Abstract
Background
In prospective studies, relationship of self-reported consumption of dairy foods with risk of diabetes mellitus is inconsistent. Few studies have assessed dairy fat, using circulating biomarkers, and incident diabetes. We tested hypothesis that circulating fatty acid biomarkers of dairy fat, 15:0, 17:0, and t-16:1n-7, are associated with lower incident diabetes.
Methods and Results
Among 3,333 adults aged 30–75 years and free of prevalent diabetes at baseline, total plasma and erythrocyte fatty acids were measured in blood collected in 1989–90 (Nurses’ Health Study) and 1993–94 (Health Professionals Follow-Up Study). Incident diabetes through 2010 was confirmed by validated supplementary questionnaire based on symptoms, diagnostic tests, and medications. Risk was assessed using Cox proportional hazards, with cohort findings combined by meta-analysis. During mean±SD follow-up of 15.2±5.6 years, 277 new cases of diabetes were diagnosed. In pooled multivariate analyses adjusting for demographics, metabolic risk-factors, lifestyle, diet, and other circulating fatty acids, individuals with higher plasma 15:0 had 44% lower risk of diabetes (quartiles 4 vs. 1, HR=0.56, 95%CI=0.37–0.86; P-trend=0.01); higher plasma 17:0, 43% lower risk (HR=0.57, 95%CI=0.39–0.83, P-trend=0.01); and higher t-16:1n-7, 52% lower risk (HR=0.48, 95%CI=0.33–0.70, P-trend <0.001). Findings were similar for erythrocyte 15:0, 17:0, and t-16:1n-7, although with broader CIs that only achieved statistical significance for 17:0.
Conclusions
In two prospective cohorts, higher plasma dairy fatty acid concentrations were associated with lower incident diabetes. Results were similar for erythrocyte 17:0. Our findings highlight need to better understand potential health effects of dairy fat; and dietary and metabolic determinants of these fatty acids.
Keywords: dairy, fatty acid, biomarkers, diabetes mellitus
INTRODUCTION
For decades, dietary recommendations for dairy foods have been based on predicted health effects of isolated individual nutrients, such as for bone health (e.g., expected benefits of calcium and vitamin D), and cardiovascular disease (CVD) (e.g., expected harms of total fat and saturated fat). Based on these considerations, major dietary guidelines recommend low-fat dairy products and avoidance of whole-fat dairy.1 However, neither low-fat nor whole-fat dairy foods have major effects on traditional cardiovascular risk factors2 nor are appreciably linked to risk of coronary heart disease events.3 Interestingly, self-reported consumption of dairy foods has mixed associations with risk of type 2 diabetes, without consistently different results for low-fat vs. whole-fat products.4–6 For instance, several studies suggest that yogurt may be beneficial for diabetes,4–6 while some large studies4, 5, but not others,6 further suggest that cheese, which is highest in dairy fat, may also be protective.
Most prior investigations have relied on self-reported dietary information from questionnaires, which can be limited by errors in memory or subjective reporting, especially for dairy fat. In addition, dairy fat is consumed not only from whole foods such as milk, cheese, yogurt, and major dishes such as pizzas, but throughout the food supply in smaller amounts in mixed dishes, bakery products, and prepared foods. This may increase challenges of accurately capturing the intake of dairy fat from all sources. In comparison, circulating blood biomarkers of fatty acids may provide a more complete measure of dairy fat consumption. These include specific fatty acids obtained primarily from dairy fat including the odd-chain saturated fats pentadecanoic acid (15:0) and heptadecanoic acid (17:0),7, 8 and the natural ruminant trans fat trans-palmitoleate (t-16:1n-7).9 These fatty acids are not endogenously synthesized and are obtained only from diet, particularly dairy, making them reasonable biomarkers of dairy fat consumption.10, 11 In addition to their role as biomarkers, it has been hypothesized that these fatty acids could have direct metabolic effects, e.g., t-16:1n-7 could mimic experimentally-observed effects of adipose-produced c-16:n-7 to increase muscle glucose uptake and suppress hepatic de novo lipogenesis.12 Yet, few prospective studies have investigated these biomarkers and incident diabetes, with mixed results (see Discussion).
Thus, effects of dairy fat, and specific dairy fatty acids, on diabetes risk remain unclear. Understanding these relationships is crucial for informing dietary guidelines as well as designing additional interventional and experimental studies to explore biological pathways and mechanisms. We tested the hypothesis that total plasma and erythrocyte biomarkers of dairy fat, 15:0, 17:0, and t-16:1n-7, are associated with lower incidence of diabetes in two large prospective cohorts.
METHODS
Study design and population
This investigation was derived from two large US prospective studies, the Nurses’ Health Study (NHS) and Health Professionals Follow-Up Study (HPFS). Details of the cohorts and blood sample collection have been reported.13
We utilized previously measured fatty acid concentrations in stored blood used for nested case-control studies of incident CVD. Among NHS and HPFS subjects who provided blood samples and were free of prevalent CVD or cancer at the time of blood sampling, we measured total plasma and erythrocyte fatty acid concentrations in 3,499 men and women. Approximately 71% of participants were fasting at time of blood collection. For present analysis focused on incident diabetes, participants with diabetes at time of blood collection were excluded (n=166), leaving a total of 3,333 participants for analysis. This included 1,364 participants who developed CVD during follow-up, and 1,969 participants who did not develop CVD during follow-up. All analyses were first conducted in these two groups separately, and then combined after confirming that all associations were similar in magnitude and direction. This investigation was approved by the human subjects committees of all participating institutions, and all participants gave implied consent by providing blood samples and returning completed questionnaires.
Fatty acids
In both cohorts, fatty acid concentrations were measured in stored total plasma and erythrocyte samples in the same laboratory using gas-liquid chromatography.13, 14 Concentrations of individual circulating fatty acids were expressed as a percentage of total fatty acids in plasma or erythrocyte membranes. Technicians and laboratory personnel were unaware of participant clinical information including disease status. Forty fatty acids in plasma and erythrocytes were quantified (Table S1). For this investigation, primary biomarkers of interest were pentadecanoic acid (15:0), heptadecanoic acid (17:0), and trans-palmitoleic acid (t-16:1n-7). Myristic acid, a much less specific biomarker for dairy fat that is found in dairy, beef, and some plant oils15 and also endogenously synthesized by the liver (e.g., in response to excess carbohydrate intake), was secondarily analyzed. All the participants had levels of these four fatty acids above detectable limits.
Reproducibility of these fatty acids (intra-class correlation coefficients) in plasma and RBC over time has been reported.13, 16 Average inter-assay CVs for quality-controls in plasma were 16.0% for 15:0, 7.2% for 17:0, 10.4% for t-16:1n-7, and 30.8% for 14:0. Corresponding CVs in erythrocyte measurements were 22.4%, 11.3%, 14.2%, and 39.0%.
