Abstract
Introduction
Significant advances have been made to understand the mechanisms involved in cardiac cell-based therapies. The early translational application of basic science knowledge has led to several animal and human clinical trials. The initial promising beneficial effect of stem cells on cardiac function restoration has been eclipsed by the inability of animal studies to translate into sustained clinical improvements in human clinical trials.
Areas covered
In this review, the authors cover an updated overview of various stem cell populations used in chronic heart failure. A critical review of clinical trials conducted in advanced heart failure patients is proposed, and finally promising avenues for developments in the field of cardiac cell-based therapies are presented.
Expert opinion
Several questions remain unanswered, and this limits our ability to understand basic mechanisms involved in stem cell therapeutics. Human studies have revealed critical unresolved issues. Further elucidation of the proper timing, mode delivery and prosurvival factors is imperative, if the field is to advance. The limited benefits seen to date are simply not enough if the potential for substantial recovery of nonfunctioning myocardium is to be realized.
Keywords: cell sources, cell-based therapy, heart failure, stem cells
1. Introduction
The use of stem cells for cardiac regeneration has been intensely studied for nearly a decade. Significant advances in stem cell biology, including better understanding of the mechanisms of stem cell plasticity and differentiation, have paralleled the evolving animal and human clinical trials. However, the promise of stem cell therapeutics in humans remains largely unrealized. The promising beneficial effect of stem cells on cardiac regeneration reported in earlier animal studies has still failed to translate into sustained clinical improvements in human clinical trials. The modest benefits of stem cell therapeutics in human trials have thus refocused inquiry in both basic and clinical aspects of stem cell therapeutics. Critical gaps remain in the understanding of the basic mechanisms involved in stem cell therapeutics, including harvesting, isolation and differentiation steps of the ideal cell type, in vitro preparation and optimization of engraftment, mobilization and in vivo strategies to enhance survival. Human studies have underscored critical unresolved clinical issues, including the vehicle, route and timing of administration and assessment of clinical efficacy. Despite these formidable challenges, the potential of stem cell therapeutics in heart failure remains provocative. In patients with congestive heart failure, progressive myocyte loss from persistent and uncorrected ischemia, sustained inflammation processes and apoptosis result in worsening fibrosis and replacement of functioning myocardium with scar. Although initially compensatory, neurohormonal activation of the adrenergic and renin–angiotensin system only serves to inexorably worsen the adverse remodeling leading to further myocardial dysfunction. Within this active microenvironment, cardiac cell-based therapies could promote or directly provide cardiac regeneration, sharply attenuating the otherwise progressive and ultimately fatal course of chronic heart failure. We propose an updated review of the various stem cell populations used in chronic heart failure. We will present a critical review of clinical trials conducted in advanced heart failure patients, and finally present promising avenues for development in the field of cardiac cell-based therapies.
2. Stem cell sources
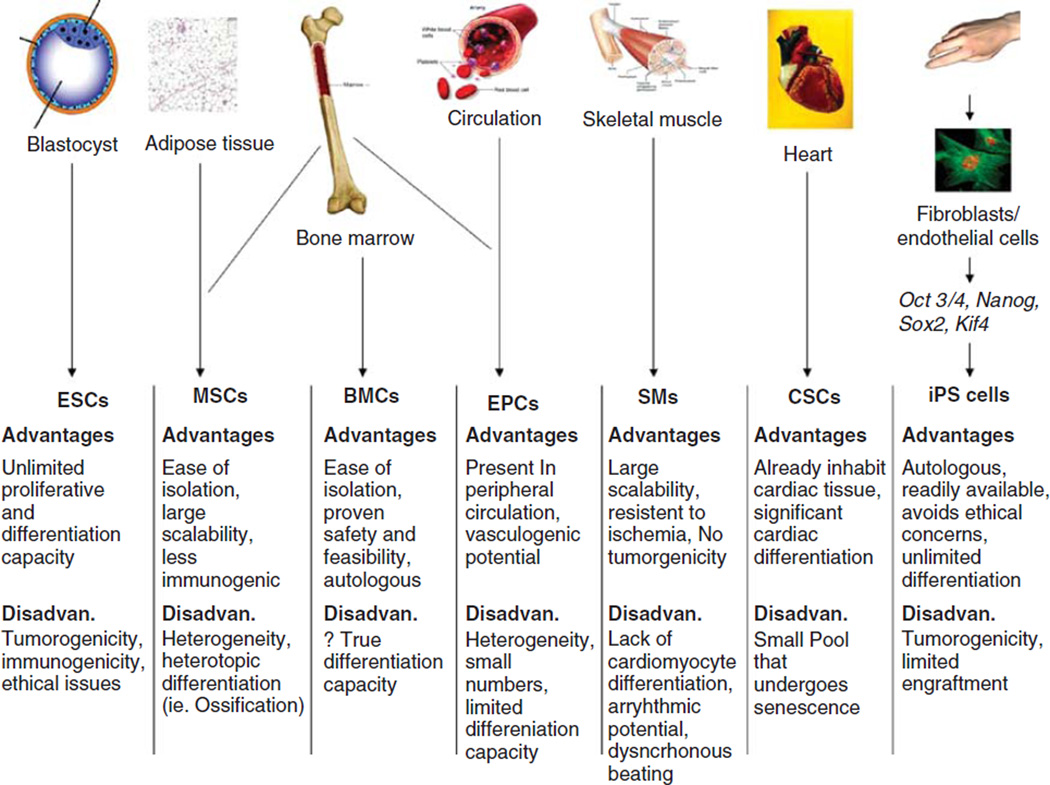
Since the initial demonstration of the regenerative capacity of progenitor cells, several various stem cell populations have been investigated. Each cell line possesses potential advantages and limitations (Figure 1). It is, thus, imperative to understand the different cell line populations and their application in cardiac-based stem cell therapy.
Figure 1. Schematic diagram of progenitor and stem cell preparations that have been used in experimental animal models and/or clinical trials for cardiac repair and regeneration.
The major advantages and disadvantages of each cell type for cardiac cell therapy are summarized below.
2.1 Fate of ‘the ideal’ stem cell
Embryonic stem cells (ESCs) have always been represented as the ideal source for stem cell therapy, as they possess a theoretical limitless proliferation and differentiation capacity. Their immense plasticity renders them an ideal stem cell source for repair of any tissue in the human body. Notably, ESCs retain the ability to form cardiomyocytes, which provides the rational for the intense focus on their use in cardiac regenerative therapy [1]. Once permitted to differentiate, the cells coalesce to form embryoid bodies and consequently develop into the cellular derivatives of all three germ layers [2]. With the aid of specific morphogens or other small molecular compounds, this differentiation can be directed along specific cell lines, namely cardiomyocytes [3]. The application of both nascent and committed ESCs in animal models of myocardial infarction has proved to be promising as it demonstrated encouraging evidence in favor of increased cell survival, prolonged engraftment and improvement in ventricular function [4,5]. There are, however, several aspects of this stem cell population that has hindered its clinical use. The ability of ESCs to differentiate into essentially any cell type can represent a double-edged sword. While ESCs indeed demonstrated robust cardiac potential, other cell types are invariably collaterally produced, leading to legitimate concerns over teratoma formation [6]. Even when differentiation is intensely directed by biomodulators, several cell types can be observed. While these implantable teratomas are largely benign, reports of transplanted cells expressing markers similar to malignant subtypes have surfaced [7]. Once considered immunoprivileged, there is now mounting evidence of human leukocyte antigen expression in ECSs, especially following differentiation [8]. Allogenic implantation could, thus, lead to cell-mediated destruction and enhanced inflammation thereby nullifying any benefits on cardiac regeneration. The immature nature of ECSs-derived cardiomyocytes often referred as early embryonic cardiac cells further limits their regenerative capacity, and it might be necessary to develop maturation protocols to guide cells to adopt the electrophysiological and mechanical properties of functional cardiomyocytes [9]. Finally, the use of embryos as a stem cell source has social and ethical ramifications, which have sparked intense debate among the scientific and lay communities. In 2009, Geron Corp. (CA, USA) has received approval from the US Food and Drug Administration (FDA) for a Phase I clinical trial of ESCs-based therapy in patients with acute spinal cord injury. Despite no reported events one year after initiation of enrollment, the trial was abruptly halted in 2012.
2.2 Promises of induced pluripotent stem cells
The notion of ESCs as a potential source for cell therapy was strengthened by the development of induced pluripotent stem (iPS) cells. The production of these cells rests on a core set of proteins (Oct 3/4, Sox2, Klf4, Nanog), which form an autoregulatory loop that maintains the pluripotency state of ESCs [10]. Exogenous overexpression of pluripotency factors alters gene expression in somatic cells resulting in reprogramming to a dedifferentiated state and expression of endogenous genetic programs of pluripotency. The cells, then, behave as ESCs and can be subsequently directed to differentiate along specific cell lines [11]. This remarkable reprogramming has now been demonstrated in both animal and human somatic cell lines [12]. Originally achieved through retroviral transfection, newer methods to induce pluripotency integration are under investigation [13]. Implantation of iPS cells into infarcted mice myocardium leads to improved structure and functions [14].
