Abstract
Purpose
Mice lacking ATP-binding cassette transporter 4 (ABCA4) and retinol dehydrogenase 8 (RDH8) mimic features of human Stargardt disease and age-related macular degeneration. RNA-sequencing of whole eyes was done to study early gene expression changes in Abca4−/−Rdh8−/− mice.
Methods
Abca4−/−Rdh8−/− mice at 4 weeks of age were exposed to intense light. Total RNA was extracted from whole eyes and used to generate RNA libraries that were paired-end sequenced on the Illumina HiSeq 2500 device. Differentially expressed genes were annotated using Gene set enrichment analysis (GSEA). Selected genes in enriched pathways exhibiting differential expression were validated using quantitative qRT-PCR and ELISA.
Results
Transcriptome analysis of the whole eye identified 200 genes that were differentially expressed 24 hours after light exposure compared to no light in Abca4−/−Rdh8−/− mice. Expression of several visual cycle and photoreceptor genes were decreased, indicative of photoreceptor/RPE cell death. Gene categories of early stress response genes, inflammatory cytokines, immune factors, and JAK STAT components were upregulated. Lipocalin 2 (Lcn2) was the most upregulated early stress response gene identified. Protein LCN2 was produced by RPE cells and the neural retina after intense light exposure as well as in cultured RPE cells from mice and humans incubated with lipopolysaccharide or photoreceptor outer segments.
Conclusions
Identification of important mediators involved in the crosstalk between the acute stress response and immune activation in RPE cells and the neural retina, such as LCN2, provide novel molecular targets for reducing cellular stress during retinal degeneration.
Keywords: visual cycle, early stress response, RPE, Abca4−; −Rdh8−; − mice
Acute stress responses are early defense mechanisms that are activated by cell damage. These responses serve as an essential component of innate immunity intended to clear potential pathogens and initiate inflammatory processes for maintenance of cell homeostasis.1 Acute phase proteins, which are synthesized in the liver, are the primary regulators of this process. Early oxidative stress and immune dysregulation in the retina caused by light damage has been considered an important driver in the progression of retinal disease, including age-related macular degeneration (AMD).2 Along with inflammation, acute stress responses are the first line of protective immune responses against external stress as light.3 Our previous study generated an Abca4−/−Rdh8−/− mouse to elucidate events in retinal degeneration caused by the disrupted visual cycle.4 ATP-binding cassette transporter 4 (ABCA4) transports all-trans-retinal from the inner to the outer leaflet of photoreceptor disc membranes5 and retinol dehydrogenase 8 (RDH8) is responsible for reduction of all-trans-retinal to all-trans-retinol in photoreceptor outer segments.6 Mutations of ABCA4 are associated with Stargardt disease and AMD.7 Abca4−/−Rdh8−/− mice display several features of human Stargardt disease and AMD-like phenotypes characterized by lipofuscin accumulation, drusen formation, complement activation, and photoreceptor/RPE atrophy under room lighting conditions.4 Intense light exposure can accelerate retinal degeneration in Abca4−/−Rdh8−/− mice and early activation of inflammatory cytokines was involved in the progression of light-induced retinal degeneration.8 Exposure to light causes photoisomerization of visual chromophore 11-cis-retinal to all-trans-retinal, however delayed clearance of all-trans-retinal in photoreceptor cells, by impaired functions of ABCA4 and/or RDH8, is associated with exacerbation of retinal degeneration as all-trans-retinal is a highly reactive toxic aldehyde.9,10 Mitochondrial associated cell death9,11 and inflammatory changes8,11 have been found as part of a cascade of cellular events caused by all-trans-retinal toxicity. However, not much is understood about the early events that ultimately lead to photoreceptor and RPE cell death in this mouse model. This mouse model coupled with RNA-sequencing (RNA-seq) technology present a unique approach to study and elucidate these early precipitating events that trigger the age-related pathology seen in human patients with Stargardt disease and AMD.
We performed a comprehensive gene expression analysis using RNA-seq to identify the early cell responses involved in retinal degeneration in Abca4−/−Rdh8−/− mice. Our study revealed a highly associated relationship between the acute stress response and immune activation in the retina.