Incident diabetes
In both cohorts, participants were asked to report physician-diagnosed diabetes and the calendar year of diagnosis. To validate self-reports, a supplementary questionnaire was sent obtaining further information on symptoms, diagnosis and drug treatment. Suspected cases were labeled as confirmed if, according to the supplementary questionnaire, they met one or more of three National Diabetes Data Group criteria: (1) classic symptoms plus fasting glucose ≥140 mg/dL or random glucose ≥200 mg/dL; (2) at least two separate elevated plasma glucose levels (fasting ≥140, random ≥200, or 2-hour challenge ≥200 mg/dL); or (3) medical prescription of oral hypoglycemic agents or insulin. These diagnostic criteria were modified after Jun 1996 to incorporate lower diagnostic cut-offs for fasting glucose of 126 mg/dL. Validity of supplementary questionnaire for diagnosing diabetes was confirmed in subsets of participants in each cohort by comparison with direct review of medical records: 98% were confirmed in NHS; and 97% in HPFS.17, 18
Covariates and other risk factors
Data on medical history, risk factors and lifestyle were obtained in both cohorts via validated self-administered questionnaires (Supplemental Material). Usual alcohol use and dietary habits were assessed through validated semi-quantitative food frequency questionnaires (FFQ)s.
Statistical analysis
Fatty acid levels were evaluated in quartiles as indicator variables, and continuously comparing the medians of the highest vs. lowest quartile (87.5th vs. 12.5th percentiles). Linear trend was assessed by assigning the median value of each quartile to participants and evaluating this as a continuous variable. Cox proportional hazards evaluated associations of each fatty acid with incident diabetes through 2010, with time at-risk from blood collection until first event, death, or censoring at return of last questionnaire. Analyses were conducted separately in each cohort and then combined using fixed effects inverse-variance-weighted meta-analysis;19 with pooled P-for-trend calculated using generalized least squares trend (GLST) meta-analysis.20 Proportional hazards assumptions were tested by an interaction term with time and not rejected for any fatty acid except for 14:0 (P-interaction=0.01 in NHS, 0.01 in HPFS). Visualization of Schoenfeld residuals for each fatty acid, including 14:0, did not suggest a non-zero slope, suggesting that any potential time interaction was small.
Multivariable models were used to minimize potential confounding, with covariates included based on biological relevance, clinical interest, strength of associations with exposure or outcome, or percent change in the risk estimate of interest (>5%). For example, because dairy fat intake could be associated with other dietary habits that may also influence diabetes, we adjusted for fruits, vegetables, fish, meats, whole grains, sugar-sweetened beverages, polyunsaturated fat, calcium, and glycemic load. We also adjusted for biomarker levels of trans-18:1 and trans-18:2, as dairy fat can contain ruminant trans fats; and for palmitic acid (16:0) and stearic acid (18:0) synthesized by hepatic de novo lipogenesis, as these fatty acids can alter relative circulating proportions of trace fatty acids and are linked to insulin resistance and diabetes in experimental and prospective studies 13, 14, 21. Dairy consumption may causally influence BMI – e.g., yogurt and cheese22, 23 (the latter, if replacing refined carbohydrates) are linked to lower long-term weight gain and dairy intake reduces weight and fat mass in short-term trials24 – suggesting BMI could partly mediate any associations between dairy intake and diabetes. Individuals with different BMIs may also select different dairy foods, which could confound associations. We therefore separately considered BMI as a potential mediator and/or confounder in an additional multivariable model.
We explored effect modification by age and BMI using the Wald test for a multiplicative interaction term, with Bonferroni-corrected significance (4 fatty acids x 2 effect modifiers: α=0.05/8=0.006). To minimize misclassification due to exposure changes over time, we performed sensitivity analyses restricted to the first 8 years of follow-up. To minimize reverse causation due to unrecognized subclinical disease or presence of clinical risk factors at the time of fatty acid measurement, we also excluded cases occurring during the first 2 years. We evaluated self-reported yogurt, cheese, and dairy fat consumption as additional covariates to assess if the associations were independent of these dietary factors outside of their specific fatty acid contents. Multiple (10-fold) imputation was performed for missing continuous, and indicator variables for missing categorical covariates. Non-linear relationships were assessed by semi-parametric restricted cubic splines, excluding participants with fatty acid levels <0.5th or >99.5th percentiles to minimize influence of outliers. Analyses were conducted using SAS 9.3 (Cary, NC), two-tailed α=0.05.
RESULTS
At baseline, mean±SD age was 64.6±8.6 years among men (range: 48–83 years) and 60.4±6.3 years among women (range: 44–70 years) (Table 1, Table S2). About half of participants were overweight or obese; 1 in 4 had hypertension or family history of diabetes; 1 in 3 had hypercholesterolemia; and 1 in 12 men and 1 in 5 women were current smokers. Average total dairy consumption was 2.1 servings/day, with similar contributions from whole-fat (~45%) and low-fat (~55%) products.
Table 1.
Baseline characteristics of 3,333 US men and women with fatty acid measurements and free of prevalent diabetes in the Nurses’ Health Study (1990) and Health Professionals Follow Up Study (1994).*
| Women (n =1,864) | Men (n =1,469) | |
|---|---|---|
| Age, years | 60.4±6.3 | 64.6±8.6 |
| Age range, years | 44 to 70 | 48 to 83 |
| Race/Ethnicity (%) | ||
| Caucasian | 99.0 | 93.6 |
| African-Americans | 0.4 | 0.1 |
| Asian/Other | 0.6 | 6.3 |
| Weight status (%) | ||
| Normal (BMI <25 kg/m2) | 55.2 | 42.4 |
| Overweight (BMI 25 to <30 kg/m2) | 31.6 | 46.8 |
| Obese (BMI ≥30 kg/m2) | 13.2 | 10.8 |
| BMI, kg/m2 | 25.3±4.5 | 25.8±3.3 |
| Smoking status (%) | ||
| Current smoker | 21.8 | 8.4 |
| Past smoker | 38.7 | 49.1 |
| Never smoker | 39.5 | 42.5 |
| Physical activity, MET-hours/week | 16.0±18.9 | 36.4±39.0 |
| Medical History (%) | ||
| Hypertension | 22.9 | 24.8 |
| Hypercholesterolemia | 35.5 | 26.7 |
| Parental MI before 60 y | 22.6 | 12.5 |
| Family history of diabetes | 27.6 | 22.7 |
| Plasma fatty acids, % of total fatty acids† | ||
| 14:0 | 0.55 (0.25, 1.01) | 0.51 (0.25, 0.98) |
| 15:0 | 0.16 (0.11, 0.22) | 0.14 (0.10, 0.20) |
| 17:0 | 0.32 (0.26, 0.39) | 0.31 (0.24, 0.38) |
| t-16:1n-7 | 0.19 (0.13, 0.28) | 0.15 (0.10, 0.23) |
| Erythrocyte fatty acids, % of total fatty acids† | ||
| 14:0 | 0.27 (0.12, 0.69) | 0.25 (0.12, 0.56) |
| 15:0 | 0.12 (0.07, 0.19) | 0.11 (0.07, 0.17) |
| 17:0 | 0.39 (0.31, 0.61) | 0.36 (0.29, 0.50) |
| t-16:1n-7 | 0.16 (0.11, 0.23) | 0.13 (0.09, 0.18) |
| Dietary factors, servings/day | ||
| Total dairy | 2.1±1.5 | 2.1±1.6 |
| Whole-fat dairy‡ | 0.95±1.17 | 0.97±1.28 |
| Low-fat dairy§ | 1.2±1.0 | 1.1±1.1 |
| Processed meats | 0.21±0.29 | 0.32±0.42 |
| Unprocessed meats | 0.90±0.49 | 0.94±0.55 |
| Fruits and vegetables | 5.5±3.0 | 5.9±3.2 |
| Fish | 0.31±0.29 | 0.27±0.25 |
| Alcohol | 0.44±0.79 | 0.98±1.28 |
Values are mean±SD for continuous variables and percent for categorical variables. Missing values range: 0.0% for age to 8.6% for parental history of MI (women), and 0.0% for age to 4.6% for smoking (men).