The use of iPS cells is a promising endeavor, and it avoids bioethical issues surrounding the use of embryos. It should, however, be noted that iPS cells present many of the same challenges observed with ESCs, such as tumorigenesis, limited in vivo cardiac differentiation and minimal engraftment. Reprogramming is also highly inefficient and independently engineered iPS cells have differences in gene expression profiles, epigenetic signatures and differentiation capacity [15]. The cardiogenic potential is also particularly poor [16], suggesting that it will be necessary to define universal standards for iPS cells and their derivatives. Recent studies show promises as it has been demonstrated that it is possible to derive cardiomyocytes through direct reprogramming of cardiac fibroblasts using cardiac-specific transcriptional regulators (Gata-4, Mef2c and Tbx5), bypassing the need to generate embryonic-like stem cells [17].
2.3 Bone marrow-derived stem cells and derivative
Because of the practical and ethical limitations of pluripotent ESCs, various adult stem cell populations have been used for cell therapy. Bone marrow-derived stem cells (BMCs) represent a readily available, ample supply of adult stem cells. They have been shown to differentiate into smooth muscle, endothelial and cardiac cells in vitro [18,19]. This availability and plasticity has fostered their role as the most extensively studied stem source for cardiac repair. BMCs can be acquired through direct extraction for later implantation or they can be mobilized from marrow stores via cytokine administration and subsequently homed to sites of injury. Both methods have shown to be effective toward infarct attenuation and improvement in ventricular function [20,21]. Early seminal work demonstrated not only a 68% regeneration of infarcted myocardium but also improved survival as well [22,23]. Unfortunately, such robust restoration has been difficult to recapitulate. While most animal models have shown benefit in overall cardiac function, there have been conflicting results with regard to the degree of cardiac differentiation and engraftment of BMCs [24]. This has called into question the in vivo plasticity of BMCs.
One subset of BMCs has been found to possess profound vasculogenic capacity. Given that revascularization is a cornerstone of current treatment for ongoing ischemia, it seems only natural that endothelial progenitor cells (EPCs) would be an ideal cell therapy source. Generally identified by their expression of CD34 or CD133 surface markers, EPCs traverse the peripheral circulation and home to sites of injury and ischemia, where they can contribute to as much as 25% of neovascular formation [25]. They also secrete proangiogenic factors resulting in improved vascular density and perfusion [26]. When exogenously administered, both vascular endothelial growth factor (VEGF) and granulocytes colony-stimulating factor (G-CSF) increase the circulating EPCs and augment neovascularization [27]. Intriguing reports of in vitro differentiation into nonvascular cell types prompted further investigation into EPCs’ potential for cardiac repair [28]. Injection of EPCs into ischemic myocardium inhibited fibrosis and augmented ventricular function [29]. Unfortunately, restricted differentiation has been a barrier to their use for cardiac regeneration. Moreover, patients with known coronary artery disease and cardiovascular risk factors, such as diabetes (the patient population most in need), demonstrate a diminished pool of circulating EPCs [30]. Finally, large heterogeneity of the stem cell population exists in circulation ranging from angioblasts to mature endothelial cells [31]. This diversity has been cited as one of the reasons for conflicting study results. Despite these obstacles EPCs indeed represent a unique stem cell source with substantial vasculogenic potential.
Mesenchymal stem cells (MSCs) can be found in the stroma of bone marrow and represent another subset of BMCs with regenerative potential [32]. MSCs are present in practically every adult tissue including adipose tissue rendering them a unique, readily available stem cell source [33]. These cells can be driven to differentiate cardiomyocytes, which has spurred their use for cardiac regeneration [34]. Preclinical models utilizing MSC implantation following myocardial infarction in rodents, canine and sheep have all demonstrated improvement in overall cardiac function with limited infarct size and reduced remodeling [35–38]. When compared to other stem cell populations, MSCs possess a couple of distinctive advantages. First, the vast scalability of mesenchymal cells coupled with their ease of isolation results in a plentiful stem cell source for exogenous administration [39]. Second, this stem cell cohort is believed to be less immunogenic than alternative cell lines alleviating the risk of rejection and allowing for allogenic transplantation [40]. However, transdifferentiation to cardiomyocytes appears limited in vivo as is engraftment and subsequent regeneration [41,42]. Concerns have also been raised as osteoblasts differentiation and concomitant ossification within myocardial tissue has been observed [43].
2.4 Skeletal myoblasts
Skeletal myoblasts are yet another potential adult stem cell source for use in cardiac repair. These ‘satellite cells’ reside in special niches beneath the basal membrane of skeletal muscle fibers. These cells can be induced to proliferate and migrate under various injurious states, such as ischemia [44]. They fuse with mature muscle cells and promote repair and regeneration of the injured tissue. Skeletal myoblasts have been shown to differentiate into nonmuscle lines in vitro and there is even some evidence that satellite cells possess the ability to become beating cardiomyocytes [45]. This particular stem cell population possesses unique qualities which render it applicable for heart failure therapy. First, satellite cells can be readily obtained and exhibit a profound proliferative capacity such that small biopsies can lead to large reservoirs of stem cells [46]. Second, skeletal myoblasts are inherently resistant to ischemia and oxidative stress which could lead to improved survival following implantation [47]. Finally, they are further differentiated than other stem cell lines and thus less prone to teratoma formation. However, their plasticity remains limited and in vivo studies have failed to demonstrate cardiac differentiation following transplantation [48]. Although a subset of implanted cells survives, skeletal myoblasts do not fuse with host tissue. Cells form myotubules which are functionally isolated from neighboring cardiac cells, and these myotubules contract to generate dyssynchronous beating with the surrounding myocardium [48]. Regardless, benefits have been observed following myoblast implantation and may be in part due to a ‘scaffolding’ effect that attenuates remodeling [49].
3. Stem cell trials: are we ‘closer’ yet?
Early clinical trials focused primarily on the safety and feasibility of stem cell implantation and as such were performed in a limited number of patients. Fuchs et al. [50] conducted a pilot study in 10 patients with a left ventricular ejection fraction (LVEF) > 30% and chronic ischemia not amenable to revascularization. Filtered BMCs were directly injected into ischemic myocardium identified by electromechanical mappings. A reduction in anginal symptoms and improvement in quality of life scores was observed at 3 months. Perin et al. [51] reported the use of stem cells in a larger feasibility cohort. The study included 21 patients with chronic ischemic cardiomyopathy injected with BMCs. Although no other serious adverse events were observed, authors did report two deaths. At 4 months, they observed an increase in LVEF (from 20 to 29%) and a significant improvement in mechanical performance in the injected segments as determined by electromechanical mapping. Silva et al. [52] used a similar technique to inject BMCs in five patients awaiting heart transplant. After 6 months, the maximal myocardial oxygen consumption improved from 10.3 ± 3 to 16.3 ± 7 mL/kg/min. Four of five patients improved enough to be removed from the transplant list. Conflicting smaller study revealed no benefit with stem cell infusion. Kuethe et al. [53] assessed BMCs benefits in five patients and reported no intergroup improvement in LVEF or peak oxygen consumption.
While these initial studies demonstrated the safety and feasibility of cell therapy in this patient population, they also revealed a number of problems inherent to stem cell-based therapies. The size of the studies renders any significant conclusions obsolete. Additionally, each study utilized different inclusion criteria, assessed different endpoints, and while most used electromechanical mapping for direct injection, this mode of delivery was not uniformly performed. Furthermore, it is difficult to determine the similarity of the cell preparations as different studies used different multipotency markers to identify the cell populations.
More recent studies have assessed more thoroughly the clinical benefit of stem cell therapy for chronic heart failure. Using intracoronary injection of BMCs, Strauer et al. [54] reported a 30% reduction in infarct size, a 15% increase in global ejection fraction and a 57% increase in infarction zone wall motion-determined left ventriculography.
In the first randomized trial, Hendrikx et al. [55] included 20 patients with a postinfarction nonviable scar who were to undergo elective coronary artery bypass grafting (CABG). BMCs were isolated, cultivated overnight and subsequently administered via direct injection at the border zone of the infarct at time of surgery. Four months after surgery, LVEF improved from 39.5 ± 5.5 to 43.1 ± 10.9% in the control group and from 42.9 ± 10.3 to 48.9 ± 9.5% in the treatment group. These differences did not reach statistical significance. In contrast, Stamm et al. [56] demonstrated positive results in patients undergoing CABG utilizing direct injection into the infarct border zone. However, in this study, CD133+ bone marrow cells were used. LVEF had a greater improvement in treated patients (from 37.4 ± 8.4 to 47.1 ± 8.3%) compared to control patients (from 37.9 ± 10.3 to 41.3 ± 9.1%).
