Abstract
OBJECTIVE
The aim of our study is to describe the changes in urinary and serum levels of novel biomarkers after gadolinium contrast administration in patients with normal renal function.
METHODS
We measured four biomarkers in 28 volunteers: interleukin-18 (IL-18), N-acetyl-glucosaminidase (NAG), neutrophil gelatinase-associated lipocalin, and cystatin C. Urinary and serum samples were collected at 0, 3, and 24 hours following gadolinium administration.
RESULTS
Baseline serum creatinine was 57.8 ± 34.5 µmol/L and remained stable. Urinary IL-18 levels increased significantly at three hours (10.7 vs. 7.3 ng/mg creatinine; P < 0.05). Similarly, urinary NAG levels increased significantly at three hours (3.9 vs. 2.2 IU/mg creatinine; P < 0.001). For both these markers, the difference was no longer significant at 24 hours. No statistically significant differences were observed for urinary and serum neutrophil gelatinase-associated lipocalin levels and for serum cystatin C levels.
CONCLUSIONS
Urinary IL-18 and NAG levels increased transiently after administration of gadolinium-based contrast agents in patients with normal renal function.
Keywords: biomarkers, contrast-induced nephropathy, gadolinium, nephrotoxicity
Introduction
Magnetic resonance imaging (MRI) has become an essential tool in current medical practice. Gadolinium-based contrast agents (GBCAs) are often administered to enhance images obtained by MRI.
Nephrotoxicity of GBCAs
Iodinated contrast agents are associated with contrast-induced nephropathy (CIN). GBCAs were initially believed to be a safe alternative to iodinated contrast agents. Their role in nephrogenic systemic fibrosis has since been well established, and their use has been limited in patients with moderate to severe kidney dysfunction.1 Recent literature has also raised legitimate concerns with regard to nephrotoxicity. Early investigations suggested a favorable renal safety profile.2–6 However, as indicated in a review by Penfield and Reilly,1 these studies had many limitations. Indeed, larger subsequent studies did detect variable degrees of nephrotoxicity.7–11 The exact mechanism of acute kidney injury (AKI) remains unknown.
Novel biomarkers of AKI
Novel biomarkers may offer many advantages over current methods of AKI assessment. In contrast to traditional markers (eg, creatinine), they could be used for earlier diagnosis as well as differential diagnosis.12 The most promising of these biomarkers include interleukin-18 (IL-18), N-acetyl-glucosaminidase (NAG), neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule-1 (KIM-1), and cystatin C.13 These have been studied in various cases of AKI, including iodinated CIN.14 Our literature review revealed very few studies relating to gadolinium-induced nephrotoxicity. Duan et al.15 reported that urinary IL-18, cystatin C, and KIM-1 might be early predictive biomarkers of nephrotoxicity in elderly patients undergoing gadolinium-enhanced MRI.
The aim of our study is to describe the changes in the urinary and serum levels of selected biomarkers after administration of GBCAs in individuals with normal renal function.
Methods
Patient population
We studied four biomarkers of AKI, IL-18, NAG, NGAL, and cystatin C, in volunteers undergoing outpatient MRI enhanced with intravenous GBCAs. From September 2007 to April 2012, all consecutive patients undergoing MRI at a single, large university-affiliated hospital were assessed for eligibility and approached for enrollment. In order to be included, patients must be over 18 years of age and have normal baseline renal function, which was defined as an estimated glomerular filtration rate ≥60 mL/min/1.73 m2 no more than three months prior to enrollment. This information was obtained using electronic medical records. Patients exposed to known nephrotoxic medications were excluded (eg, NSAIDs, lithium, aminoglycosides, cisplatin, and calcineurin inhibitors). Informed consent was obtained in all participants. The hospital’s research ethics review board approved the study protocol. The study was conducted in accordance with the principles of the Declaration of Helsinki.
Biomarker measurement
Urinary and serum levels of biomarkers were measured at baseline, three hours, and 24 hours following injection of GBCAs. All urinary biomarkers were normalized for urinary creatinine concentration. Plasmatic creatinine was also measured at the same intervals. IL-18 and cystatin C were measured using ELISA kits from R&D Systems. NAG and NGAL were measured using ELISA kits, respectively, from Bio-Quant Inc. and AntibodyShop. All procedures followed the manufacturer’s directives.16–19 All collected samples were stored at −20 °C until analysis.
Statistical analysis
Results were expressed as mean concentration ± standard deviation. Comparisons of variables before and after administration of GBCAs were realized using Student’s t-test if distribution was normal; otherwise, Wilcoxon signed-rank test was used. A P-value of less than 0.05 was considered statistically significant. In the case of missing values, analyses were performed using only the available data.
