Abstract
Purpose
To evaluate the efficacy of a controlled release dexamethasone delivery system for suppressing inflammation in an ocular burn + desiccating stress (OB+DS) model.
Methods
Nanowafers (NW) loaded with Dexamethasone (Dex, 10 μg) or vehicles (2.5% Methylcellulose; MC) were fabricated using hydrogel template strategy. C57BL/6 mice were subjected to unilateral alkali ocular burn with concomitant desiccating stress for 2 or 5 days and topically treated either with 2 μL of 0.1% Dex or vehicle four times per day and compared with mice that had MC-NW or Dex-NW placed on their corneas. Clinical parameters were evaluated daily. Mice were euthanized after 2 or 5 days. Quantitative PCR evaluated the expression of inflammatory cytokines IL-1β and IL-6 and matrix metalloproteinases (MMP) in whole cornea lysates. Myeloperoxidase activity (MPO) was measured using a commercial kit in cornea lysates.
Results
Both Dex drop and Dex-NW groups had significantly lower corneal opacity scores compared with their vehicles. Both Dex drops and Dex-NW significantly decreased expression of IL-1β, IL-6, and MMP-9 RNA transcripts compared with vehicle drops or wafers 2 and 5 days after the initial lesion. A significant lower number of neutrophils was found in both Dex treatment groups and this was accompanied by decreased MPO activity compared with vehicle controls.
Conclusions
Dex-NW has efficacy equal to Dex drops in preserving corneal clarity and decreasing expression of MMPs and inflammatory cytokines of the corneas of mice subjected to an OB+DS model.
Keywords: nanowafer, alkali injury, dry eye, neutrophils, MMPs, dexamethasone
Chemical and thermal injuries to the ocular surface have a high potential to cause blindness. Clinical prognosis depends in part on how promptly the proper treatment is initiated in the acute setting, but sight threatening sequelae may develop even in cases with optimum treatment.1 Vision impacts mobility, independence and patients' quality of life. Our modern digital society requires vision to read, use a computer, drive, and maintain independence. Although the knowledge regarding the effects of these injuries on promoting inflammation and tissue destruction has increased, the therapeutic strategies to decrease inflammation and preserve corneal clarity have not improved at the same rate.
We have developed a more severe murine model of ocular injury where the combination of alkali ocular burn with desiccating stress (OB+DS model) amplifies corneal inflammation, MMPs expression, and neutrophil infiltration resulting in greater residual corneal opacity than alkali burn alone.2
Presently, eye injuries are treated with topical ophthalmic solutions, which need to be instilled several times in a day for maintaining a therapeutic concentration of the drug at the site of injury. However, achieving therapeutic concentration of drug on the ocular surface remains a challenge due to the continuous basal production of tears, rapid blinking, and tear clearance through the nasolacrimal drainage system. These physiological mechanisms also limit the efficacy of topically applied ophthalmic solutions. Recommended therapeutic regimens for modulation the ocular surface response to trauma requires multiple dosing and this frequent dosing schedule may not be feasible in critically ill patients in intensive care. In an outpatient clinical setting, current preferred therapeutic strategies for ocular burn include instillation of topical ophthalmic solution of corticosteroid and antibiotic drugs at hourly intervals.3,4 Furthermore, patients often do not comply with this frequency of drug administration.5
Nanotechnology has been used for ocular drug delivery to improve treatment efficacy. Nanoparticles formulations loaded with different therapeutic molecules have been used to treat corneal diseases such as microbial infection and prevent corneal graft rejection.6–8 Contact lenses coated with nanospheres loaded with drugs also have been studied.9,10
Dexamethasone is an anti-inflammatory glucocorticoid commonly used after cornel injury and eye surgery. Topical 0.1% Dex treatment effectively decreased the expression of inflammation cytokines and MMPs in our OB+DS model model.11 In this study, dexamethasone nanowafers (Dex-NWs) were fabricated by a previously published procedure.12,13 The Dex-NW slowly release the drug as it dissolves. The findings of the present study showed that Dex-NW improved corneal clarity, decreased inflammatory cytokine and MMP expression in the wounded corneas, while reduced neutrophil infiltration. The efficacy of once a day Dex-NW treatment was comparable to four times a day topical Dex eyedrops treatment.
Materials and Methods
Materials
Sodium methylcellulose (MC, molecular weight 90,000) and high performance liquid chromatography (HPLC) solvents (acetonitrile) were obtained from Sigma-Aldrich Corp. (St. Louis, MO, USA). USP grade dexamethasone sodium phosphate (Dex) was obtained from Spectrum Chemicals (New Brunswick, NJ, USA). Polymerase chain reaction reagents and Oregon Green Dextran (72 KDa) were purchased from Life Technologies (Carlsbad, CA, USA). The drug release study from the Dex-NW was analyzed by HPLC method using a Shimadzu-Prominence HPLC system (Kyoto, Japan).
Animals
All animals were treated in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and the protocols were approved by the Baylor College of Medicine Institutional Animal Care and Use Committee (Houston, TX, USA). Female C57BL/6J mice (6- to 8-weeks old) were purchased from the Jackson Laboratory (Bar Harbor, ME, USA).
Two hundred and twenty-two C57BL/6J mice were used in this study. Twenty-two C57BL/6J animals were used per group (vehicle or Dex in the format of drops or NW) and per time point (2 and 5 days): 6 for histology, 12 for real-time PCR, and four for myeloperoxidase assay. Corneal opacity and wound closure rate were evaluated in 12 live mice that were also used for either histology or PCR. Twenty-two naïve animals were used for normal controls. For the dose response experiment, a separate group of 24 mice were used (four/group).
Nanowafer Fabrication
The NW were fabricated according to a previously published procedure with slight modifications.12–14 A clear MC solution (4% wt/vol, 12 mL) was transferred with a pipette onto a polydimethylsiloxane (PDMS) imprint (3″ diameter) containing square posts (of 500 × 500 nm, 500-nm high) placed on a flat glass plate and left to dry at room temperature. Thus, formed MC NW was carefully peeled away from the PDMS imprint. The concentration of the polymer solution (2.5%) was adjusted to obtain the required thickness of the NW. The MC NW obtained was approximately 3″ diameter, approximately 75-μm thick, and has arrays of wells (500 × 500 nm, 500-nm deep).
The MC wafers were filled with Dex by transferring a thick solution of Dex-MC with micropipette onto the NW followed by gently swiping with a Teflon swiper. The drug-filled wells are open on one side of the NW. The open face of the NW was placed in direct contact with cornea, so that the drug molecules can directly diffuse into the ocular tissue. The drug-filled NW were punched into 2-mm diameter discs that were used in vitro and in vivo experiments. Each 2-mm diameter NW contained 5.027 × 105 wells.