In the first trial of significant size, Assmus et al. [57] randomized 75 patients 3 months postinfarct to infusion with circulating EPCs, BMCs or no cell infusion. Cells were infused into the artery supplying the most dyskinetic area using balloon occlusion. The primary endpoint assessed was the absolute change in LVEF at 3 months. Changes in LVEF in the control group, EPC group and BMC group were −0.3 ± 3.4, 0.4 ± 3.0 and 3.7 ± 4.0%, respectively. In the subset of patients who could undergo cardiac MRI (those without defibrillators), the LVEF increased 4.8 ± 6.0% in the BMC group compared to 2.8 ± 5.2% in the EPC group. Investigators concluded that the BMCs group significantly increased LVEF, while EPC infusion had no effect.
In a randomized trial involving 24 patients, Losordo et al. [58] assessed the effect of peripheral CD34+ cells in patients suffering from intractable angina not deemed candidates for revascularization. G-CSF was administered for 5 days resulting in an increase in peripheral circulating CD34+ cells as previously discussed. The cells were subsequently isolated and injected into the ischemic zone identified by SPECT scanning using the NOGA system. There were no serious adverse events in either the treatment group or the placebo group. Although the sample size was small, they did observe a slight benefit in the treatment group as assessed by angina frequency, NTG use and exercise tolerance.
In a more recent paper van Ramshorst et al. [59], patients with chronic angina and low LVEF were randomized in a one to one fashion to isolate BMCs or saline injection utilizing the NOGA catheter system into the ischemic region identified by SPECT imaging. At 3 months, there was a considerable improvement in the Summed Stress Score, the CSS class and the Quality of Life Score in the BMCs group. Authors also noted an increase in the LVEF by 3% in the treatment group, compared to no change in the placebo group, and LVEF also improved in the treatment group.
Finally, one study utilized skeletal myoblasts as the stem cell source. Menasche et al. [60] randomized 120 patients with an LVEF > 15 and < 35% who were undergoing CABG to injection with either 400 × 106 cells, 800 × 106 cells or placebo. Six months after the procedure, there was no difference in either regional or global LV function determined by echocardiography.
While several of the aforementioned trials did demonstrate some beneficial effects of stem cell therapy, it is evident that much more work is needed to further elucidate the utility of this therapy in chronic heart failure. Results from original NIH-sponsored trials such as ‘The Transendocardial Autologous Cells in Ischemic Heart Failure (TAC-HFT)’ [61], and the ‘Prospective Randomized Study of Mesenchymal Stem Cell Therapy in Patients Undergoing Cardiac Surgery (PROMETHEUS)’ [62] bring hope for a better understanding of cardiac cell-based therapies. More importantly, large trials utilizing standardized techniques for harvest, isolation and delivery assessing pertinent clinical endpoints are imperative to better delineate the efficacy of stem cell implantation. Nevertheless, the trials to date do raise interesting questions and encourage further research.
4. Future avenues
4.1 Paracrine effect
Although many studies have demonstrated decreased infarct size, reduced remodeling and improvement in overall cardiac function, it remains to be fully understood how these favorable effects are derived. Despite the extensive number of stem cells infused, only a small fraction remains viable in the host tissue for any considerable length of time [63]. Those that do survive are typically not in large enough quantity to account for the beneficial effects seen on ventricular function. Furthermore, positive findings have been reported as early as 24 h after stem cell implantation, much too short of an interval for substantial regeneration to occur [64]. Finally, similar effects on cardiac function have been seen despite the use of different stem cell populations. Therefore, it was suggested (and now shown to have significant merit) that the implanted stem cells act as a reservoir for secretable trophic factors, which enhance cell survival, induce vasculogenesis and limit fibrosis. These so-called paracrine effects have been studied intensely in recent years and much information has been brought to bear on the subject.
When cultured in growth, media stem cells secrete a number of cytoprotective factors [65]. These factors are further upregulated upon introduction of the cells to a hypoxic environment. Interestingly, even after the stem cells have been removed, the ‘conditioned media’ alone is enough to attenuate apoptosis when compared to controls [66]. Moreover, stem cells enhance the survival of hypoxic cardiomyocytes although separated by a semipermeable membrane [64]. Cytoprotective factors are felt to be important effectors of this enhanced protection [67]. Blockade of pathways, such as PI3K, blunts the cardioprotection rendered by progenitor cells [64]. Moreover, MSCs engineered to overexpress AKT-1 greatly enhance cell survival. In both rat and pig models, injection of AKT-1 MSC conditioned the medium significantly reducing infarct size and apoptosis in ischemic border zones [68,69]. Sfrp2, a Wnt signaling antagonist, has been found to be an important regulator of this pro-survival pathway, and as such, suppression of Sfrp2 blocks the positive affect of AKT-1 MSCs [70]. Stromal cell-derived factor-1 (SDF-1) and TB4 are the downstream effectors of this pathway and have demonstrated beneficial effects as well. SDF-1 expressing MSCs improve ventricular function without evidence of significant engraftment [71]. Injection of TB4 following myocardial infarction improves ventricular function and limits scar formation [72]. There is ongoing work to further elucidate the pro-survival effects that stem cells have on neighboring myocardium.
Implantation of stem cells has also been shown to increase vasculogenesis. Although EPCs are home to sites of injury and incorporate into the microvasculature, there seems to be a paracrine-mediated affect as well [73,74]. MSCs injected into ischemic hindlimb models increased distal perfusion and vascular density, despite the lack of incorporation of stem cells into mature vessels [75]. Conditioned medium alone injected into ischemic rat hearts led to increased vascular density when compared to controls [76]. These effects are felt to be mediated at least in part by bFGF, VEGF and angiopoietin-1 which are significantly upregulated following stem cell implantation [74].
There is now compelling evidence that at least some of the benefits observed following stem cell implantation results from a paracrine effect the cells have on the surrounding tissue. Whether or not this is the principle factor at play remains controversial. Regardless, these findings prompt further elucidation. Evidence from AKT-1 overexpressing stem cells has lent credence to the notion of improving stem cells’ trophism and subsequent effect on cardiac improvement [69]. Can we recapitulate these findings in human models and is it feasible to do so in clinical practice? Second, if the favorable effects seen are largely the result of paracrine factors, then the question arises whether stem cell implantation is needed at all. Instead pharmacotherapy consisting of pro-survival, vasculogenic and antifibrotic factors could be administered alleviating the risks and labor-intensive process of stem cell harvest, growth and implantation. On the other hand is the real benefit of stem cell therapy derived from a cell population that can sense and react to an internal milieu with sustainable targeted therapy? These questions and others still remain with regard to the paracrine effects of stem cells.
4.2 Cardiac stem cells
The classically held view of the heart as a postmitotic organ possessing no regenerative capacity has shaped our therapeutic strategies in treating heart failure for the past several decades. Our current therapies focus largely on limiting infarct expansion and attenuating remodeling. However, more recent findings have challenged this long-held view and raise the possibility of cardiac myocyte turnover. The discovery of cardiac chimerism in sex-mismatched cardiac transplants prompted this paradigm shift [77]. Soon after implantation of a female donor heart into a male host, there are found resident myocytes and endothelial cells that bear Y chromosomes indicating infiltration and engraftment of host-derived cells. Moreover, cardiomyocytes undergoing mitosis have been observed in such pathologic conditions as hypertension and heart failure [78]. Ultimately pools of cells morphologically different from mature cardiomyocytes and expressing stem cell markers were discovered solidifying that at least a subset of cardiomyocytes do indeed undergo regeneration [79]. Therefore, while a large portion of the heart is in fact postmitotic, the organ as a whole can no longer be viewed as such.
Although there remains a great deal of controversy and heterogeneity in defining cardiac stem cells (CSCs), there is no doubt that there are cell populations within the adult heart that exhibit various stem cell markers. To date, a variety of markers indicating ‘stemness’ has been used to identify this cell population. These include MDR1 (found in ‘side population’ cells which retain the ability to exclude Hoechst dye), c-Kit, Sca-1 and Isl-1 [80]. Considerable debate also continues as to the origin and regenerative capacity of these stem cells. Side population and Isl-1+ cells appear to reside in cardiac tissue as remnants of cardiac morphogenesis [81]. Alternatively, evidence suggests that c-kit+ cells inhabit the bone marrow and are upregulated and migrate to cardiac tissue in the setting of ischemic injury [82]. Regardless of the origin, the degree of differentiation and engraftment appears to require neighboring viable cardiomyocytes contributing an as-yet-unknown amount and identity of paracrine factors which aid in functional maturation of the CSCs [83].