Results
A total of 28 patients (15 women and 13 men) were included in this study. The mean age was 57.8 ± 10.4 years. Musculoskeletal imaging was the main indication for MRI (43%), followed by central nervous system (18%) and liver imaging (18%).
Urinary biomarkers NGAL, IL-18, and NAG were obtained in all patients. Serum biomarkers NGAL and cystatin C were obtained in 25 patients.
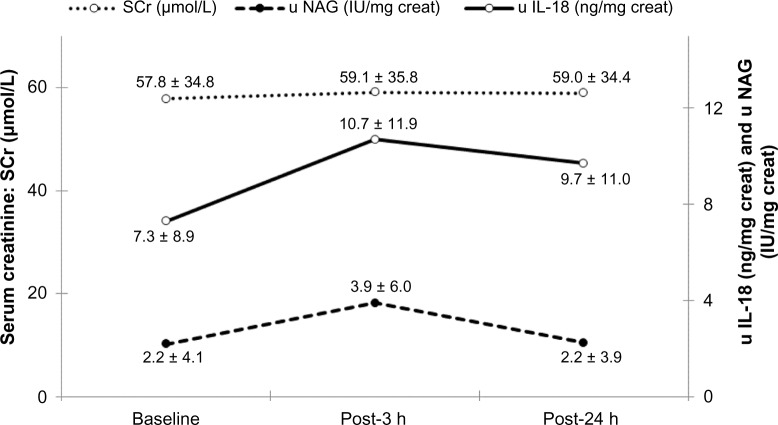
The mean baseline serum creatinine was 57.8 ± 34.5 µmol/L and remained stable throughout the first 24 hours (Table 1). As illustrated in Table 2 and Figure 1, IL-18 urinary concentrations increased significantly at three hours (10.7 vs. 7.3 ng/mg creatinine; P < 0.05). Similarly, urinary NAG levels were significantly higher at three hours (3.9 vs. 2.2 IU/mg creatinine; P < 0.001). Serum cystatin C levels decreased at three hours from 933.7 to 897.8 ng/mL but did not reach strict statistical significance (P = 0.05). For all three markers, the difference was no longer significant at 24 hours (Table 3). No differences were observed at three or 24 hours for urinary and serum NGAL levels.
Table 1.
Biomarker levels at baseline, three hours, and 24 hours.
| VARIABLE | NUMBER OF PATIENTS | BASELINE | POST–3 H | POST–24 H |
|---|---|---|---|---|
| SCr (µmol/L) | 28 | 57.8 ± 34.5 | 59.1 ± 35.8 | 59.0 ± 33.4 |
| s NGAL (ng/mL) | 25 | 88.6 ± 38.3 | 88.7 ± 36.0 | 96.5 ± 39.1 |
| s Cystatin C (ng/mL) | 25 | 933.7 ± 354.5 | 897.8 ± 349.0 | 936.3 ± 365.9 |
| u NGAL (ng/mg creat) | 28 | 3.1 ± 4.0 | 3.4 ± 4.7 | 3.9 ± 5.5 |
| u IL-18 (ng/mg creat) | 28 | 7.3 ± 8.9 | 10.7 ± 11.9 | 9.7 ± 11.0 |
| u NAG (IU/mg creat) | 28 | 2.2 ± 4.1 | 3.9 ± 6.0 | 2.2 ± 3.9 |
Note: All urinary biomarkers were corrected for urinary creatinine.
Abbreviations: SCr, serum creatinine; s NGAL, serum neutrophil gelatinase-associated lipocalin; s cystatin C, serum cystatin C; u NGAL, urine serum neutrophil gelatinase-associated lipocalin; u IL-18, urine interleukin-18; u NAG, urine N-acetyl-glucosaminidase.
Table 2.
Biomarker levels at three hours compared to baseline.
| VARIABLE | NUMBER OF PATIENTS | BASELINE | POST–3 H | DIFFERENCE | P-VALUE |
|---|---|---|---|---|---|
| SCr (µmol/L) | 28 | 57.8 | 59.1 | 1.3 | 0.18 |
| s NGAL (ng/mL) | 25 | 88.6 | 88.7 | 0.1 | 0.45 |
| s Cystatin C (ng/mL) | 25 | 933.7 | 897.8 | −35.9 | 0.05 |
| u NGAL (ng/mg creat) | 28 | 3.1 | 3.4 | 0.3 | 0.23 |
| u IL-18 (ng/mg creat) | 28 | 7.3 | 10.7 | 3.4 | 0.03 |
| u NAG (IU/mg creat) | 28 | 2.2 | 3.9 | 1.7 | 0.001 |
Figure 1.
SCr, u NAG, and u IL-18.
Table 3.