Fatty acid concentrations are reported as medians (12.5th , 87.5th percentiles, representing midpoint of bottom and top quartiles).
Whole milk, ice cream, butter, cream, sour cream, cream cheese, and other cheese.
Low-fat or skim milk, yogurt, and cottage cheese.
Within each circulating lipid compartment (plasma, erythrocyte), the different fatty acid biomarkers were modestly intercorrelated: in plasma, adjusted intercorrelations (r) ranged from 0.10 to 0.59 (Table S3); and in erythrocytes, from 0.47 to 0.69 (Table S4). As expected for these different lipid compartments, correlations between plasma and erythrocyte fatty acid biomarkers were moderate: 0.32 for 15:0, 0.44 for 17:0, 0.42 for t-16:1n-7, and 0.24 for 14:0. Partial correlations with self-reported dairy fat consumption were modest for plasma 15:0 (r=0.29), 17:0 (r=0.21), and t-16:1n-7 (r=0.22); and weaker for 14:0 (r=0.11) (Table S3; Figure S1); and similar but somewhat lower for erythrocyte fatty acids (Table S4). These correlations were similar among participants who were fasting at time of blood collection (data not shown).
Plasma fatty acid biomarkers and risk of diabetes
During mean±SD follow-up of 15.2±5.6 years, 277 new cases of diabetes mellitus were diagnosed. After adjustment for demographics, metabolic risk factors, lifestyle, dietary habits, and other circulating fatty acids, compared to the lowest quartile, individuals in the highest quartile of plasma 15:0 had 44% lower risk of diabetes (HR=0.56, 95%CI=0.37–0.86; P-trend=0.01); of plasma 17:0, 43% lower risk (HR=0.57, 95%CI=0.39–0.83, P-trend<0.01); and of t-16:1n-7, 52% lower risk (HR=0.48, 95%CI=0.33–0.70, P-trend <0.001) (Table 2). Findings were generally similar in magnitude and direction in the two separate cohorts, without statistically significant effect modification by sex. 14:0 was not significantly associated with diabetes (P-trend=0.36).
Table 2.
Risk of incident diabetes according to plasma fatty acid biomarkers of dairy fat consumption among 3,333 men and women in the NHS (N=184 cases), HPFS (N=93 cases), and both cohorts combined.
| Cohort-specific fatty acid quartiles
|
|||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | P for trend* | |
| 15:0, NHS | |||||
| % of total FA, median | 0.11 | 0.14 | 0.17 | 0.22 | |
| No. cases | 33 | 49 | 55 | 47 | |
| Person-months | 95,002 | 89,388 | 98,482 | 95,695 | |
| Multivariable HR (95%CI)† | Reference | 1.26 (0.80–2.00) | 0.89 (0.56–1.43) | 0.60 (0.36–1.01) | 0.01 |
| 15:0, HPFS | |||||
| % of total FA, median | 0.10 | 0.13 | 0.15 | 0.20 | |
| No. cases | 18 | 14 | 34 | 27 | |
| Person-months | 55,559 | 51,928 | 58,919 | 61,348 | |
| Multivariable HR (95%CI) | Reference | 0.63 (0.31–1.30) | 1.12 (0.59–2.12) | 0.49 (0.23–1.04) | 0.09 |
| 15:0, pooled | Reference | 1.03 (0.70–1.52) | 0.96 (0.66–1.41) | 0.56 (0.37–0.86) | 0.01 |
| 17:0, NHS | |||||
| % of total FA, median | 0.26 | 0.30 | 0.33 | 0.39 | |
| No. cases | 59 | 47 | 44 | 34 | |
| Person-months | 94,103 | 92,604 | 94,510 | 97,350 | |
| Multivariable HR (95%CI) | Reference | 0.79 (0.53–1.19) | 0.75 (0.49–1.14) | 0.50 (0.31–0.81) | 0.01 |
| 17:0, HPFS | |||||
| % of total FA, median | 0.24 | 0.29 | 0.32 | 0.38 | |
| No. cases | 23 | 24 | 17 | 29 | |
| Person-months | 56,574 | 55,212 | 57,264 | 58,704 | |
| Multivariable HR (95%CI) | Reference | 0.80 (0.43–1.47) | 0.48 (0.25–0.94) | 0.69 (0.38–1.26) | 0.18 |
| 17:0, pooled | Reference | 0.79 (0.57–1.11) | 0.66 (0.46–0.94) | 0.57 (0.39–0.83) | <0.01 |
| t-16:1n-7, NHS | |||||
| % of total FA, median | 0.13 | 0.17 | 0.21 | 0.28 | |
| No. cases | 54 | 38 | 44 | 48 | |
| Person-months | 90,854 | 83,770 | 95,906 | 108,037 | |
| Multivariable HR (95%CI) | Reference | 0.79 (0.51–1.22) | 0.69 (0.46–1.05) | 0.48 (0.30–0.76) | 0.002 |
| t-16:1n-7, HPFS | |||||
| % of total FA, median | 0.10 | 0.13 | 0.16 | 0.22 | |
| No. cases | 27 | 17 | 23 | 26 | |
| Person-months | 57,935 | 52,101 | 60,989 | 56,729 | |
| Multivariable HR (95%CI) | Reference | 0.66 (0.35–1.24) | 0.64 (0.36–1.16) | 0.49 (0.26–0.90) | 0.03 |
| t-16:1n-7, pooled | Reference | 0.75 (0.52–1.07) | 0.67 (0.48–0.94) | 0.48 (0.33–0.70) | <0.001 |
| 14:0, NHS | |||||
| % of total FA, median | 0.24 | 0.44 | 0.64 | 0.98 | |
| No. cases | 29 | 46 | 38 | 71 | |
| Person-months | 94,398 | 93,023 | 93,812 | 97,334 | |
| Multivariable HR (95%CI) | Reference | 1.55 (0.97–2.49) | 0.95 (0.57–1.57) | 0.93 (0.55–1.57) | 0.35 |
| 14:0, HPFS | |||||
| % of total FA, median | 0.25 | 0.40 | 0.57 | 0.90 | |
| No. cases | 10 | 22 | 27 | 34 | |
| Person-months | 53,011 | 56,008 | 56,340 | 62,395 | |
| Multivariable HR (95%CI) | Reference | 1.98 (0.90–4.33) | 1.85 (0.83–4.15) | 1.50 (0.56–3.98) | 0.92 |
| 14:0, pooled | Reference | 1.65 (1.10–2.48) | 1.15 (0.75–1.76) | 1.03 (0.65–1.64) | 0.36 |
Computed within each cohort by assigning median level in each quartile to participants and evaluating this variable continuously. Pooled P-for-trend was calculated using generalized least squares trend (GLST) meta-analysis.20
Adjusted for age (years), race (white, nonwhite), smoking status (never, former, current, missing), physical activity (METS/week), alcohol (servings/day), family history of diabetes (yes, no, missing), parental history of MI (yes, no, missing), hypercholesterolemia (yes, no), hypertension (yes, no), menopausal status in NHS (pre, post), postmenopausal hormone use in NHS (no, yes, missing), and consumption of fish (servings/day), processed meats (servings/day), unprocessed meats (servings/day), fruits (servings/day), vegetables (servings/day), whole grains (g/day), coffee (servings/day), sugar-sweetened beverages (servings/day), glycemic load (continuous), dietary calcium (mg/day), polyunsaturated fat (g/day), total energy (kcal/day), and plasma trans-18:1, trans-18:2, 16:0, and 18:0 (each as % of total fatty acids).
