Abstract
BACKGROUND & AIMS
Use of exogenous hormones, in the form of oral contraceptives (OCs), has been linked consistently to risk of Crohn’s disease (CD). Nonetheless, it is not clear how OCs might contribute to the progression of CD.
METHODS
We conducted a prospective study of female patients with CD (age, 16–51 y), identified from the inpatient and outpatient care components of the Swedish National Patient Register from January 2002 through December 2013. Information on current OC use was obtained from the Prescribed Drug Register starting in July of 2005 and updated until December of 2013. Primary outcomes were defined as first CD-related surgery and first steroid prescription. We used Cox proportional hazard modeling with time-varying covariates to estimate multivariable-adjusted hazard ratios (MV-adjusted HRs).
RESULTS
We identified 482 incident cases of surgery among 4036 patients with CD, with a median follow-up period of 58 months. Compared with nonusers, the MV-adjusted HRs for surgery were 1.14 (95% confidence interval [CI], 0.80–1.63) for past users and 1.30 (95% CI, 0.89–1.92) for current users. The risk of surgery increased with longer duration of use (Ptrend = .036) and higher prescribed daily dose (Ptrend = .016). Specifically, for women with more than 3 years of OC use, the MV-adjusted HR for surgery was 1.68 (95% CI, 1.06–2.67). The association was confined to the combination type of OC. We estimated that for every 83 patients with CD receiving the combination type of oral contraceptives for at least 1 year, 1 extra surgery is required. The rate of steroid prescriptions did not appear to increase with past or current use of OCs, compared with patients who have not taken OCs (all Pcomparisons > .20).
CONCLUSIONS
In a nationwide analysis of patients in Sweden, long-term use of OCs, particularly the combination type, was associated with an increased risk of surgery among women with established CD. Clinicians carefully should evaluate and monitor contraceptive options among women with established CD.
Keywords: Crohn’s Disease, Birth Control, Surgery, Swedish National Patient Register
Crohn’s disease (CD) is a chronic inflammatory disorder of the gastrointestinal tract with diverse clinical presentation and natural history.1 Disease presentation is characterized based on the Montreal classification, which includes age of diagnosis, disease location, and disease behavior defined by the presence of stricturing, penetrating, or perianal disease.1 Natural history studies have estimated that more than 40% of patients require surgery within 10 years of diagnosis, and nearly 50% experience stricturing or penetrating disease within 20 years of diagnosis.2,3 Because of this heterogeneity in disease presentation and course, identification of lifestyle and medications that potentially could alter clinical progression is critical to our understanding of the pathogenesis of these disorders. In addition, understanding the role of such factors may guide clinical recommendations.
Exogenous hormone use in the form of oral contraceptives has been linked previously to the risk of CD.4,5 Although the exact mechanism underlying this association is unclear, the effect is plausibly mediated by alterations in intestinal permeability, immune function, and, potentially, the gut microbiome.6–9 Nonetheless, a specific role of oral contraceptives on progression, rather than the etiology of CD, is unclear. The few prior studies that have investigated the link between oral contraceptive use and CD progression have had significant limitations including retrospective design,10 small sample size,10,11 and limited ascertainment of oral contraceptive exposure.10–12 In addition, because of a secular trend in the type and dose of oral contraceptive use,13 more recent studies to evaluate the effect of newer-generation oral contraceptives on CD progression are needed.
We therefore sought to examine the association between oral contraceptive use and risk of CD complications defined by need for surgery and steroid use using a large population-based cohort in Sweden.
Materials and Methods
Study Population
The Swedish health care system is tax-funded and offers universal access including prescription coverage. The Swedish National Board of Health and Welfare has collected individual-level data on hospital discharges on a countywide level since 1964 (nationwide since 1987).14 Each record, organized according to an individual’s personal identity number, includes date of birth, sex, dates of hospital admission, hospital department, and discharge diagnoses (including surgical procedures), coded according to the International Classification of Diseases.15 Since 2001, this registry was expanded to include specialized outpatient care.14
Our study population included women between the ages of 16 (median age of first sexual encounter in Sweden and earliest time of first oral contraceptive [OC] use) and 51 (median age of menopause) with at least 2 inpatient or outpatient encounters with a primary diagnosis of CD (International Classification of Diseases, 9th revision: 555 or International Classification of Diseases, 10th revision: K50) after January 2002. We selected January 2002 as our study starting point to allow for a 1-year lag between the introduction of an outpatient registry and accumulation of prevalent cases of CD that previously were not captured through hospitalizations. The accuracy of International Classification of Diseases coding for disease ascertainment for the inpatient Swedish registry has been validated previously, with a positive predictive value of 85%–95%.16 In addition, a recent study from this cohort showed that this definition yields a stable and reliable prevalence for CD, comparable with those reported previously in Scandinavia and Canada.17 Although there has not been a formal validation study in Sweden about the accuracy of capturing incident cases of CD, preliminary results from an ongoing validation study showed a positive predictive value greater than 93% with even a single inflammatory bowel disease–related encounter (personal communication: Jonas Ludvigsson, December 14, 2015).
