-randomized data suggest that it is safe, well-tolerated, and does not add perioperative morbidity in carefully selected cases.8 Furthermore, while post-operative RT has also historically been employed, no randomized data is available to suggest benefit from this approach, and it is associated with higher acute and long-term morbidity.7,9,10
Until prospective, randomized data are available clinicians will continue to rely upon reports of clinical experience treating RPS patients to help guide management. We retrospectively analyzed our experience at a high-volume sarcoma referral center to investigate the local control, survival outcomes, and complication rates of patients treated using aggressive surgery and RT for RPS.
METHODS
We identified 121 consecutive patients with histologically confirmed soft –tissue sarcomas of the retroperitoneum treated with surgery and RT as part of their definitive management at XXXX from 1965 through 2012. These patients were extracted from a database, maintained in the Department of Radiation Oncology, of non-metastatic soft tissue sarcoma patients treated with a combination of surgery and RT between 1960 and 2012. Patients with the following histologies were excluded from this analysis because of differing biology and treatment paradigms: desmoid fibromatoses and visceral angiosarcoma,. Medical records were reviewed in detail after obtaining approval from our institutional review board. Patients underwent a full history, complete physical examination, routine blood tests, and appropriate imaging before their treatment. All sarcoma diagnoses were confirmed at the time of presentation by pathologic review of the tissue at XXXX.
Patient and Tumor Characteristics
Patient and tumor characteristics are listed in Table 1. The median patient age was 57 years (range, 20–77) with 63 males (52%) and 58 females (48%). The most common retroperitoneal sarcoma histology was liposarcoma (n=42, 34%), with just over half of those classified as de-differentiated liposarcomas (n=24, 57% of the liposarcomas; atypical lipomatous tumor [ALT], n=13; liposarcoma not otherwise specified, n=2; myxoid liposarcoma, n=2, pleomorphic liposarcoma, n=1). All 13 ALTs were deemed low-grade, while the myxoid liposarcomas were considered intermediate grade and the remaining liposarcomas were deemed high grade. The other two most common histologies were malignant fibrous histiocytoma / undifferentiated pleomorphic sarcoma (MFH/UPS) (n=34, 28%) and leiomyosarcomas (n=28, 23%). The remaining histologies included: unclassified sarcoma (n=8, 6%), synovial sarcoma (n=3, 3%), hemangiopericytoma (n=3, 3%) neurogenic sarcoma (n=2, 2%), alveolar (n=1, 1%), extraskeletal myxoid chondrosarcoma (n=1, 1%), and Ewings sarcoma (n=1, 1%). The majority of patients had high grade tumors (n=82, 68%), with only 22 patients (18%) having intermediate grade and 17 patients (14%) having low grade tumors. Tumor size was recorded in 120 patients (99%) with a median maximal dimension of 10.3 cm (range, 1.7–36 cm). One hundred seven patients (88%) had tumors larger than 5 cm.
Table 1.
Patient and Tumor Characteristics
| Variable | All Patients (n=121) Value or No. (%) |
|---|---|
| Follow-up time, months | |
| Median | 100 |
| Range | 20–462 |
| Age, years | |
| Median | 57 |
| Range | 20–77 |
| Sex | |
| Male | 63 (52) |
| Female | 58 (48) |
| Maximum Tumor Dimension, cm | |
| Median | 10.3 |
| Range | 1.7–36 |
| Tumor size | |
| ≤ 5 cm | 13 (11) |
| > 5 cm | 107 (88) |
| Unknown | 1 (1) |
| Grade | |
| Low | 17 (14) |
| Intermediate | 22 (18) |
| High | 82 (68) |
| Histopathology | |
| Liposarcoma | 42 (34) |
| MFH/UPS | 34 (28) |
| Leiomyosarcoma | 28 (23) |
| Unclassified | 8 (6) |
| Other | 11 (9) |
| De novo at presentation | |
| Yes | 86 (71) |
| No | 35 (29) |
| Treatment Approach | |
| Preop RT | 88 (73) |
| Postop RT | 33 (27) |
| Final Surgical Resection Margin | |
| Positive/Uncertain | 63 (52) |
| Negative | 58 (48) |
| Radiation | |
| EBRT only | 100 (82) |
| EBRT + IORT | 19 (16) |
| EBRT + Brachy | 1 (1) |
| Brachy only | 1 (1) |
| Radiation Dose, Gy | |
| Median | 50.4 |
| Range | 45–56 |
| Chemotherapy | |
| Neo/Adj | 61 (50) |
| CCRT | 17 (14) |
Abbreviations: MFH/UPS, malignant fibrous histiocytoma/unclassified pleomorphic sarcoma; RT, radiation therapy; EBRT, external beam radiation therapy; IORT, intraoperative radiation therapy; Neo/Adj, neoadjuvant/adjuvant; CCRT, concurrent chemoradiation.