Methods
Animals
Abca4−/−Rdh8−/− mice were generated and genotyped as described previously.4 Only mice with leucine variation at amino acid 450 of RPE6512 and free of rd8 mutation13 were used in the study. C57BL/6J mice were used as controls. Mertk−/− mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). All mice used in the study were housed in the animal facility at the School of Medicine, Case Western Reserve University, regularly maintained in a 12-hour light (∼10 lux)/12-hour dark cycle environment. All animal procedures and experiments were approved by the Case Western Reserve University Animal Care Committees and conformed to recommendations of the American Veterinary Medical Association Panel on Euthanasia and the Association of Research for Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research.
Induction of Light Damage
Female Abca4−/−Rdh8−/− mice at 4 weeks of age were dark-adapted overnight before exposure to light. Pupils were dilated with a mixture of 0.5% tropicamide and 0.5% phenylephrine hydrochloride (Midorin-P; Santen Pharmaceutical, Japan, Osaka, Japan) 5 minutes before light exposure. Light damage was induced in mice by fluorescent light exposure at 10,000 lux (150 W spiral lamps; Commercial Electric Products, Cleveland, OH, USA) for 30 minutes in a white bucket as described previously.14 Mice were returned to the dark until further analysis.
Retinal Histology
All procedures for histologic analysis were performed as described previously.8 After enucleation, eyes were fixed in 1% glutaraldehyde/4% paraformaldehyde followed by paraffin embedding. Sections were cut at 5-μm thickness and stained with hematoxylin-eosin (HE) for visualization under a light microscope.
Spectral-Domain Optical Coherence Tomography (SD-OCT)
Ultra-high resolution SD-OCT (Bioptigen SD-OCT Envisu C2200; Bioptigen, Research Triangle Park, NC, USA) was used for in vivo imaging of mouse retinas. Mice were anesthetized by intraperitoneal injection of a mixture (20 mL/g body weight) containing ketamine (6 mg/mL) and xylazine (0.44 mg/mL) in 10 mM sodium phosphate, pH 7.2, with 100 mM NaCl. Pupils were dilated with a mixture of 0.5% tropicamide and 0.5% phenylephrine hydrochloride (Santen Pharmaceutical). Five pictures acquired in the B-scan mode were used to construct each final averaged SD-OCT image by previously established protocols.10
Total RNA Preparation and Whole Eye Library Preparation
Eyes were collected from 4-week-old female Abca4−/−Rdh8−/− mice and kept in RNA later stabilization solution (Qiagen, Valencia, CA, USA). Total RNA was extracted with the RNeasy Mini Kit (Qiagen). Mouse eye libraries were prepared using Illumina TruSeq Stranded Total RNA protocol with Ribo-Zero Gold (rRNA depletion) following the manufacturer's instructions (Illumina, San Diego, CA, USA).
Illumina RNA-Seq Runs.
Each murine eye library was sequenced using 50 base pairs (bp) paired end sequencing on Illumina HiSeq 2500. Reads were aligned with the mouse genome assembly (mm9, UCSC) using an optimized data analysis workflow ToPHat (version 2.0.12). Data analysis software Cuffdiff (version 2.2.1) was used to define differential gene expression and CummeRbund (version 2.6.1) was used for final data summary and visualization. All of the above processes were performed at the Institute for Computational Biology, Case Western Reserve University. Processed and raw FASTQ files were deposited in Gene expression Omnibus (GEO) and assigned an accession no GSE75470.
Gene Set Enrichment Analysis (GSEA).
Analysis of RNA-seq data was done using GSEA (gene set enrichment analysis software). A core set of 14,313 genes with significant gene IDs (FPKM values > 1.0) was uploaded in the GSEA database for identifying significantly enriched gene sets upon comparison with annotated genes in the data base. Annotated gene sets contain genes classified according to their GO biological processes.15
Quantitative RT-PCR (qRT-PCR)
Total RNA was isolated from eyes collected at 3 hours, 24 hours, and 7 days after light exposure of 4-week-old Abca4−/−Rdh8−/− mice. cDNA was synthesized with the QuantiTect Reverse Transcription Kit (Qiagen) following the manufacturer's instructions. Quantitative RT-PCR amplification was performed using SYBR Green I Master mix (Roche Diagnostics, Risch-Rotkreuz, Switzerland). Primers were designed using the web tool Primer 3 and synthesized by Eurofins MWG Operon (Huntsville, AL, USA). Fold changes were calculated after normalizing the data to Gapdh.
