Abstract
Objective
To evaluate whether dispositional mindfulness is associated with glucose regulation and type 2 diabetes.
Methods
Study participants (N = 399) were from the New England Family Study, a prospective birth cohort, with median age 47 years. Dispositional mindfulness was assessed using the Mindful Attention Awareness Scale (MAAS). Type 2 diabetes and “normal plasma glucose” were defined using American Diabetes Association criteria.
Results
Multivariable-adjusted regression analyses demonstrated that participants with high versus low MAAS scores were significantly more likely to have normal plasma glucose levels (prevalence ratio = 1.35 (95% confidence interval (CI): 1.08,1.87)), and were not significantly associated with type 2 diabetes (prevalence ratio = 0.83, 95% CI: 0.38,1.79), adjusted for age, sex, race/ethnicity, family history of diabetes and childhood socioeconomic status. Mediation analyses provided evidence of mediation via obesity and sense of control, where indirect effects were prevalence ratios (95% CI) of 1.03 (1.00,1.10) and 1.08 (1.00,1.21), respectively.
Conclusions
Dispositional mindfulness may be associated with better glucose regulation, in part because of a lower likelihood of obesity and greater sense of control among participants with higher levels of mindfulness. These findings need to be replicated by prospective studies to establish causality and to evaluate potential implications for mindfulness-based interventions to reduce risk of type 2 diabetes.
Keywords: mindfulness, diabetes, type 2 diabetes, glucose regulation, epidemiology
The prevalence of type 2 diabetes is increasing in the United States (US) and worldwide. In 2012, 9.3% of the US population had diabetes, of which approximately 90% was type 2 diabetes. Diabetes is the 7th leading cause of death in the US. The estimated annual economic costs in the US were $245 billion in 2012.1
Increasing prevalence of type 2 diabetes is due in large part to behavioral changes in diet and diet composition in the context of an increasingly obesogenic food environment. Strategies to halt and reverse population-level increases in type 2 diabetes have emphasized behavioral interventions, but such interventions are only modestly effective.2,3 Novel intervention targets are needed to improve their effectiveness and ultimately their population-level impact. We focus here on one such intervention target, mindfulness, for which there increasing evidence of a role in behavior and behavior change.
Mindfulness often is defined as the ability to attend in a nonjudgmental way to one’s own physical and mental processes during ordinary, everyday tasks.4 “Dispositional mindfulness” represents an inherent, yet modifiable, trait, where all people have varying capacities to attend and to be aware of what is occurring in the present moment.5 A study of twins involving 2118 adolescents suggested that dispositional mindfulness is one-third heritable and two-thirds due to non-shared (ie, individual-specific) environmental factors.6 Mindfulness interventions appear to have modest effects on altering dispositional mindfulness.7 Consequently, it is useful to understand associations of this naturally occurring trait with outcomes of health-related behaviors including type 2 diabetes, particularly because relatively low-cost interventions may be able to modify mindfulness.
Little is known about the relation of mindfulness with type 2 diabetes. Preliminary mindfulness-based intervention randomized controlled trials in diabetic patients showed reductions in fasting glucose or HbA1c in some but not all studies.8–12 The interventions that showed significant improvements in glucose regulation trained diabetic patients in mindfulness, while also directing their attention to behaviors that improve glucose regulation such as diet, physical activity, glucose monitoring, and diabetes medication use.8,9 We demonstrated associations of dispositional mindfulness with normal fasting glucose among participants in the New England Family Study, the same cohort used here.13 However, associations of dispositional mindfulness with type 2 diabetes, and the mechanisms by which mindfulness influences glucose regulation and type 2 diabetes, have not yet been explored.
There are a number of mechanisms through which mindfulness could influence type 2 diabetes. Type 2 diabetes is caused by a complex interaction of environmental factors, human behavior and genetic predisposition. Obesity is one of the primary risk factors for type 2 diabetes.14 About 85% of people with type 2 diabetes are overweight or obese.15 Evolutionarily, humans have had good cause to crave determinants of obesity, such as consumption of high caloric palatable foods and physical rest.16,17 In many calorie-rich industrialized societies, this craving no longer achieves the same adaptive benefits due to much greater food availability and sedentary occupations and pastimes.16 In these environments, craving and resulting behaviors can lead to excessive food consumption, obesity, and poor physical conditioning.16,17 In this context, mindfulness may be effective at limiting overconsumption of food and underutilizing opportunities for physical activity. Mindfulness has shown a positive association with greater self-regulation and ability to notice cravings without acting on them.18 Treating emotions and physical sensations as passing events can help people tolerate cravings and overcome addictions, whether it is for cigarettes18 or potentially for other consumption-related risks for type 2 diabetes such as overconsumption of high caloric palatable foods, or sedentary activities such as electronic screen use.19,20
Neurophysiological studies showed that regions of the prefrontal cortex, including the dorsolateral prefrontal cortex and anterior cingulate cortex, are implicated in self-regulation and inhibitory control related to limiting excessive hedonic (ie, pleasure-focused) feeding behavior.16 Mindfulness meditation interventions have been shown to influence these same regions in the prefrontal cortex,21 which is supportive evidence that mindfulness and related interventions such as mindfulness meditation, may be effective for self-regulation to limit excessive food consumption, with resulting risk for obesity and type 2 diabetes.
Overall, there are a number of particularly plausible psychological and behavioral mechanisms of how dispositional mindfulness could influence glucose regulation, discussed in a 2015 published theoretical framework for how mindfulness could influence cardiovascular disease risk.22 Plausible mechanisms include craving (eg, for palatable foods and sedentary activities),19,20,22 stress response (particularly related to diabetes risk factors, such as impacts of stress on palatable food consumption),23 sense of control (eg, a person’s sense of efficacy in carrying out goals related to diabetes prevention, such as diet or physical activity regimen adherence),18 and awareness of present moment experiences (eg, including body awareness of how the body feels after consuming certain types/amounts of foods and engaging in physical activities).24–26 Observational studies suggest that lipids, hypertension, smoking, depression and socioeconomic status also can improve risk prediction for type 2 diabetes, although their causal role in the development of type 2 diabetes is less clear.14,27 These may be additional plausible mechanisms of how mindfulness could influence glucose regulation. However, the mechanisms linking mindfulness to glucose regulation have not been established using modern analytic mediation approaches.