Primary Exposure and Other Covariates
Since July 1, 2005, information on all dispensed prescriptions for the entire Swedish population, including date of redemption, amount of drug, and dosage, has been collected in the Swedish Prescribed Drug Register.18 Specifically, sale information about each prescription for all individuals is transferred directly from the cashier to the registry. The registry is virtually complete for the entire population, with identity data missing for less than 0.3% of all items.18 Information on the defined daily dose (DDD) also is available for each prescription. The DDD is a statistical measure of drug consumption defined by the World Health Organization to standardize the comparison of drug use between different drugs or between different health care environments. OC use was identified through Anatomical Therapeutic Chemical codes G03AA or G03AB (combined hormonal contraceptives) and G03AC (progestin-only). These Anatomical Therapeutic Chemical codes have been used previously to describe patterns of OC use in Sweden.19 Information on OC use was updated monthly and entered in our models as current, past, or nonusers. In addition, we calculated and updated the cumulative duration of OC intake and the cumulative DDD of use for each participant by adding the number of months and DDD use over follow-up time, respectively.
We obtained data on education level from the longitudinal integrated database for health insurance and labor market studies, which integrates annually updated administrative information from the labor market and educational and social sectors from 1990 onward for all individuals 16 years or older registered as residents in Sweden.20 Data from the longitudinal integrated database for health insurance and labor market studies are linked to patients with a diagnosis of CD using Swedish residents’ unique personal identity numbers. Information on age, sex, dates of birth, death, or immigration were collected form the Swedish Total Population Register kept by Statistics Sweden. We also calculated and updated the number of CD-related encounters (both inpatient and outpatient) for each participant during the follow-up period. Finally, information on parity was collected from the Swedish Medical Birth Register, which includes information on more than 98% of all births in Sweden since 1973. Information is collected prospectively from standardized prenatal, obstetric, and neonatal records.21 We adjusted for parity in our sensitivity analyses because it may account for differences in disease severity because women with more severe disease are less likely to become pregnant.
Outcome Ascertainment
Information on CD-related surgery was obtained from surgical procedure codes available nationwide in the registry since 1987. The validity of surgical procedure coding in the National Patient Register has been reported to be high, with an estimated 0.1% of procedures miscoded.22 For CD-related surgeries, we used JFB and JFH codes, which refer to partial resection and colectomy, respectively. We defined the need for steroid as the first dispensed prescription for steroids (Anatomical Therapeutic Chemical codes: H02AB06, H02AB07 for oral prednisolone, and A07EA06 for oral budesonide) after the diagnosis of CD.
Statistical Analysis
At baseline, we included women with a diagnosis of CD (ages, 16–51 y) without a prior history of CD-related surgery. Person-time for each participant was calculated from July 1, 2005, or the date of diagnosis, whichever came last, until the date of first CD-related surgery, death from any cause, or December 31, 2013, whichever came first. To avoid immortal time bias, the date of the second CD-related encounter was considered the date of diagnosis of CD. We used Cox proportional hazards modeling, adjusting for covariates to calculate adjusted hazard ratios (HR) and 95% confidence interval (CIs). Because there could be differences in the duration of disease at the time of study entry in July 2005, we adjusted for duration of disease, which was calculated from the time of first CD-related encounter to the study entry, in our multivariable analyses. Finally, we explored the possibility that the effect of oral contraceptive use on risk of surgery or steroid prescription is modified by education level, parity, or number of CD-related encounters by entering oral contraceptive use and these variables in our models as a multiplicative interaction terms. We used SAS version 9.3 (Cary, NC) for these analyses. All P values were 2-sided and less than .05 was considered statistically significant. The study was approved by the regional ethics committee at Karolinska Institutet (Stockholm, Sweden).
Results
Through December 2013, we identified 482 incident cases of surgeries among 4036 CD patients who contributed 260,811 person-months with a median follow-up period of 58 months. Compared with nonusers of oral contraceptives, current and past users were similar according to age, duration of disease, county of residence, and number of encounters during follow-up evaluation (Table 1). Compared with nonusers, past or current users were less likely to have had more than 12 years of education (Table 1).
Table 1.