Evaluated continuously, 15:0 was associated with 38% lower risk (pooled HR=0.62, 95%CI=0.46–0.85); 17:0, with 32% lower risk (HR=0.68, 95%CI=0.50–0.91); and t-16:1n-7, with 46% lower risk (HR=0.54, 95%CI=0.40–0.73) (Table 3). Similar to categorical analyses, plasma 14:0 was not associated with diabetes risk (HR=0.82, 95%CI=0.60–1.11). Because BMI is a major predictor of insulin resistance and diabetes, we conducted further multivariable-adjusted analyses to evaluate the potential independent associations between BMI and dairy fatty acid biomarker levels at baseline. In both cohorts combined, higher 15:0 was not associated with BMI (Q1 vs. Q4: 25.3 vs. 25.3 kg/m2, P–trend=0.97) (Table S2). Higher 17:0 (25.8 vs. 25.0, P-trend<0.001) and t-16:1n-7 (25.9 vs. 25.2, P-trend<0.001) were associated with lower BMI, although absolute differences were modest. 14:0 was associated with higher BMI (24.9 vs. 25.9, P-trend<0.001). After further adjustment for baseline BMI as a potential mediator or confounder, associations with incident diabetes were slightly attenuated for 17:0 and not appreciably altered for other fatty acids (Table 3). Results were also similar after further multivariable adjustment for conjugated linoleic acid, another ruminant-derived fatty acid (data not shown).
Table 3.
Risk of incident diabetes according to plasma fatty acid biomarkers of dairy fat consumption, evaluated continuously, among 3,333 men and women in the NHS (N=184 cases), HPFS (N=93 cases), and both cohorts combined.
| Multivariable HR (95%CI) standardized to difference between midpoints of highest vs. lowest quartiles (87.5th minus 12.5th percentiles)
|
||||
|---|---|---|---|---|
| NHS | HPFS | Pooled | P–value | |
| 15:0 | range=0.10* | range=0.10 | ||
| Multivariable HR (95%CI)† | 0.63 (0.42–0.92) | 0.61 (0.36–1.03) | 0.62 (0.46–0.85) | <0.01 |
| + BMI‡ | 0.60 (0.40–0.89) | 0.73 (0.43–1.25) | 0.64 (0.47–0.89) | 0.01 |
| 17:0 | range=0.13 | range=0.14 | ||
| Multivariable HR (95%CI) | 0.71 (0.49–1.04) | 0.63 (0.39–1.02) | 0.68 (0.50–0.91) | 0.01 |
| + BMI | 0.76 (0.52–1.10) | 0.69 (0.42–1.11) | 0.73 (0.55–0.99) | 0.04 |
| t-16:1n-7 | range=0.15 | range=0.12 | ||
| Multivariable HR (95%CI) | 0.52 (0.36–0.76) | 0.58 (0.36–0.93) | 0.54 (0.40–0.73) | <0.001 |
| + BMI | 0.51 (0.36–0.74) | 0.61 (0.37–0.99) | 0.54 (0.41–0.73) | <0.001 |
| 14:0 | range=0.74 | range=0.65 | ||
| Multivariable HR (95%CI) | 0.92 (0.64–1.32) | 0.61 (0.35–1.09) | 0.82 (0.60–1.11) | 0.19 |
| + BMI | 0.90 (0.63–1.31) | 0.75 (0.42–1.32) | 0.85 (0.63–1.16) | 0.32 |
The difference in % of total fatty acids between midpoint of highest vs. lowest quartile.
Multivariable adjustments as in Table 2.
Further adjusted for BMI (kg/m2) as a potential mediator or confounder of the association.
We further examined the potential influence of each individual covariate in the model. Adjustment for fatty acid biomarkers of de novo lipogenesis, 16:0 and 18:0,25, 26 had the greatest effect on plasma 15:0, which was no longer significantly associated with diabetes when 16:0 and 18:0 were removed as covariates (continuous HR=1.10, 95%CI=0.85–1.42). Conversely, removal of these fatty acid covariates had smaller influence on associations with diabetes for plasma 17:0 (HR=0.67, 95%CI=0.51–0.89) or t-16:1n-7 (HR=0.68, 95%CI=0.51–0.92). With simultaneous mutual adjustment for all three dairy fat biomarkers, associations were nonsignificant for plasma 15:0 (continuous HR=0.83, 95%CI=0.53–1.29) and 17:0 (HR=0.93, 95%CI=0.64–1.36), and unchanged for t-16:1n-7 (HR=0.59, 95%CI=0.43–0.80).
Semi-parametric analyses
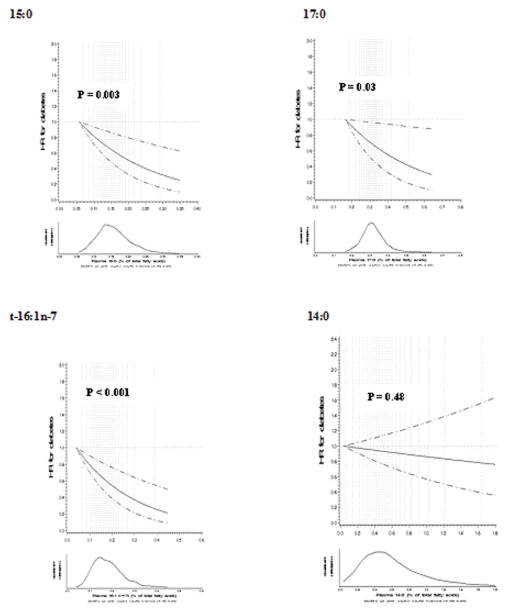
Restricted cubic splines demonstrated an inverse linear relationship for plasma 15:0, 17:0, and t-16:1n-7 and incident diabetes (Figure 1). Plasma 14:0 was not associated with risk (P-linearity=0.48).