Treatment
Eighty-six patients (71%) were treated at XXXX as de novo RPS presentation, while 35 patients (29%) presented to XXXX after a recurrence of their RPS that had been previously treated with definitive surgery at an outside institution. No patient in this analysis received previous RT for RPS. For those patients who presented to XXXX having already undergone surgery for their current presentation of disease at an outside facility, the determination of need for re-excision was made by the evaluating surgeon on the basis of prior surgical margins, location and extent of residual disease, and morbidity associated with additional surgery. Final surgical margins were negative in 58 patients (48%), while 33 (27%) had positive margins, and 30 (25%) had uncertain margins.
The decision to treat with RT in combination with surgery was made by the treating surgeon and radiation oncologist. For patients who presented to XXXX with gross tumor, preoperative RT was performed in 88 patients (73%) to a median dose of 50.4 Gy (range, 40–56 Gy). Otherwise, RT was delivered postoperatively (n=33, 27%); the median postoperative RT dose was 55 Gy (range, 44.5–65.5 Gy). All but one patient received external beam radiation therapy (EBRT), with 100 (82%) receiving only EBRT, 19 (16%) receiving combination with intraoperative radiation therapy (IORT), and 1 (1%) patient in combination with brachytherapy. One patient was treated with brachytherapy alone (total implant dose 50 Gy). For all patients receiving EBRT, the median dose was 50.4 Gy (range, 45–56 Gy). For 16 patients (13%) the EBRT technique was intensity modulated radiation therapy (IMRT). IORT doses ranged from 10–15 Gy (15 Gy: 12 patients; 12.5 Gy: 1 patient; 10 Gy: 6 patients). The patient who received both brachytherapy and EBRT was treated to brachytherapy dose of 20 Gy in addition to 55 Gy EBRT.
A total of 61 (50%) patients were treated with chemotherapy either neoadjuvantly or adjuvantly at the discretion of the treating medical oncologist, typically administered for larger, high grade tumors. Seventeen (14%) patients were treated with concurrent chemoradiation.
Follow-up and Statistical Analysis
The median follow-up time for patients alive at last follow up from the completion of RT was 100 months (range, 20–467 months). Differences between proportions of categorical data were analyzed by using Fisher’s exact test and chi-squared analyses as appropriate. Survival times were calculated from the RT completion date to the first occurrence of the outcome of interest. The Kaplan-Meier method was used to estimate actuarial rates of overall survival (OS), disease-specific survival (DSS), intra-abdominal recurrence, and distant metastatic free survival (DMFS). Log-rank tests were applied to assess significance of differences between actuarial curves. A 5% significance level was used for analyses. The Cox proportional hazards model was used for multivariate analysis to assess the adjusted effects of numerous factors on the outcomes of interest. Significant (P ≤ 0.05) estimated hazard ratios (HR) are reported. IBM SPSS Statistics 22 was used for data analysis.
RESULTS
Survival
The 5-year and 10-year OS rates were 57% and 40%, respectively, with a higher 5-year OS among patients presenting with de novo RPS compared to those presenting with recurrent disease (59% vs. 53%, P=0.04). Sixty four (53%) deaths were attributable to RPS resulting in a 5-year and 10-year DSS rate of 61% and 44%, respectively.
Factors associated with DSS in the univariate analysis included having high grade tumors (P=0.003), positive or uncertain surgical margins (P=0.04), or having recurrent presentation of disease after prior definitive treatment (P=0.047) (Table 2). On multivariate analysis, only high grade (P=0.04, HR 2.5; 95% CI 1.1–5.9) and positive or uncertain margins (P=0.02, HR 1.8; 95% CI 1.1–3.1) remained significantly associated with poorer DSS.
Table 2.
