Abstract
Spontaneous isolated superior mesenteric artery dissection (SISMAD) has been known as a rare vascular disease. However it is increasingly reported in these days with the development of advanced imaging technology. Underlying etiology, natural course or an optimal management strategy of SISMAD is not exactly known at the moment. During the past 10 years, we have had an interest in this rare vascular disease and collected clinical and image data in 100 or more patients with SISMAD. In this review article, I would like to describe my current understanding of SISMAD on the base of our recent publications in the major vascular surgery journals.
Keywords: Dissection, Superior mesenteric artery, Natural course
INTRODUCTION
Spontaneous isolated superior mesenteric artery dissection (SISMAD) is a rare vascular disorder. The incidence of SISMAD reported in an autopsy series was 0.06% [1]. Since Bauersfeld [2] reported the first case series of SISMAD in 1947, this rare vascular disorder has been anecdotally reported as case reports or case series. The development of advanced imaging technology, in particular abdominal computed tomography (CT) scan, appears to have increased detection of SISMAD. By reviewing previous reports, we came to know that SISMAD is more prevalent in males in their 50s and often develop at the proximal part of the superior mesenteric artery (SMA). Though we don’t know the exact reasons, SISMAD seems to be more frequently reported in Asian countries including Korea, Japan and China [3–11].
ETIOLOGY
Some authors suggested an arterial wall pathology as the underlying cause of SISMAD, which includes fibromuscular dysplasia, cystic medial necrosis, arterial mediolysis, adventitial inflammation, disruption of the internal elastic lamina, penetrating arterial ulcer, pseudoaneurysm, and aneurysm [12,13]. Although several authors described various connective tissue diseases as possible causes of SISMAD, no specific underlying cause of SISMAD was identified in the majority of reports. Furthermore, it is not easy to differentiate those connective tissue disorders from the secondary changes following arterial dissection. One Chinese group reported familial cases of SISMAD with genetic heterogeneity of chromosome locus 5q13–14 [14].
To find any differences in patient characteristics between SISMAD and combined aortic and SMA dissection (CASMAD), we investigated demographic (age, gender), clinical (concurrent disease, mode of symptom onset, and time relation between meal and symptom onset), and lesion characteristics (angiographic type, SMA branching angle from the aorta, location of entry tear site, and length of SMA dissection) of SISMAD and CASMAD after excluding patients with Marfan’s syndrome. By such comparison, we found some difference between SISMAD (n=51) and CASMAD (n=38). SISMAD was more prevalent in male gender (90% vs. 71%, P=0.02) and symptomatic patients were more common in SISMAD (77% vs. 0%, P<0.001). However, hypertension was more common in patients with CASMAD (66% vs. 31%, P=0.001).
The other hypothesis to explain the pathogenesis of SISMAD is “shear stress injury” of the SMA. It is assumed that an abnormal shear stress can develop at the transitional zone of SMA from a fixed (under the pancreas body) to a relatively unfixed (in the mesenteric root) segment. This abnormal shear stress can cause SMA dissection just like the development of type B aortic dissection at the level of the left subclavian artery. As the first step to prove the “shear stress hypothesis” of SISMAD, we retrospectively measured the distance between the lower margin of the pancreas to the entry site of SISMAD on CT images (Fig. 1). The entry sites of SISMAD were located at a mean distance of 11.2±9.61 mm from the lower margin of the pancreas [3,15].
Fig. 1.
Measurement of distance from the lowest margin of the pancreas to an entry site of dissection on computed tomography scan. Cross-sectional view at the level of (A) the most proximal entry site and (B) lowest margin of pancreas. (C) Three-dimensional reconstructed lateral view showing the relative position of an entry site of spontaneous isolated superior mesenteric artery dissection and pancreas body (P).