Accordingly, the primary objective of this study was to evaluate associations of dispositional mindfulness with glucose regulation and type 2 diabetes. Furthermore, the study aimed to evaluate plausible mechanisms that may help explain how dispositional mindfulness could influence glucose regulation, such as obesity, perceived stress, sense of control, hypertension, HDL cholesterol, physical activity, smoking, depressive symptoms, and educational attainment.
METHODS
Sample
Study participants were from the New England Family Study (NEFS), which comprises a series of adult follow-up studies of 17,921 offspring born to pregnant women enrolled in the New England (Providence and Boston) sites of the Collaborative Perinatal Project (CPP) between 1959 and 1974.13 The current NEFS study, named the Longitudinal Effects on Aging Perinatal (LEAP) Project, enrolled Providence-born participants between 2010 and 2011. From the entire Providence cohort (N = 4062), a random stratified sample of 1400 members was selected, with preference (ie, a higher sampling fraction) for racial/ethnic minorities. The Providence CPP was predominantly white race/ethnicity at study onset, reflective of the demographics in the community at that time in history. Racial/ethnic minorities were oversampled in the LEAP Project to increase generalizability of findings to other racial/ethnic groups. Of the 1400 cohort members randomly selected, participants were eligible for assessment (ie, not deceased, not incarcerated, had assessments taken at age 7 years, were located, and lived within 100 miles of the clinical assessment site). Of the 796 eligible participants, we were able to establish contact with 522 (76%) participants within the relatively brief 13-month data collection period, and invited them to participate in the study. Of these 522 participants, 19% (N = 95) refused to participate in the study, and an additional 5% (N = 27) agreed to participate but were unable to schedule assessments within the data collection period. Of the 400 final participants, one was excluded due to being diagnosed with diabetes at age <20 years (at age 2), suggesting type 1 diabetes, and therefore, removed from the risk set for incident type 2 diabetes.
Independent Variable
Brown and Ryan5 describe dispositional mindfulness as an inherent state of consciousness characterized by the presence or absence of attention to, or awareness of, what is occurring in the present moment. The Mindful Attention Awareness Scale (MAAS) is a 15-item questionnaire of dispositional mindfulness in which respondents indicate, on a 6-point Likert-type scale (1=almost always to 6=almost never), their level of awareness and attention to present events and experiences.5 Sample MAAS items include: “I find it difficult to stay focused on what’s happening in the present,” “I tend not to notice feelings of physical tension or discomfort until they really grab my attention” and “I could be experiencing some emotion and not be conscious of it until some time later.”5 The MAAS has been shown to have a single-factor structure,5 and appears to emphasize an element related to dissociation and absent-mindedness.28 A mean score is calculated (range 1–6), where higher scores reflect greater self-reported attention and awareness, or “dispositional mindfulness.” The scale exhibits good internal consistency (Cronbach’s α = 0.82–0.87) and high test-retest reliability over a 1-month period (intraclass correlation = 0.81).5,28
The MAAS score has been shown to have a positive association with long-term meditation experience, with Zen meditators having higher MAAS scores than age- and sex-matched community members,5 and Thai monks having higher MAAS scores than Thai or American students.29 A systematic review and meta-analysis showed randomized controlled trials that evaluate impacts of mindfulness training on self-reported mindfulness scores, including the MAAS, exhibit overall improvements in self-reported mindfulness in relation to wait-list control groups, but not in relation to active control groups.7 The convergent and discriminant validity of the MAAS has been evaluated, and it appears to measure a distinct construct where higher scorers on the MAAS tend to be more aware of and receptive to inner experiences and are more mindful of their overt behavior.5 They are more aware of their emotional states and able to alter them, and they are more likely to fulfill basic psychological needs.5 Furthermore, higher MAAS scorers are less likely to be self-conscious, socially anxious and ruminative than low scorers.5 There has been minimal research done on MAAS content validity, structural validity and responsiveness; consequently these psychometric properties of the MAAS are not well understood.30 The assessment of mindfulness is without a gold standard, and there is current debate on the accuracy of self-reported mindfulness, including using the MAAS.7,30 Thus, these measures should be considered instruments of a developing science, not finished products.
Primary analyses utilized a categorical exposure variable called “MAAS score level.” The numbers of participants with MAAS score of 1–2, >2–3, >3–4, >4–5 and >5–6 were 7 (1.8%), 17 (4.3%), 59 (15.1%), 129 (33.0%) and 179 (45.8%), respectively. Consequently we applied MAAS score cut points to evaluate associations of low (MAAS score<4; N = 77) and medium MAAS levels (MAAS score 4–5; N = 131), in relation to high MAAS levels (MAAS score >5, N = 174), all with reasonable sample sizes to allow for adequate statistical power, similar to prior research.13,31 This approach allowed for exploration of threshold effects for low versus high MAAS scores. These are the same cut-points used in prior studies of the MAAS score in relation to cardiovascular disease risk factors.13,31
Dependent Variables
Type 2 diabetes and “normal plasma glucose” were defined using American Diabetes Association criteria.32,33 Specifically, participants were considered to have type 2 diabetes if they were taking diabetes medication, or had 8-hour fasting plasma glucose ≥126 mg/dL or non-fasting glucose ≥200 mg/dL without diabetes medication. “Normal plasma glucose” was defined as plasma glucose <100 mg/dL and not taking diabetes medication. Medication use was assessed directly from participants’ medication bottles brought in during clinical assessments, and coded by 2 physicians according to the medical condition for which the medications were used. Glucose was measured enzymatically in plasma samples at CERLab (Harvard Medical School, Boston, MA), described elsewhere.13,34 Glucose at the concentrations of 90 and 312 mg/dL showed day-to-day variability (CV) of 1.7% and 1.6%, respectively, using this assay.