The role of CSCs in nature has been the subject of intense research. Recent evidence dating cardiomyocytes using DNA integration of Carbon-14 in individuals born prior to Cold War era nuclear testing indicate that almost half of all cardiac cells undergo replacement during the typical lifespan [84]. This appears to be part of the organ’s homeostasis to protect against the normal ‘wear and tear’ experienced throughout one’s lifetime. As mentioned, myocyte turnover is accelerated in such conditions as ischemia, hypertension and heart failure [78]. Unfortunately, the innate ability for regeneration appears inadequate to repair the organ following profound insults. There are several possible reasons for this inconsistency. First, an ischemic insult leads to attrition of the local stem cell pool as well as the surrounding mature myocytes [85]. Second, evidence suggests that cardiomyocyte repopulation and regeneration is largely relegated to the infarct border zone [86]. It appears that the stem cells are unable to survive in the injured myocardium, which is likely due to various factors, including persistent ischemia, production of reactive metabolites and ongoing inflammation. Furthermore, stem cells appear to require viable neighboring cardiomyocytes in order to differentiate into mature functioning cardiac cells. Paradoxically, although the absolute numbers of CSCs is increased in injurious states, a larger proportion is rendered senescent and is unable to undergo further differentiation [85]. In addition, there is a reduction in the CSC pool with age [87]. Therefore, it seems that at times when the stem cells are needed most, the regenerative capacity is ineffective in bringing about true recovery.
While the innate regenerative capacity of the heart may be limited, CSCs represent another potential exogenous stem cell source for cardiac repair. CSCs can be acquired from endomyocardial biopsies and subsequently isolated via enzymatic digestion. The cells can then be grown in culture as cardiospheres and later implanted into injured myocardium [88]. Preclinical studies utilizing exogenous CSCs have resulted in regeneration, vasculogenesis and improved ventricular function [89]. However, thus far, the benefits appear to be confined to the border zones of infarcts. The first Phase I clinical study using cardiosphere-derived cells (CArdiosphere-Derived aUtologous stem Cells to reverse ventricular dysfunction [CADUCEUS]) randomly allocated patients to assess the reparative capacity of this special stem cell cohort in human patients. Positive unprecedented results showing increase in myocardial viability and therapeutic regeneration has revived the clinical enthusiasm for cardiac cell-based therapies [90]. Although many obstacles remain, CSCs represent a unique population that is ideally suited for regeneration. Perhaps with improved methods of delivery and enhanced survival, this stem cell population may provide the best hope of complete regeneration. Furthermore, the notion of enhancing the innate ability of the heart to repair itself has the potential to alleviate the need for exogenous stem cell implantation altogether. It is only through a further understanding of this stem cell population that these questions may be answered.
4.3 Regeneration
Although the paracrine actions of stem cells are likely playing a role in the modest benefits seen on ventricular function, the principal goal of stem cell therapy remains true regeneration or prevention of scar tissue. This can only be brought about through prolonged survival, differentiation and engraftment of the implanted cell population. In vitro evidence suggests that a variety of stem cell populations can differentiate into functional cardiomyocytes and endothelial cells. Orlic et al. demonstrated an impressive 68% regeneration when these cells were implanted into mouse models of myocardial infarction [22]. However, over a decade of research has since shown that the translation of in vitro findings to real clinical benefit has been difficult. Several key barriers still exist. Among those are ways to better enhance survival, differentiation and engraftment of the exogenous stem cell population.
As previously mentioned many studies demonstrate only temporary inhabitance of exogenous stem cells in host tissue [26]. Thus, early benefits in cardiac function can be lost over time. In order to have prolonged improvement in cardiac function and true regeneration the infused cells must be able to survive. The first way to enhance survival is to ensure the best delivery method. As discussed previously, there are several methods for the implantation of stem cells. However, the ideal route of delivery remains to be identified. Many of the current methods result in the mechanical destruction of a large portion of the injected cells. Intracoronary and intravascular methods lead to substantial washout and entrapment in non-target tissues. There is ongoing research for ways to increase the efficiency of stem cell delivery. One new interesting method of enhancing cell survival involves the engineering of collagen ‘scaffolds’. Sheets of stem cells can be grown on culture-enriched substrates and subsequently delivered to injured myocardium. This network of stem cells provides the structural support for continued growth and nutrient distribution to promote cell survival in a harsh environment [91].
Another area of obscurity relates to the optimal number of cell and timing of delivery. The variations in cell counts and timing have been cited for the disparity in trial results. Meluzin et al. [92] found a cell number-dependent incremental improvement in left ventricular function. Early after myocardial injury there is clearly an upregulation of cell homing mechanism, which may enhance stem cell recruitment [93]. However, ongoing ischemia, production of reactive metabolites and inflammation may render the local milieu too toxic for the survival of transplanted cells. The randomized TIME trial focuses on timing to evaluate early (3 days) versus intermediate (7 days) delivery of stem cells [94].
The optimal growth environment remains to be identified. As mentioned, upregulation of pro-survival factors, such as AKT-1, increases endurance of stem cells and subsequent effect on cardiac function [69]. Other such studies of factor-enriched stem cell populations have echoed these results [95]. The more we understand what factors lead to prolonged survival, the more we may be able to enrich the stem cell population prior to or even after infusion. If the ‘holy grail’ of stem cell therapy remains true regeneration of injured or nonviable tissue, then we must find ways to better enhance stem cell survival and engraftment. Further elucidation of the proper timing, mode delivery and pro-survival factors is imperative, if the field is to advance. The limited benefits seen to date are simply not enough if the potential for substantial recovery of non-functioning myocardium is to be realized.
4.4 Biomedical-engineered stem cells and ‘personalized’ heart machinery
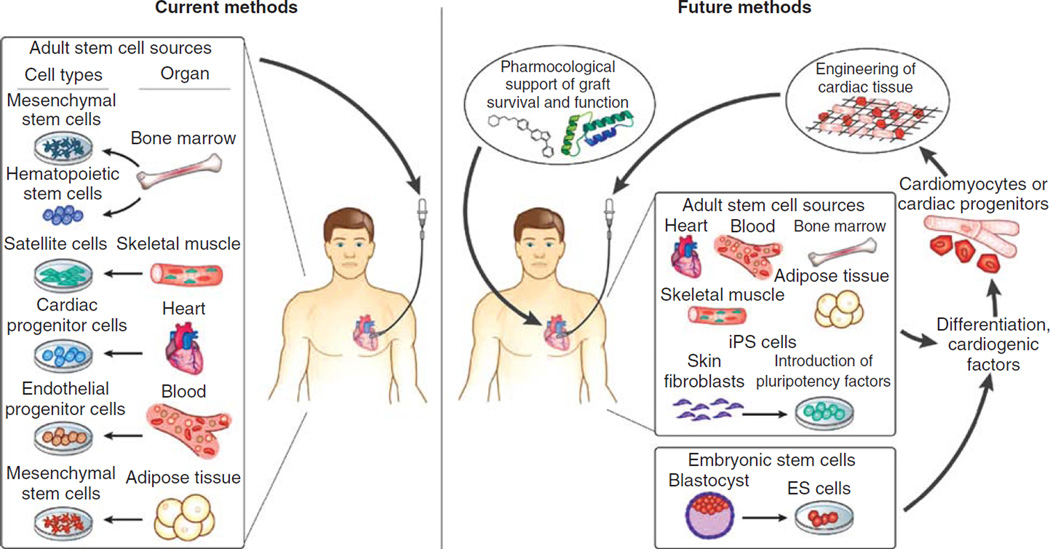
By recognizing the important role of the dynamic physical environment of the heart, external stimuli can be produced to better mimic and promote the differentiation of stem cells to cardiomyocytes in vitro. Thus, the structurally and functionally mature cardiomyocytes could be obtained by exposing human iPSCs, BMCs and/or other types of stem cells to an engineering system which models the ‘heart machinery’ outside the body. This engineering system provides stimuli at physiological levels similar to those found in the heart, such as using biochemical signaling [96], electrical stimulation and mechanical stretching [97] in a user-specific way to guide stem cell differentiation to cardiomyocytes [98]. The differentiation and maturation of cardiomyocytes is subject to patient-to-patient variations. Therefore, external stimuli have to be patient-specific to achieve high efficiency and functionality of differentiation. This can be realized by refining the chemical cues of the substrate, in combination of controlling mechanical strain and electrical pulse within the heart machinery in a user-specified manner, thereby enabling ‘personalized’ medicine. Figure 2 presents a schematic diagram of current and putative future approaches to repair infarcted myocardium.
Figure 2. Schematic diagram of current and putative future approaches to repair infarcted myocardium.
The left panel depicts potential sources of various adult progenitor and stem cell preparations that have been used in clinical trials. Future approaches (right panel) may focus on cardiac cell preparations from ESCs, induced pluripotent cells or direct transdifferentiation of adult stem cells from heterologous tissues to cardiac cells using cardiogenic regulatory factors. Bioengineering of cardiac tissue and/or pharmacological support of grafted cells might be necessary to ensure long-term regeneration of cardiac tissue.