Univariate Analysis of Factors Potentially Affecting Actuarial Rates of Disease Specific Survival at 5 years
| Variable | DSS 5-year control, % |
DSS P Value |
|---|---|---|
| Age | ||
| > 65 | 51 | 0.13 |
| ≤ 65 | 63 | |
| Tumor Size | ||
| > 5 cm | 60 | 0.95 |
| ≤ 5 cm | 67 | |
| De Novo at presentation | ||
| Yes | 64 | 0.047 |
| No | 55 | |
| Histology | ||
| Liposarcoma | 71 | 0.60 |
| Other | 56 | |
| Grade | ||
| High | 54 | 0.006 |
| Intermediate | 70 | |
| Low | 86 | |
| Margin Status | ||
| Positive/Uncertain | 59 | 0.04 |
| Negative | 63 | |
| Treatment Plan | ||
| Preop RT | 65 | 0.78 |
| Postop RT | 51 | |
| Radiation Dose | ||
| > 50 Gy | 61 | 0.91 |
| ≤ 50 Gy | 59 | |
| IORT | ||
| Yes | 63 | 0.78 |
| No | 60 | |
| Neo or Adj. Chemo | ||
| Yes | 66 | 0.61 |
| No | 56 | |
| Concurrent Chemoradiation | ||
| Yes | 47 | 0.34 |
| No | 63 |
Abbreviations: DSS, disease specific survival; RT, radiation therapy; IORT, intraoperative radiation therapy; Neo/Adj, neoadjuvant/adjuvant.
Intra-abdominal Recurrences
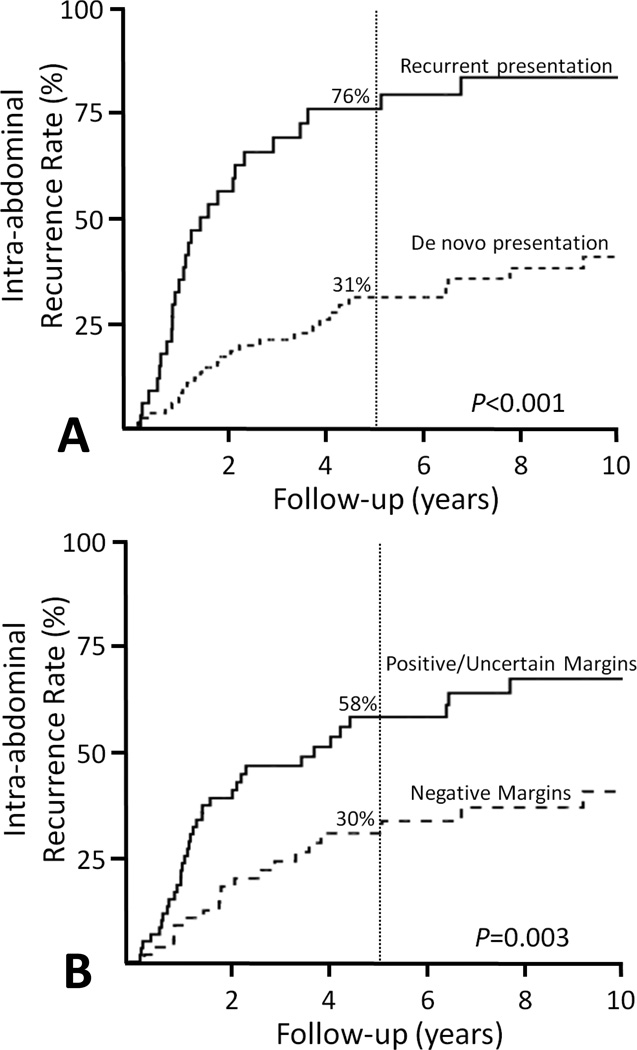
The 5-year and 10-year intra-abdominal recurrence rates were 44% and 54%, respectively, with only 31% of the recurring patients having presented with de novo RPS. Fifty-five patients (crude rate of 45%) had intra-abdominal recurrences with a median time to intra-abdominal failure of 18 months (range, 3–173 months). Approximately half of the intra-abdominal recurrences were in the RT field (n=28, 51%; out-of-field n=27, 49%). Several factors resulted in a higher 5-year intra-abdominal recurrence rate including patients with a histologic diagnosis of liposarcoma (54% vs. 39% for other histologies, P=0.008), presenting with recurrent disease (76% vs. 31% with de novo RPS, P<0.001), and having positive or uncertain margins (58% vs. 30% for negative margins, P=0.003) (Table 3) (Figure 1). Additionally, the median time to intra-abdominal recurrence was shorter among patients presenting with recurrent versus de novo RPS (14 vs. 24 months, P=0.047).
Table 3.