Enzyme-Linked Immunosorbent Assay (ELISA)
Expression of lipocalin 2 (LCN2) was quantified in whole eye cell lysates by ELISA (Mouse Lipocalin-2 Quantikine Elisa Kit) purchased from R&D systems (Minneapolis, MN, USA). Female Abca4−/−Rdh8−/− mice and WT mice at 4 weeks of age were exposed to light of 10,000 lux for 30 minutes. Mouse eyes were collected 24 hours, and 3 and 7 days after light exposure, and were homogenized in 500 μL of PBS (10 mM sodium phosphate, pH 7.2, with 100 mM NaCl) with protease inhibitor cocktails (Roche Diagnostics) using a glass–glass homogenizer. Homogenates (50 μL) were used for quantification. A single data point was obtained from each mouse (2 eyes). Pluripotent stem cell–derived human RPE cells were stimulated with LPS and LCN2 expression in the cell lysate and medium were determined by Human Lipocalin-2 Quantikine ELISA kit (R&D Systems).
Isolation of the Retina and RPE Cells After Light Exposure in Abca4−/−Rdh8−/− Mice for RNA Analyses
Abca4−/−Rdh8−/− mice at 4 weeks of age (n = 6) were exposed to light at 10,000 lux for 30 minutes. Eye cups were made by removing the lens, cornea, and vitreous, and neural retina was peeled off leaving the eye cup with the RPE-choroid. Total RNA was extracted from the retina and RPE-choroid as described.
Stimulation of Mouse Primary RPE Cells by Photoreceptor Outer Segment (POS)
Primary RPE cells were isolated from 2-week-old Abca4−/−Rdh8−/− mice based on published methods.8 Enucleated eyes were incubated in 2% dispase (Invitrogen) solution for 45 minutes in a 37°C water bath with gentle mixing every 15 minutes. After removal of the neural retina, RPE sheets were peeled away from the choroid and pipetted into a tube containing Dulbecco's modified Eagle's medium (DMEM) plus streptomycin/penicillin and 10% fetal bovine serum (FBS). Primary isolated RPE cells were plated into a 96-well plate and grown in DMEM containing minimal essential medium nonessential amino acids (Invitrogen), 100 units/mL penicillin (Invitrogen), 100 μg/mL streptomycin (Invitrogen), 20 mM HEPES, pH 7.0, and 10% FBS at 37°C. These cells were checked for their attachment and medium change was given every 2 days. At approximately 2 weeks when the cells achieved 70% to 80% confluency, they were coincubated with POS at a final concentration of 6 mg/mL for 24 hours at 37°C.
Stimulation of Human RPE Cells With Lipopolysaccharide (LPS)
Human RPE cells differentiated from pluripotent stem cells were cultured according to procedures reported previously.16 Lipopolysaccharide from InvivoGen (San Diego, CA, USA) was added to RPE cells at a final concentration of 1 μg/mL at 37°C for 24 hours. Lipopolysaccharide is a well characterized endotoxin known to be a ligand of TLR4 involved in activation of several proinflammatory cytokines.17
Data Analysis
Data representing the mean ± SD of at least three independent experiments were compared by the 1-way ANOVA test. A P value of < 0.05 was considered statistically significant.