5. Conclusion
More than a decade after the paradigm shift in cardiac cell-based therapies, several unanswered questions remain. The variability among cell populations used, the modest benefits of stem cell therapeutics in human trials, and our inability to promote sustained myocardial recovery have refocused inquiry in both basic and clinical aspects of stem cell therapeutics. Critical gaps still remain to be bridged in the understanding of the basic mechanisms involved in stem cell therapeutics, including harvesting, isolation and differentiation steps of the ideal cell type, in vitro preparation and optimization of engraftment, mobilization and in vivo strategies to enhance survival.
6. Expert opinion
About five million individuals in the United States suffer from heart failure, the cardiovascular disease with the steepest rise in incidence. While current pharmacologic and device therapies reduce mortality, these therapies largely serve to attenuate progression rather than to reverse the fundamental pathologic processes and restore normal cardiac function. Over a decade of research has shed much light on the field of stem cell therapy for cardiac repair. Though many animal and several clinical studies have shown favorable effects on overall cardiac function, the true benefits of stem cell therapy have yet to be realized. In the wake of past and ongoing clinical trials, several areas of research need to be advanced and questions need to be addressed. Clinical trials evaluating the benefit of stem cells for chronic heart failure have been limited compared to those assessing the impact following acute myocardial infarction (Table 1). Nevertheless, the heart failure trials have been plagued by many of the same problems. Some of the difficulties include the use of different cell types, use of various delivery methods, diverse inclusion criteria and differing endpoints. These extensive intertrial variations have made comparisons and conclusions difficult. Regardless of these pitfalls, a discussion of the stem cell trials in chronic heart failure that have been conducted thus far is worthwhile if we are to better understand what is currently known and what is yet needed. More needs to be known about the paracrine effects involved in stem cell therapy, the utility of CSCs as a source and their capacity in innate regeneration, and the best way to enhance stem cell survival, differentiation and engraftment in host tissue.
Table 1.
Summary of trials assessing the effects of stem cell therapy in cardiomyopathy.
| Patient characteristics | Design | Delivery methods | Cell type/# | Results | Refs. |
|---|---|---|---|---|---|
| Ischemic cardiomyopathy, LVEF ≤ 30% |
Non-randomized, 8 patients | Direct injection via NOGA electro-mechanical mapping |
Autologous BMCs (n = 0.7 to 8.8 × 106 CD34+ cells) |
Improved symptoms, myocardial perfusion and function of ischemic regions by MRI |
[73] |
| Ischemic cardiomyopathy, LVEF ≤ 30% |
Non-randomized, 10 patients | Direct injections (n = 12) via biosense electro-mechanical mapping |
Autologous BMCs (n = 32.6 ± 27.5 × 106 cells/mL) |
Improved angina class, reduced stress-induced ischemia in injected territories |
[50] |
| Ischemic cardiomyopathy, LVEF ≤ 40%, reversibility on SPECT |
Non-randomized, 21 patients (first 14 treated, last 7 control) |
Direct injection via NOGA electro-mechanical mapping |
Autologous BMCs (n = 25.5 ± 6.3 × 106) |
Improvement in LVEF (5%), reduction in end systolic volume |
[51] |
| Ischemic cardiomyopathy, LVEF ≤ 40%, heart Tx candidates |
Non-randomized, 5 patients | Direct injection via NOGA electro-mechanical mapping |
Autologous BMCs (n = not listed) |
Increase in myocardial oxygen consumption (10.6 ± 3 to 16.3 ± 7 mL/kg/min) |
[52] |
| History of transumural anterior MI with LAD stenting |
Non-randomized, 5 consecutive patients |
Intracoronary with balloon occlusion in infarct related artery |
Autologous BMCs (n = 2.9 ± 0.9 × 107 cells) |
No improvement in myocardial function or physical performance |
[53] |
| History of transmural MI 2 7 ± 3.1 months prior treated by PCTA and/or stent |
Non-randomized, 18 consecutive patients with 18 patients in parallel as controls |
Intracoronary with balloon occlusion in infarct related artery |
Autologous BMCs (n = 2.9 ± 0.9 × 107 cells) |
Reduction in infarct size by 30%, increase in LVEF by 15% and infarct wall movement by 57% |
[54] |
| History of MI, nonviability, scheduled for elective CABG |
Randomized 1:1, open label, 20 patients |
Direct injection into infarct border zone at time of CABG |
Autologous BMCS (n = 60.1 ± 31.6 × 106 cells) |
Increase in global LVEF, improvement in systolic thickening |
[55] |
| History of MI 14 days prior, akinetic region, need for CABG |
15 consecutive patients (safety and feasibility) followed by 40 patients randomized, open label |
Direct injection at time of CABG into region of perfusion defect |
Autologous BMCs isolated for CD133 (median number = 5.8 × 106) |
Increase in LVEF compared to CABG group alone |
[56] |
| History of MI > 3 months prior, infarct region supplied by patent coronary artery |
Randomized, open label, peripheral EPCs (n = 75), BMCs (n = 24), no infusion (n = 23) |
Intracoronary with balloon occlusion in infarct related artery |
Autologous EPCs (n = 22 ± 11 × 10 6) or BMCs (n = 20.3 ± 11.0 × 106) |
Increase in ejection fraction (2.9%) in BMC cohort, no effect in CPC cohort |
[57] |
| History of MI > 3 months prior, regional LV dysfunction |
Non-randomized, 121 consecutive patients |
Intracoronary with balloon occlusion in infarct related artery |
Autologous BMCs (n = 214 ± 98 × 106 cells) |
Decrease in BNP levels from baseline |
[99] |
| Class III or class IV angina, not candidate for revasulcarization, reversiblity on perfusion scan |
Randomized, double-blinded to 3 doses of GCSF |
Direct injection via NOGA electro-mechanical mapping |
Leukapheresis after GCSF infusion with CD34+ Cells |
No adverse events, trend toward decrease in angina and nitroglycerin use |
[58] |
| History of MI > 4 weeks prior, 35% ≤ EF ≥ 15%, akinetic nonviable by DSE |
Randomized, double-blinded, placebo-controlled, 3 arms, 120 patients, 97 patients received cells (33,400 × 106 cells, 34,800 × 106 cells) and 30 placebo |
Direct injection (mean of 30 sites) around akinetic segment at time of CABG |
Cells expanded from muscle biopsy of approximately 10 g |
No improvement in LV function, high dose cohort did have a decrease in LV volume |
[59] |
| MI > 6 months prior, class III or class IV Angina, LVEF ≥ 35%, reversiblity by SPECT |
Randomized 1:1, double- blinded, placebo-controlled, 50 patients |
Direct injection via NOGA electro-mechanical mapping into ischemic region. |
Autologous BMCs (n = 98 × 106 cells) |
Significant but modest improvement perfusion by SPET, improvement in angina class |
[60] |
| Non-ischemic cardiomyopathy | Randomized, 44 patients (n = 24 treated) |
Intracoronary (2/3 into left Main, 1/3 into RCA) with balloon occlusion of coronary sinus for 3 min |
Autologous BMCs | Significant improvement in NYHA class and LVEF (5.4%), reduction in end-systolic volumes |
[100] |
BMCs: Bone marrow stem cells; CABG: Coronary artery bypass; LVEF: Left ventricular ejection fraction; MI: Myocardial infarction; MRI: Magnetic resonance imaging; NOGATM:; PCI: Percutaneous coronary intervention; PCTA: Percutaneous transluminal angioplasty; SPECT: Single-photon emission computed tomography.
Still, stem cell therapy offers hope of restorative heart function. However, after almost a decade of intense study, it has been difficult to reproduce in human studies the substantial regeneration seen in earlier animal studies. In vitro and preclinical evidence of stem cell differentiation and myocardial regeneration has proven difficult to translate to clinical benefit and raised novel and stimulating lines of inquiry: is the ‘already failed’ heart in fact unsuitable for stem cell therapy? Perhaps, as argued by some, the toxic heart failure milieu into which the stem cells are instilled is too inhospitable an environment for substantial engraftment. Since the human heart appears capable of minor repair and regeneration during the normal ‘wear and tear’ of everyday life, significant or ongoing injury seems to outstrip the organ’s regenerative reserve. Perhaps this lack of regenerative capacity is not related merely to the number of available stem cells alone (in which case ex vivo expansion and implantation of stem cells would seemingly restore regenerative capacity), but rather to the local cellular and tissue milieu for which no amount of exogenous stem cells would suffice. Finally, signs and symptoms of clinical heart failure evolve only after the underlying cellular and neurohormonal processes are advanced the intact organisms reserve is exhausted. By the time clinical heart failure is diagnosed in such a late disease stage, has too much cellular reprogramming and tissue remodeling taken place to expect considerable changes with stem cell therapies? Would stem cell therapies have greater potential if given earlier? This fundamental question regarding the timing of stem cell therapy, like the other important questions highlighted in this review, will hopefully be answered with additional future well-designed studies.