Biomarker levels at 24 hours compared to baseline.
| VARIABLE | NUMBER OF PATIENTS | BASELINE | POST–24 H | DIFFERENCE | P-VALUE |
|---|---|---|---|---|---|
| SCr (µmol/L) | 28 | 57.8 | 59.0 | 1.2 | 0.22 |
| s NGAL (ng/mL) | 25 | 88.6 | 96.5 | 7.9 | 0.11 |
| s Cystatin C (ng/mL) | 25 | 933.7 | 936.3 | 2.5 | 0.90 |
| u NGAL (ng/mg creat) | 28 | 3.1 | 3.9 | 0.8 | 0.49 |
| u IL-18 (ng/mg creat) | 28 | 7.3 | 9.7 | 2.4 | 0.17 |
| u NAG (IU/mg creat) | 28 | 2.2 | 2.2 | 0.0 | 0.40 |
Discussion
IL-18 is a proinflammatory cytokine found in a broad range of diseases. In animal models of ischemic AKI, it is induced in the proximal tubule and becomes measurable in urine.20 Ensuing studies have shown significant increase in urinary levels of IL-18 in various settings of acute tubular necrosis, including iodinated CIN.21 Duan et al.15 showed that urinary IL-18 was significantly increased 24 hours after administration of GBCAs and could be used as an early predictor of gadolinium-induced nephrotoxicity in elderly patients. In our study, there was a significant rise in IL-18 as early as three hours after contrast administration in patients with normal baseline creatinine. As we have not measured creatinine later than 24 hours, clinical significance of this finding is unknown. The following decrease in IL-18 at 24 hours may suggest that this rise is of no clinical importance.
NAG is a proximal tubule lysosomal enzyme that has been extensively studied.13 Increased urinary concentration is a sensitive marker for loss of lysosomal integrity by proximal tubule injury.22 Similar to urinary IL-18, our study showed statistically significant rise at three hours and return to baseline at 24 hours.
Limitations
This study has some limitations including small sample size and short follow-up. Furthermore, it is possible that we did not account for all other causes of variations in biomarker concentrations. Ideally, a control group would have helped provide better evidence for causality. Moreover, it would have been interesting to measure KIM-1 given the available literature but we did not have the necessary tools.
Conclusions
In this study, we found that urinary IL-18 and NAG levels increased transiently after administration of GBCAs in patients with normal renal function. No statistically significant differences were observed for urinary and serum NGAL levels and for serum cystatin C levels. The clinical significance of these findings remains to be determined.
Acknowledgments
The authors thank Naoual Elftouh from Maisonneuve-Rosemont Hospital for her help in the statistical analysis of the data.
Abbreviations
- MRI
magnetic resonance imaging
- GBCA
gadolinium-based contrast agent
- ICA
iodinated contrast agent
- CIN
contrast-induced nephropathy
- AKI
acute kidney injury
- IL-18
interleukin-18
- NAG
N-acetyl-glucosaminidase
- NGAL
neutrophil gelatinase-associated lipocalin
- KIM-1
kidney injury molecule-1
- GFR
glomerular filtration rate
Footnotes
ACADEMIC EDITOR: Karen Pulford, Editor in Chief
PEER REVIEW: Four peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1,044 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Contributed to the conception of the study: HM, L-PL, J-FN, FAL, NH, MV, VP, ML. Contributed to the acquisition of data: FAL, NH. Drafted the work: HM, L-PL. Contributed to the critical revision of the study: HM, L-PL, J-FN, FAL, NH, MV, VP, ML. All authors reviewed and approved the final manuscript.