Univariate Analysis of Factors Potentially Affecting Actuarial Rates of Intra-abdominal Recurrence and Distant Metastatic Free Survival Rates at 5 years
| Variable | Intra-abdominal Recurrence Rate at 5-year, % |
Intra-abdominal Recurrence P Value |
DMFS 5-year rate, % |
DMFS P value |
|---|---|---|---|---|
| Age | ||||
| > 65 | 62 | 0.047 | 73 | 0.48 |
| ≤ 65 | 40 | 68 | ||
| Tumor Size | ||||
| > 5 cm | 42 | 0.13 | 70 | 0.51 |
| ≤ 5 cm | 65 | 62 | ||
| De Novo at presentation | ||||
| Yes | 31 | <0.001 | 66 | 0.28 |
| No | 76 | 78 | ||
| Histology | ||||
| Liposarcoma | 54 | 0.008 | 84 | 0.08 |
| Other | 39 | 61 | ||
| Grade | ||||
| High | 46 | 0.32 | 60 | 0.003 |
| Intermediate | 40 | 81 | ||
| Low | 41 | 94 | ||
| Margin Status | ||||
| Positive/Uncertain | 58 | 0.003 | 70 | 0.86 |
| Negative | 30 | 68 | ||
| Treatment Plan | ||||
| Preop RT | 44 | 0.60 | 70 | 0.62 |
| Postop RT | 47 | 68 | ||
| Radiation Dose | ||||
| > 50 Gy | 43 | 0.85 | 67 | 0.71 |
| ≤ 50 Gy | 49 | 57 | ||
| IORT | ||||
| Yes | 44 | 0.82 | 67 | 0.46 |
| No | 45 | 70 | ||
| Neo or Adj. Chemo | ||||
| Yes | 37 | 0.38 | 71 | 0.98 |
| No | 51 | 67 | ||
| Concurrent Chemoradiation | ||||
| Yes | 45 | 0.96 | 50 | 0.02 |
| No | 44 | 72 |
Abbreviations: DMFS, distant metastatic free survival; RT, radiation therapy; IORT, intraoperative radiation therapy; Neo/Adj, neoadjuvant/adjuvant.
Figure 1.
Intra-abdominal recurrence rates for patients with retroperitoneal sarcomas stratified by (A) de novo versus recurrent disease at presentation and (B) surgical margin status.
Several RT-related treatment factors were evaluated for a correlation with intra-abdominal tumor control, but none were significant: treatment era (≤1989 vs. 1990–1999 vs. ≥ 2000, P=0.10), RT dose (>50 Gy vs. ≤50 Gy, P=0.85), location of recurrence (in-field vs. out-of-field, P=0.16), pattern of recurrence (solitary vs. multifocal, P=0.20), timing of RT (pre- vs. post-op, P=0.6), the use of IORT (P=0.82), or the use of concurrent chemoradiation (P=0.96).
Multivariate analysis of intra-abdominal recurrence risk revealed only two factors associated with a poorer outcome: positive or uncertain margin status (P<0.001, HR 2.7 95% CI 1.6–4.8) and recurrent presentation of disease after having undergone previous surgery (P<0.001, HR 4.4 95% CI 2.5–7.5) (Figure 1).
We examined factors associated with in-field recurrences. Those tumors that recurred in the RT field were treated to a median dose of 50.4 Gy (range, 40–60 Gy), and there was no difference in the median time to failure when compared to out-of-field recurrences (21 vs. 16 months, respectively P=0.16). Liposarcoma histology (P=0.17), tumor grade (P=0.06), tumor size (P=0.41), margin status (P=0.65), RT dose (>50 Gy vs. ≤50 Gy; P=0.48), and use of concurrent chemotherapy (P=0.11) were not associated with in-field versus out-of-field recurrences (Table 4).
Table 4.
Characteristics of Intra-abdominal Recurrences and a Comparison between those Recurring In-field versus Out-of-Field
| Variable |
All Intra-abdominal (n=55) Value or No. (%) |
In-field (n=28) Value or No (%) |
Out-of-field (n=27) Value or No (%) |
P value |
|---|---|---|---|---|
| Tumor size | ||||
| ≤ 5 cm | 8 (15) | 3 (11) | 5 (19) | 0.41 |
| > 5 cm | 47 (86) | 25 (89) | 22 (81) | |
| Grade | ||||
| Low | 8 (15) | 7 (25) | 1 (4) | 0.06 |
| Intermediate | 8 (15) | 3 (11) | 5 (18) | |
| High | 39 (70) | 18 (64) | 21 (78) | |
| Histopathology | ||||
| Liposarcoma | 26 (47) | 11 (39) | 15 (56) | 0.17 |
| Other | 29 (53) | 17 (61) | 12 (44) | |
| De novo at presentation | ||||
| Yes | 28 (51) | 18 (64) | 10 (37) | 0.04 |
| No | 27 (49) | 10 (36) | 17 (63) | |
| Treatment Approach | ||||
| Preop RT | 39 (71) | 16 (57) | 23 (85) | 0.02 |
| Postop RT | 16 (29) | 12 (43) | 4 (15) | |
| Final Surgical Resection Margin | ||||
| Positive/Uncertain | 35 (64) | 17 (61) | 18 (67) | 0.65 |
| Negative | 20 (36) | 11 (39) | 9 (33) | |
| Radiation Dose, Gy | ||||
| ≤ 50 | 23 (42) | 13 (46) | 10 (37) | 0.48 |
| > 50 | 32 (58) | 15 (54) | 17 (63) | |
| Concurrent Chemotherapy | ||||
| Yes | 8 (15) | 2 (7) | 6 (22) | 0.11 |
| No | 47 (86) | 26 (93) | 21 (78) | |
| Recurrence Pattern | ||||
| Solitary | 43 (78) | 27 (96) | 16 (59) | <0.001 |
| Multifocal | 12 (22) | 1 (4) | 11 (41) |
Abbreviations: RT, radiation therapy.