Figure 1.
Semi-parametric multivariable-adjusted associations of plasma 15:0, 17:0, t-16:1n-7, and 14:0 with incident diabetes among 3,333 US men and women in two separate cohorts, evaluated using restricted cubic splines with covariate adjustments as in Table 2. Solid and dashed lines represent hazard ratios (HRs) and 95% confidence intervals, respectively; dotted vertical lines represent 21 knots. P-values for linear association are shown. P-values for nonlinearity were nonsignificant in all analyses; the automatic selection process did not identify any significant spline variables.
Erythrocyte fatty acid biomarkers
When we evaluated erythrocyte fatty acids, lower incidence of diabetes was seen with higher levels of 17:0 (HR=0.54, 95%CI=0.34–0.87, P-trend <0.001), and t-16:1n-7 (HR=0.78, 95%CI=0.51–1.18, P-trend=0.05). (Table S5). Erythrocyte 15:0 was not significantly associated with incident diabetes (HR=0.83, 95%CI=0.55–1.25, P-trend=0.63), and erythrocyte 14:0 was associated with a nonsignificant trend toward higher risk (HR=1.56, 95%CI=0.98–2.49, P-trend=0.13). The latter association was driven by findings in HPFS.
When these erythrocyte fatty acids were evaluated continuously, similar findings were seen for 15:0 and 17:0; t-16:1n-7 was no longer associated with lower risk (HR=0.80, 95%CI=0.57–1.13); and 14:0 was associated with 36% higher risk (HR=1.36, 95%CI=1.11–1.67) (Table S6). As with the categorical analyses, the latter association was driven by findings in HPFS only (data not shown). As seen with plasma 17:0, further adjustment for BMI as a mediator or confounder partly attenuated the observed inverse association for erythrocyte 17:0. Among other fatty acid covariates in the multivariable model, removal of adjustment for fatty acid biomarkers of de novo lipogenesis had little effect on these risk estimates (data not shown).
Sensitivity analyses
When we evaluated potential effect modification, the associations of plasma and erythrocyte fatty acids with incident diabetes did not significantly vary according to differences in age or BMI (P > Bonferroni-corrected α=0.006 each). Associations were also similar after further adjustment for self-reported consumption of yogurt, cheese, or dairy fat (Table S7). For example, compared with the association of plasma t-16:1n-7 with diabetes in the main model (continuous HR=0.54, 95%CI=0.40–0.73), findings were not appreciably altered by additional adjustment for reported consumption of yogurt (HR=0.55, 95%CI=0.41–0.73), cheese (HR=0.53, 95%CI=0.40–0.71) or dairy fat (HR=0.52, 95%CI=0.38–0.70). Results were also generally similar after excluding cases occurring within the first 2 years; and restricting to first 8 years of follow-up (Table S8).
DISCUSSION
In two separate cohorts of US men and women, three plasma biomarkers of dairy fat, 15:0, 17:0 and trans-16:1 n-7, were associated with lower risk of incident diabetes. Findings were generally similar for erythrocyte fatty acids, although with smaller and not always significant magnitudes of association. These results provide new evidence on associations of dairy-derived circulating fatty acids and risk of diabetes.
Our findings suggest that either these dairy fatty acids themselves or other correlated factors in dairy fat could reduce risk of diabetes. For instance, short- and medium- chain saturated fats, vitamin D (a fat-soluble vitamin), omega-3 fats, or gangliosides in dairy fat could each play a role27. The magnitudes of our associations, linear dose-response, independence from established diabetes risk factors and other dietary habits, and consistency of findings across sensitivity analyses argue against residual confounding as the sole explanation for our findings. These results highlight the need for intensive experimental and mechanistic evaluation on health effects of dairy fat; as well as dietary and potential metabolic determinants of these circulating fatty acids, including their sensitivity to indicators of de novo lipogenesis.
In observational analyses, self-reported consumption of high-fat dairy foods such as cheese have beneficial or neutral associations with diabetes;4–6 while dairy fat consumption and dairy fat biomarkers correlate with improved hepatic and systemic insulin sensitivity and lower hepatic steatosis.28, 29 When we included all three plasma fatty acids in one model, only t-16:1n-7 retained independent significant association with diabetes. We have hypothesized12 that benefits could be due to isomeric similarities between t-16:1 n-7, a natural ruminant trans fat, and cis-16:1n-7 that when derived from adipose tissue through de novo lipogenesis may act in feedback loops to reduce hepatic fat synthesis and increase muscle insulin sensitivity.30, 31 We wonder whether t-16:1n-7, consumed in the diet, might have similar effects.
15:0, 17:0, and t-16:1n-7 are predominantly obtained from diet and not synthesized, making reverse causation due to abnormal metabolism at baseline unlikely. One small study (n=12) found that providing a mixed oil high in trans-18:1 (vaccenic acid) also increased serum t-16:1n-7; while human peripheral blood mononuclear cells cultured with t-18:1 incorporated small amounts of t-16:1n-7 into cellular lipids.32 These findings suggest small amounts of t-16:1n-7 could derive from partial beta-oxidation (endogenous chain shortening) of t-18:1; labelled fatty acid tracer studies are needed to confirm this result. In the present study, among all dietary factors, circulating t-16:1n-7 correlated most with dairy fat rather than sources of industrial trans fat (vaccenic acid), suggesting that direct dairy consumption remains a major source. Genome-wide association studies have not identified significant genetic determinants of circulating t-16:1n-7,33 further suggesting absence of strong endogenous influences. Body weight and insulin resistance produce no known effects on levels of these circulating fatty acids, and findings were generally similar following adjustment for BMI. Reverse causation could play a role in behaviors, for example if higher-risk participants with subclinical prediabetes elected to avoid whole-fat dairy products. Yet, the prospective nature of our analysis minimizes this possibility; and 15:0, 17:0 and t-16:1n-7 also remained inversely associated with diabetes after excluding cases occurring in the first two years.