In Vitro Drug Release Study
Accurately weighed Dex-NWs were placed inside dialysis tubes (MWCO 2000; Pierce Biotechnology, Rockford, IL, USA). Each loaded dialysis tube was placed inside a 5 mL Eppendorf tube containing PBS (pH 7.4) and constantly shaken at 37°C. Aliquots were obtained at different time points and analyzed using a Shimadzu Prominence UV–HPLC system (Kyoto, Japan) with a Kinetex 5uXB-C18 100A (150 × 4.6 mm) column from Phenomenex (Torrance, CA, USA). Fresh PBS was added to replace the aliquot extracted.
Each sample was filtered through a 0.2-μm syringe filter, and drug concentration was calculated by comparing the peak area of standards and sample detected at 240 nm. The UV–HPLC system was equipped with an autosampler, in line degasser, and column oven set at room temperature.
The mobile phase for Dex analysis was a mixture of 0.1 M monosodium phosphate (90%) at pH 4.6 and acetonitrile (10%). Injection volume was 5 μL, the flow rate was 0.8 mL/min, and the pressure was lower than 2500 psi. The total drug content in the NW was determined by dissolving an accurately weighed NW in 1 mL PBS solution and 2 mL of ethanol to precipitate the polymer. The suspension was centrifuged to remove the polymer. The clear solution was filtered through a 0.2-μm syringe filter followed by UV–HPLC analysis. The total drug content in the NW was quantified by comparing with the standard curve. This experiment was performed in triplicate.
Ocular Burn and Desiccating Stress (OB+DS) Model
Unilateral alkali burn was created on the right eye of 6- to 8-week-old female C57BL/6J after systemic anesthesia with isoflurane using a vaporizer (SomnoSuite, Kent Scientific, Torrington, CT, USA), by placing one 2.0-mm diameter filter paper disc presoaked with 1 N NAOH on the central cornea for 10 seconds, followed by extensive rinsing with balanced salt solution (Alcon, Fort Worth, TX, USA), as previously described.2 Precautions were taken to avoid damage to the peripheral cornea, conjunctiva, and lids. After anesthesia recovery, mice were subjected to desiccating stress (DS) to create an OB+DS model. Desiccating stress was induced by sterile subcutaneous injection of 0.5 mg/mL scopolamine hydrobromide (Sigma-Aldrich Corp.) four times per day (QID) into alternating flanks and exposure to a drafty low humidity (<30% relative humidity) environment for 2 or 5 days as previously described.15
Dosing Regimens
Mice subjected to OB+DS model were topically treated as described below. Tissues were collected after 2 and 5 days of creating the alkali burn and dry eye.
1. Dose response experiment:
To test the effective dose of Dex-NW, C57BL/6 mice subjected to the OB+DS model received a corneal application of MC NW loaded with 2, 4, or 10 μg Dex once per day (QD) or 10 μg every other day (QOD) and were compared with naïve control corneas and 2.5% MC blank nanowafers.
2. NW drug delivery system:
Mice subjected to the OB+DS model for 2 and 5 days received a corneal application of either 10 μg Dex-NW or vehicle (2.5% MC wafer) QD.
3. Conventional topical anti-inflammatory therapy:
Mice subjected to the OB+DS model for 2 and 5 days were topically treated either with 2 μL sodium phosphate Dex (1mg/mL; Spectrum Laboratory, Gardena, CA, USA), or vehicle (2.5% MC) QID and compared with Dex-NW QD–treated corneas. Each drop delivers 2 μg/eye/application, for a total of 8 μg/day/eye.
Clinical Findings
Opacity Score.
Corneal edema and opacity were graded biomicroscopically by two masked observers in images taken by a color digital camera DS-Fi1 (Nikon, Melville, New York, USA) as previously described.16 Corneal opacity was scored using a scale of 0 to 4 (grade 0 = completely clear, grade 1 = slightly hazy, iris and pupils easily visible, grade 2 = slightly opaque, iris and pupils still detectable, grade 3 = opaque, pupils hardly detectable, and grade 4 = completely opaque with no view of the pupils).
Measurement of Corneal Epithelial Defect.
Corneal epithelial healing was assessed daily by fluorescein staining. After instilling 1 μL of 0.1% liquid sodium fluorescein onto the ocular surface, corneas were rinsed with PBS and photographed with a stereoscopic zoom microscope (SMZ 1500; Nikon) under fluorescence excitation at 470 nm (DS-Qi1Mc, Nikon). Corneal epithelial defects were graded in digital images by two masked observers in a categorical manner (present/absent) to generate a survival curve. Biological replicate scores were transferred to an excel database where the results were analyzed.
Histology and Immunostaining
A total of six mice per experimental group at 2 and 5 days post injury were euthanized. For immunohistochemistry, eyes and adnexa were excised, embedded in optimal cutting temperature compound (VWR, Suwanee, GA, USA), and flash frozen in liquid nitrogen. Sagittal 8-μm tissue sections were cut with a cryostat (HM 500; Micron, Waldorf, Germany) and placed on glass slides that were stored at −80°C. Immunohistochemistry was performed to detect neutrophils using rat anti-Gr-1 antibody (Ly6G, 1:250, clone 1A8l; BD Pharmingen, San Diego, CA, USA). Cryosections were stained with the primary antibody and appropriate biotinylated secondary antibody (1:100 biotin goat α-rat; BD Pharmingen) using a Vectastain Elite ABC kit and Nova Red reagents (Vector, Burlingame, CA, USA). Sections from each experimental group were examined and photographed with a microscope equipped with a digital camera (Eclipse E400 with a DS-Qi1Mc; Nikon). The numbers of Gr-1 positive (+) cells were counted in cornea sections from each animal at ×20 magnification and results averaged and expressed as the number of positive cells per cornea.
Immunofluorescent staining was performed in frozen tissue sections using rabbit polyclonal antibody anti–MMP-1 (1:100, ab-137332; Abcam, MA, USA) and anti-MMP-9 (1:100; Santa Cruz Biotechnology, Dallas, TX, USA). Secondary goat anti-rabbit Alexa-Fluor 488 conjugated IgG antibodies were used, as previously described.2 The images were captured and photographed by a Nikon fluorescence microscope (Eclipse E400 equipped with a DS–F1 digital camera). Mean fluorescent intensity/area was measured in the corneal epithelium and analyzed using the NIS Elements Software (Nikon).