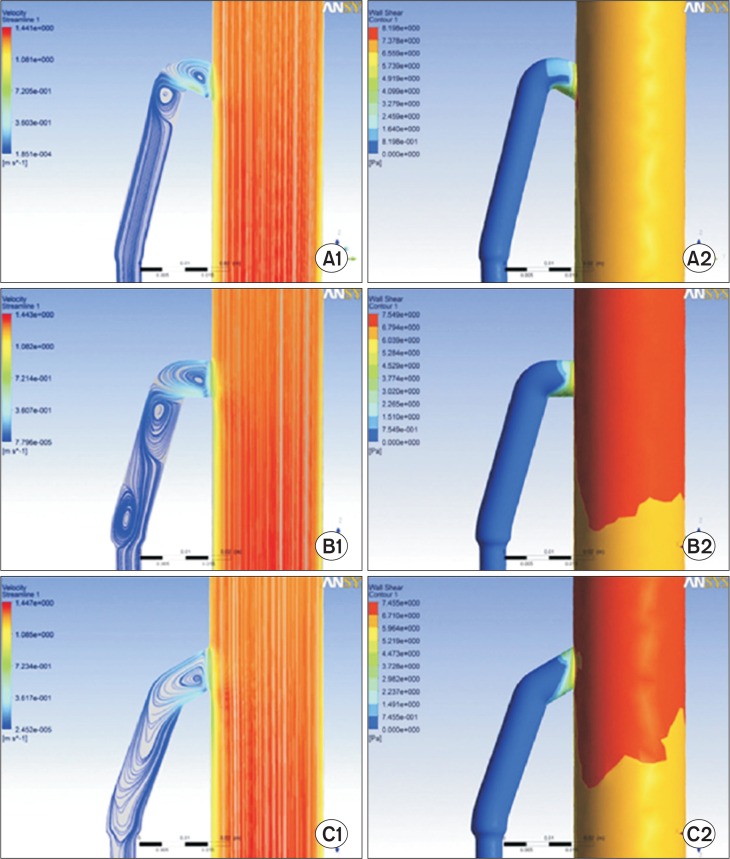
On the assumption that mesenteric blood flow augmentations may change shear stress at a specific part of the SMA wall and lead to the development of the arterial dissections, we conducted computer simulation studies using fluid dynamic models in collaboration with a specialist in fluid dynamics. To investigate flow dynamics around the origin of the SMA and its convex curvature, we conducted a flow structure investigation using computational fluid dynamics. The commercial software CFX (V.12; ANSYS Inc., Canonsburg, PA, USA), which uses a finite volume method, was used to simulate blood flow by solving the Navier-Stokes equations. Fig. 2 shows the streamline patterns of blood flow within the SMA and wall shear stress distributions at the midsection of the SMA during the systolic phase using various branching angles of the SMA from the abdominal aorta. When we focused on the streamline patterns, we could see the rotating vortical flow close to the ostium of the SMA. We also observed an accelerated flow from the posterior wall towards the anterior wall of the SMA with strong momentum independent of the SMA branching angle. On a computer simulation model, we observed abnormal mechanical stresses to the anterior wall of the SMA at the transition zone of the SMA from a fixed (under the pancreas) to a relatively unfixed portion (in the mesentery root), but the location of dissection entry was not consistent with the lower margin of the pancreas. We concluded that the development of SISMAD seems more likely due to abnormal hemodynamic forces caused by convex curvature and running of the SMA from a fixed to a relatively mobile part.
Fig. 2.
Limiting streamline patterns (left panels; A1, B1, and C1) and wall shear stress (WSS) distributions (right panels; A2, B2, and C2) according to three branching angles (60°, 90°, and 120°) of the superior mesenteric artery from the aorta.