Potential Mediators
As described above, some of the most plausible mediators between mindfulness and glucose regulation, for which we also had data available, are body mass index, sense of control, and perceived stress. Body mass index (kg/m2) was directly assessed. Weight and height measures were obtained from participants wearing light clothing without shoes, using a calibrated stadiometer and weighing scale operated by trained nurse researchers. Heads were positioned in the Frankfurt plane. Obesity was defined as BMI≥30 kg/m2. Perceived stress was assessed using the 4-item Perceived Stress Scale with established validity/reliability described elsewhere (range: 4–20).35 Sense of control was assessed via the Pearlin and Schooler Mastery Scale (range 7–35) with good internal consistency (Cronbach’s a = 0.71), where higher levels represent lower perceived control.36
Further plausible mediators, for which data also were available, include blood pressure, lipids, physical activity, smoking, depression, and socioeconomic status (eg, educational attainment). Systolic and diastolic blood pressure were assessed by certified research nurses using mercury sphygmomanometers, in seated participants with arms at heart level, resting 5 minutes prior to assessment, consistent with American Heart Association guidelines. The mean of the second and third blood pressure readings was used. Hypertension was defined as systolic/diastolic blood pressure ≥140/90 mmHg and participants not taking antihypertensive medication. HDL cholesterol was measured enzymatically in plasma samples at CERLab (Harvard Medical School, Boston, MA), described elsewhere.37 HDL-C at the concentrations of 27.0 and 54.9 mg/dL showed day-to-day variability (CV) of 3.3% and 1.7%, respectively, using this assay. Physical activity was assessed using the International Physical Activity Questionnaire with reasonable measurement properties, described in more detail else-where.38 Smoking was assessed by self-report, and dichotomized as current smoker versus non-smoker. Depressive symptomatology was computed as the sum of responses from the 10-item Center for Epidemiologic Studies Depression Scale (CES-D) (range: 0–30).39 Educational attainment was categorized as ≤high school versus >high school.
Potential Cofounders
Age was directly assessed via date of birth (recorded directly in this birth cohort), subtracted from clinic visit date. Sex was self-reported. Race/ethnicity was self-reported in adulthood. In rare cases that it was missing (N = 11), maternal reports of race/ethnicity recorded during pregnancy were used. Family history of diabetes was obtained by self-report, asking participants whether their biological father or mother ever had diabetes that first appeared as an adult. Childhood socioeconomic status (SES) was assessed directly from parents when offspring were 7 years old, using a weighted percentile of both parents’ educational attainment, occupation, and income relative to the USA population.40
Analytic Methods
Multivariable-adjusted regression analyses evaluated associations of MAAS score level with likelihood of having type 2 diabetes or normal plasma glucose. Estimated prevalence ratios were calculated utilizing log-binomial regressions. Analyses were adjusted for potential confounders including age, sex, race/ethnicity, family history of diabetes, and childhood SES.
Mediation analyses assessed whether obesity, physical activity, smoking, hypertension, HDL cholesterol, depressive symptoms, perceived stress, sense of control, and educational attainment accounted for the association between mindfulness and having normal fasting glucose. Analyses used formal mediation methods based on the counterfactual framework, which allows for decomposition of a total effect into direct and indirect effects, even in models with interactions and nonlinearities.41,42 Examining indirect effects provides evidence of whether mindfulness may exert its effects uniquely through any of the potential mediators examined in this study. Percentile-based 95% confidence intervals were estimated using the bootstrap method with 1000 samples.43 We utilized log-binomial regression analyses to evaluate the associations of MAAS level, and each potential mediator, with having normal fasting glucose.
For some participants, certain covariates were missing. Assuming that the covariates are missing at random, we used multiple imputation44 to create 100 complete datasets. Multivariable regression analyses was performed on each of the 100 complete datasets, each comprising 399 participants, and results were combined using Rubin’s Rule for multiple imputation.44
We performed the mediation analyses, using normal plasma glucose as the primary outcome, in the sample with complete data (N = 331) because methods for mediation analyses with multiply imputed data are not available. Comparisons between participants with complete data (N = 331) versus incomplete data (N = 68) showed no significant mean differences for the independent and dependent variables, covariates and potential mediators (p > .05).
The following numbers of LEAP participants provided data for each independent variable, dependent variable, and covariate: Mindful Attention Awareness Scale (MAAS) score (N = 391), plasma glucose (N = 385), medication use (N = 399), age (N = 399), sex (N = 399), race/ethnicity (N = 399), family history of diabetes (N = 393), childhood socioeconomic status (SES) (N = 385), body mass index (N = 399), blood pressure (N = 396), HDL cholesterol (N = 385), physical activity (N = 376), smoking (N = 399), perceived stress (N = 392), sense of control (N = 393), depressive symptomatology (N = 392) and education (N = 392). Statistical analyses were conducted using SAS version 9.2 (Cary, NC).
RESULTS
Initial unadjusted analyses demonstrated significant associations between MAAS level and normal plasma glucose levels, age, smoking, depressive symptoms, perceived stress, and sense of control (Table 1). Further unadjusted analyses demonstrated significant associations of diabetes status with childhood socioeconomic index, obesity, hypertension, HDL cholesterol, family history of diabetes, sense of control, and educational attainment (Table 2).
Table 1.