RNA Isolation and Quantitative PCR
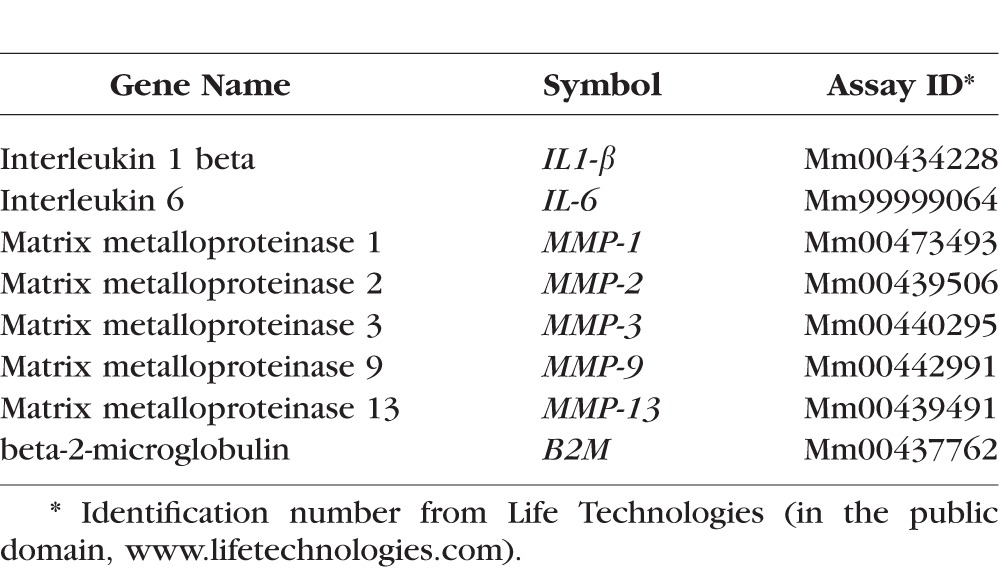
Four whole corneas (including stroma) per group at 2 and 5 days post injury were excised, minced, and total RNA was extracted using a Qiagen MicroPlus RNeasy isolation Kit (Qiagen, Valencia, CA, USA) according to the manufacturer's instructions, quantified by a NanoDrop ND-2000 Spectrophotometer (Thermo Scientific, Wilmington, DE, USA) and stored at −80°C. First-strand cDNA was synthesized with random hexamers by M-MuLV reverse transcription (Ready-To-Go You-Prime First-Strand Beads; GE Healthcare, Inc., Arlington Heights, NJ, USA), as previously described.17 Real-time PCR was performed with specific Taqman MGB probes (Applied Biosystems, Inc., Foster City, CA, USA) and PCR master mix (Taqman Gene Expression Master Mix), in a commercial thermocycling system (StepOnePlus Real-Time PCR System, Applied Biosystems), according to the manufacturer's recommendations. Quantitative real-time PCR was performed using gene expression assay primers and MGB probes specific for murine targets described in the Table. The beta-2-microglobulin (B2M) gene was used as an endogenous reference for each reaction to correct for differences in the amount of total RNA added. The results of quantitative PCR were analyzed by the comparative CT method where target change = 2–ΔΔCT. The results were normalized by the CT value of B2M and the levels of relative RNA transcripts in the untreated group was used as the calibrator.
Table.
Oligonucleotide Primers Used for Real-Time PCR
Myeloperoxidase Assay
Myeloperoxidase (MPO) activity was measured using a myeloperoxidase colorimetric activity assay kit as described by the manufacturer (Sigma-Aldrich Corp.). Briefly, whole-cornea lysates from experimental groups (n = 4/group) were homogenized in MPO assay buffer and the homogenate was centrifuged at 14,000g for 20 minutes at 4°C. Total protein concentration was measured by the BCA protein assay as previously described.18 A 50 μg/sample was mixed with MPO assay buffer and MPO substrate, incubated at room temperature for 2 hours, and then mixed with tetramethylbenzidine probe. Fluorescence was measured at 412 nm using a Tecan Infinite M200 (Teacan, Inc.) plate reader equipped with Magellan V6.55 software (San Jose, CA, USA). Biologic replicate samples were averaged. Results are presented as mean ± SEM milliunits.
Statistical Analysis
Results are presented as the mean ± SEM. Two-way ANOVA with Bonferroni post hoc testing was used for statistical comparisons of gene expression. P less than or equal to 0.05 was considered statistical significant. These tests were performed using GraphPad Prism 6.0 software (GraphPad Incorporation, San Diego, CA, USA).
Results
Sustained Release of Dexamethasone From Nanowafer
In this study, methylcellulose (MC) polymer was used for NW fabrication because of its water solubility, transparency, and mucoadhesive properties. Methylcellulose is nontoxic, and because of its good biocompatibility, it is frequently used as a component of artificial tears. The NW contains patterned arrays of nanoreservoirs loaded with drug.13,14 The drug will be slowly released from these nanoreservoirs for several hours. Dex was chosen for these studies as a prototype corticosteroid. We fabricated Dex-NW using a previously published procedure.12–14
Dex is an anti-inflammatory glucocorticoid commonly used after cornel injury and eye surgery. Topical 0.1% Dex treatment effectively decreased the expression of inflammation cytokines and MMPs in our OB+DS model.11 We hypothesized that daily application of a Dex-NW can control inflammation to a similar degree as conventional Dex drops administrated at least four times a day in an OB+DS model. Using this model, we investigated the efficacy of Dex-NW in ameliorating clinical signs and decreasing expression of inflammatory cytokines and MMPs, and limiting neutrophil influx compared with conventional Dex drops.