Article highlights.
Various cell types have been used to restore cardiac function and promote myocardial recovery in congestive heart failure.
Each cell type possesses potential advantages and limitations.
Clinical trials have demonstrated promising results for cardiac-based stem cell therapies.
Conflicting clinical trial reports still raise the need to conduct standardized larger studies to address the efficacy of stem cell therapy in advanced heart failure.
Our ability to understand future mechanisms involved in cell-based therapies will allow us to improve clinical outcomes with this potential treatment.
This box summarizes key points contained in the article.
Footnotes
Declaration of interest
The authors state no conflict of interest and have received no payment in preparation of this manuscript.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Kehat I, Kenyagin-Karsenti D, Snir M, et al. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest. 2001;108:407–414. doi: 10.1172/JCI12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 3.Tran TH, Wang X, Browne C, et al. Wnt3a-induced mesoderm formation and cardiomyogenesis in human embryonic stem cells. Stem Cells. 2009;27:1869–1878. doi: 10.1002/stem.95. [DOI] [PubMed] [Google Scholar]
- 4.Laflamme MA, Chen KY, Naumova AV, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 5.van Laake LW, Passier R, Monshouwer-Kloots J, et al. Human embryonic stem cell-derived cardiomyocytes survive and mature in the mouse heart and transiently improve function after myocardial infarction. Stem Cell Res. 2007;1:9–24. doi: 10.1016/j.scr.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Nussbaum J, Minami E, Laflamme MA, et al. Transplantation of undifferentiated murine embryonic stem cells in the heart: teratoma formation and immune response. FASEB J. 2007;21:1345–1357. doi: 10.1096/fj.06-6769com. [DOI] [PubMed] [Google Scholar]
- 7.Blum B, Benvenisty N. The tumorigenicity of human embryonic stem cells. Adv Cancer Res. 2008;100:133–158. doi: 10.1016/S0065-230X(08)00005-5. [DOI] [PubMed] [Google Scholar]
- 8.Draper JS, Fox V. Human embryonic stem cells: multilineage differentiation and mechanisms of self-renewal. Arch Med Res. 2003;34:558–564. doi: 10.1016/j.arcmed.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Gupta MK, Walthall JM, Venkataraman R, et al. Combinatorial polymer electrospun matrices promote physiologically-relevant cardiomyogenic stem cell differentiation. PLoS One. 2011;6:e28935. doi: 10.1371/journal.pone.0028935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takahashi K, Okita K, Nakagawa M, Yamanaka S. Induction of pluripotent stem cells from fibroblast cultures. Nat Protoc. 2007;2:3081–3089. doi: 10.1038/nprot.2007.418. [DOI] [PubMed] [Google Scholar]
- 11.Narazaki G, Uosaki H, Teranishi M, et al. Directed and systematic differentiation of cardiovascular cells from mouse induced pluripotent stem cells. Circulation. 2008;118:498–506. doi: 10.1161/CIRCULATIONAHA.108.769562. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J, Wilson GF, Soerens AG, et al. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ Res. 2009;104:e30–e41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu J, Hu K, Smuga-Otto K, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nelson TJ, Martinez-Fernandez A, Yamada S, et al. Repair of acute myocardial infarction by human stemness factors induced pluripotent stem cells. Circulation. 2009;120:408–416. doi: 10.1161/CIRCULATIONAHA.109.865154. • Landmark study showing cardiac function improvement by iPS cell implantation.
- 15.Stadtfeld M, Hochedlinger K. Induced pluripotency: history, mechanisms, and applications. Genes Dev. 2010;24:2239–2263. doi: 10.1101/gad.1963910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanna JH, Saha K, Jaenisch R. Pluripotency and cellular reprogramming: facts, hypotheses, unresolved issues. Cell. 2010;143:508–525. doi: 10.1016/j.cell.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ieda M, Fu JD, Delgado-Olguin P, et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belema Bedada F, Technau A, Ebelt H, et al. Activation of myogenic differentiation pathways in adult bone marrow-derived stem cells. Mol Cell Biol. 2005;25:9509–9519. doi: 10.1128/MCB.25.21.9509-9519.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flaherty MP, Dawn B. Noncanonical Wnt11 signaling and cardiomyogenic differentiation. Trends Cardiovasc Med. 2008;18:260–268. doi: 10.1016/j.tcm.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Subramanian K, Geraerts M, Pauwelyn KA, et al. Isolation procedure and characterization of multipotent adult progenitor cells from rat bone marrow. Methods Mol Biol. 2010;636:55–78. doi: 10.1007/978-1-60761-691-7_4. [DOI] [PubMed] [Google Scholar]
- 21.Rota M, Kajstura J, Hosoda T, et al. Bone marrow cells adopt the cardiomyogenic fate in vivo. Proc Natl Acad Sci USA. 2007;104:17783–17788. doi: 10.1073/pnas.0706406104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orlic D, Kajstura J, Chimenti S, et al. Bone marrow stem cells regenerate infarcted myocardium. Pediatr Transplant. 2003;7(Suppl 3):86–88. doi: 10.1034/j.1399-3046.7.s3.13.x. [DOI] [PubMed] [Google Scholar]
- 23.Orlic D, Kajstura J, Chimenti S, et al. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci USA. 2001;98:10344–10349. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kajstura J, Rota M, Whang B, et al. Bone marrow cells differentiate in cardiac cell lineages after infarction independently of cell fusion. Circ Res. 2005;96:127–137. doi: 10.1161/01.RES.0000151843.79801.60. [DOI] [PubMed] [Google Scholar]
- 25.Murayama T, Tepper OM, Silver M, et al. Determination of bone marrow-derived endothelial progenitor cell significance in angiogenic growth factor-induced neovascularization in vivo. Exp Hematol. 2002;30:967–972. doi: 10.1016/s0301-472x(02)00867-6. [DOI] [PubMed] [Google Scholar]
- 26.Kupatt C, Horstkotte J, Vlastos GA, et al. Embryonic endothelial progenitor cells expressing a broad range of proangiogenic and remodeling factors enhance vascularization and tissue recovery in acute and chronic ischemia. FASEB J. 2005;19:1576–1578. doi: 10.1096/fj.04-3282fje. [DOI] [PubMed] [Google Scholar]
- 27.Fukuhara S, Tomita S, Nakatani T, et al. G-CSF promotes bone marrow cells to migrate into infarcted mice heart, and differentiate into cardiomyocytes. Cell Transplant. 2004;13:741–748. doi: 10.3727/000000004783983486. [DOI] [PubMed] [Google Scholar]
- 28.Yeh HI, Lai YJ, Lee YN, et al. Differential expression of connexin43 gap junctions in cardiomyocytes isolated from canine thoracic veins. J Histochem Cytochem. 2003;51:259–266. doi: 10.1177/002215540305100215. [DOI] [PubMed] [Google Scholar]
- 29.Jujo K, Ii M, Losordo DW. Endothelial progenitor cells in neovascularization of infarcted myocardium. J Mol Cell Cardiol. 2008;45:530–544. doi: 10.1016/j.yjmcc.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwasaki H, Kawamoto A, Ishikawa M, et al. Dose-dependent contribution of CD34-positive cell transplantation to concurrent vasculogenesis and cardiomyogenesis for functional regenerative recovery after myocardial infarction. Circulation. 2006;113:1311–1325. doi: 10.1161/CIRCULATIONAHA.105.541268. [DOI] [PubMed] [Google Scholar]
- 31.Miyamoto Y, Suyama T, Yashita T, et al. Bone marrow subpopulations contain distinct types of endothelial progenitor cells and angiogenic cytokine-producing cells. J Mol Cell Cardiol. 2007;43:627–635. doi: 10.1016/j.yjmcc.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Alhadlaq A, Mao JJ. Mesenchymal stem cells: isolation and therapeutics. Stem Cells Dev. 2004;13:436–448. doi: 10.1089/scd.2004.13.436. [DOI] [PubMed] [Google Scholar]
- 33.Porada CD, Zanjani ED, Almeida-Porad G. Adult mesenchymal stem cells: a pluripotent population with multiple applications. Curr Stem Cell Res Ther. 2006;1:365–369. doi: 10.2174/157488806778226821. [DOI] [PubMed] [Google Scholar]