Unadjusted Participant Characteristics for Entire Sample (Overall) and Stratified by Mindful Attention Awareness Scale (MAAS) Levela
| Mass Levelb | |||||
|---|---|---|---|---|---|
| Overall | Low | Medium | High | Pc | |
| Diabetes Measures | |||||
| Fasting glucose, mg/dL | 98 (92, 108) | 101 (94, 108) | 98 (90, 109) | 97 (92, 107) | .20 |
| Diabetes medication, % taking medication | 7.2 | 9.0 | 7.5 | 5.6 | .31 |
| Diabetes, % | 9.9 | 11.7 | 10.9 | 7.6 | .26 |
| Normal, % | 49 | 37 | 49 | 54 | .04 |
| Confounders | |||||
| Age, years | 47 (46, 48) | 47 (46, 48) | 47 (45, 48) | 47 (46, 49) | .03 |
| Sex, % female | 57 | 63 | 58 | 53 | .11 |
| Race/ethnicity, % white | 65 | 68 | 70 | 61 | .20 |
| Family history of diabetes, % yes | 41 | 40 | 47 | 36 | .35 |
| Childhood socioeconomic index | 41 (26, 57) | 39 (24, 58) | 39 (24, 54) | 44 (28, 58) | .35 |
| Potential Mediators | |||||
| Obesity, % obese | 44 | 55 | 43 | 41 | .06 |
| Hypertension, % | 32 | 32 | 29 | 36 | .41 |
| HDL Cholesterol | 53 (42, 65) | 52 (40, 62) | 51 (42, 64) | 54 (42, 68) | .37 |
| Physical activity, % low activity | 38 | 49 | 33 | 36 | .15 |
| Smoking, % current smoker | 36 | 49 | 35 | 31 | .009 |
| Depressive symptoms, CESD score | 6 (3, 10) | 12 (8, 16) | 7 (4, 11) | 4 (2, 8) | <.0001 |
| Perceived Stress, score | 9 (6, 12) | 12 (9, 13) | 9 (7, 11) | 7 (5, 10) | <.0001 |
| Sense of control, score | 14 (12, 18) | 17 (15, 23) | 14 (13, 18) | 14 (10, 16) | <.0001 |
| Educational attainment, % >high school | 30 | 27 | 30 | 31 | .61 |
Note.
= Point estimates represent median (interquartile range) or percentage.
= MAAS levels represent the following MAAS scores (range 1–6): Low:, <4, Medium: 4–5, High: >5.
= p values are derived from Mantel-Haenszel chi-square tests (categorical variables) or Kruskal Wallis tests (continuous variables).
BMI, body mass index; CESD, Center for Epidemiologic Studies Depression Scale; FSIQ, Full Scale Intelligence Quotient
N = 399
Table 2.
Participant Characteristics of Confounders and Potential Mediators, Stratified by Diabetes Statusa
| Diabetes Status | ||||
|---|---|---|---|---|
| Normal (glucose <100 mg/dL) |
Glucose ≥100 and <200 mg/dL |
Diabetes | pb | |
| Confounders | ||||
| Age, years | 47 (45, 49) | 47 (46, 48) | 48 (47, 49) | .13 |
| Sex, % female | 64 | 43 | 66 | .11 |
| Race/ethnicity, % white | 70 | 61 | 59 | .06 |
| Family history of diabetes, % yes | 38 | 41 | 59 | .02 |
| Childhood socioeconomic index | 42 (27, 61) | 41 (26, 54) | 35 (19, 46) | .004 |
| Potential Mediators | ||||
| Obesity, % obese | 34 | 53 | 77 | <.0001 |
| Hypertension, % | 20 | 38 | 68 | <.0001 |
| HDL Cholesterol | 57 (44, 70) | 51 (40, 59) | 46 (38, 60) | <.0001 |
| Physical activity, % low activity | 34 | 37 | 52 | .06 |
| Smoking, % current smoker | 33 | 36 | 42 | .59 |
| Depressive symptoms, CESD score | 6 (3, 11) | 6 (4, 10) | 8 (4, 13) | .38 |
| Perceived Stress, score | 9 (6, 11) | 9 (7, 12) | 9 (7, 12) | .58 |
| Sense of control, score | 14 (11, 17) | 15 (12, 18) | 16 (14, 18) | .007 |
| Educational attainment, % ≤high school | 35 | 24 | 19 | .006 |
Note.
= Point Estimates Represent Median (Interquartile Range) or Percentage
CESD, Center for Epidemiologic Studies Depression Scale; FSIQ, Full Scale Intelligence Quotient
= p values are derived from chi-square tests (categorical variables) or Kruskal Wallis tests (continuous variables).
Diabetes defined as fasting glucose>126 mg/dL or non-fasting glucose>200 mg/dL, or taking diabetes medication
N = 399
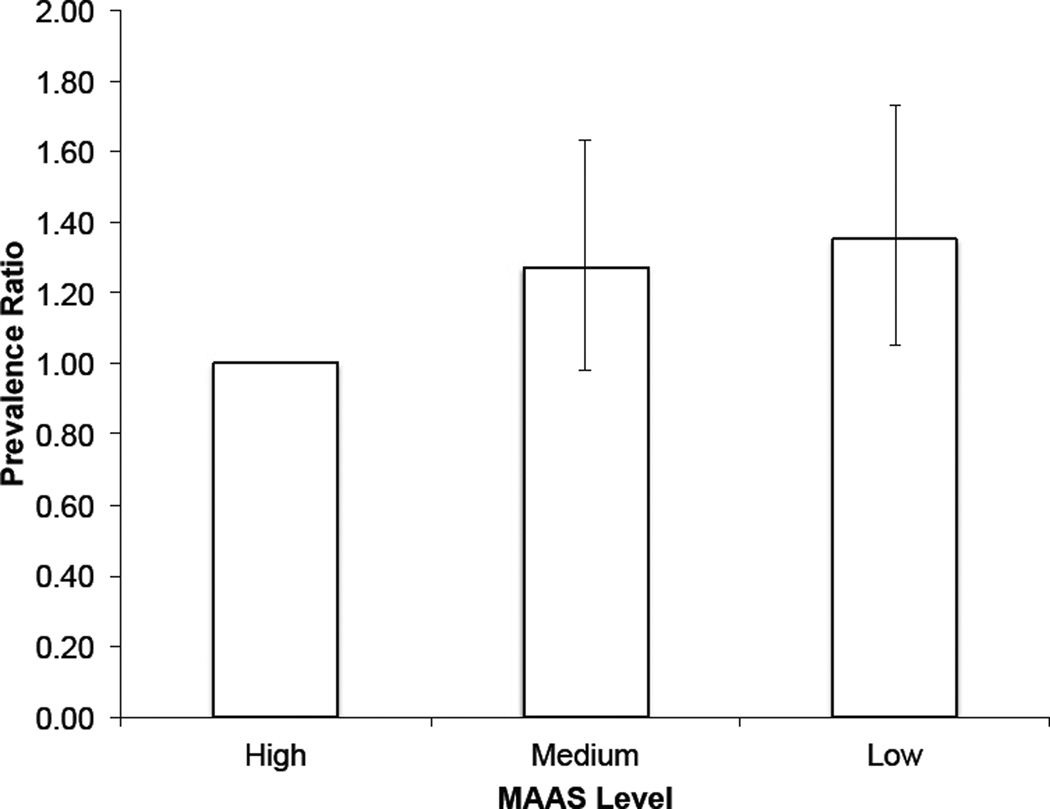
Multivariable regression analyses demonstrated that participants with high versus low MAAS levels were more likely (prevalence ratio = 1.42; 95% CI: 1.08, 1.87) to have normal plasma glucose levels, adjusting for age, sex, race/ethnicity and family history of diabetes (Table 3); after adjusting for childhood SES, the prevalence ratio was 1.35 (95% CI: 1.05, 1.73) (Table 3, Figure 1). Similar trends were seen with type 2 diabetes as the dependent variable, but effect estimates were not statistically significant, where participants with high versus low MAAS were less likely to have type 2 diabetes (prevalence ratio = 0.83; 95% CI: 0.38, 1.79), after adjusting for age, sex, race/ethnicity, family history of diabetes, and childhood SES (Table 3). Formal statistical tests of an interaction between mindfulness and sex, and mindfulness and race/ethnicity, for the relationship between MAAS score and normal plasma glucose prevalence demonstrated no evidence for effect measure modification (p = .88 and p = .97, respectively).