REFERENCES
- 1.Penfield JG, Reilly RF. What nephrologists need to know about gadolinium. Nat Clin Pract Nephrol. 2007;3(12):654–68. doi: 10.1038/ncpneph0660. [DOI] [PubMed] [Google Scholar]
- 2.Rofsky NM, Weinreb JC, Bosniak MA, Libes RB, Birnbaum BA. Renal lesion characterization with gadolinium-enhanced MR imaging: efficacy and safety in patients with renal insufficiency. Radiology. 1991;180(1):85–9. doi: 10.1148/radiology.180.1.2052729. [DOI] [PubMed] [Google Scholar]
- 3.Bellin MF, Deray G, Assogba U, et al. Gd-DOTA: evaluation of its renal tolerance in patients with chronic renal failure. Magn Reson Imaging. 1992;10(1):115–8. doi: 10.1016/0730-725x(92)90380-i. [DOI] [PubMed] [Google Scholar]
- 4.Arsenault TM, King BF, Marsh JW, et al. Systemic gadolinium toxicity in patients with renal insufficiency and renal failure: retrospective analysis of an initial experience. Mayo Clin Proc. 1996;71(12):1150–4. doi: 10.4065/71.12.1150. [DOI] [PubMed] [Google Scholar]
- 5.Townsend RR, Cohen DL, Katholi R, et al. Safety of intravenous gadolinium (Gd-BOPTA) infusion in patients with renal insufficiency. Am J Kidney Dis. 2000;36(6):1207–12. doi: 10.1053/ajkd.2000.19836. [DOI] [PubMed] [Google Scholar]
- 6.Rieger J, Sitter T, Toepfer M, Linsenmaier U, Pfeifer KJ, Schiffl H. Gadolinium as an alternative contrast agent for diagnostic and interventional angiographic procedures in patients with impaired renal function. Nephrol Dial Transplant. 2002;17(5):824–8. doi: 10.1093/ndt/17.5.824. [DOI] [PubMed] [Google Scholar]
- 7.Sam AD, Morasch MD, Collins J, Song G, Chen R, Pereles FS. Safety of gadolinium contrast angiography in patients with chronic renal insufficiency. J Vasc Surg. 2003;38(2):313–8. doi: 10.1016/s0741-5214(03)00315-x. [DOI] [PubMed] [Google Scholar]
- 8.Erley CM, Bader BD, Berger ED, et al. Gadolinium-based contrast media compared with iodinated media for digital subtraction angiography in azotaemic patients. Nephrol Dial Transplant. 2004;19(10):2526–31. doi: 10.1093/ndt/gfh272. [DOI] [PubMed] [Google Scholar]
- 9.Ergün I, Keven K, Uruç I, et al. The safety of gadolinium in patients with stage 3 and 4 renal failure. Nephrol Dial Transplant. 2006;21(3):697–700. doi: 10.1093/ndt/gfi304. [DOI] [PubMed] [Google Scholar]
- 10.Kane GC, Stanson AW, Kalnicka D, et al. Comparison between gadolinium and iodine contrast for percutaneous intervention in atherosclerotic renal artery stenosis: clinical outcomes. Nephrol Dial Transplant. 2008;23(4):1233–40. doi: 10.1093/ndt/gfm725. [DOI] [PubMed] [Google Scholar]
- 11.Perazella MA. Current status of gadolinium toxicity in patients with kidney disease. Clin J Am Soc Nephrol. 2009;4(2):461–9. doi: 10.2215/CJN.06011108. [DOI] [PubMed] [Google Scholar]
- 12.Herget-Rosenthal S. One step forward in the early detection of acute renal failure. Lancet. 2005;365(9466):1205–6. doi: 10.1016/S0140-6736(05)74787-5. [DOI] [PubMed] [Google Scholar]
- 13.Charlton JR, Portilla D, Okusa MD. A basic science view of acute kidney injury biomarkers. Nephrol Dial Transplant. 2014;29(7):1301–11. doi: 10.1093/ndt/gft510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vanmassenhove J, Vanholder R, Nagler E, Van Biesen W. Urinary and serum biomarkers for the diagnosis of acute kidney injury: an in-depth review of the literature. Nephrol Dial Transplant. 2013;28(2):254–73. doi: 10.1093/ndt/gfs380. [DOI] [PubMed] [Google Scholar]
- 15.Duan S-B, Liu G-L, Yu Z-Q, Pan P. Urinary KIM-1, IL-18 and Cys-c as early predictive biomarkers in gadolinium-based contrast-induced nephropathy in the elderly patients. Clin Nephrol. 2013;80(5):349–54. doi: 10.5414/CN107829. [DOI] [PubMed] [Google Scholar]
- 16.IL-18, ELISA (IL-1F4) Kit. R&D Systems; USA: 2007. [Google Scholar]
- 17.NAG . Instructions du Manufacturier. California: Bio-Quant Inc.; 2007. Colorimetric Assay Kit. [Google Scholar]
- 18.GAL . ELISA Kit 036 inlay, Instructions du Manufacturier. Antibody Shop; Denmark: 2007. [Google Scholar]
- 19.Quantikine, Human Cystatin C Immunoassay. R&D Systems; USA: 2007. [Google Scholar]
- 20.Melnikov VY, Ecder T, Fantuzzi G, et al. Impaired IL-18 processing protects caspase-1-deficient mice from ischemic acute renal failure. J Clin Invest. 2001;107(9):1145–52. doi: 10.1172/JCI12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ling W, Zhaohui N, Ben H, et al. Urinary IL-18 and NGAL as early predictive biomarkers in contrast-induced nephropathy after coronary angiography. Nephron Clin Pract. 2008;108(3):c176–81. doi: 10.1159/000117814. [DOI] [PubMed] [Google Scholar]
- 22.Waring WS, Moonie A. Earlier recognition of nephrotoxicity using novel biomarkers of acute kidney injury. Clin Toxicol. 2011;49(8):720–8. doi: 10.3109/15563650.2011.615319. [DOI] [PubMed] [Google Scholar]