Characteristics of Participants at Midpoint of the Study
| Oral contraceptive use |
|||
|---|---|---|---|
| Never (N = 1338) |
Past (N = 542) |
Current (N = 641) |
|
| Age, mean (SD), y | 37 (10) | 37 (10) | 35 (11) |
| Duration of disease, mean (SD), y |
5 (1) | 5 (1) | 5 (1) |
| Medications (ever use), % | |||
| Mesalamine | 53 | 52 | 57 |
| Steroids | 82 | 85 | 85 |
| Immunomodulatorsa | 53 | 52 | 53 |
| Anti-TNFb | 15 | 16 | 16 |
| Disease location,c % | |||
| TI | 24 | 26 | 28 |
| Colon | 31 | 31 | 29 |
| Ileocolon | 45 | 43 | 46 |
| Health care region of residence, % |
|||
| Northern Sweden | 9 | 9 | 6 |
| Stockholm-Gotland | 24 | 18 | 24 |
| Southeastern Sweden | 12 | 13 | 10 |
| Southern Sweden | 20 | 24 | 22 |
| Uppsala-Örebro | 21 | 21 | 25 |
| Western Sweden | 14 | 15 | 13 |
| Education, % | |||
| ≤9 y | 16 | 11 | 8 |
| 9–12 y | 38 | 57 | 60 |
| >12 y | 46 | 32 | 32 |
| Number of encounters, mean (SD) |
12 (12) | 13 (11) | 12 (10) |
NOTE. January 2008 was used as the midpoint of the study. TI, terminal ileum; TNF, tumor necrosis factor.
Consists of methotrexate, azathioprine, and 6-mercaptopurine.
Data on infliximab therapy were available only in the following counties: Jämtland, Jönköping, Gotland, Skåne, Västmanland, and Gävleborg.
25% of participants had a missing disease location.
Oral Contraceptives and Risk of Surgery
The most common indication for surgery was ileocecal resection (N = 239), followed by small-bowel resection (N = 54), and total colectomy with ileostomy (N = 54). Overall, 80% of the surgeries were performed for bowel resection and 20% were performed for perianal disease. Compared with nonusers, the multivariable (MV)-adjusted HRs of surgery were 1.14 (95% CI, 0.80–1.63) among past users and 1.30 (95% CI, 0.89–1.92) among current users (Table 2). The risk of surgery appeared to increase with longer duration of use (Ptrend = .036) and higher number of DDD prescribed (Ptrend = .016) (Table 2). Compared with nonusers, the MV-adjusted HRs of surgery were 0.90 (95% CI, 0.56–1.45) with 1 year or less of use, 1.25 (95% CI, 0.83–1.90) with more than 1 and 3 years or less of use, and 1.68 (95% CI, 1.06–2.67) with more than 3 years of use (Table 2). Similarly, compared with nonusers, the MV-adjusted HRs of surgery were 0.94 (95% CI, 0.56–1.58) with 300 or fewer DDDs, 1.25 (95% CI, 0.82–1.89) with more than 300 and 900 or less DDDs, and 1.60 (95% CI, 1.10–2.34) for more than 900 DDDs of use (Table 2). We also explored the link between the number of prescriptions and the duration of oral contraceptive use and the risk of perianal surgery and observed no associations (all Ptrend > .56).
Table 2.
Oral Contraceptive Use and Risk of Surgery
| Never | Past | Current | P trend | ||
|---|---|---|---|---|---|
| Cases/person-months | 420/230,491 | 34/17,938 | 28/12,382 | ||
| Incident rate/100,000 person-months | 18.2 | 19.0 | 22.6 | ||
| Age-adjusted HR (95% CI) | 1.00 | 1.16 (0.81–1.65) | 1.23 (0.84–1.81) | ||
| MV-adjusted HR (95% CI)a | 1.00 | 1.14 (0.80–1.63) | 1.30 (0.89–1.92) | ||
| Cumulative duration of use, y | |||||
|
|
|||||
| 0 | ≤1 | > 1 and ≤3 | >3 | ||
|
|
|||||
| Cases/person-months | 420/230,491 | 18/10,738 | 24/11,588 | 20/7994 | |
| Incident rate/100,000 person-months | 18.2 | 16.8 | 20.7 | 25.0 | |
| Age-adjusted HR (95% CI) | 1.00 | 0.92 (0.57–1.47) | 1.21 (0.80–1.82) | 1.60 (1.02–2.53) | .057 |
| MV-adjusted HR (95% CI)a | 1.00 | 0.90 (0.56–1.45) | 1.25 (0.83–1.90) | 1.68 (1.06–2.67) | .036 |
| Cumulative DDD use | |||||
|
|
|||||
| 0 | ≤300 | >300 and ≤900 | >900 | ||
|
|
|||||
| Cases/person-months | 420/230,491 | 15/8608 | 24/11,346 | 30/12,902 | |
| Incident rate/100,000 person-months | 18.2 | 17.4 | 21.2 | 23.3 | |
| Age-adjusted HR (95% CI) | 1.00 | 0.96 (0.57–1.61) | 1.21 (0.80–1.82) | 1.45 (1.00–2.12) | .046 |
| MV-adjusted HR (95% CI)a | 1.00 | 0.94 (0.56–1.58) | 1.25 (0.82–1.89) | 1.60 (1.10-2.34) | .016 |
Adjusted for age (years), number of encounters, duration of disease (years), and county.