Results
In Vivo Imaging Reveals Changes in Retinal Morphology 24 Hours After Light Damage
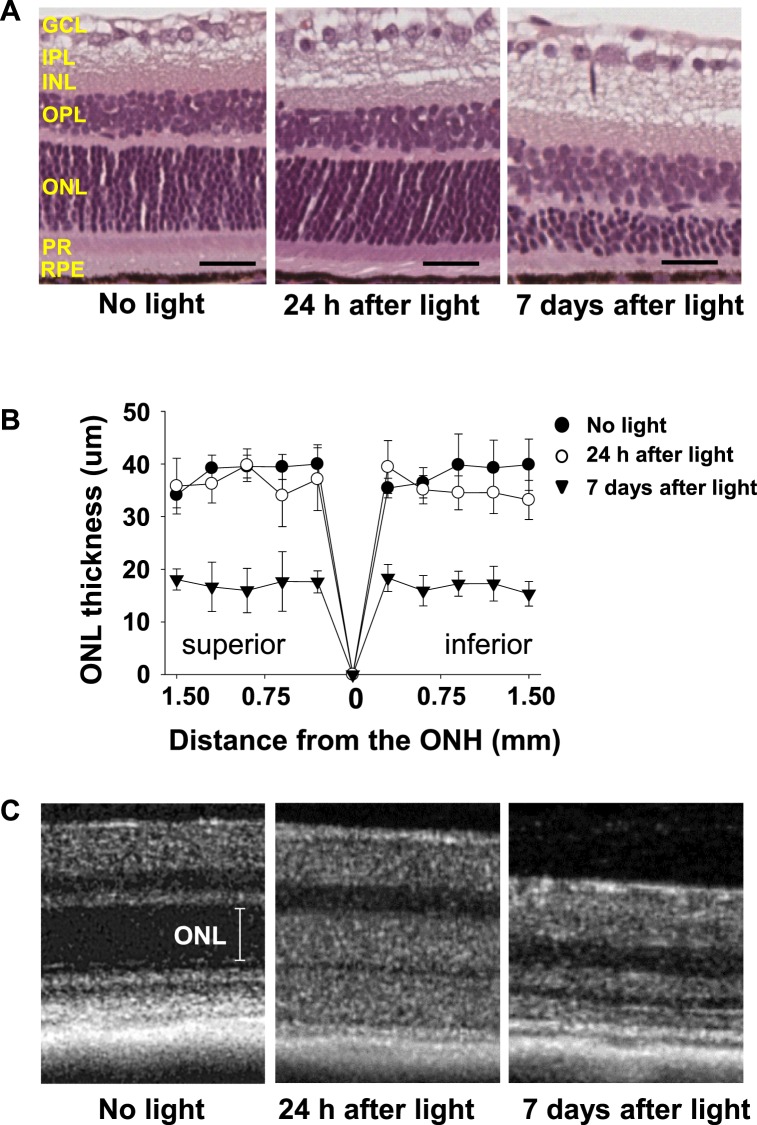
To examine morphologic changes of the retina after light exposure, retinal histology was analyzed with eyes of 4-week-old Abca4−/−Rdh8−/− mice 24 hours and 7 days after light exposure at 10,000 lux for 30 minutes (Fig. 1A). No notable retinal degeneration was visible 24 hours after light exposure in hematoxylin-eosin stained sections. Retinal degeneration with reduction of outer nuclear layer (ONL) thickness was observed 7 days after illumination (Fig. 1B). On the other hand, in vivo OCT imaging revealed noticeable changes in the ONL layer 24 hours after illumination (Fig. 1C). Therefore, morphologic changes observed in the OCT results indicated prominent underlying cellular events, associated with photoreceptor degeneration, occur as early as 24 hours after illumination.
Figure 1.
Retinal changes after light exposure in Abca4−/−Rdh8−/− mice. (A) Representative retinal images of the inferior retina at 750 to 1000 μm from the optic nerve head (ONH) of 4-week-old Abca4−/−Rdh8−/− mice after light exposure at 10,000 lux for 30 minutes are shown. No apparent morphologic alterations are visible 24 hours after light exposure whereas severe retinal degeneration is observed 7 days after light exposure (n = 3 for each group). PR, photoreceptors; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. Scale bar: 20 μm. (B) Outer nuclear layer thickness of 4-week-old Abca4−/−Rdh8−/− mice after light exposure at 10,000 lux for 30 minutes is plotted (n = 3 for each group). Sections were prepared with the paraffin embedment. Error bars: Mean ± SD. (C) Representative in vivo SD-OCT imaging of the inferior retina at 500 to 750 μm from the ONH show changes in ONL thickness 24 hours after light exposure and by 7 days there is an observed loss of ONL indicating severe retinal degeneration (n = 3 for each group).