- 34.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 35.Colter DC, Class R, DiGirolamo CM, Prockop DJ. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci USA. 2000;97:3213–3218. doi: 10.1073/pnas.070034097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dai W, Hale SL, Martin BJ, et al. Allogeneic mesenchymal stem cell transplantation in postinfarcted rat myocardium: short- and long-term effects. Circulation. 2005;112:214–223. doi: 10.1161/CIRCULATIONAHA.104.527937. [DOI] [PubMed] [Google Scholar]
- 37.Kudo M, Wang Y, Wani MA, et al. Implantation of bone marrow stem cells reduces the infarction and fibrosis in ischemic mouse heart. J Mol Cell Cardiol. 2003;35:1113–1119. doi: 10.1016/s0022-2828(03)00211-6. [DOI] [PubMed] [Google Scholar]
- 38.Silva GV, Litovsky S, Assad JA, et al. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005;111:150–156. doi: 10.1161/01.CIR.0000151812.86142.45. [DOI] [PubMed] [Google Scholar]
- 39.Hamamoto H, Gorman JH, III, Ryan LP, et al. Allogeneic mesenchymal precursor cell therapy to limit remodeling after myocardial infarction: the effect of cell dosage. Ann Thorac Surg. 2009;87:794–801. doi: 10.1016/j.athoracsur.2008.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miyahara Y, Nagaya N, Kataoka M, et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. 2006;12:459–465. doi: 10.1038/nm1391. [DOI] [PubMed] [Google Scholar]
- 41.Rose RA, Jiang H, Wang X, et al. Bone marrow-derived mesenchymal stromal cells express cardiac-specific markers, retain the stromal phenotype, and do not become functional cardiomyocytes in vitro. Stem Cells. 2008;26:2884–2892. doi: 10.1634/stemcells.2008-0329. [DOI] [PubMed] [Google Scholar]
- 42.Gojo S, Gojo N, Takeda Y, et al. In vivo cardiovasculogenesis by direct injection of isolated adult mesenchymal stem cells. Exp Cell Res. 2003;288:51–59. doi: 10.1016/s0014-4827(03)00132-0. [DOI] [PubMed] [Google Scholar]
- 43.Breitbach M, Bostani T, Roell W, et al. Potential risks of bone marrow cell transplantation into infarcted hearts. Blood. 2007;110:1362–1369. doi: 10.1182/blood-2006-12-063412. [DOI] [PubMed] [Google Scholar]
- 44.Buckingham M, Montarras D. Skeletal muscle stem cells. Curr Opin Genet Dev. 2008;18:330–336. doi: 10.1016/j.gde.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 45.Arsic N, Mamaeva D, Lamb NJ, Fernandez A. Muscle-derived stem cells isolated as non-adherent population give rise to cardiac, skeletal muscle and neural lineages. Exp Cell Res. 2008;314:1266–1280. doi: 10.1016/j.yexcr.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 46.Pagani FD, DerSimonian H, Zawadzka A, et al. Autologous skeletal myoblasts transplanted to ischemia-damaged myocardium in humans. Histological analysis of cell survival and differentiation. J Am Coll Cardiol. 2003;41:879–888. doi: 10.1016/s0735-1097(03)00081-0. [DOI] [PubMed] [Google Scholar]
- 47.Menasche P. Skeletal myoblast transplantation for cardiac repair. Expert Rev Cardiovasc Ther. 2004;2:21–28. doi: 10.1586/14779072.2.1.21. [DOI] [PubMed] [Google Scholar]
- 48.Leobon B, Garcin I, Menasche P, et al. Myoblasts transplanted into rat infarcted myocardium are functionally isolated from their host. Proc Natl Acad Sci USA. 2003;100:7808–7811. doi: 10.1073/pnas.1232447100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Farahmand P, Lai TY, Weisel RD, et al. Skeletal myoblasts preserve remote matrix architecture and global function when implanted early or late after coronary ligation into infarcted or remote myocardium. Circulation. 2008;118:S130–S137. doi: 10.1161/CIRCULATIONAHA.107.757617. [DOI] [PubMed] [Google Scholar]
- 50.Fuchs S, Satler LF, Kornowski R, et al. Catheter-based autologous bone marrow myocardial injection in no-option patients with advanced coronary artery disease: a feasibility study. J Am Coll Cardiol. 2003;41:1721–1724. doi: 10.1016/s0735-1097(03)00328-0. [DOI] [PubMed] [Google Scholar]
- 51.Perin EC, Dohmann HF, Borojevic R, et al. Transendocardial, autologous bone marrow cell transplantation for severe, chronic ischemic heart failure. Circulation. 2003;107:2294–2302. doi: 10.1161/01.CIR.0000070596.30552.8B. [DOI] [PubMed] [Google Scholar]
- 52.Silva GV, Perin EC, Dohmann HF, et al. Catheter-based transendocardial delivery of autologous bone-marrow-derived mononuclear cells in patients listed for heart transplantation. Tex Heart Inst J. 2004;31:214–219. [PMC free article] [PubMed] [Google Scholar]
- 53.Kuethe F, Richartz BM, Kasper C, et al. Autologous intracoronary mononuclear bone marrow cell transplantation in chronic ischemic cardiomyopathy in humans. Int J Cardiol. 2005;100:485–491. doi: 10.1016/j.ijcard.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 54.Strauer BE, Brehm M, Zeus T, et al. Regeneration of human infarcted heart muscle by intracoronary autologous bone marrow cell transplantation in chronic coronary artery disease: the IACT Study. J Am Coll Cardiol. 2005;46:1651–1658. doi: 10.1016/j.jacc.2005.01.069. [DOI] [PubMed] [Google Scholar]
- 55.Hendrikx M, Hensen K, Clijsters C, et al. Recovery of regional but not global contractile function by the direct intramyocardial autologous bone marrow transplantation: results from a randomized controlled clinical trial. Circulation. 2006;114:I101–I107. doi: 10.1161/CIRCULATIONAHA.105.000505. [DOI] [PubMed] [Google Scholar]
- 56.Stamm C, Kleine HD, Choi YH, et al. Intramyocardial delivery of CD133+ bone marrow cells and coronary artery bypass grafting for chronic ischemic heart disease: safety and efficacy studies. J Thorac Cardiovasc Surg. 2007;133:717–725. doi: 10.1016/j.jtcvs.2006.08.077. [DOI] [PubMed] [Google Scholar]
- 57.Assmus B, Honold J, Schachinger V, et al. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–1232. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 58.Losordo DW, Schatz RA, White CJ, et al. Intramyocardial transplantation of autologous CD34+ stem cells for intractable angina: a phase I/IIa double-blind, randomized controlled trial. Circulation. 2007;115:3165–3172. doi: 10.1161/CIRCULATIONAHA.106.687376. [DOI] [PubMed] [Google Scholar]
- 59.van Ramshorst J, Bax JJ, Beeres SL, et al. Intramyocardial bone marrow cell injection for chronic myocardial ischemia: a randomized controlled trial. JAMA. 2009;301:1997–2004. doi: 10.1001/jama.2009.685. [DOI] [PubMed] [Google Scholar]
- 60.Menasche P, Alfieri O, Janssens S, et al. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation. 2008;117:1189–1200. doi: 10.1161/CIRCULATIONAHA.107.734103. [DOI] [PubMed] [Google Scholar]
- 61.Trachtenberg B, Velazquez DL, Williams AR, et al. Rationale and design of the Transendocardial Injection of Autologous Human Cells (bone marrow or mesenchymal) in Chronic Ischemic Left Ventricular Dysfunction and Heart Failure Secondary to Myocardial Infarction (TAC-HFT) trial: A randomized, double-blind, placebo-controlled study of safety and efficacy. Am Heart J. 2011;161:487–493. doi: 10.1016/j.ahj.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 62.Mazo M, Arana M, Pelacho B, Prosper F. Mesenchymal stem cells and cardiovascular disease: a bench to bedside roadmap. Stem Cells Int. 2012;2012:175979. doi: 10.1155/2012/175979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jahangir E, Fazio S, Sampson UK. Incident diabetes and statins: the blemish of an undisputed heavy weight champion? Br J Clin Pharmacol. 2012 doi: 10.1111/j.1365-2125.2012.04445.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kupatt C, Hinkel R, Lamparter M, et al. Retroinfusion of embryonic endothelial progenitor cells attenuates ischemia-reperfusion injury in pigs: role of phosphatidylinositol 3-kinase/AKT kinase. Circulation. 2005;112:I117–I122. doi: 10.1161/CIRCULATIONAHA.104.524801. [DOI] [PubMed] [Google Scholar]
- 65. Finlay MC, Hunter RJ, Baker V, et al. A randomised comparison of Cartomerge vs. NavX fusion in the catheter ablation of atrial fibrillation: the CAVERN Trial. J Interv Card Electrophysiol. 2012;33:161–169. doi: 10.1007/s10840-011-9632-7. •• Important study related to cytoprotective effect of stem cells.