Table 3.
Multivariable-adjusted Regression Analyses Showing Prevalence Ratios (95% Confidence Intervals) for Having Normal Plasma Glucose and Diabetes According to Mindfulness Awareness Attention Scale (MAAS) Level.a
| Model Adjustment | |||
|---|---|---|---|
| Outcome | MAAS Level | Age, sex, race/ethnicity, family history of diabetes |
Age, sex, race/ethnicity, family history of diabetes, childhood SES |
| Normal Plasma Glucose | Low | 1.00 (ref) | 1.00 (ref) |
| Medium | 1.29 (0.97, 1.72) | 1.27 (0.98, 1.63) | |
| High | 1.42 (1.08, 1.87) | 1.35 (1.05, 1.73) | |
| Diabetes | Low | 1.00 (ref) | 1.00 (ref) |
| Medium | 1.02 (0.47, 2.20) | 1.03 (0.44, 2.43) | |
| High | 0.79 (0.37, 1.70) | 0.83 (0.38, 1.79) | |
Note.
= Low MAAS level is referent category.
MAAS levels represent the following MAAS scores (range 1–6): Low: <4, Medium: 4–5, High: >5.
SES = socioeconomic status
N = 399
Figure 1. Prevalence Ratios (95% Confidence Intervals) of Having Normal Plasma Glucose Levels (<100 mg/dL).
Prevalence ratios of having normal plasma glucose according to mindfulness level, adjusted for age, sex, race/ethnicity, family history of diabetes, and childhood socioeconomic status. MAAS levels represent the following MAAS scores (range 1–6): Low: <4, Medium: 4–5, High: >5.
In mediation analyses, obesity and sense of control explained part of the association between mindfulness and the likelihood of having a normal fasting glucose. The total effect of high versus low MAAS level on having normal fasting glucose in the complete case mediation analyses was a prevalence ratio of 1.31 (95% CI: 1.03, 1.67), adjusted for age, race/ethnicity, sex, family history of diabetes, and childhood SES. Indirect effects for obesity and sense of control were prevalence ratios of 1.03 (95% CI: 1.00, 1.10), and 1.08 (95% CI: 1.00, 1.21), respectively. This demonstrated that 0.03 and 0.08 of the 0.31 additional prevalence ratio total effects due to high versus low MAAS may be mediated through obesity and sense of control, respectively. All other tested mediators (ie, hypertension, HDL cholesterol, physical activity, smoking, depressive symptoms, perceived stress and educational attainment) showed no significant evidence of mediation.
DISCUSSION
Study findings demonstrated that participants with higher dispositional mindfulness were significantly more likely to have normal plasma glucose, after adjusting for age, race/ethnicity, sex, family history of diabetes, and childhood SES. Participants with higher levels of mindfulness were about 20% less likely to have type 2 diabetes, although confidence intervals were wide and these associations were not statistically significant. Mediation analyses between mindfulness and glucose regulation suggested potential mediating roles of obesity and sense of control.
Prior Literature
Several studies have investigated the role of mindfulness-based interventions in glucose regulation in diabetic patients. Of the 5 RCTs to our knowledge, 2 studies showed significant reductions in glucose regulation measures including HbA1C and fasting glucose,8,9 and 3 studies demonstrated null findings.10–12 Both interventions that showed significant glucose regulation improvements specifically trained participants in mindfulness, and also providing training in behaviors that improve glucose regulation such as diet, physical activity, glucose monitoring, and diabetes medication use.8,9 Studies that did not show improvements in glucose regulation typically tested standardized mindfulness-based interventions, namely Mindfulness-Based Cognitive Therapy11,12 and Mindfulness-Based Stress Reduction.10 These standardized interventions provide some training in mindful eating and mindful movements, but do not deliberately link the importance of these factors to diabetes control, and do not address diabetes medication adherence or glucose monitoring.45,46 The aforementioned Mindfulness-Based Stress Reduction study did provide some customization of the mindfulness intervention to difficult thoughts and feelings related to diabetes.10 Perhaps not unsurprisingly, this latter intervention demonstrated significant improvements in depression in the intervention versus control group, but only weak non-significant (p = .09) improvements in HbA1c.10 Mindfulness-based interventions targeted towards improving mindfulness skills for glucose regulation may increase effect sizes of mindfulness interventions for diabetes management. Examples of plausible targeted interventions include mindfulness modules addressing thoughts of craving for high caloric palatable foods or sedentary activities. Interventions targeting self-compassion and self-care applied to glucose regulation behaviors such as diet, physical activity, weight maintenance, and diabetes medication adherence also may be effective. To our knowledge, there have been no mindfulness-based interventions to date targeted towards preventing diabetes incidence.