Oral Contraceptives and Risk of Steroid Prescription
We also assessed the effect of oral contraceptive prescription on steroid use. Compared with nonusers, the rate of steroid prescription did not appear to increase with past or current use of oral contraceptive (Table 3). Compared with nonusers, the MV-adjusted HRs of steroid prescription were 0.98 (95% CI, 0.66–1.44) among past users and 1.09 (95% CI, 0.73–1.64) among current users. Similarly, the risk of steroid prescription did not appear to increase with longer duration of use (Ptrend = .88) or higher number of DDDs prescribed (Ptrend = .20) (Table 3).
Table 3.
Oral Contraceptive Use and Risk of Steroid Prescription
| Never | Past | Current | P trend | ||
|---|---|---|---|---|---|
| Cases/person-months | 405/268,059 | 28/20,995 | 25/14,225 | ||
| Incident rate/100,000 person-months | 15.1 | 13.3 | 17.6 | ||
| Age-adjusted HR (95% CI) | 1.00 | 1.01 (0.69–1.49) | 1.18 (0.79–1.76) | ||
| MV-adjusted HR (95% CI)a | 1.00 | 0.98 (0.66–1.44) | 1.09 (0.73–1.64) | ||
| Cumulative duration of use, y | |||||
|
|
|||||
| 0 | ≤1 | >1 and ≤3 | >3 | ||
|
|
|||||
| Cases/person-months | 405/268,059 | 19/12,497 | 22/13,470 | 12/9253 | |
| Incident rate/100,000 person-months | 15.1 | 17.1 | 16.3 | 13.0 | |
| Age-adjusted HR (95% CI) | 1.00 | 1.02 (0.64–1.61) | 1.18 (0.77–1.82) | 1.04 (0.58–1.86) | .58 |
| MV-adjusted HR (95% CI)a | 1.00 | 0.99 (0.63–1.58) | 1.11 (0.72–1.71) | 0.95 (0.53-1.71) | .88 |
| Cumulative DDD use | |||||
|
|
|||||
| 0 | ≤300 | >300 and ≤900 | >900 | ||
|
|
|||||
| Cases/person-months | 405/268,059 | 19/10,104 | 26/13,090 | 22/14,847 | |
| Incident rate/100,000 person-months | 15.1 | 18.8 | 19.9 | 14.8 | |
| Age-adjusted HR (95% CI) | 1.00 | 1.28 (0.81–2.03) | 1.43 (0.96–2.13) | 1.18 (0.77–1.83) | .09 |
| MV-adjusted HR (95% CI)a | 1.00 | 1.27 (0.80–2.02) | 1.33 (0.89–1.99) | 1.11 (0.72–1.72) | .20 |
Adjusted for age (years), number of encounters, duration of disease (years), and county.
Types of Oral Contraceptives and Risk of Steroid Prescription and Surgery
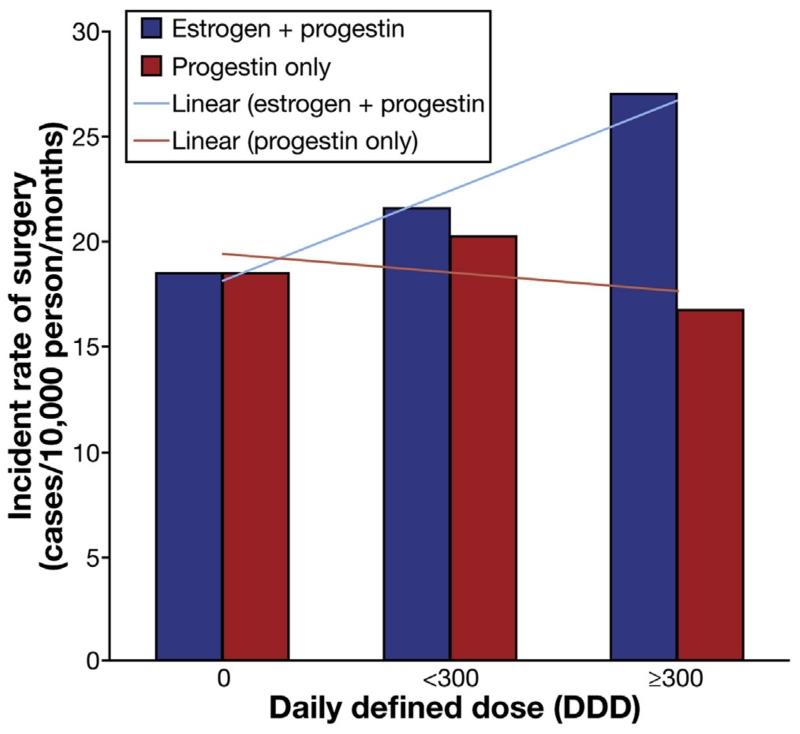
We explored the possibility that the effect of oral contraceptive use on risk of surgery may vary by types of hormones and evaluated the risk according to the progestin-only or combination of estrogen and progestin oral contraceptives. Although similar to our main analyses, longer duration (Ptrend = .041) and higher DDDs (Ptrend = .020) were associated with an increased risk of surgery with combination oral contraceptives, there were no associations between progestin-only–containing pills and risk of surgery (all Ptrend > .50). Specifically, with every 1 year of combination oral contraceptive use, the risk of surgery increased by nearly 30% (MV-adjusted HR, 1.29; 95% CI, 1.05–1.57). Similarly, with every 300 increase in DDD prescription, the risk of surgery increased by 24% (MV-adjusted HR, 1.24; 95% CI, 1.01–1.52) (Figure 1). We estimated that for every 83 CD patients treated for at least 1 year with combination-type oral contraceptives, 1 extra surgery is required. We also explored the link between progestin-containing intrauterine device, which are becoming increasingly popular, and risk of surgery and steroid prescription. Compared with nonusers, the MV-adjusted HR of surgery and steroid prescriptions were 0.98 (95% CI, 0.69–1.40) and 1.03 (95% CI, 0.71–1.60), respectively.
Figure 1.
Type of oral contraceptive use and risk of surgery.
Exploratory and Sensitivity Analyses