- 66.Fermann GJ, Lindsell CJ, Storrow AB, et al. Galectin 3 complements BNP in risk stratification in acute heart failure. Biomarkers. 2012;17(8):706–713. doi: 10.3109/1354750X.2012.719037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Collins SP, Lindsell CJ, Yealy DM, et al. A comparison of criterion standard methods to diagnose acute heart failure. Congest Heart Fail. 2012;18:262–271. doi: 10.1111/j.1751-7133.2012.00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roden DM, Xu H, Denny JC, Wilke RA. Electronic medical records as a tool in clinical pharmacology: opportunities and challenges. Clin Pharmacol Ther. 2012;91:1083–1086. doi: 10.1038/clpt.2012.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Monahan K, Brewster J, Wang L, et al. Relation of the severity of obstructive sleep apnea in response to anti-arrhythmic drugs in patients with atrial fibrillation or atrial flutter. Am J Cardiol. 2012;110:369–372. doi: 10.1016/j.amjcard.2012.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Di Salvo TG. Pulmonary hypertension and right ventricular failure in left ventricular systolic dysfunction. Curr Opin Cardiol. 2012;27:262–272. doi: 10.1097/HCO.0b013e3283522098. [DOI] [PubMed] [Google Scholar]
- 71.Dong Y, Teoh HL, Chan BP, et al. Changes in cerebral hemodynamic and cognitive parameters after external carotid-internal carotid bypass surgery in patients with severe steno-occlusive disease: a pilot study. J Neurol Sci. 2012;322(1–2):112–116. doi: 10.1016/j.jns.2012.07.034. [DOI] [PubMed] [Google Scholar]
- 72.Tse V, Xu L, Yung YC, et al. The temporal-spatial expression of VEGF, angiopoietins-1 and 2, and Tie-2 during tumor angiogenesis and their functional correlation with tumor neovascular architecture. Neurol Res. 2003;25:729–738. doi: 10.1179/016164103101202084. [DOI] [PubMed] [Google Scholar]
- 73.Tse HF, Kwong YL, Chan JK, et al. Angiogenesis in ischaemic myocardium by intramyocardial autologous bone marrow mononuclear cell implantation. Lancet. 2003;361:47–49. doi: 10.1016/S0140-6736(03)12111-3. [DOI] [PubMed] [Google Scholar]
- 74.Kamihata H, Matsubara H, Nishiue T, et al. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation. 2001;104:1046–1052. doi: 10.1161/hc3501.093817. [DOI] [PubMed] [Google Scholar]
- 75.Kalka C, Masuda H, Takahashi T, et al. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci USA. 2000;97:3422–3427. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bartsch T, Brehm M, Falke T, et al. Rapid healing of a therapy-refractory diabetic foot after transplantation of autologous bone marrow stem cells. Med Klin (Munich) 2005;100:676–680. doi: 10.1007/s00063-005-1093-2. [DOI] [PubMed] [Google Scholar]
- 77.Klein I, Cornejo JC, Polakos NK, et al. Kupffer cell heterogeneity: functional properties of bone marrow derived and sessile hepatic macrophages. Blood. 2007;110:4077–4085. doi: 10.1182/blood-2007-02-073841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eapen M, Rubinstein P, Zhang MJ, et al. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet. 2007;369:1947–1954. doi: 10.1016/S0140-6736(07)60915-5. [DOI] [PubMed] [Google Scholar]
- 79.Martin CM, Meeson AP, Robertson SM, et al. Persistent expression of the ATP-binding cassette transporter, Abcg2, identifies cardiac SP cells in the developing and adult heart. Dev Biol. 2004;265:262–275. doi: 10.1016/j.ydbio.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 80.Challen GA, Little MH. A side order of stem cells: the SP phenotype. Stem Cells. 2006;24:3–12. doi: 10.1634/stemcells.2005-0116. [DOI] [PubMed] [Google Scholar]
- 81.Laugwitz KL, Moretti A, Lam J, et al. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–653. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thiele J, Varus E, Wickenhauser C, et al. Mixed chimerism of cardiomyocytes and vessels after allogeneic bone marrow and stem-cell transplantation in comparison with cardiac allografts. Transplantation. 2004;77:1902–1905. doi: 10.1097/01.tp.0000127591.34203.8e. [DOI] [PubMed] [Google Scholar]
- 83.Rosenblatt-Velin N, Lepore MG, Cartoni C, et al. FGF-2 controls the differentiation of resident cardiac precursors into functional cardiomyocytes. J Clin Invest. 2005;115:1724–1733. doi: 10.1172/JCI23418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bergmann O, Bhardwaj RD, Bernard S, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Maltais S, Perrault LP, Ly HQ. The bone marrow-cardiac axis: role of endothelial progenitor cells in heart failure. Eur J Cardiothorac Surg. 2011;39:368–374. doi: 10.1016/j.ejcts.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 86.Quevedo HC, Hatzistergos KE, Oskouei BN, et al. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci USA. 2009;106:14022–14027. doi: 10.1073/pnas.0903201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Heiss C, Keymel S, Niesler U, et al. Impaired progenitor cell activity in age-related endothelial dysfunction. J Am Coll Cardiol. 2005;45:1441–1448. doi: 10.1016/j.jacc.2004.12.074. [DOI] [PubMed] [Google Scholar]
- 88.van Vliet P, Roccio M, Smits AM, et al. Progenitor cells isolated from the human heart: a potential cell source for regenerative therapy. Neth Heart J. 2008;16:163–169. doi: 10.1007/BF03086138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Smith RR, Barile L, Cho HC, et al. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 90.Siu CW, Tse HF. Cardiac regeneration: messages from CADUCEUS. Lancet. 2012;379:870–871. doi: 10.1016/S0140-6736(12)60236-0. [DOI] [PubMed] [Google Scholar]
- 91.Kofidis T, Lenz A, Boublik J, et al. Bioartificial grafts for transmural myocardial restoration: a new cardiovascular tissue culture concept. Eur J Cardiothorac Surg. 2003;24:906–911. doi: 10.1016/s1010-7940(03)00577-3. [DOI] [PubMed] [Google Scholar]
- 92.Meluzin J, Mayer J, Groch L, et al. Autologous transplantation of mononuclear bone marrow cells in patients with acute myocardial infarction: the effect of the dose of transplanted cells on myocardial function. Am Heart J. 2006;152:975, e9–e915. doi: 10.1016/j.ahj.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 93.Lee SP, Youn SW, Cho HJ, et al. Integrin-linked kinase, a hypoxia-responsive molecule, controls postnatal vasculogenesis by recruitment of endothelial progenitor cells to ischemic tissue. Circulation. 2006;114:150–159. doi: 10.1161/CIRCULATIONAHA.105.595918. [DOI] [PubMed] [Google Scholar]
- 94.Traverse JH, Henry TD, Vaughan DE, et al. Rationale and design for TIME: a phase II, randomized, double-blind, placebo-controlled pilot trial evaluating the safety and effect of timing of administration of bone marrow mononuclear cells after acute myocardial infarction. Am Heart J. 2009;158:356–363. doi: 10.1016/j.ahj.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cheng Z, Ou L, Zhou X, et al. Targeted migration of mesenchymal stem cells modified with CXCR4 gene to infarcted myocardium improves cardiac performance. Mol Ther. 2008;16:571–579. doi: 10.1038/sj.mt.6300374. [DOI] [PubMed] [Google Scholar]
- 96.Wei F, Wang T, Liu J, et al. The subpopulation of mesenchymal stem cells that differentiate toward cardiomyocytes is cardiac progenitor cells. Exp Cell Res. 2011;317:2661–2670. doi: 10.1016/j.yexcr.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 97.Christoforou N, Oskouei BN, Esteso P, et al. Implantation of mouse embryonic stem cell-derived cardiac progenitor cells preserves function of infarcted murine hearts. PLoS One. 2010;5:e11536. doi: 10.1371/journal.pone.0011536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Storrow AB, Lindsell CJ, Collins SP, et al. Standardized reporting criteria for studies evaluating suspected acute heart failure syndromes in the emergency department. J Am Coll Cardiol. 2012;60:822–832. doi: 10.1016/j.jacc.2012.03.072. [DOI] [PubMed] [Google Scholar]
- 99.Assmus B, Fischer-Rasokat U, Honold J, et al. Transcoronary transplantation of functionally competent BMCs is associated with a decrease in natriuretic peptide serum levels and improved survival of patients with chronic postinfarction heart failure: results of the TOPCARE-CHD Registry. Circ Res. 2007;100:1234–1241. doi: 10.1161/01.RES.0000264508.47717.6b. [DOI] [PubMed] [Google Scholar]
- 100.van der Bogt KE, Schrepfer S, Yu J, et al. Comparison of transplantation of adipose tissue- and bone marrow-derived mesenchymal stem cells in the infarcted heart. Transplantation. 2009;87:642–652. doi: 10.1097/TP.0b013e31819609d9. [DOI] [PMC free article] [PubMed] [Google Scholar]