The role of dispositional mindfulness in diabetes risk has received little study. One prior investigation by our group in the New England Family Study cohort found significant associations of mindfulness with having normal glucose levels, adjusting for age, race/ethnicity and sex.13 The current study extended explorations of confounding by adjusting for early life and family factors including childhood SES and family history of diabetes. Furthermore, this the first study to our knowledge to investigate associations of dispositional mindfulness with type 2 diabetes, and to explore potential mediators of the relation between dispositional mindfulness and glucose regulation. Dispositional mindfulness is of interest in and of itself, as there may be various routes to mindfulness. A twin study in 2118 adolescents published in 2015 suggested that dispositional mindfulness is one-third heritable and two-thirds due to non-shared environmental factors.6 Mindfulness interventions such as mindfulness meditation are some of the best studied routes to improve mindfulness, and may indeed be the most effective.30 However, there may be other influences on dispositional mindfulness, such as role modeling by family and community members, and genetic differences in neurophysiological abilities to be aware of thoughts, emotions, and physical sensations.6
Mechanisms
This study demonstrated evidence of mediation by obesity and sense of control. There was no evidence of potential mediation by other plausible factors including hypertension, HDL cholesterol, physical activity, smoking, depressive symptoms, perceived stress, and educational attainment.
Prior studies found associations of mindfulness with measures of adiposity. Studies have found significant associations of dispositional mindfulness with body mass index, obesity and regional fat distribution,13,31 and evidence of effects of mindfulness-based interventions on weight loss in participants seeking to lose weight.20,47 Obesity itself is likely the most important contributor to type 2 diabetes, where 85% of diabetics are overweight or obese.15 Mindful awareness brought to experiences of food overconsumption and inadequate physical activity may help participants be aware of the long reach of their immediate food and exercise decisions on their long-term well-being.20,48
Studies have shown early evidence that mindfulness is related to improved sense of control and self-efficacy. For example, a mindful eating intervention in participants with type 2 diabetes demonstrated improved eating-related self-efficacy in participants following the intervention.49 A prospective study found that participants with greater dispositional mindfulness were more likely to enact their physical activity intentions than those with lesser dispositional mindfulness.50 A study by our group demonstrated evidence that sense of control is a significant mediator between dispositional mindfulness and cardiovascular health.13 Self-efficacy, a similar construct to sense of control, is related to positive diabetes-related health behaviors including diet, physical activity and medication adherence.51,52 Further evaluation of plausible mediators in intervention studies and prospective observational studies will improve etiologic knowledge.
Strengths and Limitations
Limitations of the study included cross-sectional analyses where the independent and dependent variables, and potential mediators, were assessed at the same time point. Cross-sectional analyses limit causal inference compared to prospective studies. Future prospective studies should provide stronger evidence on whether dispositional mindfulness is associated with glucose regulation and type 2 diabetes. Secondly, dispositional mindfulness was assessed using a self-report questionnaire. The assessment of mindfulness is without a gold standard, and there is current debate on the accuracy of self-reported mindfulness, including using the MAAS.7,30 Thus, whereas these tools should be considered tools of a developing science, they have attained psychometric properties that justify their use in associational studies such as the current one (described in more detail in the Methods section above). Future research that uses triangulation of methods will allow better causal inference. For example, this would include studies that evaluate the effects of mindfulness-based interventions on glucose regulation, as well as studies investigating the role of dispositional mindfulness in glucose regulation and type 2 diabetes. Thirdly, results should be interpreted with the knowledge that total effect analyses were performed on the multiply imputed data (N = 399), which reduce bias due to observed covariates, whereas the mediation analyses were performed using complete case data (N = 331) due to mediation methods not currently available for multiply imputed data. However, effect sizes were similar for both approaches, where prevalence ratios for high versus low MAAS on normal plasma glucose was 1.35 (95% CI: 1.05, 1.73) using multiple imputation (N = 399) and 1.31 (95% CI: 1.03, 1.67) using complete case analyses (N = 331) in fully adjusted models. Furthermore, comparisons between participants with complete data (N = 331) versus incomplete data (N = 68) showed no statistically significant mean differences for the independent and dependent variables, covariates, and potential mediators (p > .05), suggesting minimal differences in study population characteristics using either approach. Fourthly, as detailed in the Methods section, 1400 participants were randomly selected from the Providence Collaborative Perinatal Project with preference for racial/ethnic minorities, of which 796 were eligible, contact was established with 522, and 400 were assessed. This limitation in generalizability should be considered when interpreting the findings. Strengths of the study included direct assessments of plasma glucose, fasting time, and diabetes medication, as well as the ability to adjust statistically for prospectively assessed plausible confounders including childhood SES.
Conclusions
This study demonstrated a significant, association of dispositional mindfulness with glucose regulation, and provided novel evidence that obesity and sense of control may serve as potential mediators of this association. As mindfulness is likely a modifiable trait,7 this study provides preliminary evidence for a fairly novel and modifiable potential determinant of diabetes risk. If future observational and intervention studies confirm a role of mindfulness in diabetes risk, mindfulness may serve as an intervention target and risk stratification variable to improve prevention and treatment of diabetes.
Acknowledgments
This study was funded by National Institutes of Health/National Institute on Aging grants RC2AG036666 and R01AG048825. It was further supported by grant number UH2AT009145 from the National Institutes of Health (NIH), specifically the National Center for Complementary and Integrative Health (NCCIH) and the Office of Behavioral and Social Sciences Research (OBSSR). Dr. Gilman was funded by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Human Subjects Statement
The study protocol was approved by the institutional review boards at Brown University and Memorial Hospital of Rhode Island (Brown University approval document #0908000028).
Conflict of Interest Statement
The authors declare no conflicts of interest.
Contributor Information
Eric B. Loucks, Brown University School of Public Health, Department of Epidemiology, Providence, RI.
Stephen E. Gilman, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD.
Willoughby B. Britton, Brown University Warren Alpert Medical School, Department of Psychiatry and Human Behavior, Providence, RI.
Roee Gutman, Brown University School of Public Health, Department of Biostatistics, Providence, RI.