Distant metastases
The 5-year and 10-year DMFS rates were 69% and 64%, respectively. Ultimately, 37 patients (31%) developed distant metastases with a median time to distant failure of 18 months (range, 0–102 months), and the most common distant metastatic site was lung (n=25, 68%). As expected, patients with high grade sarcomas (P=0.003) had significantly poorer DMFS. On univariate analysis, none of the following variables were significantly associated with DMFS: liposarcoma histology (P=0.08), tumor size (P=0.51), the use of (neo)adjuvant chemotherapy (P=0.98), or timing of RT (pre- vs. post-op, P=0.62) (Table 3). On multivariate analysis, high grade (P=0.04, HR 8.3; 95% CI 1.1–60.9) remained the only variable significantly associated with poorer DMFS.
After Relapse
For patients with relapse, the 3-year and 5-year DSS rates after relapse were 36% and 24%, respectively, with a median survival time of 30 months (range, 4–172 months). There was no difference in DSS for patients who relapsed only locally compared to only distantly (P=0.53). For the 46 patients that relapsed within the abdomen only (no concurrent distant disease), the 3-year and 5-year DSS rates were 44% and 27%. Thirty two patients (70%) underwent salvage surgery and 27 (59%) received salvage chemotherapy. Salvage surgery was associated with a significantly longer median DSS (47 vs. 8 months, P=0.002) and 5-year DSS (35% vs. 8%, P=0.002).
For the 37 patients that relapsed distantly, 12 patients (32%) underwent surgical salvage and 32 patients (87%) received salvage systemic therapy. Surgical salvage of metastatic disease was significantly associated with a prolonged median DSS (81 vs. 17 months, P<0.001) and 5-year DSS (68% vs. 0%, P<0.001). There also was a prolonged median survival (26 vs. 8 months, P=0.01) and 5-year DSS (22% vs. 0%, P=0.01) with the use of salvage chemotherapy.
Treatment complications
Five patients (4%) had clinically significant RT-related complications, resulting in a 10-year actuarial complication rate of 5%. All of the complications occurred in patients who were treated between 1965 and 1985. One complication was asymptomatic (fibrosis), 2 required medical interventions managed on an outpatient basis (necrosis and fibrosis), and 2 required surgical intervention. All the complications were in patients treated with RT post-operatively; therefore, the 10 year complication rate for patients treated with post-operative RT was 20% compared to 0% in patients receiving pre-operative RT (P<0.001). The median dose delivered in patients who had RT-related complications was 60 Gy; only one patient had a complication with a dose < 60 Gy (P<0.001).
DISCUSSION
Our analyses confirmed that presentation with recurrent RPS after having undergone previous surgery is a particularly adverse prognostic factor for patients with this disease. This finding corroborates data from other institutions regarding the difficulty of managing RPS in the recurrent setting.11 Similar to soft-tissue sarcomas occurring in the extremities and superficial trunk, positive or uncertain resection margins in operated RPS is also associated with a high risk for intra-abdominal recurrences. In our study, we report these factors reinforce the previously documented importance of aggressive surgical management for RPS.12,13 We report few late RT-related complications but continue to observe high rates of intra-abdominal recurrences despite multidisciplinary therapy, which emphasizes the need for better strategies to improve outcomes for patients with RPS.
