Abstract
Chikungunya virus (CHIKV) is a mosquito-borne alphavirus showing a recent resurgence and rapid spread worldwide. While vaccines are under development, there are currently no therapies to treat this disease, except for over-the-counter (OTC) analgesics, which alleviate the devastating arthritic and arthralgic symptoms. To identify novel inhibitors of the virus, analogues of the natural product bryostatin 1, a clinical lead for the treatment of cancer, Alzheimer’s disease, and HIV eradication, were investigated for in vitro antiviral activity and were found to be among the most potent inhibitors of CHIKV replication reported to date. Bryostatin-based therapeutic efforts and even recent anti-CHIKV strategies have centered on modulation of protein kinase C (PKC). Intriguingly, while the C ring of bryostatin primarily drives interactions with PKC, A- and B-ring functionality in these analogues has a significant effect on the observed cell-protective activity. Significantly, bryostatin 1 itself, a potent pan-PKC modulator, is inactive in these assays. These new findings indicate that the observed anti-CHIKV activity is not solely mediated by PKC modulation, suggesting possible as yet unidentified targets for CHIKV therapeutic intervention. The high potency and low toxicity of these bryologs make them promising new leads for the development of a CHIKV treatment.
Bryostatin 1 (1, henceforth bryostatin) is a densely functionalized marine macrolide that has been a target of interest for decades due to its synthetic challenges and the numerous clinically relevant activities it elicits.1 Its structure was elucidated by Pettit and co-workers in 19822 after having been identified as the active compound in marine bryozoan extracts that showed efficacy against a lymphocytic leukemia cell line.3 This activity sparked multiple studies on its potential utility as an antineoplastic agent. To date, bryostatin has been the subject of 37 cancer clinical trials, either alone or in combination with other oncolytics.4 Furthermore, the biological activity associated with bryostatin is not limited to cancer. Its ability to speed recovery after ischemic damage5 and its capacity to induce synaptogenesis6 have attracted interest in its use in reducing the debilitating effects of stroke and in treating neurodegenerative diseases.7 It is currently under clinical evaluation for the treatment of Alzheimer’s disease.8 With respect to its application in HIV therapy, bryostatin exhibits a unique ability to activate latently infected reservoir cells, which harbor replication-competent provirus.9,10 These reservoir cells are responsible for the viral rebound that occurs upon cessation of antiretroviral therapy and are the cause of chronic infection, which then necessitates chronic antiretroviral therapy. The elimination of these reservoirs upon activation, through cytopathic effects or immunotoxin treatment, is a leading strategy in efforts to eradicate HIV/AIDS.11
This diverse range of bryostatin activities (from pro-proliferative to pro-apoptotic) is thought to arise from its ability to bind to and modulate the activity of various isoforms of protein kinase C (PKC),12 a family of serine/threonine kinases integral to cellular signal transduction.13 Two of the three PKC subclasses (conventional PKCs: α, βI/βII, γ; novel PKCs: δ, ε, η, θ) are activated by diacylglycerol (DAG), the endogenous PKC modulator, upon binding to the PKC C1 domains. This allosteric regulation by DAG can be similarly induced by bryostatin at concentrations that are orders of magnitude lower than those needed for DAG. Other natural product scaffolds that modulate PKC by binding to the C1 domains include tiglianes (e.g., phorbol esters, prostratin), ingenanes, daphnanes, aplysiatoxin, and teleocidin.