Charles B. Eaton, Brown University Warren Alpert Medical School, Department of Family Medicine, Providence, RI.
Stephen L. Buka, Brown University School of Public Health, Department of Epidemiology, Providence, RI.
References
- 1.Centers for Disease Control and Prevention (CDC) National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta, GA: CDC, US Department of Health and Human Services; 2014. [Google Scholar]
- 2.Dunkley AJ, Bodicoat DH, Greaves CJ, et al. Diabetes prevention in the real world: effectiveness of pragmatic lifestyle interventions for the prevention of type 2 diabetes and of the impact of adherence to guideline recommendations: a systematic review and meta-analysis. Diabetes Care. 2014;37(4):922–933. doi: 10.2337/dc13-2195. [DOI] [PubMed] [Google Scholar]
- 3.Aguiar EJ, Morgan PJ, Collins CE, et al. Efficacy of interventions that include diet, aerobic and resistance training components for type 2 diabetes prevention: a systematic review with meta-analysis. Int J Behav Nutr Phys Act. 2014;11:2. doi: 10.1186/1479-5868-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Epstein RM. Mindful practice. JAMA. 1999;282(9):833–839. doi: 10.1001/jama.282.9.833. [DOI] [PubMed] [Google Scholar]
- 5.Brown KW, Ryan RM. The benefits of being present: mindfulness and its role in psychological well-being. J Pers Soc Psychol. 2003;84(4):822–848. doi: 10.1037/0022-3514.84.4.822. [DOI] [PubMed] [Google Scholar]
- 6.Waszczuk MA, Zavos HM, Antonova E, et al. A multivariate twin study of trait mindfulness, depressive symptoms, and anxiety sensitivity. Depress Anxiety. 2015;32(4):254–261. doi: 10.1002/da.22326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Visted E, Jollestad J, Nielsen MB, Nielsen GH. The impact of group-based mindfulness training on self-reported mindfulness: a systematic review and meta-analysis. Mindfulness. 2015;6:501–522. [Google Scholar]
- 8.Gregg JA, Callaghan GM, Hayes SC, Glenn-Lawson JL. Improving diabetes self-management through acceptance, mindfulness, and values: a randomized controlled trial. J Consult Clin Psychol. 2007;75(2):336–343. doi: 10.1037/0022-006X.75.2.336. [DOI] [PubMed] [Google Scholar]
- 9.Youngwanichsetha S, Phumdoung S, Ingkathawornwong T. The effects of mindfulness eating and yoga exercise on blood sugar levels of pregnant women with gestational diabetes mellitus. Appl Nurs Res. 2014;27(4):227–230. doi: 10.1016/j.apnr.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Hartmann M, Kopf S, Kircher C, et al. Sustained effects of a mindfulness-based stress-reduction intervention in type 2 diabetic patients: design and first results of a randomized controlled trial (the Heidelberger Diabetes and Stress-study) Diabetes Care. 2012;35(5):945–947. doi: 10.2337/dc11-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Son J, Nyklicek I, Pop VJ, et al. Mindfulness-based cognitive therapy for people with diabetes and emotional problems: long-term follow-up findings from the DiaMind randomized controlled trial. J Psychosom Res. 2014;77(1):81–84. doi: 10.1016/j.jpsychores.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 12.Tovote KA, Schroevers MJ, Snippe E, et al. Long-term effects of individual mindfulness-based cognitive therapy and cognitive behavior therapy for depressive symptoms in patients with diabetes: a randomized trial. Psychother Psychosom. 2015;84(3):186–187. doi: 10.1159/000375453. [DOI] [PubMed] [Google Scholar]
- 13.Loucks EB, Britton WB, Howe CJ, et al. Positive associations of dispositional mindfulness with cardiovascular health: the New England Family Study. Int J Behav Med. 2015;22(4):540–550. doi: 10.1007/s12529-014-9448-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu FB, Manson JE, Stampfer MJ, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl. J Med. 2001;345(11):790–797. doi: 10.1056/NEJMoa010492. [DOI] [PubMed] [Google Scholar]
- 15.Eberhardt MS, Ogden CL, Engelgau M, et al. Prevalence of overweight and obesity among adults with diagnosed diabetes: United States, 1988–1994 and 1999–2002. MMWR Morb Mortal Wkly Rep. 2004;53(45):1066–1068. [PubMed] [Google Scholar]
- 16.Appelhans BM. Neurobehavioral inhibition of reward-driven feeding: implications for dieting and obesity. Obesity. 2009;17(4):640–647. doi: 10.1038/oby.2008.638. [DOI] [PubMed] [Google Scholar]
- 17.Lieberman LS. Dietary, evolutionary, and modernizing influences on the prevalence of type 2 diabetes. Ann Rev Nutr. 2003;23:345–377. doi: 10.1146/annurev.nutr.23.011702.073212. [DOI] [PubMed] [Google Scholar]
- 18.Brewer JA, Mallik S, Babuscio TA, et al. Mindfulness training for smoking cessation: results from a randomized controlled trial. Drug Alcohol Depend. 2011;119(1–2):72–80. doi: 10.1016/j.drugalcdep.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brand M, Young KS, Laier C. Prefrontal control and internet addiction: a theoretical model and review of neuropsychological and neuroimaging findings. Front Hum Neurosci. 2014;8:375. doi: 10.3389/fnhum.2014.00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olson KL, Emery CF. Mindfulness and weight loss: a systematic review. Psychosom Med. 2015;77(1):59–67. doi: 10.1097/PSY.0000000000000127. [DOI] [PubMed] [Google Scholar]
- 21.Tang YY, Holzel BK, Posner MI. The neuroscience of mindfulness meditation. Nat Rev Neurosci. 2015;16(4):213–225. doi: 10.1038/nrn3916. [DOI] [PubMed] [Google Scholar]
- 22.Loucks EB, Schuman-Olivier Z, Britton WB, et al. Mindfulness and cardiovascular disease risk: state of the evidence, plausible mechanisms, and theoretical framework. Curr Cardiol Rep. 2015;17(12):112. doi: 10.1007/s11886-015-0668-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maniam J, Morris MJ. The link between stress and feeding behaviour. Neuropharmacol. 2012;63(1):97–110. doi: 10.1016/j.neuropharm.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 24.Holzel BK, Lazar SW, Gard T, et al. How does mindfulness meditation work? Proposing mechanisms of action from a conceptual and neural perspective. Perspect Psychol Sci. 2011;6(6):537–559. doi: 10.1177/1745691611419671. [DOI] [PubMed] [Google Scholar]