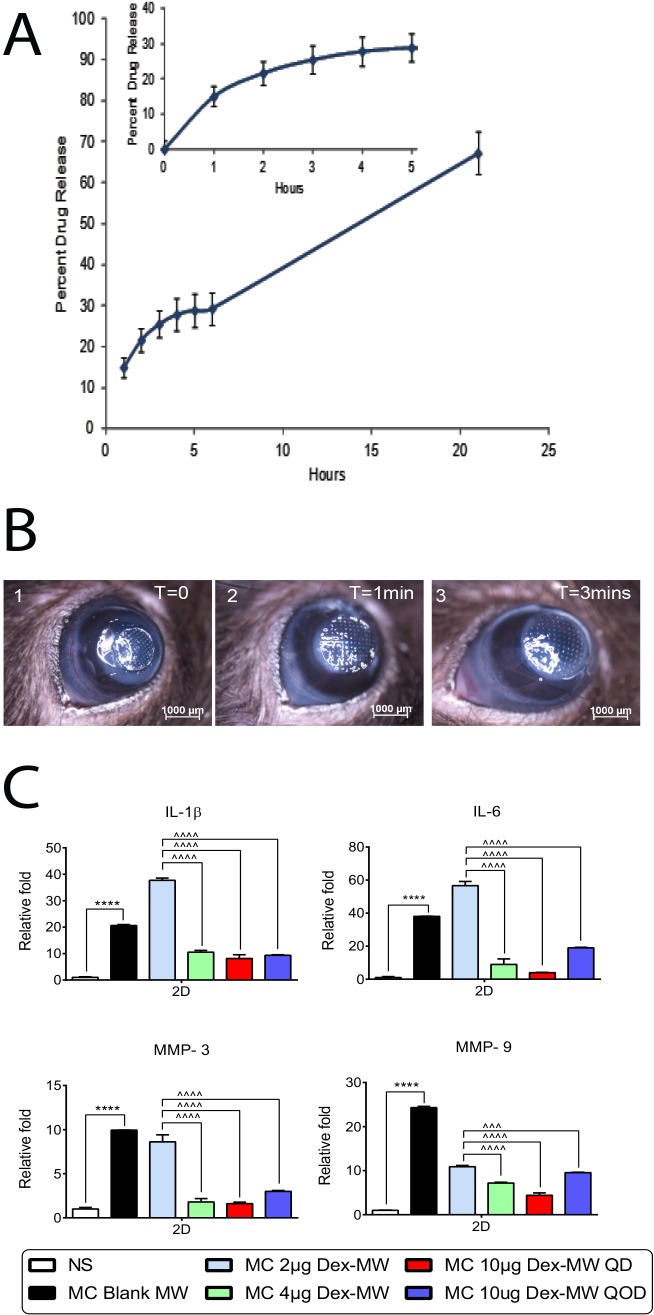
The in vitro drug release from Dex-NW was analyzed by HPLC method. The in vitro drug release profile of Dex-NW revealed a sustained Dex release for up to 24 hours (Fig. 1A). In the first hour, approximately 15% of the drug was released followed by 5% to 7% release per hour for the next 5 hours. As can be seen from the Figure 1A (inset), there was a very small burst release in the first hour followed by a sustained release for up to 24 hours. In practical terms, when a Dex-NW is applied on the injured cornea, a small initial burst release of the drug from the Dex-NW is followed by a prolonged release for sustained anti-inflammatory activity.
Figure 1.
(A) In vitro drug release of Dex from NW. The in vitro drug release profile of Dex-NW revealed a sustained Dex release for up to 24 hours. In the first hour, approximately 15% (inset) of the drug was released followed by 5% to 7% release per hour for the next 5 hours for up to 24 hours. (B) Nanowafer placement. Representative bright field digital images of burned eyes immediately after placement of NW in the central cornea up to 3 minutes. The NW was grasped gently by a jeweler's forceps and then applied to the cornea. A10-μl drop of BSS was applied to hydrate the NW and facilitate its uniform adhesion to the cornea. After hydration, the NW expanded. Careful application using this technique provided good adhesion of the NW to the cornea that did not dislodge with blinking. T, time. (C) Dose response of the MC Dex-NW on expression of inflammatory cytokine and MMP genes. Corneal expression of inflammatory cytokines (IL-1β and IL-6) and matrix metalloproteinase (MMP-3 and -9) are significantly increased in the combined model of alkali burn and dry eye. Ten micrograms of Dex-NW applied every day at 2 days post injury has greater anti-inflammatory potency to suppress the expression of inflammatory cytokines (IL-1β and IL-6) and matrix metalloproteinase (MMP-3 and -9) compared with vehicle controls. n = 4 right corneas/group. ****P < 0.0001: NS versus MC blank NW; ^^^P < 0.001, ^^^^P < 0.0001: 2 μg Dex-NW NW versus 4 μg Dex-NW, 10 μg Dex-NW. NS, nonstressed.
To test the maximum effective dose for suppression of inflammation, Dex-NWs loaded with 2, 4, and 10 μg of Dex and tested in our OB+DS model.2 Four mice per group were subjected to alkali burn and desiccating stress and received daily applications of the different NW (2, 4, 10 μg) for 2 days. After the creation of a unilateral alkali burn, the NW was grasped gently by a jeweler's forceps and applied to the cornea surface. A 10-μl drop of PBS was applied to hydrate the NW and facilitate its uniform adhesion to the cornea. After hydration, the NW expanded (Fig. 1B). Careful application using this technique provided good adhesion of the NW to the cornea that did not dislodge with blinking.
Another group of four mice received the 10 μg NW QOD. Whole corneas were harvested and gene expression investigated by real-time PCR. We observed that the 2 μg Dex-NW failed to reduce the expression levels of IL-1β, IL-6, and MMP-3 in the wounded corneas (Fig. 1C), while the 10 μg Dex QD had the lowest expression of IL-1β, IL-6, MMPs-3, and -9 in the wounded tissue compared with 4 or 10 μg Dex-NW QOD. Therefore, due to its greater anti-inflammatory potency, 10 μg Dex-NW was chosen as the drug concentration for the remainder of the experiments.
Dexamethasone Loaded-Nanowafer has Equal Efficacy to Conventional Dexamethasone Drops
Dex has been used to treat eye inflammation caused by injury, surgery, or other conditions. Our previous studies showed that Dex decreased corneal opacity scores and inflammatory cytokine and MMP expression in eyes subjected to our OB+DS model.11 Following unilateral alkali burn, mice were placed in a desiccating environment and treatment was initiated (either topical eyedrops or NW placement on the central cornea over the lesion). Clinical parameters (wound healing and corneal opacity) were evaluated daily for the duration of the experiments. We found that both Dex-NW and Dex drops treatments resulted in lower corneal opacity scores compared with their respective vehicle (Fig. 2A). Five days post injury, Dex-NW and Dex drops treatment yielded similarly lower corneal opacity scores, indicating that once daily Dex-NW had equal efficacy to Dex drops applied QID in preserving corneal clarity. Dex treatment, either by conventional drops or by NW, had similar effects on wound closure rate compared with their vehicle (Fig. 2B).
Figure 2.