Transcriptome Profiling in Eyes of Abca4−/−Rdh8−/− Mice After Light Exposure
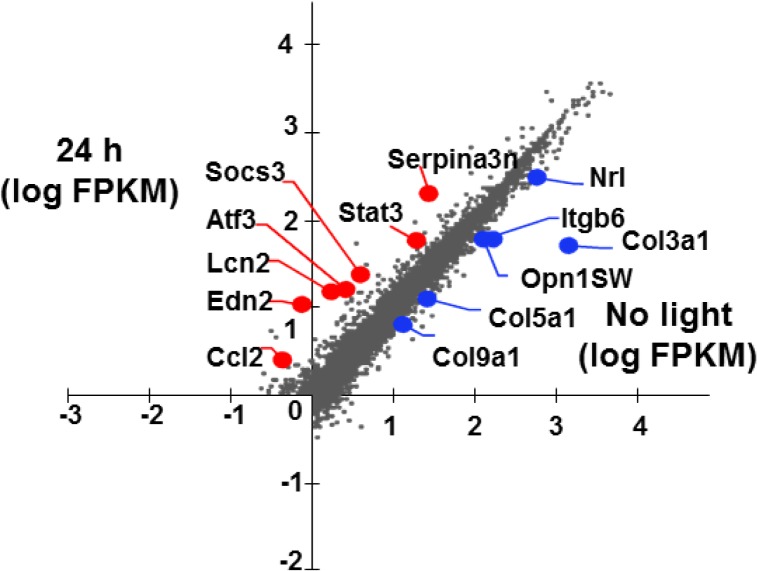
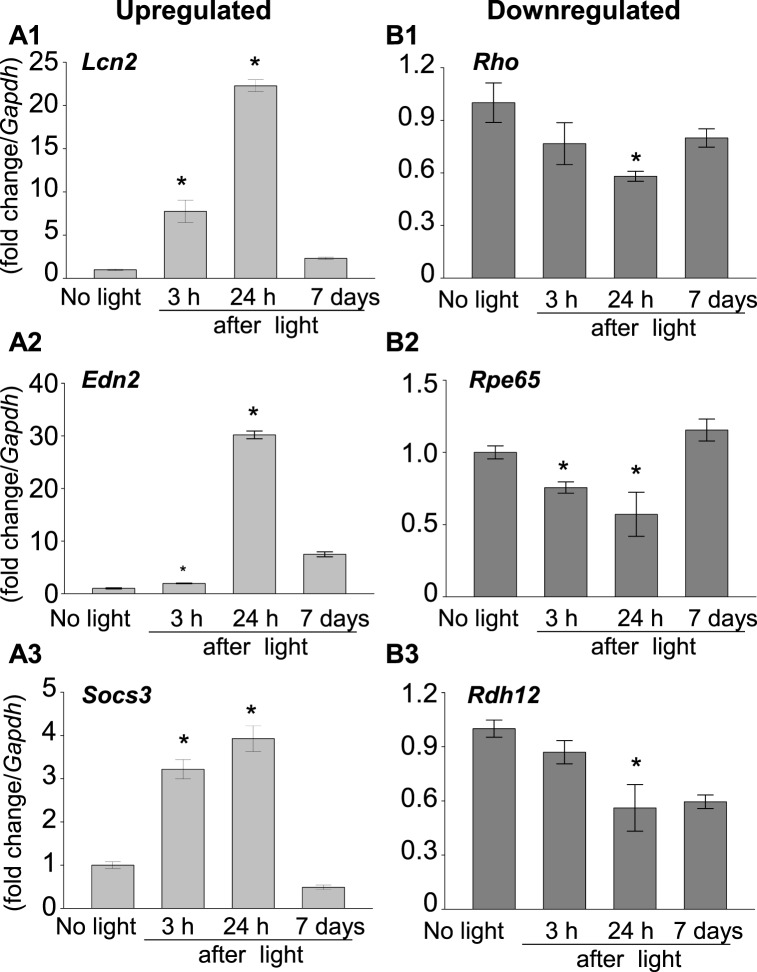
To investigate changes in gene expression after light exposure in Abca4−/−Rdh8−/− mice, total RNA was extracted from eyes collected 3 and 24 hours, and 7 days after light exposure and subjected to RNA-seq. Samples were taken in triplicate for each time point and no light exposed mice were used as controls. Libraries were generated, then tagged with unique index sequences and sequenced on a single lane of the HiSeq 2500 platform (Illumina). Reads generated were mapped to the mouse reference genome (mm9, UCSC). Abundance of assembled transcripts was reported as fragments per kilobase of exon per million fragments mapped (FPKM). Fold change (FC) differences between light exposed and no light exposed samples were calculated. Applying a fold cutoff of 2-fold and P < 0.05, 101 genes were found differentially expressed after 3 hours, 200 genes after 24 hours, and 34 genes after 7 days. Most of the genes had a bipartite pattern of expression found increasing at 3 hours, peaking at 24 hours and most of the genes returned to levels comparable to the no light exposed condition by 7 days. Genes found differentially expressed at 24 hours were analyzed using the GSEA database.18 Those 200 genes were found