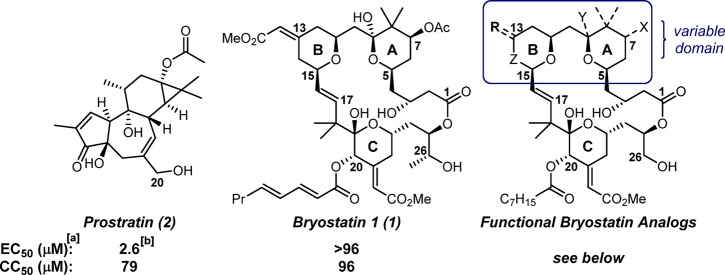
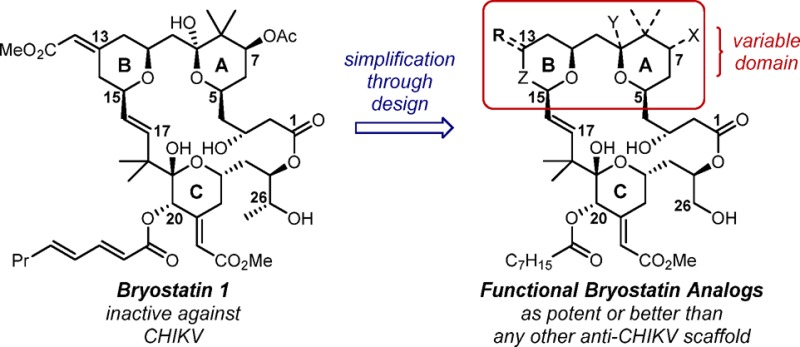
Interestingly, several of these scaffolds have recently been shown to protect BGM (buffalo green monkey) cells from chikungunya virus (CHIKV)-induced cell death,14 with the most recent report specifically implicating a PKC-dependent pathway.14c CHIKV is a mosquito-borne arbovirus that is becoming an increasingly prominent public health threat.15 Since its first appearances in the Americas in late 2013, over one million confirmed or suspected cases have been reported.16 CHIKV induces debilitating arthritis and arthralgia in the acute phase; in approximately 20% of CHIKV-infected patients, intense joint pain can persist for months to years. To date, there are no therapies for CHIKV, and symptoms can only be managed through use of OTC analgesics. Several vaccination strategies have performed well in a clinical setting (phase I and II trials in some cases), not only demonstrating safety and tolerability but also eliciting production of the neutralizing antibodies necessary for protection against natural viral challenges.17 However, given the potential of CHIKV to reach pandemic levels (a 2006–2007 outbreak in India led to an estimated 1.4–6.5 million infections15e), developing agents to be given prophylactically or to target the virus early after infection is an important complementary goal. Prostratin (2), a modulator of conventional and novel PKC C1 domains, has been reported by the Rega Institute for Medical Research and the Institut de Chimie des Substances Naturelles to show promising antiviral activity against CHIKV.14a A synthesis of prostratin has been reported by the Wender group in connection with their HIV eradication studies, and this synthesis has since been used to produce analogues that exhibit pan-PKC selectivity similar to prostratin but are up to 130-fold more potent in a variety of in vitro and ex vivo assays.10,18 The Wender group has similarly produced designed bryostatin analogues (bryologs) that are more synthetically accessible, more effective, and better tolerated than bryostatin in numerous assays performed thus far, including cellular, animal, and ex vivo studies on samples from HIV-infected individuals.19 Given that bryostatin and bryologs outperform prostratin in various PKC-based assays, it was expected that they would exhibit similar activity against CHIKV if the replication of this virus depends on a PKC-modulated pathway. Interestingly, however, as reported here, bryostatin was ineffective in antiviral CHIKV cell-based assays, showing no cell-protective effect before reaching cytotoxic doses (ca. 100 μM; Figure 1). In contrast, several bryologs were shown to be selective inhibitors of CHIKV replication.
Figure 1.
Comparison of natural PKC-activating scaffolds prostratin and bryostatin as cell-protective agents against CHIKV. aEC50 = concentration of compound that reduces the CHIKV-induced cytopathic effect by 50%; CC50 = concentration at which cell viability is 50% relative to untreated cells as a result of treatment with compound alone. bValues taken from ref (14a).