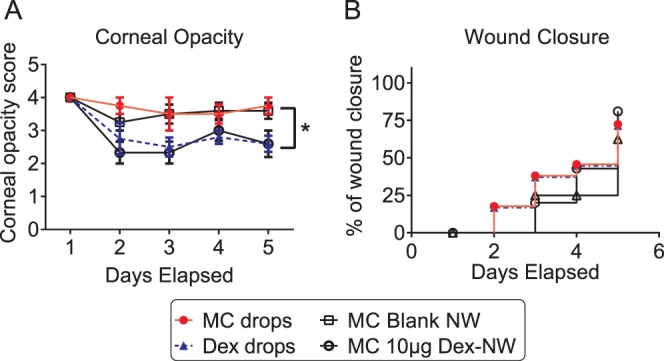
Dex drops or Dex-NW treatment decreases corneal opacity score. (A, B) Corneal opacity ([A] mean ± SEM) and wound closure rate ([B] categorical) in corneas subjected to ocular burn with concomitant desiccating stress and treated with Dex drops or Dex-NW and compared with its controls. Both Dex-NW and Dex drops treatments resulted in lower corneal opacity scores compared with their respective vehicle. n = 12 animals/group. *P < 0.05. Dex drops or Dex-NW treatment had similar effects on wound closure rate compared with their vehicle.
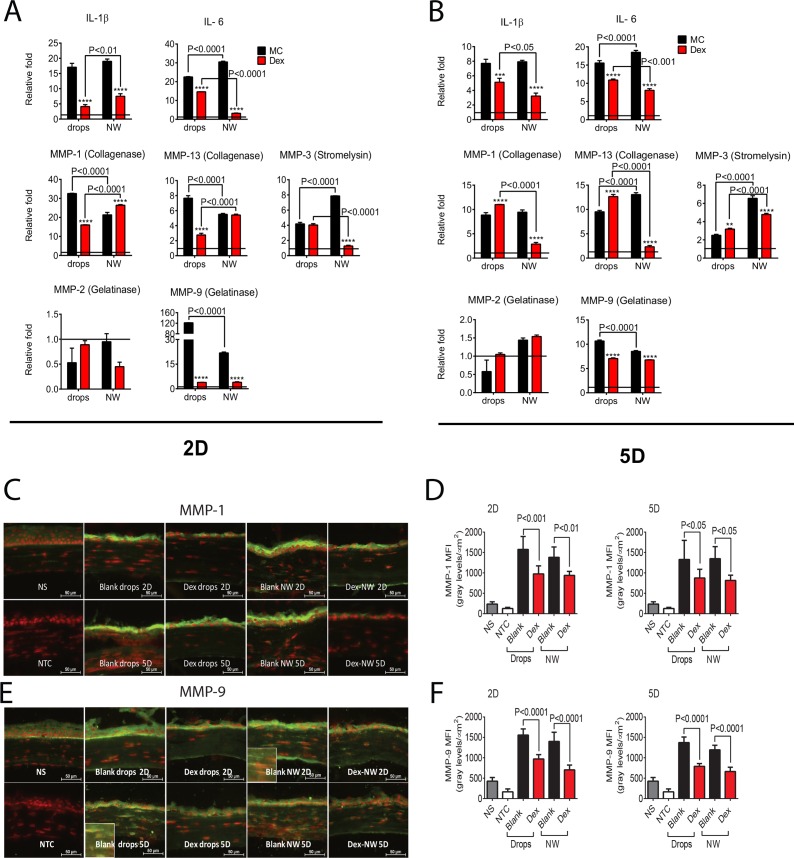
We have shown that expression of inflammatory cytokines and MMPs in the OB+DS model is greater than either of the individual treatment.2 Next, we compared the expression of IL-1β, IL-6, MMPs-1, -2, -3, -9, and -13 in the Dex-NW–treated corneas subjected to the OB+DS model by PCR to corneas treated with conventional Dex drops. Dex topical treatment significantly decreased IL-1β, IL-6, MMPs-1, -9, and -13 transcripts at 2 days post injury and significantly decreased IL-1β, IL-6, and MMP-9 at 5 days. In contrast, Dex-NW QD–treated corneas had lower expression of IL-1β, IL-6, and MMP-9 at both 2 and 5 days while it showed a significant decrease in MMPs-1 and -13 at 5 days post initial burn (Figs. 3A, 3B). Interestingly, significantly lower IL-6 and MMP-3 at 2 days and lower IL-1β, IL-6, MMPs-1, and -13 transcripts were measured in the Dex-NW compared with Dex drops at 5 days post initial burn, indicating that sustained Dex release produced greater anti-inflammatory and anti-MMP activity especially in the late stage of disease. There was no change in MMP-2 expression with any of the treatment groups. These data support our hypothesis that this sustained release formulation promotes diffusion of drug molecules directly into ocular tissues to produce greater anti-inflammatory effects in the late stage of the injury.
Figure 3.
Methylcellulose NW loaded with 10 μg Dex decrease inflammatory cytokines and MMPs in corneas of the combined model of alkali burn and dry eye. (A, B) Mean ± SEM of results of gene expression analysis of inflammatory cytokines (IL-1β and IL-6) and MMPs-1, -2, -3, -9, and -13 RNA transcripts in whole corneas from animals subjected to ocular burn + desiccating stress for 2 (A) or 5 (B) days and topically treated with Dex drops or Dex-NW and compared with its vehicle controls. The horizontal line at each figure represents the level of the mRNA expression for untreated group, which was used as the calibrator and normalized as 1. n = 4–5 right corneas/group. (C, D) Representative merged pictures of MMP-1 (C) and -9 (D) immunofluorescent staining shown in green of central cornea cryosections from animals subjected to a combined model of alkali burn and dry eye topically treated with Dex drops or Dex-NW and compared with its vehicle controls. Counterstaining was PI = red; n = 6 right corneas/group. (E, F) Quantification of mean fluorescence intensity (MFI) values from MMP-1 (E) and MMP-9 (E) staining are displayed. n = 6 right corneas/group. n = 4–5 right corneas/group. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001: MC blank wafers versus MC Dex-NW, MC blank drops versus MC Dex drops. MC blank-NW versus MC Dex-NW; NS = nonstressed; NTC, negative control; MFI, mean fluorescence intensity.
Immunoreactivity of corneas to MMP-1 and -9 were evaluated by immunostaining. Minimal levels of MMP-1 and -9 were present in the control corneas (Figs. 3C, 3E) and increased reactivity to both MMPs in the corneal epithelium was seen when they were treated with MC vehicle, either in drop or NW format. Matrix metalloproteinase-9–positive cells were also observed in the corneal stroma of vehicle treated groups (Fig. 3E). Consistent with the PCR results, both Dex-NW and Dex drops decreased MMP-1 and MMP-9 immunoreactivity in the corneal epithelium at 2 and 5 days. Quantification data for the mean fluorescence intensity of MMP-1 and -9 staining are shown in Figures 3D and 3F.