DIAGNOSIS
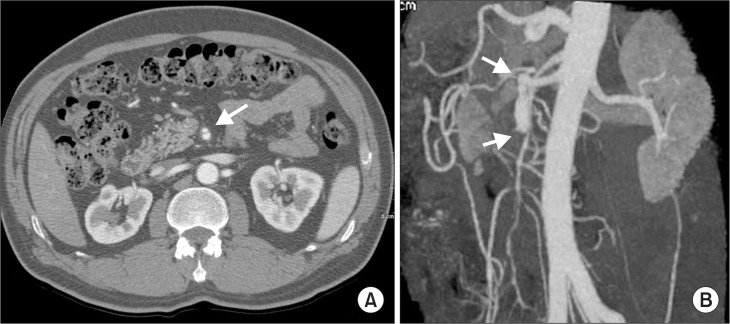
Most patients with SISMAD are easily diagnosed with the characteristic finding of a “double lumen sign” of the SMA on axial views of CT images and/or dissected segment of SMA on selective mesenteric angiography or CT angiography (CTA) (Fig. 3). To detect arterial dissection on CT image, it requires a high level of suspicion when viewing visceral arterial images. In practice, catheter-based arteriography is not recommended for the diagnosis of SISMAD. On CT images of SISMAD, it is important to see the entry and reentry sites of the arterial dissection, the diameter and length of the dissected segment, the patency of the false and true lumens, the major collateral branches of the SMA, the presence of mesentery hematoma, any signs of bowel ischemia, and the presence of other concurrent arterial dissections.
Fig. 3.
Diagnosis of superior mesenteric artery (SMA) dissection with the characteristic finding of a “double lumen sign” of the SMA on axial views of computed tomography (CT) images (A) and/or dissected segment of SMA on selective mesenteric angiography or CT angiography (B).
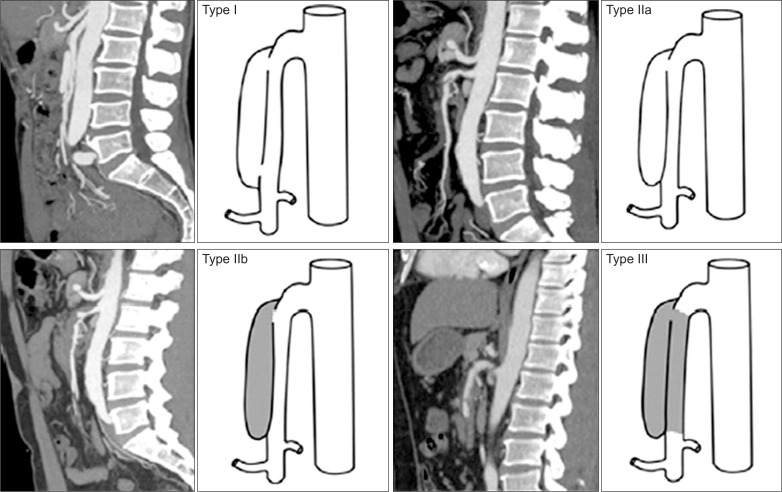
According to the patency of the entry and reentry sites, and false and true lumens, SISMAD was stratified based on their angiographic features [16]. We previously reported a simplified and modified angiographic classification of SISMAD (Table 1, Fig. 4) [4]. When we retrospectively categorized SISMAD patients by their angiographic types on CT angiography, they were found to be distributed as type I in 37%, type II in 55%, and type III in 8%.
Table 1.
Angiographic types of spontaneous isolated superior mesenteric artery dissection
| Type | Angiographic finding |
|---|---|
| Type I | Patent true and false lumens; visible entry and reentry sites |
| Type IIa | Patent true and false lumens; visible entry, no visible reentry |
| Type IIb | Patent true lumen but thrombosed false lumen; no visible reentry |
| Type III | SMA dissection confirmed; occluded true and false lumens |
SMA, superior mesenteric artery.
Data from the article of Yun, et al. (Eur J Vasc Endovasc Surg 2009;37:572–577) [4].
Fig. 4.
Angiographic classification of spontaneous isolated superior mesenteric artery dissection.