Results and Discussion
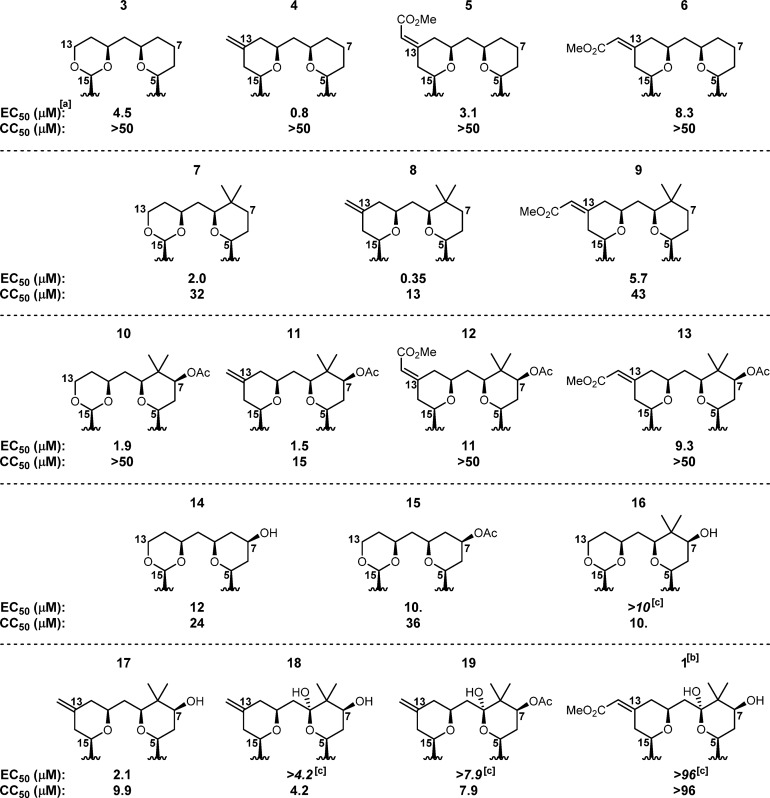
In an attempt to define the specific structural feature(s) of bryostatin that contribute to its activity or lack thereof, several representative compounds from the Wender group’s B-ring dioxane and B-ring pyran analogue classes19 were initially evaluated (see Figure 2). Previous studies indicated that the C-ring functionality of bryostatin contacts PKC and thus influences affinity. The A- and B-ring fragments are proposed to enhance affinity by controlling the alignment of C-ring binding elements and to influence function by affecting PKC translocation and/or membrane insertion. Intriguingly, there appears to be a clear dependence on northern fragment (A- and B-ring) substitution patterns in this in vitro CHIKV assay. Northern fragment functionality had previously been shown by the Wender group to impact PKC affinity20 and translocation,21 but in higher order assays relevant to specific indications (e.g., HIV latency reversal10), these analogues and bryostatin typically performed on similar levels. In contrast, distinct trends are observed here between northern fragment substitution and CHIKV inhibition.
Figure 2.
In vitro cell-protective effects of B-ring dioxane and B-ring pyran bryostatin analogues against CHIKV (full structure provided in Figure 1). aSee Figure 1 for a description of EC50 and CC50 values; standard deviations for all values can be found in the Supporting Information. bNote that bryostatin 1 (1) contains a slightly different southern fragment than the analogues above, namely, the C-20 octadienoate (rather than octanoate) and the presence of a C-26 methyl group. cValues in italics indicate that toxicity was observed before the EC50 value could be generated.