Because oral contraceptives are used most commonly among women between the ages of 16 and 40, we performed a sensitivity analysis, limiting our cohort to women in this age range and observed similar association. Compared with nonusers, MV-adjusted HR of surgery for more than 3 years of oral contraceptives use was 1.68 (95% CI, 1.06–2.67; Ptrend = .036).
Since the prescription registry in Sweden was started in 2005, we considered the possibility that women who were oral contraceptive users in 2005 may have been using OCs for varying durations. Thus, we conducted analyses in which we excluded women who were OC users during the first year the prescription registry was started (July 2005 to July 2006), and restricted analyses to follow-up evaluation after July 2006. Compared with nonusers, the MV-adjusted HRs of surgery were 1.17 (95% CI, 0.75–1.82) among past users and 1.41 (95% CI, 0.75–2.64) among current users. The risk of surgery appeared to increase with longer duration of use (Ptrend = .035) and higher number of DDDs prescribed (Ptrend = .012). Specifically, compared with nonusers, the MV-adjusted HRs of need for surgery were 2.73 (95% CI, 1.39–5.36) for women with more than 3 years of use and 2.20 (95% CI, 1.28–3.80) for more than 900 DDD use. Similarly, in these analyses we observed an increase in risk of steroid prescriptions with higher numbers of DDD use (Ptrend = .017).
We considered the possibility that our observed association may be the result of differential use of oral contraceptives among patients with more severe disease and therefore performed a sensitivity analysis further adjusting our models for use of immunosuppressive and anti–tumor necrosis factor medications, which could be associated with more severe disease, and observed no significant change in our effect estimates. Compared with nonusers, the MV-adjusted HR of surgery for more than 3 years of oral contraceptive use was 1.65 (95% CI, 1.01–2.62).
We also evaluated potential differences on the influence of oral contraceptive use on risk of surgery according to parity and observed no effect modification (Pinteraction = .86) (Supplementary Table 1). In addition, the effect of oral contraceptive use on risk of surgery was not modified by education level and number of encounters during follow-up evaluation (all Pinteraction > .20).
Discussion
In a prospective nationwide cohort study, we show that long-term use of oral contraceptives (eg, >900 DDD or >3 years of use) in patients with established CD is associated with an increased likelihood for surgery. In addition, we show that the association is confined to a combination type of oral contraceptives.
Our findings are supported by a prior study. By using the placebo-treated arm of the Canadian Mesalamine for Remission of Crohn’s Disease Study, Timmer et al11 showed that oral contraceptive use is associated with an increased risk of relapse among CD patients. Because of the small sample size (n = 152) and less than 1 year follow-up time of this study, our large nationwide cohort with a median duration of 58 months significantly extends this finding. In contrast, 2 prior studies have shown no effect of oral contraceptive use on risk of surgery or flare-ups.10,12 In a study of 138 CD patients, oral contraceptive use was not associated with an increased risk of recurrent surgery among women with at least one previous surgery.10 However, oral contraceptive use was ascertained only at a single time point and the pattern of use may have changed according to disease behavior (eg, previous surgery). Finally, Cosnes et al12 showed that oral contraceptive use was not associated with an increased risk of CD flare. However, the follow-up time was less than 1 year and a more definitive end point such as surgery was not evaluated.