CLINICAL FEATURES AND NATURAL COURSE
On our retrospective review of SISMAD patients in Samsung Medical Center, the mean age of patients was 54.6±11.7 years (range 40–85 years) and 90% was male gender. Clinically, 23% of the patients were incidentally detected on CT images while 77% of SISMAD patients presented with abdominal pain [15]. In 92% of the patients, pain developed with sudden onset, with an epigastric or periumbilical presentation in 66%, severity of 7–10 on a 0-to-10 analogue scale in 79% and postprandial aggravation only in 16% of SISMAD patients [3].
We found a positive correlation between pain severity and dissection length (P=0.03; ρ=0.50, Spearman’s partial correlation analysis) [4]. When we compared their clinical features by the angiographic types, there was no difference between types. However dissection length was positively associated with more severe clinical symptoms in SISMAD patients [4].
We reported the natural course of SISMAD derived from follow-up of 46 SISMAD patients who underwent conservative treatment and periodic follow-up with CTA [3]. Among a total of 58 patients with SISMAD, endovascular (n=1) or surgical (n=4) treatment were performed for patients with persistent abdominal pain despite conservative treatment or signs of bowel ischemia.
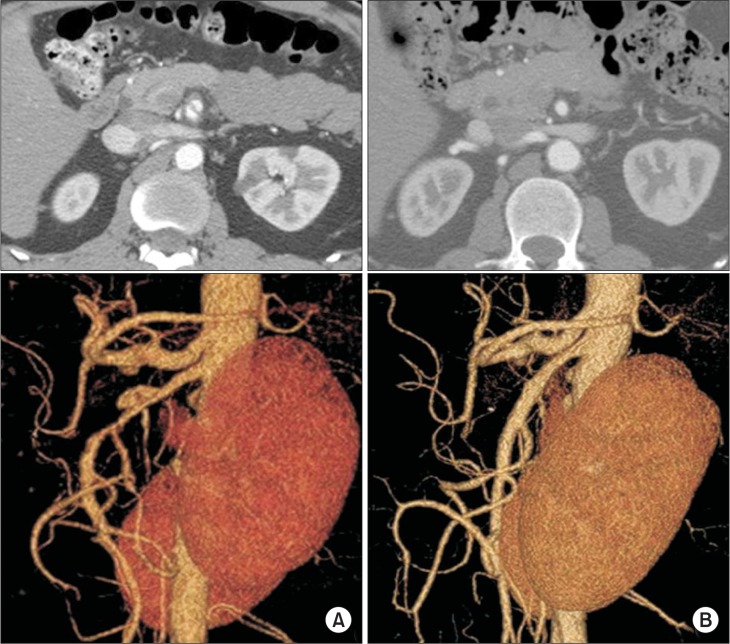
We retrospectively investigated changes in length, type, or remodeling of the dissection and SMA patency on multi-detector CT scan. To evaluate clinical course, presence of persistent or recurrent abdominal symptoms was queried on an outpatient basis. After a median of 23.0 months (range 6.5–74.2 months), follow-up CT angiograms showed diminished extent of the false lumen size in 19 (41%), no change in 20 (44%), diminished length of dissection in 11 (24%), and complete remodeling of dissection in 7 patients (15%) (Fig. 5). No patient showed progression of the dissection on follow-up angiograms.
Fig. 5.
Complete remodeling of superior mesenteric artery (SMA) dissection on follow-up computed tomography (CT) angiogram. (A) Initial CT scan showing double-lumen sign on an axial view (top) and a wind-sock-shaped dissection lesion on the anterior wall of the SMA (type IIa spontaneous isolated superior mesenteric artery dissection) (bottom), (B) follow-up CT angiogram at 7 months after conservative treatment shows disappearance of the double-lumen sign on axial view (top) and complete remodeling of the SMA on a reconstructed view of the SMA (bottom).
During the follow-up period, 10 patients (26%) reported mild abdominal discomfort; however, no patient developed recurrent abdominal pain following conservative treatment of the recurrent pain. During the follow-up period, one patient required small bowel resection due to late development of bowel stricture; however, no mortality related to SISMAD occurred [3].
TREATMENT
Treatment options for patients with SISMAD include conservative management with or without antithrombotic therapy, endovascular therapy with SMA stenting or open surgery such as bypass or direct surgical reconstruction of the SMA lesion.