In these cohorts, plasma fatty acid biomarkers correlated more strongly with dairy fat intake than did erythrocyte biomarkers, and were also more strongly inversely associated with diabetes. Correlations of all these fatty acids with self-reported dairy consumption were modest. This may be due to random or systematic errors in self-reported diet, variability of these fatty acids in different dairy foods,34 laboratory error in fatty acid measures, within-person variation in diet or circulating fatty acids, or other unknown influences on bioavailability, metabolism, or incorporation into specific lipid-compartments of these fatty acids. Notably, dairy fat is consumed not just as whole foods (milk, cheese, yogurt, butter) but mixed into numerous foods, dishes, and recipes as major and minor ingredients. In our analysis of NHANES data based on detailed, product-specific dietary recalls (2005–2012), 51% of cheese and 30% of total dairy is consumed in mixed dishes, especially grain products but also mixed with meats, sweets, vegetables, and eggs (data not shown). FFQs that estimate dairy fat intakes from whole foods and major mixed sources (e.g., pasta dishes, burritos, pizza) may not accurately capture quantities in these mixed dishes nor the multitude of smaller amounts in many other products. Thus, the observed modest correlations of 15:0, 17:0 and t-16:1n-7 with self-reported dairy fat may appropriately reflect the challenges in fully estimating dairy fat from questionnaires. 14:0 had weak correlations with self-reported intakes of dairy fat, meat, and carbohydrate-rich foods (the latter being drivers of endogenous hepatic fatty acid synthesis). Plasma 14:0 was unassociated with diabetes risk; erythrocyte 14:0 was associated with increased risk in one cohort. Higher laboratory imprecision in 14:0 measures could limit ability to detect associations. Trend toward higher risk could also relate to derivation of 14:0 from endogenous de novo lipogenesis, a correlate of insulin resistance and diabetes.21 This could also be a chance finding, and should be interpreted with caution. Recent analysis in EPIC identified a positive association between plasma phospholipid 14:0 and incident diabetes.21 Our findings support the need for further studies on 14:0, especially on endogenous metabolic determinants of its circulating levels.
In pooled analyses of observational studies, self-reported intakes of total dairy, low-fat dairy, whole-fat dairy, milk, and cheese have each shown mixed (beneficial or neutral) associations with diabetes; yogurt consumption more consistently associated with lower risk.4–6 Interestingly, none of these studies identified harms of dairy, including whole-fat dairy, for diabetes. A handful of prior prospective studies have evaluated circulating biomarkers of dairy fat consumption in relation to incident diabetes (Table 4). Only two 12, 35 reported on t-16:1 n-7: both found substantially lower risk, consistent with our findings. Three of 5 prior studies evaluating 15:0 and 2 of 3 studies evaluating 17:0 observed significant inverse associations with diabetes; none observed higher risk. In the present analysis, t-16:1 n-7 remained associated with diabetes after adjustment for the other dairy fat biomarkers, suggesting it may be particularly relevant for further investigation of its determinants and biologic effects. Our findings build upon and extend prior studies by evaluating these fatty acid biomarkers, including t-16:1 n-7, in total plasma and erythrocytes in two large, well-established cohorts of US men and women and including adjustment for a range of potential confounding factors.
Table 4.
Summary of prior prospective studies evaluating fatty acid biomarkers of dairy fat and incidence of diabetes.
| Study | Design | Lipid compartment | No. of cases | Multivariable-Adjusted Hazard Ratio (95%CI)
|
|||
|---|---|---|---|---|---|---|---|
| 15:0 | 17:0 | t-16:1n-7 | 14:0 | ||||
| CHS12* | Prospective cohort | Plasma phospholipid | 304 | NS | NS | 0.38 (0.24–0.62)* | NS† |
| EPIC- Interact21 | Nested case- subcohort | Plasma phospholipid | 12,403 | 0.79 (0.73–0.85)‡ | 0.67 (0.63–0.71)‡ | - | 1.15 (1.09–1.22)‡ |
| MCCS36 | Case-cohort | Plasma phospholipid | 364 | 0.26 (0.17–0.40)§ | - | - | - |
| MESA35 | Prospective cohort | Plasma phospholipid | 205 | NS | - | 0.52 (0.32–0.85)* | NS |
| VIP37 | Nested case- control study | Erythrocyte membrane | 237 | 0.65 (0.50–0.85)¶ | 0.47 (0.35–0.63)¶ | - | 1.25 (1.01–1.53)¶ |
Comparing the highest vs. lowest quintile of levels.
Separately reported by Ma et al., Am J Clin Nutr 2015;101:153–63.
Per 1 SD increment in levels.
Odds ratio comparing the highest vs. lowest quintile of levels.
Odds ratio per 1 SD increment in levels.
NS=not significant, CHS=Cardiovascular Health Study, EPIC=European Prospective Investigation into Cancer and Nutrition, MCCS=Melbourne Collaborative Cohort Study, MESA=Multi-Ethnic Study of Atherosclerosis, VIP=Vasterbotten Intervention Programme. Previous smaller subset reports from EPIC are not shown.
Our investigation has several strengths. We assessed circulating fatty acids, which may more fully capture all dietary sources of dairy fat. We evaluated two separate lipid compartments, in which generally similar associations support validity of the findings, especially in light of their potential differential temporal and metabolic responses to dietary fats intake. The prospective cohort design minimized reverse causation, selection bias, and recall bias. Inclusion of two separate cohorts including both men and women provided for replication as well as pooling of cohort findings.
Potential limitations should be considered. Misclassification due to laboratory error and within-person changes over time would attenuate results, making it more difficult to detect true associations. Despite this, we were able to detect associations with diabetes, suggesting true relationships could be even stronger. Residual confounding due to unmeasured or mismeasured covariates cannot be excluded. However, magnitudes of associations and linear dose-responses suggest residual confounding is unlikely to fully explain the results. These cohorts comprised educated US health professionals. Yet, although absolute levels of fatty acids and rates of diabetes may vary by education, geography, or race/ethnicity, we do not suspect that biological effects of these fatty acids should differ on a relative scale across different populations. In summary, we found that plasma 15:0, 17:0, and trans-16:1 n-7 were associated with lower incidence of diabetes in two separate prospective cohort studies.
Supplementary Material
Clinical Perspectives.
Diabetes is a major preventable cause of morbidity and mortality. Growing evidence suggests dairy foods, and even dairy fat, could reduce risk of diabetes. Most prior studies utilized self-reported dietary questionnaires, which could be prone to subjective reporting and might not fully capture dairy fat intake, particularly from mixed dishes. Circulating fatty acid biomarkers, such as odd-chain saturated fats (15:0, 17:0) and certain natural trans fats (t-16:1n-7), may provide more objective measures of dairy fat intake. We prospectively evaluated the relationship between plasma concentrations of 15:0, 17:0, and t-16:1n-7 and new-onset diabetes among 3,333 men and women aged 30–75 years in two separate US cohorts. Incident diabetes was confirmed by validated methods based on symptoms, diagnostic tests, and medical therapy. After adjusting for demographics, metabolic risk factors, lifestyle, diet, and other circulating fatty acids, we found that all three of these circulating fatty acids were associated with substantially lower risk of diabetes, with about 45–50% lower risk comparing the top vs. bottom quartiles of their levels. Potential limitations include misclassification of fatty acid measurements due to laboratory error and within-person variation over time, which would attenuate results toward the null; and residual confounding due to unmeasured or mismeasured covariates, although this would seem unlikely to fully explain the magnitudes of observed associations. Our findings suggest that dairy foods, and specifically dairy fat, could help prevent diabetes, highlighting the need for intensive experimental and mechanistic evaluation on health effects of dairy fat as well as determinants of these circulating fatty acids.