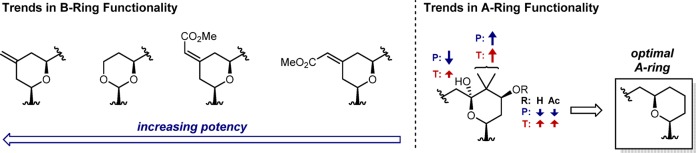
For each of the two different regions of the northern fragment, a structure–activity relationship can be derived (see Figure 3). Within the B ring, C-13-methylenyl analogues were the most potent, outperforming the B-ring dioxane and C-13 E- and Z-enoate classes at all points that could be directly compared (see rows 1 and 3 in Figure 2 for comparisons across the entire series). This series also produced the most potent compound known to date, analogue 8 (C-8 gem-dimethyl + C-13-methylenyl substitutions; EC50 = 0.35 μM), outperforming the previously reported jatrophanes22 (EC50 = 0.8 μM). However, other than the unsubstituted A-ring version of the C-13-methylenyl series (4), these compounds typically exhibited high cytotoxicity, with the more elaborated A-ring analogues 18 and 19 inducing toxicity before any observable cell-protective effect. The B-ring dioxane series also displayed generally high potency (unsubstituted A-ring analogue 3 and C-8 gem-dimethyl analogue 7) coupled with higher toxicities (see row 4 in Figure 2), although compound 10 was a notable exception, as it proved to be both potent and nontoxic. The C-13 E-enoate series (analogues 5 and 12) showed good activity, but additional analogues with this enoate moiety will need to be evaluated to determine its effect. The C-13 Z-enoate series (mimicking the natural system) proved to be the least potent in all direct comparisons, although it also displayed little observable toxicity.
Figure 3.
Trends in CHIKV inhibition as controlled through northern fragment substitution patterns of bryostatin-like analogues. (Left) Trends observed based on B-ring functionality of B-ring dioxane and the pyran series. (Right) Effects of A-ring functionality with P representing potency (in terms of cell-protective effects) and T representing toxicity. See text and Figure 1 for description of EC50 and CC50 values.
The A-ring functionality also displayed a distinct level of control over activity. The effect of introducing the natural C-9-hemiketal was difficult to assess because of the high toxicity of C-13-methylenyl analogues 18 and 19, although they were both less efficacious and more toxic than their des-C-9-OH variants 17 and 8, respectively, suggesting that introduction of this functionality (also seen in bryostatin 1) was deleterious to activity. The C-8 gem-dimethyl moiety appeared to improve function (compare rows 1 and 2 in Figure 2), although it also introduced toxic effects. The C-7 hydroxy and C-7 acetate functionality slightly reduced potency and showed mixed effects on toxicity, with the C-7-acetate analogues being slightly less toxic than the C-7-hydroxy compounds. Ultimately, the unfunctionalized A-ring compounds (see row 1 in Figure 2) proved to be the most potent and best tolerated of all the variations on A-ring functionality.
This distinct dependence of activity on northern fragment functionality is in contrast to trends typically observed in the evaluation of bryostatin and its analogues in cultured and primary cell lines. While A- and B-ring functionality have been shown to elicit differing modulation of PKC isoforms in vitro (pan-PKC vs novel PKC-only activation19−21) and to play a role in controlling PKC-based tumor promotion,23 these differences have not led to vastly different phenotypic outcomes when moving toward higher order models of disease (e.g., HIV latency reversal models10). More strikingly, while recapitulation of bryostatin-like activity has been achieved on several accounts both in vitro and ex vivo through the Wender group’s function-oriented synthesis approach, the current study is a rare instance in which bryostatin-inspired analogues are a few orders of magnitude more efficacious than the natural product,24 as seen in comparing 8 with 1 (0.35 vs >96 μM, respectively). These observations do not necessarily rule out PKC as a contributing factor for the observed activity (as has been recently suggested14c), but the stark contrast in behavior between minimally simplified analogues and the natural product suggests that there are additional pathways operating independent of PKC. As of yet, secondary pathways that might contribute to the observed cell-protective effect have not been identified but are actively being pursued and will be reported in due course. The significance of a PKC-independent pathway contributing to anti-CHIKV activity is heightened by the fact that the top performers in the above library are among the most potent compounds evaluated in this assay and thus stand as important leads for efforts aimed at developing the first clinically relevant anti-CHIKV agent.
Experimental Section
CHIKV CPE Reduction Assays
CHIKV Indian Ocean strain 899 (GenBank FJ959103.1) was generously provided by Prof. S. Günther (Bernhard Nocht Institute for Tropical Medicine, Hamburg, Germany). BGM cells were maintained in cell growth medium composed of minimum essential medium (MEM Rega-3, Gibco, Belgium) supplemented with 10% fetal bovine serum (FBS, Integro, The Netherlands), 1% l-glutamine (Gibco), and 1% sodium bicarbonate (Gibco). The antiviral assays were performed in virus growth medium, which is the respective cell growth medium supplemented with 2% (instead of 10%) FBS. Cell cultures were maintained at 37 °C in an atmosphere of 5% CO2 and 95–99% humidity.