Gobble et al. [17] thoroughly reviewed the literature and reported 106 cases of SISMAD. Excluding 10 asymptomatic cases, 96 symptomatic patients were treated with either expectant management, anticoagulation, open surgery, or endovascular stent placement. Of these patients, 48% were managed successfully with conservative management. In whom conservative management was not successful in relieving symptoms, they were treated with open surgery or SMA stenting. They reported a 17.7% mortality rate in SISMAD patients.
In our experience at Samsung Medical Center, in Seoul, Korea, among 58 patients with SISMAD, 49 patients (84.5%) were symptomatic. Excluding five patients who underwent open or endovascular therapy due to prolonged unrelieved pain, 91.4% (53/58) of all SISMAD and 89.8% (44/49) of symptomatic patients showed symptomatic improvement with conservative treatment. Among them, 75.5% (40/53) were not prescribed anticoagulants or antiplatelet agents. We experienced that there was no difference in clinical course in patients with and without adjuvant antithrombotic therapy.
Our recommendations for symptomatic SISMAD patients are conservative management as first-line treatment, consisting of bowel rest to reduce demand of mesenteric blood supply, intravenous fluids, and nutritional support. With conservative therapy, abdominal pain subsided within 7 days (median 4 days, range 1–7 days) in the majority of patients. According to our recent data of long-term results of conservative treatment of 100 or more patients with SISMAD (unpublished data), we have experienced results similar to the above-described results. Regarding the indications for intervention, we recommend interventional therapy for patients with prolonged abdominal pain >7 days or with signs of acute bowel ischemia.
When we reviewed the follow-up results after conservative treatment of SISMAD, 90% showed complete relief of abdominal pain whereas 10% showed residual abdominal discomfort. Among the patients with complete relief of abdominal pain, 26% showed recurrent abdominal discomfort in later days but were spontaneously relieved unless they were followed by structural deformity such as ischemic stricture of the bowel. Follow-up CTAs of SMA dissection showed no change in 44%, complete remodeling of the SMA dissection in 15% and improvement of dissection (e.g., reduced false lumen diameter or reduced dissection length) in 41% of patients [3].
Various surgical treatments such as combined SMA thrombectomy, intimectomy and patch angioplasty; SMA interposition graft, and aorto-SMA bypass have been reported [18–23]. In SISMAD patients, mesenteric anastomosis is technically demanding due to a thin dissected SMA wall, risk of distal or proximal progression of the arterial dissection, and difficult proximal control of the SMA. In our current practice, open surgery is not used for patients with SISMAD unless there is presence of bowel gangrene or late bowel stricture.
Endovascular treatment with SMA stenting has been reported with successful outcomes [10,17,24]. However there has been no report describing long-term results of endovascular treatment for patients with SISMAD. As described above, most of the SISMAD patients are in age of 50s. Unlike the atherosclerotic SMA disease, SMA stenting in acute SMA dissection carries the risk of complications such as arterial rupture or progression of arterial dissection. Endovascular therapy is an excellent option when it comes to length of hospital stay and promptness of pain relief. However, I personally worry about the risk of late development of in-stent restenosis or thrombotic occlusion of the SMA stent. In my personal experience, SMA stents used for patients with atherosclerotic SMA disease was not so durable in patency compared to stents used in other arteries. Additionally “leave nothing behind” is a current trend of endovascular therapy. Therefore, despite the expected prompt efficacy of SMA stenting, I would like to recommend conservative treatment for patients with SISMAD, considering the possible late complications of SMA stenting. Only in specific cases of unrelieved abdominal pain for >7 days after conservative treatment, or in patients with a short life expectancy such as very old patients or in patients with coexisting malignant disease, should SMA stenting be recommended as primary treatment. Otherwise, I would like to recommend an expectant, conservative treatment as the first line treatment of SISMAD.