The exact mechanism by which oral contraceptive use may alter disease progression is unclear. However, a number of hypotheses are biologically plausible. First, oral contraceptive use has been linked to alteration in the intestinal barrier function, a key biologic pathway involved in the pathogenesis of CD.6,8 This alteration also could have prognostic significance among individuals with established disease. Second, oral contraceptive use, through its effect in altering endogenous levels of sex hormones, particularly estrogen, enhances the cellular proliferation and the humoral immune system,7 which in turn may alter disease progression. This hypothesis is supported further by our observation that the effect appears to be limited to estrogen-containing oral contraceptives. In addition, the level of androgens, particularly that of testosterone, also is affected significantly by oral contraceptive use. Testosterone has been shown to modulate immune function, including cytokine production. In animal models, endogenous levels of testosterone are linked to a reduction in the expression of Toll-like receptor 4 on macrophages, which play a fundamental role in pathogen recognition and innate immunity.23 Recent human data also have suggested a link between prediagnostic endogenous levels of testosterone and the risk of CD.24 Therefore, oral contraceptive use through modulating levels of testosterone may alter CD progression. In addition to the potential effect of oral contraceptive use on intestinal barrier function and innate and adaptive immunity, recent animal data have suggested that gut commensal microbes may modulate levels of endogenous testosterone, leading to the development and progression of autoimmune diseases.9 These observations suggest a complex interaction between endogenous levels of sex hormone, immune function, and the gut microbiome in regulating the development and progression of immune-mediated disorders.
Regardless of the potential mechanism, the effect of oral contraceptives on CD progression appears to be related to consistent and long-term use of these medications. A similar pattern of associations also has been reported with other chronic illnesses such as breast cancer and cardiovascular diseases.25 The apparent lack of a statistically significant association between current use of oral contraceptives and risk of surgery may be explained by prior observation in this registry that a substantial percentage of women (~25%) either completely stop using oral contraceptives or switch to a nonhormonal type, particularly intrauterine devices, within 6 months of first oral contraceptive prescription.19 In addition, nearly 15% of women switch from one type of oral contraceptive to another within the first 6 months of the first oral contraceptive prescription.19 Therefore, a large percentage of current users include individuals who only briefly use oral contraceptives.
Our study had several strengths that are worth noting. First, our prospective study minimized the possibility of recall and selection biases that typically are seen in many case-control and cross-sectional studies. Second, because of the excellent nationwide coverage of the Swedish inpatient and outpatient registries, our results represent a more precise estimate of the true effect of oral contraceptives on the risk of CD progression and could be used to make clinical recommendations in patients with established CD. Finally, in our analysis, we updated information on oral contraceptive use every month and used time-varying exposures in our models. Thus, we were able to account for changes in the use or type of oral contraceptive, minimizing the possibility of exposure misclassification.
We acknowledge several limitations. First, differences in disease severity may impact the decision to prescribe oral contraceptives (eg, women with more severe disease are more likely to be prescribed an oral contraceptive). Specifically, in our exploratory analysis (Supplementary Table 1), the effect of combination-type oral contraceptives on risk of surgery was stronger among nulliparous women, thus raising the possibility that voluntary childlessness among women with more severe disease may alter patterns of oral contraceptive use. However, such a bias likely would have led to spurious associations between short-term use of oral contraceptives and risk of surgery and/or steroid prescription. In contrast, we did not observe an association between short term use of oral contraceptives and risk of short-term changes in disease activity as assessed by the need for steroid prescription. In addition, in sensitivity analysis, further adjusting our models for immunosuppressive and anti–tumor necrosis factor medications, which also may be associated with disease severity, did not materially alter our effect estimates. Finally, the specificity of our finding to combination-type oral contraceptives makes it unlikely that the observed association is related to channeling bias. Second, we used surgery and steroid prescriptions as a proxy for disease flare and/or complication and were unable to specifically evaluate the short-term effect of oral contraceptive use on disease activity using various clinical and endoscopic activity indices. In addition, steroid prescriptions may not fully capture all cases of CD flares, particularly because some individuals may have had prior steroid prescriptions and therefore did not require new prescriptions for new episodes of flares. Therefore, the lack of robust associations between oral contraceptive use and disease flare as measured by steroid prescription may be related to nondifferential misclassification of outcomes. Third, we did not have information on smoking, which previously was linked to poor outcomes in CD.11,26–29 However, considering that smoking generally is discouraged in patients using oral contraceptives, particularly the combination type, adjusting for smoking likely would have strengthened our observed associations. Fourth, our cohort consisted almost entirely of Scandinavian women, and therefore it is possible that our findings may not be generalizable to other races/ethnicities. However, there are no data to suggest that the putative biological mechanisms by which oral contraceptives increase the risk and progression of CD would be expected to differ by ethnicities. Finally, we acknowledge that because data on oral contraceptive use before 2005 were not available, we may not be able to fully evaluate the effect of duration of use on the risk of CD surgery. However, in our sensitivity analysis, we show that starting our follow-up period in June 2006 (1 year after the prescription registry was started) actually strengthened our observed association, making it less likely that this may have altered our results.