BGM cells were seeded in 96-well tissue culture plates (Becton Dickinson, Aalst, Belgium) at a density of 2.5 × 104 cells/well in 100 μL of assay medium and were allowed to adhere overnight. Next, a compound dilution series was prepared in the medium on top of the cells, after which the cultures were infected with 0.001 MOI of CHIKV 899 inoculum in 100 μL of assay medium. On day 5 postinfection (p.i.), the plates were processed using the MTS/PMS method as described by the manufacturer (Promega, The Netherlands). The 50% effective concentration (EC50), which is defined as the compound concentration that is required to inhibit virus-induced cell death by 50%, was determined using logarithmic interpolation. Potential cytotoxic/cytostatic effects of the compound were also evaluated in uninfected cells by means of the MTS/PMS method. The 50% cytotoxic concentration (CC50; i.e., the concentration that reduces the overall metabolic activity of the cells by 50%) was calculated using logarithmic interpolation. All assay wells were checked microscopically for minor signs of virus-induced CPE or possible alterations to the cell or monolayer morphology caused by the compound.
Acknowledgments
This research was supported by the National Institutes of Health (CA031845 and CA031841 to P.A.W.). Additional funding was provided by the Stanford University Center for Molecular Analysis and Design (B.L.), the National Science Foundation (K.N.), and the Amgen Graduate Fellowship (D.S.). L.D. is funded by the Research Foundation of Flanders (FWO). This work was also supported by KU Leuven Geconcerteerde Onderzoeksactie (GOA 10/014) and by the BELSPO IUAP consortium BELVIR (Belgium).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.jnatprod.5b01016.
The authors declare no competing financial interest.
Supplementary Material
References
- For an overview and clinical summary, see: Pettit G. R.; Herald C. L.; Hogan F. In Anticancer Drug Development; Baguley B. C.; Kerr D. J., Eds.; Academic Press: San Diego, 2002; pp 203–235. [Google Scholar]
- Pettit G. R.; Herald C. L.; Doubek D. L.; Herald D. L.; Arnold E.; Clardy J. J. Am. Chem. Soc. 1982, 104, 6846–6848. 10.1021/ja00388a092. [DOI] [Google Scholar]
- Pettit G. R.; Day J. F.; Hartwell J. L.; Wood H. B. Nature 1970, 227, 962–963. 10.1038/227962a0. [DOI] [PubMed] [Google Scholar]
- a For current clinical information, see: http://clinicaltrials.gov.; b For a review of bryostatins’ clinical efficacy prior to 2003, see: Kortmansky J.; Schwartz G. K. Cancer Invest. 2003, 21, 924–936. 10.1081/CNV-120025095. [DOI] [PubMed] [Google Scholar]
- Sun M. K.; Hongpaisan J.; Nelson T. J.; Alkon D. L. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 13620–13625. 10.1073/pnas.0805952105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Sun M. K.; Hongpaisan J.; Alkon D. L. Proc. Natl. Acad. Sci. U. S. A. 2009, 106, 14676–14680. 10.1073/pnas.0907842106. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Sun M.-K.; Alkon D. L. Pharmacol. Ther. 2010, 127, 66–77. 10.1016/j.pharmthera.2010.03.001. [DOI] [PubMed] [Google Scholar]; c Zohar O.; Lavy R.; Zi X.; Nelson T.; Hongpaisan J.; Pick C.; Alkon D. Neurobiol. Dis. 2011, 41, 329–337. 10.1016/j.nbd.2010.10.001. [DOI] [PubMed] [Google Scholar]; d Tan Z.; Turner R.; Leon R.; Li X.; Hongpaisan J.; Zheng W.; Logsdon A.; Naser Z.; Alkon D.; Rosen C.; Huber J. Stroke 2013, 44, 3490–3497. 10.1161/STROKEAHA.113.002411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan T. K.; Nelson T. J.; Verma V. A.; Wender P. A.; Alkon D. L. Neurobiol. Dis. 2009, 34, 332–339. 10.1016/j.nbd.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurotrope, Inc. (2015). Neurotrope Announces Positive Top-Line Results from Its Phase 2a Study of Bryostatin-1 in Alzheimer’s Disease. [Press release]. Retrieved from http://www.neurotropebioscience.com/Welcome_to_Neurotrope_BioScience/Bryostatin-1.html.