Complete resection, which often includes adjacent organs, offers the only reasonable chance at long-term survival. However, the reported 5-year local control rates using surgery alone are between 23% and 59% (Table 5),5,14–19 with most series reporting outcomes for only primary RPS patients, not recurrent presentations. These poor local control rates contribute to the 75% incidence of sarcoma-related deaths for these patients.20 Therefore, a multidisciplinary approach with integration of adjuvant therapies is often considered in an attempt to improve outcomes. When surgery is combined with RT, series show that the 5-year local control rate increases to 38% to 80%.14,16,18,21 We observed an intra-abdominal recurrence rate of 44% at 5 years (31% for de novo RPS). This compares favorably to surgical resection alone, especially given that this series reports on a population that is selected for being at a higher risk of relapse and almost a third of patients were treated for recurrent disease.
Table 5.
Local control rates in series analyzing outcomes for surgery alone
| First Author | Year | No. of Patients | 5-year LC | Excluded Recurrent RPS |
|---|---|---|---|---|
| Lewis5 | 1998 | 231 | 59 | Yes |
| Stoeckle18 | 2000 | 34 | 23 | Yes |
| van Dalen24 | 2001 | 77 | 37 | Yes |
| Ferrario14 | 2003 | 98 | 47 | Yes |
| Lehnert17 | 2009 | 50 | 59 | Yes |
| Bonvalot22 | 2009 | 279 | 51 | Yes |
| Strauss19 | 2010 | 170 | 55 | Yes |
Abbreviations: LC, local control; RPS, retroperitoneal sarcomas.
We observed a higher frequency of multifocal disease in patients who received previous surgery, but more importantly, having a recurrent presentation after prior resection was one of the major factors associated with intra-abdominal recurrence. These findings reinforce that immediate referral for patients with RPS to a high-volume sarcoma center may be beneficial. The surgical community has adopted the concept of concentrating infrequently performed and complex procedures in high-volume specialist centers.12,13 Gutierrez and colleagues evaluated 4205 patients to determine the prognostic significance of surgical center volume on outcomes for soft tissue sarcomas and reported a lower post-operative mortality and better survival for patients with RPS treated at high-volume centers.22 Similarly, another study found that tumor rupture among 382 patients with RPS was associated with low surgical volume and that better local control was associated with higher volume.23
Long-term tumor control and survival is also highly dependent on the ability of the surgeon to obtain negative margins. This correlation is also well-described in the surgical literature.4,5,23–26 A large series by Lewis and colleagues from Memorial Sloan-Kettering Cancer Center reported a significantly better median survival in patients with RPS who underwent a complete resection (103 months vs. 18 months for an incomplete resection), and they observed no difference in survival between patients who underwent an incomplete resection compared to those whose disease was not resectable (P = 0.4).5 Furthermore, Van Dalen and colleagues reported a higher rate of incomplete resections at low-volume centers (38% vs. 18% at high-volume centers, P=0.002), which was associated with poorer outcome.25 Not only is referral to a specialty center important for more surgical experience with multivisceral en bloc resections, but referral centers also have greater access to possible clinical trials and adjuvant treatments22,27 that require input from sarcoma-specialists in the fields of pathology, radiology, radiation and medical oncology.
The use of RT to manage RPS remains controversial due to uncertainty about the different radiosensitivities of the histologic subtypes and the proximity of the target to radiosensitive normal tissues. Many of the larger historical studies used postoperative RT to treat RPS.7 However, there has been a shift in later years favoring preoperative therapy.27 The main reason for the shift towards neoadjuvant therapy is out of concern for increased complication rates with the higher postoperative RT doses (≥60 Gy vs. 45–50.4 Gy for preoperative RT).28 Our findings support these concerns. While there were only five treatment-related toxicities in our cohort, all were treated with postoperative RT in an older era where imaging and RT techniques were more limited; also, four of the patients were treated to doses of 60 Gy or above. Based on these observations, we would not recommend the use of postoperative RT outside of a clinical trial. Our study suggests an acceptable risk-benefit ratio for the use of preoperative RT, and we recommend a dose of 50.4 Gy in 28 daily fractions in cases where RT is used.
We acknowledge several limitations to our study that warrant consideration when interpreting the results. First, as with any retrospective study, there are inherent selection biases. Specifically, since not all patients with RPS received adjuvant RT, the patients in this series were likely perceived to be at a higher risk of relapse at the time of treatment disposition, which cannot be quantified retrospectively. Second, despite our having a relatively large cohort compared to other RT-focused RPS studies, the number of events was still limited and the treatment of these patients spanned several decades over which there have been changes to imaging and techniques in the delivery of RT, as well as changes in the surgical approach to these tumors. Finally, identifying RT-induced complications retrospectively has limited applicability. Fortunately, a Phase III multicenter randomized trial (STRASS trial) comparing surgery alone to preoperative RT and surgery will offer the most appropriate study design to investigate the use of RT for RPS, and it is currently accruing to aid in determining if preoperative radiation reduces the risk of local recurrence.