In conclusion, we show that consistent and long-term use of oral contraceptives, particularly the combination type, is associated with an increased risk of surgery among women with established CD. Future studies should focus on mechanisms by which oral contraceptive use alters the risk and progression of CD. Finally, our data suggest the importance of carefully evaluating contraceptive options among women with established CD.
Supplementary Material
Acknowledgments
This study was approved by the regional ethics committee at Karolinska Institutet (Stockholm, Sweden).
Funding
Supported by a senior investigator grant from the Crohn’s and Colitis Foundation of America and by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; K24 DK098311) (A.C.), and by a career development award from the American Gastroenterological Association and by the National Institute of Diabetes and Digestive and Kidney Diseases (K23 DK099681) (H.K.). This work also was supported by a travel grant from the American College of Gastroenterology.
Abbreviations used in this paper
- CI
confidence interval
- DDD
defined daily dose
- HR
hazard ratio
- MV
multivariable
- OC
oral contraceptive
Footnotes
This article has an accompanying continuing medical education activity, also eligible for MOC credit, on page e13. Learning Objective: At the conclusion of this exercise, the learner will be able to: (a) discuss whether oral contraceptives are associated with an increased risk of developing Crohn’s disease; (b) identify medications associated with an increased risk of surgery to treat Crohn’s disease; and (c) quantify the increased risk of surgery among Crohn’s patients on combination-type oral contraceptives.
Conflicts of interest
These authors disclose the following: Hamed Khalili has received consulting fees from AbbVie, Inc; and Andrew Chan has served as a consultant for Bayer Healthcare, Pfizer, Inc, and Pozen, Inc. The remaining authors disclose no conflicts.
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at http://dx.doi.org/10.1053/j.gastro.2016.02.041.
References
- 1.Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19(Suppl A):5A–36A. doi: 10.1155/2005/269076. [DOI] [PubMed] [Google Scholar]
- 2.Solberg IC, Vatn MH, Hoie O, et al. Clinical course in Crohn’s disease: results of a Norwegian population-based ten-year follow-up study. Clin Gastroenterol Hepatol. 2007;5:1430–1438. doi: 10.1016/j.cgh.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Peyrin-Biroulet L, Loftus EV, Jr, Colombel JF, et al. The natural history of adult Crohn’s disease in population-based cohorts. Am J Gastroenterol. 2010;105:289–297. doi: 10.1038/ajg.2009.579. [DOI] [PubMed] [Google Scholar]
- 4.Khalili H, Higuchi LM, Ananthakrishnan AN, et al. Oral contraceptives, reproductive factors and risk of inflammatory bowel disease. Gut. 2013;62:1153–1159. doi: 10.1136/gutjnl-2012-302362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cornish JA, Tan E, Simillis C, et al. The risk of oral contraceptives in the etiology of inflammatory bowel disease: a meta-analysis. Am J Gastroenterol. 2008;103:2394–2400. doi: 10.1111/j.1572-0241.2008.02064.x. [DOI] [PubMed] [Google Scholar]
- 6.Braniste V, Jouault A, Gaultier E, et al. Impact of oral bisphenol A at reference doses on intestinal barrier function and sex differences after perinatal exposure in rats. Proc Natl Acad Sci U S A. 2010;107:448–453. doi: 10.1073/pnas.0907697107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cutolo M, Capellino S, Straub RH. Oestrogens in rheumatic diseases: friend or foe? Rheumatology. 2008;47(Suppl 3):iii2–iii5. doi: 10.1093/rheumatology/ken150. [DOI] [PubMed] [Google Scholar]
- 8.Looijer-van Langen M, Hotte N, Dieleman LA, et al. Estrogen receptor-beta signaling modulates epithelial barrier function. Am J Physiol Gastrointest Liver Physiol. 2011;300:G621–G626. doi: 10.1152/ajpgi.00274.2010. [DOI] [PubMed] [Google Scholar]
- 9.Markle JG, Frank DN, Mortin-Toth S, et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339:1084–1088. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- 10.Sutherland LR, Ramcharan S, Bryant H, et al. Effect of oral contraceptive use on reoperation following surgery for Crohn’s disease. Dig Dis Sci. 1992;37:1377–1382. doi: 10.1007/BF01296007. [DOI] [PubMed] [Google Scholar]
- 11.Timmer A, Sutherland LR, Martin F. Oral contraceptive use and smoking are risk factors for relapse in Crohn’s disease. The Canadian Mesalamine for Remission of Crohn’s Disease Study Group. Gastroenterology. 1998;114:1143–1150. doi: 10.1016/s0016-5085(98)70419-6. [DOI] [PubMed] [Google Scholar]
- 12.Cosnes J, Carbonnel F, Carrat F, et al. Oral contraceptive use and the clinical course of Crohn’s disease: a prospective cohort study. Gut. 1999;45:218–222. doi: 10.1136/gut.45.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans G, Sutton EL. Oral contraception. Med Clin North Am. 2015;99:479–503. doi: 10.1016/j.mcna.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Socialstyrelsen [Accessed April 26, 2016];In English - the National Patient Register. http://www.socialstyrelsen.se/english. Published 2011.