- 25.Silverstein RG, Brown AC, Roth HD, Britton WB. Effects of mindfulness training on body awareness to sexual stimuli: implications for female sexual dysfunction. Psychosom Med. 2011;73(9):817–825. doi: 10.1097/PSY.0b013e318234e628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bornemann B, Herbert BM, Mehling WE, Singer T. Differential changes in self-reported aspects of interoceptive awareness through 3 months of contemplative training. Frontier Psychol. 2014;5:1504. doi: 10.3389/fpsyg.2014.01504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson PW, Meigs JB, Sullivan L, et al. Prediction of incident diabetes mellitus in middle-aged adults: the Framingham Offspring Study. Arch Intern Med. 2007;167(10):1068–1074. doi: 10.1001/archinte.167.10.1068. [DOI] [PubMed] [Google Scholar]
- 28.Baer RA, Smith GT, Hopkins J, et al. Using self-report assessment methods to explore facets of mindfulness. Assessment. 2006;13(1):27–45. doi: 10.1177/1073191105283504. [DOI] [PubMed] [Google Scholar]
- 29.Christopher MS, Christopher V, Charoensuk S. Assessing “Western” mindfulness among Thai Theravada Buddhist Monks. Mental Health Relig Culture. 2009;12(3):303–314. [Google Scholar]
- 30.Park T, Reilly-Spong M, Gross CR. Mindfulness: a systematic review of instruments to measure an emergent patient-reported outcome (PRO) Qual Life Res. 2013;22(10):2639–2659. doi: 10.1007/s11136-013-0395-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loucks EB, Britton WB, Howe CJ, et al. Associations of dispositional mindfulness with obesity and central adiposity: the New England Family Study. Int J Behav Med. 2015 doi: 10.1007/s12529-015-9513-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.American Diabetes A. Standards of medical care in diabetes – 2014. Diabetes Care. 2014;37(Suppl 1):S14–S80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 33.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34(Suppl 1):S62–S69. doi: 10.2337/dc11-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loucks EB, Britton WB, Howe CJ, et al. Positive associations of dispositional mindfulness with cardiovascular health: the New England Family Study. Int J Behav Med. 2015;22(4):540–550. doi: 10.1007/s12529-014-9448-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 36.Pearlin LI, Schooler C. The structure of coping. J Health Soc Behav. 1978;19(1):2–21. [PubMed] [Google Scholar]
- 37.Rifai N, Cole TG, Iannotti E, et al. Assessment of interlaboratory performance in external proficiency testing programs with a direct HDL-cholesterol assay. Clin Chem. 1998;44(7):1452–1458. [PubMed] [Google Scholar]
- 38.Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 39.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10(2):77–84. [PubMed] [Google Scholar]
- 40.Myrianthopoulos NC, French KS. An application of the U.S. Bureau of the Census socioeconomic index to a large, diversified patient population. Soc Sci Med. 1968;2(3):283–299. doi: 10.1016/0037-7856(68)90004-8. [DOI] [PubMed] [Google Scholar]
- 41.Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods. 2013;18(2):137–150. doi: 10.1037/a0031034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.VanderWeele TJ. Mediation analysis: a practitioner’s guide. In: Efron B, Tibshirani RJ, editors. Annu Rev Public Health. Boca Raton, FL: Chapman & Hall/CRC; 1994. [2015 Nov 30]. [Epub ahead of print]. An Introduction to the Bootstrap. [Google Scholar]
- 43.Rubin DB. Multiple Imputation for Nonresponse in Surveys. Hoboken, NJ: Wiley; 2004. [Google Scholar]
- 44.Segal ZV, Williams JMG, Teasdale JD, Kabat-Zinn J. Mindfulness-Based Cognitive Therapy for Depression. New York, NY: The Guildford Press; 2012. [Google Scholar]
- 45.Kabat-Zinn J. Full Catastrophe Living. New York, NY: Bantam Books; 2013. [Google Scholar]
- 46.Fulwiler C, Brewer JA, Loucks EB. Mindfulness-based interventions for weight loss and CVD risk management. Curr Cardiovasc Dis Risk Rep. 2015;9(10) doi: 10.1007/s12170-015-0474-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsafou KE, De Ridder DT, van Ee R, Lacroix JP. Mindfulness and satisfaction in physical activity: a cross-sectional study in the Dutch population. J Health Psychol. 2015 Jan 28; doi: 10.1177/1359105314567207. pii: 1359105314567207. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 48.Miller CK, Kristeller JL, Headings A, Nagaraja H. Comparison of a mindful eating intervention to a diabetes self-management intervention among adults with type 2 diabetes: a randomized controlled trial. Health Educ Behav. 2014;41(2):145–154. doi: 10.1177/1090198113493092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chatzisarantis NL, Hagger MS. Mindfulness and the intention-behavior relationship within the theory of planned behavior. Personal Soc Psychol Bull. 2007;33(5):663–676. doi: 10.1177/0146167206297401. [DOI] [PubMed] [Google Scholar]
- 50.Walker RJ, Smalls BL, Hernandez-Tejada MA, et al. Effect of diabetes self-efficacy on glycemic control, medication adherence, self-care behaviors, and quality of life in a predominantly low-income, minority population. Ethnicity Dis. 2014;24(3):349–355. [PMC free article] [PubMed] [Google Scholar]
- 51.Davies MJ, Gagliardino JJ, Gray LJ, et al. Real-world factors affecting adherence to insulin therapy in patients with Type 1 or Type 2 diabetes mellitus: a systematic review. Diabet Med. 2013;30(5):512–524. doi: 10.1111/dme.12128. [DOI] [PubMed] [Google Scholar]