In conclusion, intra-abdominal RPS recurrences continue to be a significant challenge despite the use of aggressive surgery and RT. Recurrent RPS tumors following prior resection and positive or uncertain margins continue to be the primary prognostic factors associated with intra-abdominal recurrences, which emphasize the importance of immediate assessment and potential treatment at high-volume sarcoma centers. There were no clear modifiable radiotherapeutic treatment factors associated with decrease in the risk of in-field recurrences, but given the complications associated with postoperative RT, we recommend that preoperative RT is the preferred strategy when combined modality therapy is recommended.
Acknowledgments
Supported in part by Cancer Center Support (Core) Grant CA016672 to The University of Texas MD Anderson Cancer Center.
Footnotes
Disclaimers: The authors declare no conflicts of interest.
REFERENCES
- 1.Clark MA, Fisher C, Judson I, et al. Soft-tissue sarcomas in adults. N Engl J Med. 2005;353:701–711. doi: 10.1056/NEJMra041866. [DOI] [PubMed] [Google Scholar]
- 2.Tseng WW, Madewell JE, Wei W, et al. Locoregional disease patterns in well-differentiated and dedifferentiated retroperitoneal liposarcoma: implications for the extent of resection? Ann Surg Oncol. 2014;21:2136–2143. doi: 10.1245/s10434-014-3643-4. [DOI] [PubMed] [Google Scholar]
- 3.Trans-Atlantic RPSWG. Management of Primary Retroperitoneal Sarcoma (RPS) in the Adult: A Consensus Approach From the Trans-Atlantic RPS Working Group. Ann Surg Oncol. 2014 doi: 10.1245/s10434-014-3965-2. [DOI] [PubMed] [Google Scholar]
- 4.Gronchi A, Miceli R, Shurell E, et al. Outcome prediction in primary resected retroperitoneal soft tissue sarcoma: histology-specific overall survival and disease-free survival nomograms built on major sarcoma center data sets. J Clin Oncol. 2013;31:1649–1655. doi: 10.1200/JCO.2012.44.3747. [DOI] [PubMed] [Google Scholar]
- 5.Lewis JJ, Leung D, Woodruff JM, et al. Retroperitoneal soft-tissue sarcoma: analysis of 500 patients treated and followed at a single institution. Ann Surg. 1998;228:355–365. doi: 10.1097/00000658-199809000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ballo MT, Zagars GK, Pollock RE, et al. Retroperitoneal soft tissue sarcoma: an analysis of radiation and surgical treatment. Int J Radiat Oncol Biol Phys. 2007;67:158–163. doi: 10.1016/j.ijrobp.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 7.Tuan J, Vitolo V, Vischioni B, et al. Radiation therapy for retroperitoneal sarcoma. Radiol Med. 2014;119:790–802. doi: 10.1007/s11547-013-0350-3. [DOI] [PubMed] [Google Scholar]
- 8.Nussbaum DP, Speicher PJ, Gulack BC, et al. The effect of neoadjuvant radiation therapy on perioperative outcomes among patients undergoing resection of retroperitoneal sarcomas. Surg Oncol. 2014;23:155–160. doi: 10.1016/j.suronc.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Pechoux C, Musat E, Baey C, et al. Should adjuvant radiotherapy be administered in addition to front-line aggressive surgery (FAS) in patients with primary retroperitoneal sarcoma? Ann Oncol. 2013;24:832–837. doi: 10.1093/annonc/mds516. [DOI] [PubMed] [Google Scholar]
- 10.Paryani NN, Zlotecki RA, Swanson EL, et al. Multimodality local therapy for retroperitoneal sarcoma. Int J Radiat Oncol Biol Phys. 2012;82:1128–1134. doi: 10.1016/j.ijrobp.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Gyorki DE, Brennan MF. Management of recurrent retroperitoneal sarcoma. J Surg Oncol. 2014;109:53–59. doi: 10.1002/jso.23463. [DOI] [PubMed] [Google Scholar]
- 12.Chowdhury MM, Dagash H, Pierro A. A systematic review of the impact of volume of surgery and specialization on patient outcome. Br J Surg. 2007;94:145–161. doi: 10.1002/bjs.5714. [DOI] [PubMed] [Google Scholar]