Dexamethasone Nanowafer Decreases Neutrophil Infiltration in Alkali-Burned Corneas Associated With Dry Eye
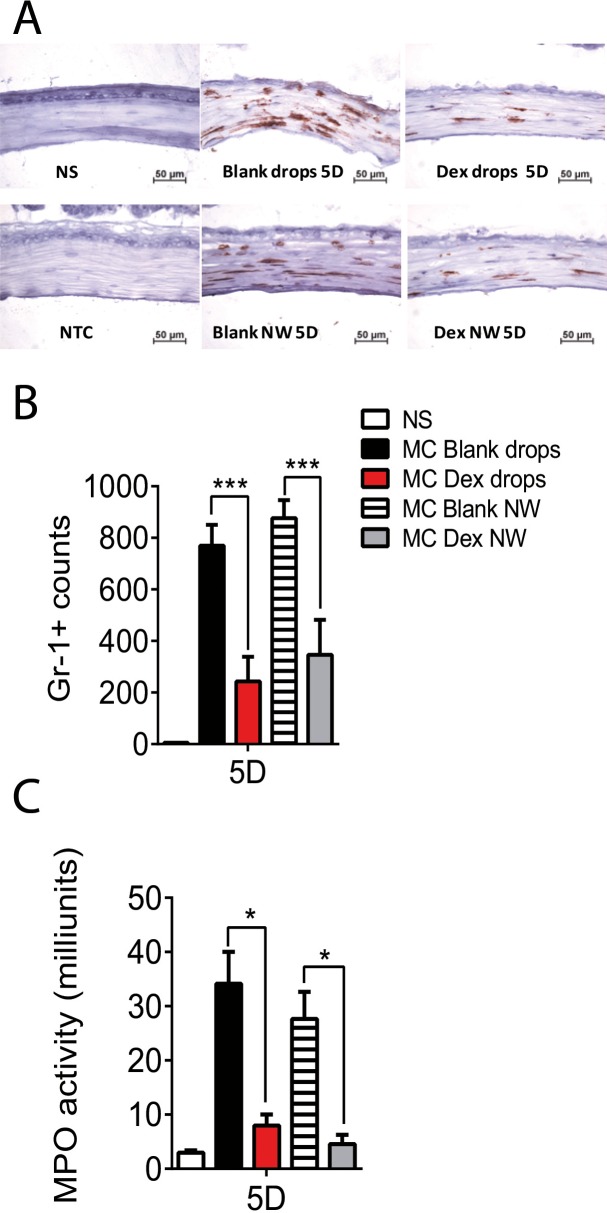
Neutrophils play an important role in the host defense against infection and neutrophil infiltration is the hallmark of acute inflammation. Dex has been reported to inhibit neutrophil migration to a site of inflammation.19 To test if Dex-NW can inhibit the influx of neutrophils, histologic sections of corneas were analyzed at different time points using neutrophil marker Gr-1 antibody (Fig. 4A). A significantly influx of Gr-1 positive (+) cells into the wound site was observed in both vehicle wafer and vehicle drops groups while both Dex-NW and Dex drops significantly decreased neutrophil infiltration (Fig. 4B).
Figure 4.
Dex NW decreases neutrophil infiltration. (A) Representative pictures of Gr-1+ cells (red) in cryosections of the central cornea from animals subjected to a combined model of alkali burn and dry eyes for 5 days (5D) and topically treated with Dex drops or Dex-NW and compared with its vehicle controls. n = 6 right corneas/group. (B) Bar graphs (mean ± SEM) of Gr-1+ cell counts in whole cornea/groups. n = 6 right corneas/group. (C) Myeloperoxidase activity in whole-corneas lysates from corneas subjected to ocular burn with concomitant desiccating stress for 5 days (5D) and topically treated with Dex drops or Dex-NW and compared with its vehicle controls (mean ± SEM). n = 4 right corneas/group. *P < 0.05; ***P < 0.001.
Myeloperoxidase (MPO), an enzyme mostly abundantly expressed in neutrophil granulocytes, has been used as a marker of neutrophil infiltration into tissue.20 As shown in Figure 4C, increased MPO levels were noted in the control groups (MC eyedrops and MC wafers) at 5 days. Consistent with the Gr-1+ staining results, both Dex-NW and Dex drops treatment significantly decreased MPO activity at 5 days post injury.
Taken together, these results demonstrated that a single application of Dex-NW had similar or greater effects to conventional Dex eyedrops applied QID, suggesting that slow release of drug content from the wafer improves biological outcomes and would facilitate compliance.
Discussion
Eye injuries due to chemical spills cause corneal epithelial damage, opacification, and corneal scarring, which often result in loss of vision and eventual blindness. After chemical injury, the therapeutic goal is to suppress ocular inflammation, to promote corneal epithelial healing and to restore corneal clarity by using anti-inflammatory drugs during the acute stage.1 Currently, eye injuries are treated with topical eyedrops of ophthalmic solutions. Although topical eye drop treatment is a simple and noninvasive mode of drug delivery, because of rapid blinking and tear turnover, most of the drug will be cleared from the ocular surface within a few seconds resulting in unsatisfactory treatment outcomes. In this study, we have presented the fabrication of Dex-NW and its therapeutic potential evaluated in an OB+DS murine model representing a severe ocular burn condition.2
Previous studies have shown that either corneal alkali burn21–23 or dry eye stress24–26 induce the production of inflammatory cytokines and increase MMPs activity in the ocular surface. In clinic, some patients with ocular burns also undergo dry eye stress: (1) there are many patients with ocular burns who also have damaged conjunctival goblet cells and lacrimal gland dysfunction, leading to decreased tear production, (2) patients with extensive facial burns have eyelids defects which can lead to corneal exposure, (3) some patients stay in environmentally controlled intensive care units (ICU) that have low humidity drafty environments. Therefore, we have recently established a mouse model that combines corneal alkali burns and desiccating stress that represents more severe ocular injury.2
Delayed or impaired wound healing has been an issue for Dex application to open wounds by blocking the fibrogenic effect of TGF-β.27 Conflicting data were reported on corneal wound healing following topical Dex treatment,28 with corneal epithelial healing retarded, but the basement membrane was well maintained. In our previous study, no wound healing was observed in the mice that receiving topical Dex treatment during OB+DS.11 Because the Dex-NW has longer drug retention time and high tissue drug absorbance, we asked whether Dex-NW affects corneal wound healing. After monitoring corneal epithelial healing on a daily basis, Dex-NW and Dex drops showed the same wound healing rate at 5 days post injuries, indicating that Dex-NW did not impair corneal wound healing. Another important clinical parameter, corneal clarity, is essential for vision and has also been evaluated after treatment with Dex-NW. As expected, administration of Dex-NW QD preserved corneal clarity at the same level as four times a day–administered Dex drops.