differentially expressed at 24 hours compared to the no light exposed control condition and were analyzed to further categorize statistically significant enriched gene sets. Gene sets in this collection are derived from the controlled vocabulary of the Gene Ontology (GO) project.15 The GO annotated gene collection included 3 functional clusters (biologic process, cellular component, and molecular function). Differentially expressed genes were classified according to their GO biologic processes (see Table). Genes found upregulated were overrepresented in categories, such as early stress response, immune factors, inflammatory cytokines and JAK STAT pathway. The analysis lists the top upregulated genes (Lcn2, Serpina3n, Atf3, Edn2, Ccl2, Socs3, Stat3) and downregulated genes (Nrl, Opn1SW, Col9a1, Col5a1, Col3a1, Itgb6; Fig. 2). To confirm the results of RNA-seq, qRT-PCR for select genes upregulated (Lcn2, Edn2, Socs3; Figs. 3A1–A3) and downregulated (Rho, Rpe65, Rdh12; Figs. 3B1–B3) was done for eyes collected after light exposure from distinct Abca4−/−Rdh8−/− mice other than those used for RNA-seq analysis. Quantitative RT-PCR results confirmed our findings from RNA-seq. Primers used in the study are listed in Supplementary Table S1.
Table.
Selected Differentially Expressed Genes 24 Hours After Light Exposure
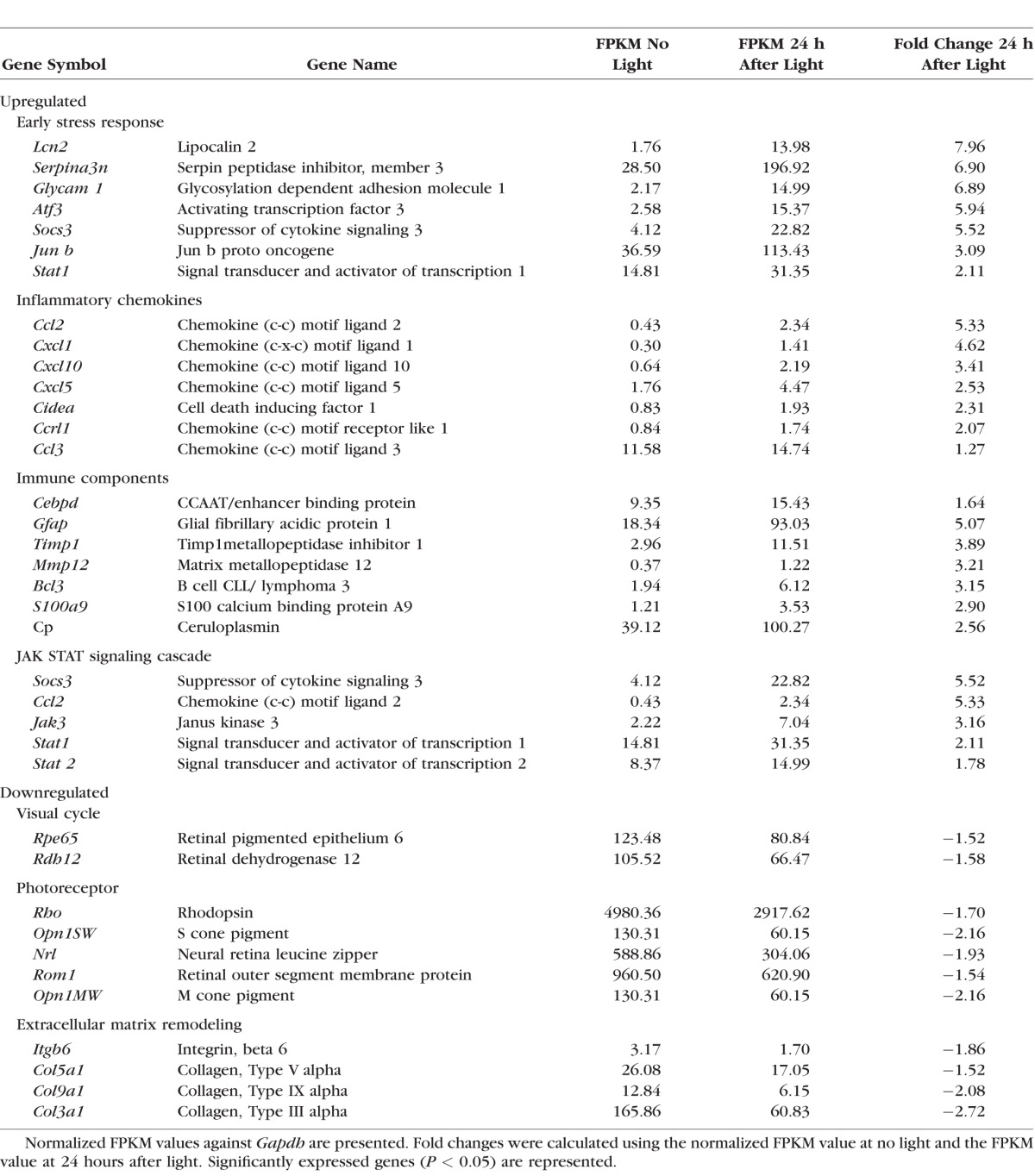
Figure 2.