- 15.Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, et al. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24:659–667. doi: 10.1007/s10654-009-9350-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. doi: 10.1186/1471-2458-11-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Busch K, Ludvigsson JF, Ekstrom-Smedby K, et al. Nationwide prevalence of inflammatory bowel disease in Sweden: a population-based register study. Aliment Pharmacol Ther. 2014;39:57–68. doi: 10.1111/apt.12528. [DOI] [PubMed] [Google Scholar]
- 18.Wettermark B, Hammar N, Fored CM, et al. The new Swedish Prescribed Drug Register—opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf. 2007;16:726–735. doi: 10.1002/pds.1294. [DOI] [PubMed] [Google Scholar]
- 19.Josefsson A, Wirehn AB, Lindberg M, et al. Continuation rates of oral hormonal contraceptives in a cohort of first-time users: a population-based registry study, Sweden 2005-2010. BMJ Open. 2013;3:e003401. doi: 10.1136/bmjopen-2013-003401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.StatisticsSweden [Accessed April 26, 2016];Background facts, labor and education statistics, integrated database for labor market research. http://www.scb.se/Pages/PublishingCalendarViewInfo____259923.aspx?PublObjId=11454. Published 2009.
- 21. [Accessed April 26, 2016];The Swedish Medical Birth Register: a summary of content and quality. http://www.socialstyrelsen.se/publikationer2003/2003-112-3. Published 2003.
- 22.Spetz CL, Carlsson CL, Engqvist M, et al. Reintroduction of social security numbers gives better basis for evaluation. The patient registry is open for research. Lakartidningen. 1996;93:1844–1847. [PubMed] [Google Scholar]
- 23.Rettew JA, Huet-Hudson YM, Marriott I. Testosterone reduces macrophage expression in the mouse of toll-like receptor 4, a trigger for inflammation and innate immunity. Biol Reprod. 2008;78:432–437. doi: 10.1095/biolreprod.107.063545. [DOI] [PubMed] [Google Scholar]
- 24.Khalili H, Ananthakrishnan AN, Konijeti GG, et al. Endogenous levels of circulating androgens and risk of Crohn’s disease and ulcerative colitis among women: a nested case-control study from the nurses’ health study cohorts. Inflamm Bowel Dis. 2015;21:1378–1385. doi: 10.1097/MIB.0000000000000385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manson JE. Current recommendations: what is the clinician to do? Fertil Steril. 2014;101:916–921. doi: 10.1016/j.fertnstert.2014.02.043. [DOI] [PubMed] [Google Scholar]
- 26.Cosnes J, Carbonnel F, Beaugerie L, et al. Effects of cigarette smoking on the long-term course of Crohn’s disease. Gastroenterology. 1996;110:424–431. doi: 10.1053/gast.1996.v110.pm8566589. [DOI] [PubMed] [Google Scholar]
- 27.Cottone M, Rosselli M, Orlando A, et al. Smoking habits and recurrence in Crohn’s disease. Gastroenterology. 1994;106:643–648. doi: 10.1016/0016-5085(94)90697-1. [DOI] [PubMed] [Google Scholar]
- 28.Duffy LC, Zielezny MA, Marshall JR, et al. Cigarette smoking and risk of clinical relapse in patients with Crohn’s disease. Am J Prev Med. 1990;6:161–166. [PubMed] [Google Scholar]
- 29.Nunes T, Etchevers MJ, Merino O, et al. Does smoking influence Crohn’s disease in the biologic era? The TABACROHN study. Inflamm Bowel Dis. 2013;19:23–29. doi: 10.1002/ibd.22959. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.