The NW drug delivery is novel in the sense that it can be fabricated with mucoadhesive polymers, such as polyvinyl alcohol, carboxymethyl cellulose, hydroxypropyl cellulose, and polyvinyl pyrrolidone, in addition to methylcellulose.12,13 Because of the mucoadhesive nature of the NW, it readily adheres to the ocular surface and releases the drug in a tightly controlled fashion. The NW increases the drug residence time on the cornea, which was found to increase diffusion of drug into the cornea stroma.12,13 On the contrary, drugs delivered as topical eye drop formulations are rapidly cleared from the ocular surface through tear production and drainage, thus limiting therapeutic effect.29 In addition to small molecules such as Dex, the NW can deliver hydrophilic and hydrophobic drugs, and also macromolecules such as antibodies, growth factors, and siRNA. The NW after application on the cornea will remain for up to 3 hours before it completely dissolves as described in one of our previous studies.12,13 Because the NW is fabricated with a mucoadhesive polymer, it will tightly adhere to the corneal surface and withstand blinking while the mouse is awake.
Dex, an anti-inflammatory glucocorticoid, is commonly used after cornel injury and eye surgery. In an outpatient clinical setting, current preferred treatment regimens for ocular burn include multiple topical administrations, including Dex every 6 hours. Hamill and colleagues4 proposed hourly administration of corticosteroids after corneal burn, which lead to improved visual acuity in patients with corneal alkali burns.1 Frequency of eyedrop administration is a main factor influencing patient compliance. Most children are uncooperative with eyedrops,30 older patients have even lower compliance due to motor disabilities and reduced visual acuity31 and critically ill patients depend on caregivers to properly administer drops. However, the outcomes of many eye diseases largely depend on the compliance of the patient to follow the treatment regimens. Hermann and colleagues5 have used electronic compliance monitoring of topical treatment for 28 patients after ophthalmic surgery, and a mean dose compliance of 50.2% was observed. Moreover, dose compliance was below 25% in approximately one of five patients. The observed mean dosage interval for each patient ranged from 4.6 to 19.7 hours with 30% of dosage intervals exceeding 12 hours. These results implied that the necessity to improve compliance with topical ophthalmic treatment regimens. Therefore, a controlled sustained release drug delivery system for administration of anti-inflammatory agents would be a major advance in the management of the blinding eye injuries and infections.32–34
In conclusion, our data show that NW provided sustained release of Dex, improved clinically graded corneal clarity while decreasing neutrophil infiltration and MMP expression in the cornea. Treatment with Dex-NW QD is as efficacious as four times a day Dex drops. The sustained drug release provided with Dex-NW may improve patient outcomes by improving efficacy of the drug as well as compliance.
Acknowledgments
The authors thank Kevin Christopher Tesareski and Mahira Zaheer for technical assistance.
Supported by grants from W81XWH-12-1-0616 (CSDP; Department of Defense, Fort Detrick, MD, USA), National Institutes of Health (NIH; Bethesda, MD, USA) Training Grant T32-AI053831 (FB; NIH, Bethesda, MD, USA), National Eye Institute/NIH Core Grant EY-002520 (Bethesda, MD, USA), Research to Prevent Blindness (New York, NY, USA), the Oshman Foundation (Houston, TX, USA), William Stamps Farish Fund (Houston, TX, USA), and the Hamill Foundation (Houston, TX, USA).
Presented in part as abstract at the annual meeting of the Association for Research in Vision and Ophthalmology, Denver, 2015.
Baylor College of Medicine has filed for intellectual property rights for the use of nanowafers in the treatment of ocular diseases. S.C. Pflugfelder, G. Acharya, and C.S. De Paiva are co-inventors of a patent application.
Disclosure: F. Bian, None; C.S. Shin, None; C. Wang, None; S.C. Pflugfelder, P; G. Acharya, P; C.S. De Paiva, P
References
- 1. Al-Moujahed A,, Chodosh J. Outcomes of an algorithmic approach to treating mild ocular alkali burns. JAMA Ophthalmol. 2015; 133: 1214–1216. [DOI] [PubMed] [Google Scholar]
- 2. Bian F,, Pelegrino FS,, Pflugfelder SC,, Volpe EA,, Li DQ,, de Paiva CS. Desiccating stress-induced MMP production and activity worsens wound healing in alkali-burned corneas. Invest Ophthalmol Vis Sci. 2015; 56: 4908–4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Donshik PC,, Berman MB,, Dohlman CH,, Gage J,, Rose J. Effect of topical corticosteroids on ulceration in alkali-burned corneas. Arch Ophthalmol. 1978; 96: 2117–2120. [DOI] [PubMed] [Google Scholar]
- 4. Hamill CE,, Bozorg S, PeggyChang HY,, et al. Corneal alkali burns: a review of the literature and proposed protocol for evaluation and treatment. Int Ophthalmol Clin. 2013; 53: 185–194. [DOI] [PubMed]
- 5. Hermann MM,, Ustundag C,, Diestelhorst M. Electronic compliance monitoring of topical treatment after ophthalmic surgery. Int Ophthalmol. 2010; 30: 385–390. [DOI] [PubMed] [Google Scholar]
- 6. Sharma A,, Tandon A,, Tovey JC,, et al. Polyethylenimine-conjugated gold nanoparticles: gene transfer potential and low toxicity in the cornea. Nanomedicine. 2011; 7: 505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pan Q,, Xu Q,, Boylan NJ,, et al. Corticosteroid-loaded biodegradable nanoparticles for prevention of corneal allograft rejection in rats. J Control Release. 2015; 201: 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mohammadpour M,, Jabbarvand M,, Karimi N. Therapeutic possibilities of ceftazidime nanoparticles in devastating pseudomonas ophthalmic infections; keratitis and endophthalmitis. Med Hypothesis Disc Innov Ophthalmol. 2012; 1: 6–9. [PMC free article] [PubMed] [Google Scholar]