Acknowledgments
MYY, FBH, DM participated in project conception and development of research methods; FBH and DM obtained funding and provided oversight; MYY, PS and EJO analyzed data and performed analysis; MYY and DM drafted the manuscript; and WCW, KMR, HC, EJO, FBH, DM provided critical feedback on revisions and other intellectual input.
Funding Sources: This study was supported by the National Institute of Environmental Health Sciences, NIH (R01-ES014433 and ES013692), as well as NIH research grants HL-60712, HL-034594, HL-088521, HL-35464, DK-58845, CA-186107, CA-49449, CA-87969, CA-55075, and CA-167552. Dr. Yakoob was supported by a Harvard University Scholarship, Founders Affiliate American Heart Association Pre-Doctoral Training Fellowship 2013–14, and Harvard Lown Cardiovascular Research Foundation Scholarship.
Footnotes
Disclosures: Dr. Mozaffarian reports ad hoc honoraria from Amarin, Astra Zeneca, Haas Avocado Board, Bunge, and Life Sciences Research Organization. Harvard University holds a patent, listing Dr. Mozaffarian among co-inventors, for use of trans-palmitoleic acid to prevent and treat insulin resistance, type 2 diabetes, and related conditions. Other authors declare no conflict of interest.
References
- 1.Dietary Guidelines Advisory Committee. Scientific Report of the 2015 Dietary Guidelines Advisory Committee. 2015. [Google Scholar]
- 2.Benatar JR, Sidhu K, Stewart RA. Effects of high and low fat dairy food on cardio-metabolic risk factors: a meta-analysis of randomized studies. PLoS One. 2013;8:e76480. doi: 10.1371/journal.pone.0076480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soedamah-Muthu SS, Ding EL, Al-Delaimy WK, Hu FB, Engberink MF, Willett WC, Geleijnse JM. Milk and dairy consumption and incidence of cardiovascular diseases and all-cause mortality: dose-response meta-analysis of prospective cohort studies. Am J Clin Nutr. 2011;93:158–71. doi: 10.3945/ajcn.2010.29866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aune D, Norat T, Romundstad P, Vatten LJ. Dairy products and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis of cohort studies. Am J Clin Nutr. 2013;98:1066–83. doi: 10.3945/ajcn.113.059030. [DOI] [PubMed] [Google Scholar]
- 5.Sluijs I, Forouhi NG, Beulens JW, van der Schouw YT, Agnoli C, Arriola L, Balkau B, Barricarte A, Boeing H, Bueno-de-Mesquita HB, Clavel-Chapelon F, Crowe FL, de Lauzon-Guillain B, Drogan D, Franks PW, Gavrila D, Gonzalez C, Halkjaer J, Kaaks R, Moskal A, Nilsson P, Overvad K, Palli D, Panico S, Quiros JR, Ricceri F, Rinaldi S, Rolandsson O, Sacerdote C, Sanchez MJ, Slimani N, Spijkerman AM, Teucher B, Tjonneland A, Tormo MJ, Tumino R, van der AD, Sharp SJ, Langenberg C, Feskens EJ, Riboli E, Wareham NJ, InterAct C. The amount and type of dairy product intake and incident type 2 diabetes: results from the EPIC-InterAct Study. Am J Clin Nutr. 2012;96:382–90. doi: 10.3945/ajcn.111.021907. [DOI] [PubMed] [Google Scholar]
- 6.Chen M, Sun Q, Giovannucci E, Mozaffarian D, Manson JE, Willett WC, Hu FB. Dairy consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. BMC Med. 2014;12:215. doi: 10.1186/s12916-014-0215-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smedman AE, Gustafsson IB, Berglund LG, Vessby BO. Pentadecanoic acid in serum as a marker for intake of milk fat: relations between intake of milk fat and metabolic risk factors. Am J Clin Nutr. 1999;69:22–9. doi: 10.1093/ajcn/69.1.22. [DOI] [PubMed] [Google Scholar]
- 8.Wolk A, Furuheim M, Vessby B. Fatty acid composition of adipose tissue and serum lipids are valid biological markers of dairy fat intake in men. J Nutr. 2001;131:828–33. doi: 10.1093/jn/131.3.828. [DOI] [PubMed] [Google Scholar]
- 9.Micha R, King IB, Lemaitre RN, Rimm EB, Sacks F, Song X, Siscovick DS, Mozaffarian D. Food sources of individual plasma phospholipid trans fatty acid isomers: the Cardiovascular Health Study. Am J Clin Nutr. 2010;91:883–93. doi: 10.3945/ajcn.2009.28877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brevik A, Veierod MB, Drevon CA, Andersen LF. Evaluation of the odd fatty acids 15:0 and 17:0 in serum and adipose tissue as markers of intake of milk and dairy fat. Eur J Clin Nutr. 2005;59:1417–22. doi: 10.1038/sj.ejcn.1602256. [DOI] [PubMed] [Google Scholar]
- 11.Hirahatake KM, Slavin JL, Maki KC, Adams SH. Associations between dairy foods, diabetes, and metabolic health: potential mechanisms and future directions. Metabolism. 2014;63:618–27. doi: 10.1016/j.metabol.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mozaffarian D, Cao H, King IB, Lemaitre RN, Song X, Siscovick DS, Hotamisligil GS. Trans-palmitoleic Acid, metabolic risk factors, and new-onset diabetes in U.S. adults: a cohort study. Ann Intern Med. 2010;153:790–9. doi: 10.1059/0003-4819-153-12-201012210-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yakoob MY, Shi P, Hu FB, Campos H, Rexrode KM, Orav EJ, Willett WC, Mozaffarian D. Circulating biomarkers of dairy fat and risk of incident stroke in U.S. men and women in 2 large prospective cohorts. Am J Clin Nutr. 2014;100:1437–47. doi: 10.3945/ajcn.114.083097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun Q, Ma J, Campos H, Hu FB. Plasma and erythrocyte biomarkers of dairy fat intake and risk of ischemic heart disease. Am J Clin Nutr. 2007;86:929–37. doi: 10.1093/ajcn/86.4.929. [DOI] [PubMed] [Google Scholar]
- 15.Hu FB, Stampfer MJ, Manson JE, Ascherio A, Colditz GA, Speizer FE, Hennekens CH, Willett WC. Dietary saturated fats and their food sources in relation to the risk of coronary heart disease in women. Am J Clin Nutr. 1999;70:1001–8. doi: 10.1093/ajcn/70.6.1001. [DOI] [PubMed] [Google Scholar]
- 16.Kotsopoulos J, Tworoger SS, Campos H, Chung FL, Clevenger CV, Franke AA, Mantzoros CS, Ricchiuti V, Willett WC, Hankinson SE, Eliassen AH. Reproducibility of plasma and urine biomarkers among premenopausal and postmenopausal women from the Nurses' Health Studies. Cancer Epidemiol Biomarkers Prev. 2010;19:938–46. doi: 10.1158/1055-9965.EPI-09-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manson JE, Rimm EB, Stampfer MJ, Colditz GA, Willett WC, Krolewski AS, Rosner B, Hennekens CH, Speizer FE. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet. 1991;338:774–8. doi: 10.1016/0140-6736(91)90664-b. [DOI] [PubMed] [Google Scholar]