CONCLUSION
SISMAD is a rare vascular disease and an uncommon cause of abdominal pain. Though the pathogenesis of SISMAD is still elusive, anatomic and hemodynic characteristics of SMA may be a causative factor for the development of SISMAD. Most patients with SISMAD showed benign clinical course. I recommend conservative treatment for all SISMAD patients as the first line therapy reserving primary intervention for selected patients.
Footnotes
Conflict of interest: None.
REFERENCES
- 1.Foord AG, Lewis RD. Primary dissecting aneurysms of peripheral and pulmonary arteries: dissecting hemorrhage of media. Arch Pathol. 1959;68:553–577. [PubMed] [Google Scholar]
- 2.Bauersfeld SR. Dissecting aneurysm of the aorta; a presentation of 15 cases and a review of the recent literature. Ann Intern Med. 1947;26:873–889. doi: 10.7326/0003-4819-26-6-873. [DOI] [PubMed] [Google Scholar]
- 3.Park YJ, Park KB, Kim DI, Do YS, Kim DK, Kim YW. Natural history of spontaneous isolated superior mesenteric artery dissection derived from follow-up after conservative treatment. J Vasc Surg. 2011;54:1727–1733. doi: 10.1016/j.jvs.2011.07.052. [DOI] [PubMed] [Google Scholar]
- 4.Yun WS, Kim YW, Park KB, Cho SK, Do YS, Lee KB, et al. Clinical and angiographic follow-up of spontaneous isolated superior mesenteric artery dissection. Eur J Vasc Endovasc Surg. 2009;37:572–577. doi: 10.1016/j.ejvs.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 5.Kim HK, Jung HK, Cho J, Lee JM, Huh S. Clinical and radiologic course of symptomatic spontaneous isolated dissection of the superior mesenteric artery treated with conservative management. J Vasc Surg. 2014;59:465–472. doi: 10.1016/j.jvs.2013.07.112. [DOI] [PubMed] [Google Scholar]
- 6.Cho BS, Lee MS, Lee MK, Choi YJ, Kim CN, Kang YJ, et al. Treatment guidelines for isolated dissection of the superior mesenteric artery based on follow-up CT findings. Eur J Vasc Endovasc Surg. 2011;41:780–785. doi: 10.1016/j.ejvs.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 7.Takayama T, Miyata T, Shirakawa M, Nagawa H. Isolated spontaneous dissection of the splanchnic arteries. J Vasc Surg. 2008;48:329–333. doi: 10.1016/j.jvs.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Yasuhara H, Shigematsu H, Muto T. Self-limited spontaneous dissection of the main trunk of the superior mesenteric artery. J Vasc Surg. 1998;27:776–779. doi: 10.1016/S0741-5214(98)70250-2. [DOI] [PubMed] [Google Scholar]
- 9.Ozaki T, Kimura M, Yoshimura N, Hori Y, Takano T, Kamura T, et al. Endovascular treatment of spontaneous isolated dissecting aneurysm of the superior mesenteric artery using stent-assisted coil embolization. Cardiovasc Intervent Radiol. 2006;29:435–437. doi: 10.1007/s00270-005-0067-3. [DOI] [PubMed] [Google Scholar]
- 10.Dong Z, Fu W, Chen B, Guo D, Xu X, Wang Y. Treatment of symptomatic isolated dissection of superior mesenteric artery. J Vasc Surg. 2013;57(2 Suppl):69S–76S. doi: 10.1016/j.jvs.2012.07.060. [DOI] [PubMed] [Google Scholar]
- 11.Jia ZZ, Zhao JW, Tian F, Li SQ, Wang K, Wang Y, et al. Initial and middle-term results of treatment for symptomatic spontaneous isolated dissection of superior mesenteric artery. Eur J Vasc Endovasc Surg. 2013;45:502–508. doi: 10.1016/j.ejvs.2013.01.039. [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto T, Deguchi J, Endo H, Miyata T. Successful treatment tailored to each splanchnic arterial lesion due to segmental arterial mediolysis (SAM): report of a case. J Vasc Surg. 2008;48:1338–1341. doi: 10.1016/j.jvs.2008.05.056. [DOI] [PubMed] [Google Scholar]