- 9. Xu J,, Li X,, Sun F. Preparation and evaluation of a contact lens vehicle for puerarin delivery. J Biomat Sci Polym Ed. 2010; 21: 271–288. [DOI] [PubMed] [Google Scholar]
- 10. Hiratani H,, Fujiwara A,, Tamiya Y,, Mizutani Y,, Alvarez-Lorenzo C. Ocular release of timolol from molecularly imprinted soft contact lenses. Biomaterials. 2005; 26: 1293–1298. [DOI] [PubMed] [Google Scholar]
- 11. Bian F,, Pelegrino FS, TuklerHenriksson J,, et al. Differential effects of dexamethasone and doxycycline on inflammation and MMP production in alkali-burned corneas associated with dry eye. Ocul Surf. 2016; 14: 242–254. [DOI] [PMC free article] [PubMed]
- 12. Yuan X,, Marcano DC,, Shin CS,, et al. Ocular drug delivery nanowafer with enhanced therapeutic efficacy. ACS Nano. 2015; 9: 1749–1758. [DOI] [PubMed] [Google Scholar]
- 13. Coursey TG,, Henriksson JT,, Marcano DC,, et al. Dexamethasone nanowafer as an effective therapy for dry eye disease. J Control Release. 2015; 213: 168–174. [DOI] [PubMed] [Google Scholar]
- 14. Acharya G,, McDermott M,, Shin SJ,, Park H,, Park K. Hydrogel templates for the fabrication of homogeneous polymer microparticles. Methods Mol Biol. 2011; 726: 179–185. [DOI] [PubMed] [Google Scholar]
- 15. Chotikavanich S,, de Paiva CS, Li DQ, et al. Production and activity of matrix metalloproteinase-9 on the ocular surface increase in dysfunctional tear syndrome. Invest Ophthalmol Vis Sci. 2009; 50: 3203–3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yoeruek E,, Ziemssen F,, Henke-Fahle S,, et al. Safety, penetration and efficacy of topically applied bevacizumab: evaluation of eyedrops in corneal neovascularization after chemical burn. Acta Ophthalmol. 2008; 86: 322–328. [DOI] [PubMed] [Google Scholar]
- 17. De Paiva CS,, Chotikavanich S,, Pangelinan SB,, et al. IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunol. 2009; 2: 243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. De Paiva CS,, Corrales RM,, Villarreal AL,, et al. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eye. Exp Eye Res. 2006; 83: 526–535. [DOI] [PubMed] [Google Scholar]
- 19. Cornelio Favarin D,, MartinsTeixeira M, Lemos de Andrade E, et al. . Anti-inflammatory effects of ellagic acid on acute lung injury induced by acid in mice. Mediators Inflamm. 2013; 2013: 164202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ji KA,, Eu MY,, Kang SH,, Gwag BJ,, Jou I,, Joe EH. Differential neutrophil infiltration contributes to regional differences in brain inflammation in the substantia nigra pars compacta and cortex. Glia. 2008; 56: 1039–1047. [DOI] [PubMed] [Google Scholar]
- 21. Herretes S,, Suwan-Apichon O,, Pirouzmanesh A,, et al. Use of topical human amniotic fluid in the treatment of acute ocular alkali injuries in mice. Am J Ophthalmol. 2006; 142: 271–278. [DOI] [PubMed] [Google Scholar]
- 22. Sosne G,, Christopherson PL,, Barrett RP,, Fridman R. Thymosin-beta4 modulates corneal matrix metalloproteinase levels and polymorphonuclear cell infiltration after alkali injury. Invest Ophthalmol Vis Sci. 2005; 46: 2388–2395. [DOI] [PubMed] [Google Scholar]
- 23. Takahashi H,, Igarashi T,, Fujimoto C,, Ozaki N,, Ishizaki M. Immunohistochemical observation of amniotic membrane patching on a corneal alkali burn in vivo. Jpn J Ophthalmol. 2007; 51: 3–9. [DOI] [PubMed] [Google Scholar]
- 24. De Paiva CS,, Corrales RM,, Villarreal AL,, et al. Apical corneal barrier disruption in experimental murine dry eye is abrogated by methylprednisolone and doxycycline. Invest Ophthalmol Vis Sci. 2006; 47: 2847–2856. [DOI] [PubMed] [Google Scholar]
- 25. Corrales RM,, Stern ME,, de Paiva CS,, Welch J,, Li DQ,, Pflugfelder SC. Desiccating stress stimulates expression of matrix metalloproteinases by the corneal epithelium. Invest Ophthalmol Vis Sci. 2006; 47: 3293–3302. [DOI] [PubMed] [Google Scholar]
- 26. Pflugfelder SC,, Farley W,, Luo L,, et al. Matrix metalloproteinase-9 knockout confers resistance to corneal epithelial barrier disruption in experimental dry eye. Am J Pathol. 2005; 166: 61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Meisler N,, Keefer KA,, Ehrlich HP,, Yager DR,, Myers-Parrelli J,, Cutroneo KR. Dexamethasone abrogates the fibrogenic effect of transforming growth factor-beta in rat granuloma and granulation tissue fibroblasts. J Invest Dermatol. 1997; 108: 285–289. [DOI] [PubMed] [Google Scholar]
- 28. Kim JC,, Chung H,, Tseng SCG. Botulinum toxin treatment for filamentary keratitis associated with corneal occlusion by lids. : Lass JH, Advances in Corneal Research. New York: Plenum Press; 1998; 105–115. [Google Scholar]
- 29. Urtti A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv Drug Deliv Rev. 2006; 58: 1131–1135. [DOI] [PubMed] [Google Scholar]
- 30. Sujuan JL,, Handa S,, Perera C,, Chia A. The psychological impact of eyedrops administration in children. J AAPOS. 2015; 19: 338–343. [DOI] [PubMed] [Google Scholar]
- 31. Dietlein TS,, Rosentreter A,, Lappas A. Complexities of medical glaucoma therapy - the elderly patient in focus [in German]. Klinische Monatsblatter fur Augenheilkunde. 2016; 233: 138–142. [DOI] [PubMed] [Google Scholar]
- 32. Lo R,, Li PY,, Saati S,, Agrawal R,, Humayun MS,, Meng E. A refillable microfabricated drug delivery device for treatment of ocular diseases. Lab Chip. 2008; 8: 1027–1030. [DOI] [PubMed] [Google Scholar]
- 33. Singh K,, Nair AB,, Kumar A,, Kumria R. Novel approaches in formulation and drug delivery using contact lenses. J Basic Clin Pharm. 2011; 2: 87–101. [PMC free article] [PubMed] [Google Scholar]
- 34. Mack BC,, Wright KW,, Davis ME. A biodegradable filament for controlled drug delivery. J Control Release. 2009; 139: 205–211. [DOI] [PubMed] [Google Scholar]