- 18.Hu FB, Manson JE, Stampfer MJ, Colditz G, Liu S, Solomon CG, Willett WC. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345:790–7. doi: 10.1056/NEJMoa010492. [DOI] [PubMed] [Google Scholar]
- 19.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 20.Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. Stata J. 2006;6:40–57. [Google Scholar]
- 21.Forouhi NG, Koulman A, Sharp SJ, Imamura F, Kroger J, Schulze MB, Crowe FL, Huerta JM, Guevara M, Beulens JW, van Woudenbergh GJ, Wang L, Summerhill K, Griffin JL, Feskens EJ, Amiano P, Boeing H, Clavel-Chapelon F, Dartois L, Fagherazzi G, Franks PW, Gonzalez C, Jakobsen MU, Kaaks R, Key TJ, Khaw KT, Kuhn T, Mattiello A, Nilsson PM, Overvad K, Pala V, Palli D, Quiros JR, Rolandsson O, Roswall N, Sacerdote C, Sanchez MJ, Slimani N, Spijkerman AM, Tjonneland A, Tormo MJ, Tumino R, van der AD, van der Schouw YT, Langenberg C, Riboli E, Wareham NJ. Differences in the prospective association between individual plasma phospholipid saturated fatty acids and incident type 2 diabetes: the EPIC-InterAct case-cohort study. Lancet Diabetes Endocrinol. 2014;2:810–8. doi: 10.1016/S2213-8587(14)70146-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364:2392–404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith JD, Hou T, Ludwig DS, Rimm EB, Willett WC, Hu FB, Mozaffarian D. Changes in intake of protein foods, carbohydrate amount and quality, and long-term weight change: results from 3 prospective cohorts. Am J Clin Nutr. 2015;101:1216–24. doi: 10.3945/ajcn.114.100867. Epub 2015 Apr 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen M, Pan A, Malik VS, Hu FB. Effects of dairy intake on body weight and fat: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2012;96:735–47. doi: 10.3945/ajcn.112.037119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu JH, Lemaitre RN, Imamura F, King IB, Song X, Spiegelman D, Siscovick DS, Mozaffarian D. Fatty acids in the de novo lipogenesis pathway and risk of coronary heart disease: the Cardiovascular Health Study. Am J Clin Nutr. 2011;94:431–8. doi: 10.3945/ajcn.111.012054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu JH, Lemaitre RN, Manichaikul A, Guan W, Tanaka T, Foy M, Kabagambe EK, Djousse L, Siscovick D, Fretts AM, Johnson C, King IB, Psaty BM, McKnight B, Rich SS, Chen YD, Nettleton JA, Tang W, Bandinelli S, Jacobs DR, Jr, Browning BL, Laurie CC, Gu X, Tsai MY, Steffen LM, Ferrucci L, Fornage M, Mozaffarian D. Genome-wide association study identifies novel loci associated with concentrations of four plasma phospholipid fatty acids in the de novo lipogenesis pathway: results from the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) consortium. Circ Cardiovasc Genet. 2013;6:171–83. doi: 10.1161/CIRCGENETICS.112.964619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mozaffarian D. Natural trans fat, dairy fat, partially hydrogenated oils, and cardiometabolic health: the Ludwigshafen Risk and Cardiovascular Health Study. Eur Heart J. 2015 Nov 17; doi: 10.1093/eurheartj/ehv595. pii: ehv595 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kratz M, Marcovina S, Nelson JE, Yeh MM, Kowdley KV, Callahan HS, Song X, Di C, Utzschneider KM. Dairy fat intake is associated with glucose tolerance, hepatic and systemic insulin sensitivity, and liver fat but not beta-cell function in humans. Am J Clin Nutr. 2014;99:1385–1396. doi: 10.3945/ajcn.113.075457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nestel PJ, Straznicky N, Mellett NA, Wong G, De Souza DP, Tull DL, Barlow CK, Grima MT, Meikle PJ. Specific plasma lipid classes and phospholipid fatty acids indicative of dairy food consumption associate with insulin sensitivity. Am J Clin Nutr. 2014;99:46–53. doi: 10.3945/ajcn.113.071712. [DOI] [PubMed] [Google Scholar]
- 30.Dimopoulos N, Watson M, Sakamoto K, Hundal HS. Differential effects of palmitate and palmitoleate on insulin action and glucose utilization in rat L6 skeletal muscle cells. Biochem J. 2006;399:473–81. doi: 10.1042/BJ20060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 2008;134:933–44. doi: 10.1016/j.cell.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jaudszus A, Kramer R, Pfeuffer M, Roth A, Jahreis G, Kuhnt K. trans Palmitoleic acid arises endogenously from dietary vaccenic acid. Am J Clin Nutr. 2014;99:431–5. doi: 10.3945/ajcn.113.076117. [DOI] [PubMed] [Google Scholar]
- 33.Mozaffarian D, Kabagambe EK, Johnson CO, Lemaitre RN, Manichaikul A, Sun Q, Foy M, Wang L, Wiener H, Irvin MR, Rich SS, Wu H, Jensen MK, Chasman DI, Chu AY, Fornage M, Steffen L, King IB, McKnight B, Psaty BM, Djousse L, Chen IY, Wu JH, Siscovick DS, Ridker PM, Tsai MY, Rimm EB, Hu FB, Arnett DK. Genetic loci associated with circulating phospholipid trans fatty acids: a meta-analysis of genome-wide association studies from the CHARGE Consortium. Am J Clin Nutr. 2015;101:398–406. doi: 10.3945/ajcn.114.094557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.United States Department of Agriculture NAL. [Accessed Nov. 22, 2015];USDA Nutrient Laboratory. ( http://fnic.nal.usda.gov/food-composition/usda-nutrient-data-laboratory.
- 35.Mozaffarian D, de Oliveira Otto MC, Lemaitre RN, Fretts AM, Hotamisligil G, Tsai MY, Siscovick DS, Nettleton JA. trans-Palmitoleic acid, other dairy fat biomarkers, and incident diabetes: the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Clin Nutr. 2013;97:854–61. doi: 10.3945/ajcn.112.045468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hodge AM, English DR, O'Dea K, Sinclair AJ, Makrides M, Gibson RA, Giles GG. Plasma phospholipid and dietary fatty acids as predictors of type 2 diabetes: interpreting the role of linoleic acid. Am J Clin Nutr. 2007;86:189–97. doi: 10.1093/ajcn/86.1.189. [DOI] [PubMed] [Google Scholar]
- 37.Krachler B, Norberg M, Eriksson JW, Hallmans G, Johansson I, Vessby B, Weinehall L, Lindahl B. Fatty acid profile of the erythrocyte membrane preceding development of Type 2 diabetes mellitus. Nutr Metab Cardiovasc Dis. 2008;18:503–10. doi: 10.1016/j.numecd.2007.04.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.