- Mehla R.; Bivalkar-Mehla S.; Zhang R.; Handy I.; Albrecht H.; Giri S.; Nagarkatti P.; Nagarkatti M.; Chauhan A. PLoS One 2010, 5, e11160. 10.1371/journal.pone.0011160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For a demonstration of similar efficacies from non-natural, design analogues, see:DeChristopher B.; Loy B.; Marsden M.; Schrier A.; Zack J.; Wender P. Nat. Chem. 2012, 4, 705–710. 10.1038/nchem.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For the current status of latency research as applied to therapies along with challenges of this treatment method, see:; a Chan C. N.; Dietrich I.; Hosie M.; Willet B. J. Gen. Virol. 2013, 94, 917–932. 10.1099/vir.0.049296-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Archin N.; Margolis D. Curr. Opin. Infect. Dis. 2014, 27, 29–35. 10.1097/QCO.0000000000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Several C1-domain-containing proteins are possible targets for bryostatin; see:Kazanietz M. G. Mol. Pharmacol. 2002, 61, 759–767. 10.1124/mol.61.4.759. [DOI] [PubMed] [Google Scholar]
- For reviews of PKC, see:; a Newton A. C. Chem. Rev. 2001, 101, 2353–2364. 10.1021/cr0002801. [DOI] [PubMed] [Google Scholar]; b Gallegos L. L.; Newton A. C. IUBMB Life 2008, 60, 782–789. 10.1002/iub.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Bourjot M.; Delang L.; Hung Nguyen V.; Neyts J.; Gueritte F.; Leyssen P.; Litaudon M. J. Nat. Prod. 2012, 75, 2183–2187. 10.1021/np300637t. [DOI] [PubMed] [Google Scholar]; b Corlay N.; Delang L.; Girard-Valenciennes E.; Neyts J.; Clerc P.; Smadja J.; Gueritte F.; Leyssen P.; Litaudon M. Fitoterapia 2014, 97, 87–91. 10.1016/j.fitote.2014.05.015. [DOI] [PubMed] [Google Scholar]; c Nothias-Scaglia L.-F.; Pannecouque C.; Renucci F.; Delang L.; Neyts J.; Roussi F.; Costa J.; Leyssen P.; Litaudon M.; Paolini J. J. J. Nat. Prod. 2015, 78, 1277–1283. 10.1021/acs.jnatprod.5b00073. [DOI] [PubMed] [Google Scholar]; d Gupta D.; Kaur P.; Leong S.; Tan L.; Prinsep M.; Chu J. Mar. Drugs 2014, 12, 115–127. 10.3390/md12010115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For recent reviews on the spread of CHIKV, its epidemiology, and methods to treat it, see:; a Solignat M.; Gay B.; Higgs S.; Briant L.; Devaux C. Virology 2009, 393, 183–197. 10.1016/j.virol.2009.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Schwartz O.; Albert M. Nat. Rev. Microbiol. 2010, 8, 491–500. 10.1038/nrmicro2368. [DOI] [PubMed] [Google Scholar]; c Thiberville S.-D.; Moyen N.; Dupuis-Maguiraga L.; Nougairede A.; Gould E.; Roques P.; de Lamballerie X. Antiviral Res. 2013, 99, 345–370. 10.1016/j.antiviral.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Leyssen P.; Smadja J.; Rasoanaivo P.; Gurib-Fakim A.; Mahomoodally M.; Canard B.; Guillemot J.-C.; Litaudon M.; Gueritte F. In Novel Plant Bioresources: Applications in Food, Medicine, and Cosmetics; Gurib-Fakim A., Ed.; John Wiley & Sons: Hoboken, 2014; pp 151–161. [Google Scholar]; e Rashad A.; Mahalingam S.; Keller P. J. Med. Chem. 2014, 57, 1147–1166. 10.1021/jm400460d. [DOI] [PubMed] [Google Scholar]; f Parashar D.; Cherian S. BioMed Res. Int. 2014, 2014, 631–642. 10.1155/2014/631642. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Higgs S.; Vanlandingham D. Int. Health 2015, 7, 1–3. 10.1093/inthealth/ihu092. [DOI] [PubMed] [Google Scholar]; h Powers A. Res. Rep. Trop. Med. 2015, 6, 11–19. 10.2147/RRTM.S53698. [DOI] [Google Scholar]; i Petitdemange C.; Wauquier N.; Vieillard V. J. Allergy Clin. Immunol. 2015, 135, 846–855. 10.1016/j.jaci.2015.01.039. [DOI] [PubMed] [Google Scholar]; j Abdelnabi R.; Neyts J.; Delang L. Antiviral Res. 2015, 121, 59–68. 10.1016/j.antiviral.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M. Trends Parasitol. 2015, 31, 43–45. 10.1016/j.pt.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For perspectives and reviews on CHIKV vaccination, see:; a Weaver S.; Osorio J.; Livengood J.; Chen R.; Stinchcomb D. Expert Rev. Vaccines 2012, 11, 1087–1101. 10.1586/erv.12.84. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Salazar-Gonzalez J.; Angulo C.; Rosales-Mendoza S. Vaccine 2015, 33, 3650–3658. 10.1016/j.vaccine.2015.05.104. [DOI] [PubMed] [Google Scholar]; c Rezza G. Pathog. Global Health 2015, 109, 170–173. 10.1179/2047773215Y.0000000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Direct analogues of prostratin have also been shown to outperform the natural tigliane; see:Beans E.; Fournogerakis D.; Gauntlett C.; Heumann L.; Kramer R.; Marsden M.; Murray D.; Chun T.; Zack J.; Wender P. Proc. Natl. Acad. Sci. U. S. A. 2013, 110, 11698–11703. 10.1073/pnas.1302634110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For a review on bryostatin-based analogue strategies within the Wender group, see:Wender P.; Donnelly A.; Loy B.; Near K.; Staveness D. In Natural Products in Medicinal Chemistry; Hanessian S., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, 2014; pp 475–544. [Google Scholar]
- Wender P.; Verma V. Org. Lett. 2008, 10, 3331–3334. 10.1021/ol801235h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wender P.; Baryza J.; Brenner S.; DeChristopher B.; Loy B.; Schrier A.; Verma V. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 6721–6726. 10.1073/pnas.1015270108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothias-Scaglia L.-F.; Retailleau P.; Paolini J.; Pannecouque C.; Neyts J.; Dumontet V.; Roussi F.; Leyssen P.; Costa J.; Litaudon M. J. Nat. Prod. 2014, 77, 1505–1512. 10.1021/np500271u. [DOI] [PubMed] [Google Scholar]
- For recent evaluations of this behavior and key references detailing the role of northern functionality on tumor promotion, see:; a Kraft M.; Poudel Y.; Kedei N.; Lewin N.; Peach M.; Blumberg P.; Keck G. J. Am. Chem. Soc. 2014, 136, 13202–13208. 10.1021/ja5078188. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Andrews I.; Ketcham J.; Blumberg P.; Kedei N.; Lewin N.; Peach M.; Krische M. J. Am. Chem. Soc. 2014, 136, 13209–13216. 10.1021/ja507825s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Growth inhibitory screens of human cancer cell lines performed by the NCI with analogue 3 represent another example for which the analogue is 2–3 orders of magnitude more effective than the natural product. A more detailed account can be found in ref (19).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.