- 13.Learn PA, Bach PB. A decade of mortality reductions in major oncologic surgery: the impact of centralization and quality improvement. Med Care. 2010;48:1041–1049. doi: 10.1097/MLR.0b013e3181f37d5f. [DOI] [PubMed] [Google Scholar]
- 14.Ferrario T, Karakousis CP. Retroperitoneal sarcomas: grade and survival. Arch Surg. 2003;138:248–251. doi: 10.1001/archsurg.138.3.248. [DOI] [PubMed] [Google Scholar]
- 15.Storm FK, Mahvi DM. Diagnosis and management of retroperitoneal soft-tissue sarcoma. Ann Surg. 1991;214:2–10. doi: 10.1097/00000658-199107000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Doorn RC, Gallee MP, Hart AA, et al. Resectable retroperitoneal soft tissue sarcomas. The effect of extent of resection and postoperative radiation therapy on local tumor control. Cancer. 1994;73:637–642. doi: 10.1002/1097-0142(19940201)73:3<637::aid-cncr2820730322>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 17.Lehnert T, Cardona S, Hinz U, et al. Primary and locally recurrent retroperitoneal soft-tissue sarcoma: local control and survival. Eur J Surg Oncol. 2009;35:986–993. doi: 10.1016/j.ejso.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Stoeckle E, Coindre JM, Bonvalot S, et al. Prognostic factors in retroperitoneal sarcoma: a multivariate analysis of a series of 165 patients of the French Cancer Center Federation Sarcoma Group. Cancer. 2001;92:359–368. doi: 10.1002/1097-0142(20010715)92:2<359::aid-cncr1331>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 19.Strauss DC, Hayes AJ, Thway K, et al. Surgical management of primary retroperitoneal sarcoma. Br J Surg. 2010;97:698–706. doi: 10.1002/bjs.6994. [DOI] [PubMed] [Google Scholar]
- 20.Miah AB, Hannay J, Benson C, et al. Optimal management of primary retroperitoneal sarcoma: an update. Expert Rev Anticancer Ther. 2014;14:565–579. doi: 10.1586/14737140.2014.883279. [DOI] [PubMed] [Google Scholar]
- 21.Catton CN, O'Sullivan B, Kotwall C, et al. Outcome and prognosis in retroperitoneal soft tissue sarcoma. Int J Radiat Oncol Biol Phys. 1994;29:1005–1010. doi: 10.1016/0360-3016(94)90395-6. [DOI] [PubMed] [Google Scholar]
- 22.Gutierrez JC, Perez EA, Moffat FL, et al. Should soft tissue sarcomas be treated at high-volume centers? An analysis of 4205 patients. Ann Surg. 2007;245:952–958. doi: 10.1097/01.sla.0000250438.04393.a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonvalot S, Rivoire M, Castaing M, et al. Primary retroperitoneal sarcomas: a multivariate analysis of surgical factors associated with local control. J Clin Oncol. 2009;27:31–37. doi: 10.1200/JCO.2008.18.0802. [DOI] [PubMed] [Google Scholar]
- 24.Bonvalot S, Miceli R, Berselli M, et al. Aggressive surgery in retroperitoneal soft tissue sarcoma carried out at high-volume centers is safe and is associated with improved local control. Ann Surg Oncol. 2010;17:1507–1514. doi: 10.1245/s10434-010-1057-5. [DOI] [PubMed] [Google Scholar]
- 25.van Dalen T, Hennipman A, Van Coevorden F, et al. Evaluation of a clinically applicable post-surgical classification system for primary retroperitoneal soft-tissue sarcoma. Ann Surg Oncol. 2004;11:483–490. doi: 10.1245/ASO.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 26.Gronchi A, Lo Vullo S, Fiore M, et al. Aggressive surgical policies in a retrospectively reviewed single-institution case series of retroperitoneal soft tissue sarcoma patients. J Clin Oncol. 2009;27:24–30. doi: 10.1200/JCO.2008.17.8871. [DOI] [PubMed] [Google Scholar]
- 27.Sherman KL, Wayne JD, Chung J, et al. Assessment of multimodality therapy use for extremity sarcoma in the United States. J Surg Oncol. 2014;109:395–404. doi: 10.1002/jso.23520. [DOI] [PubMed] [Google Scholar]
- 28.Sindelar WF, Kinsella TJ, Chen PW, et al. Intraoperative radiotherapy in retroperitoneal sarcomas. Final results of a prospective, randomized, clinical trial. Arch Surg. 1993;128:402–410. doi: 10.1001/archsurg.1993.01420160040005. [DOI] [PubMed] [Google Scholar]