- 13.Attia C, Villard J, Boussel L, Farhat F, Robin J, Revel D, et al. Endovascular repair of localized pathological lesions of the descending thoracic aorta: midterm results. Cardiovasc Intervent Radiol. 2007;30:628–637. doi: 10.1007/s00270-007-9030-9. [DOI] [PubMed] [Google Scholar]
- 14.Jia Z, Zhang X, Wang W, Tian F, Jiang G, Li M. Spontaneous isolated superior mesenteric artery dissection: genetic heterogeneity of chromosome locus 5q13–14 in 2 male familial cases. Ann Vasc Surg. 2015;29:1019.e1–e5. doi: 10.1016/j.avsg.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 15.Park YJ, Park CW, Park KB, Roh YN, Kim DI, Kim Y W. Inference from clinical and fluid dynamic studies about underlying cause of spontaneous isolated superior mesenteric artery dissection. J Vasc Surg. 2011;53:80–86. doi: 10.1016/j.jvs.2010.07.055. [DOI] [PubMed] [Google Scholar]
- 16.Sakamoto I, Ogawa Y, Sueyoshi E, Fukui K, Murakami T, Uetani M. Imaging appearances and management of isolated spontaneous dissection of the superior mesenteric artery. Eur J Radiol. 2007;64:103–110. doi: 10.1016/j.ejrad.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 17.Gobble RM, Brill ER, Rockman CB, Hecht EM, Lamparello PJ, Jacobowitz GR, et al. Endovascular treatment of spontaneous dissections of the superior mesenteric artery. J Vasc Surg. 2009;50:1326–1332. doi: 10.1016/j.jvs.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 18.Krupski WC, Effeney DJ, Ehrenfeld WK. Spontaneous dissection of the superior mesenteric artery. J Vasc Surg. 1985;2:731–734. doi: 10.1016/0741-5214(85)90046-1. [DOI] [PubMed] [Google Scholar]
- 19.Vignati PV, Welch JP, Ellison L, Cohen JL. Acute mesenteric ischemia caused by isolated superior mesenteric artery dissection. J Vasc Surg. 1992;16:109–112. doi: 10.1016/0741-5214(92)90426-9. [DOI] [PubMed] [Google Scholar]
- 20.Solis MM, Ranval TJ, McFarland DR, Eidt JF. Surgical treatment of superior mesenteric artery dissecting aneurysm and simultaneous celiac artery compression. Ann Vasc Surg. 1993;7:457–462. doi: 10.1007/BF02002130. [DOI] [PubMed] [Google Scholar]
- 21.Sparks SR, Vasquez JC, Bergan JJ, Owens EL. Failure of nonoperative management of isolated superior mesenteric artery dissection. Ann Vasc Surg. 2000;14:105–109. doi: 10.1007/s100169910019. [DOI] [PubMed] [Google Scholar]
- 22.Gouëffic Y, Costargent A, Dupas B, Heymann MF, Chaillou P, Patra P. Superior mesenteric artery dissection: case report. J Vasc Surg. 2002;35:1003–1005. doi: 10.1067/mva.2002.122152. [DOI] [PubMed] [Google Scholar]
- 23.Picquet J, Abilez O, Pénard J, Jousset Y, Rousselet MC, Enon B. Superficial femoral artery transposition repair for isolated superior mesenteric artery dissection. J Vasc Surg. 2005;42:788–791. doi: 10.1016/j.jvs.2005.05.048. [DOI] [PubMed] [Google Scholar]
- 24.Casella IB, Bosch MA, Sousa WO., Jr Isolated spontaneous dissection of the superior mesenteric artery treated by percutaneous stent placement: case report. J Vasc Surg. 2008;47:197–200. doi: 10.1016/j.jvs.2007.07.051. [DOI] [PubMed] [Google Scholar]