RNA-seq revealed differential expression of genes after light exposure in Abca4−/−Rdh8−/− mice. Scatter plot of log FPKM values showing the most upregulated (in red) and downregulated (in blue) genes 24 hours after light exposure. Four-week-old Abca4−/−Rdh8−/− mice were used (n = 3 for each group).
Figure 3.
Large changes in expression of early stress response genes after light exposure. mRNA levels of select genes ([A1–A3] upregulated, Lcn2, Edn2, Socs3; [B1–B3] downregulated, Rho, Rpe65, Rdh12) were analyzed by qRT-PCR in whole eyes of 4-week-old Abca4−/−Rdh8−/− mice collected 3 and 24 hours, and 7 days after light exposure. Results were consistent with RNA-seq data. Fold changes in expression are compared to no light exposed Abca4−/−Rdh8−/− mice. The expression of each gene was normalized to the housekeeping gene Gapdh. Error bars: SD of the means (n = 3). *P < 0.05, light versus no light exposed.
Early Stress Response Gene Lipocalin 2 Was Upregulated 24 Hours After Light Exposure
Lipocalin 2 (Lcn2) mRNA expression was 22-fold higher 24 hours after light exposure in Abca4−/−Rdh8−/− mice compared to no light exposed mice (Fig. 3A1). Protein expression of LCN2 displayed a similar pattern as mRNA with the highest expression occurring at 24 hours after light exposure (Fig. 4A). To further evaluate the likely source of LCN2, qRT-PCR of Lcn2 was performed with RPE cells and neural retinas isolated from 4-week-old Abca4−/−Rdh8−/− mice 24 hours after light exposure. Lcn2 was found enriched in the RPE and the retina after normalization with Gapdh, and the RPE displayed significantly more elevated changes compared to the retina after light exposure (Fig. 4B). Retinal pigment epithelial cells phagocytize old POS and excessive amounts of POS debris are produced after light exposure in vivo.19 To further explore whether LCN2 production is associated with RPE phagocytosis of POS, RPE cells isolated from 2-week-old Abca4−/−Rdh8−/− mice were coincubated with POS for 24 hours. A 47-fold increase in LCN2 production was observed with POS compared to nontreated cells (Fig. 4C).
Figure 4.
Lipocalin 2 expression in the retinal cells in mice. (A) Protein levels of LCN2 measured in 4-week-old Abca4−/−Rdh8−/− mice by ELISA. *P < 0.05 versus no light exposed (n = 12). (B) Retinal pigment epithelial cells and neural retinas were isolated from 4-week-old Abca4−/−Rdh8−/− mice. RNA levels of Lcn2 were found significantly increased in the RPE compared to the retina after light exposure. **P < 0.05 versus the retina, *P < 0.05 versus no light exposed (n = 12). (C) Retinal pigment epithelial cells isolated from a 2-week-old Abca4−/−Rdh8−/− mice was coincubated with photoreceptor outer segment (POS, 6 mg/mL) for 24 hours. Gene Lcn2 expression was increased after stimulation with POS. *P < 0.05 versus no POS treatment (n = 15).
LCN2 Expression in a Mertk−/− Mouse, a Model of Retinitis Pigmentosa
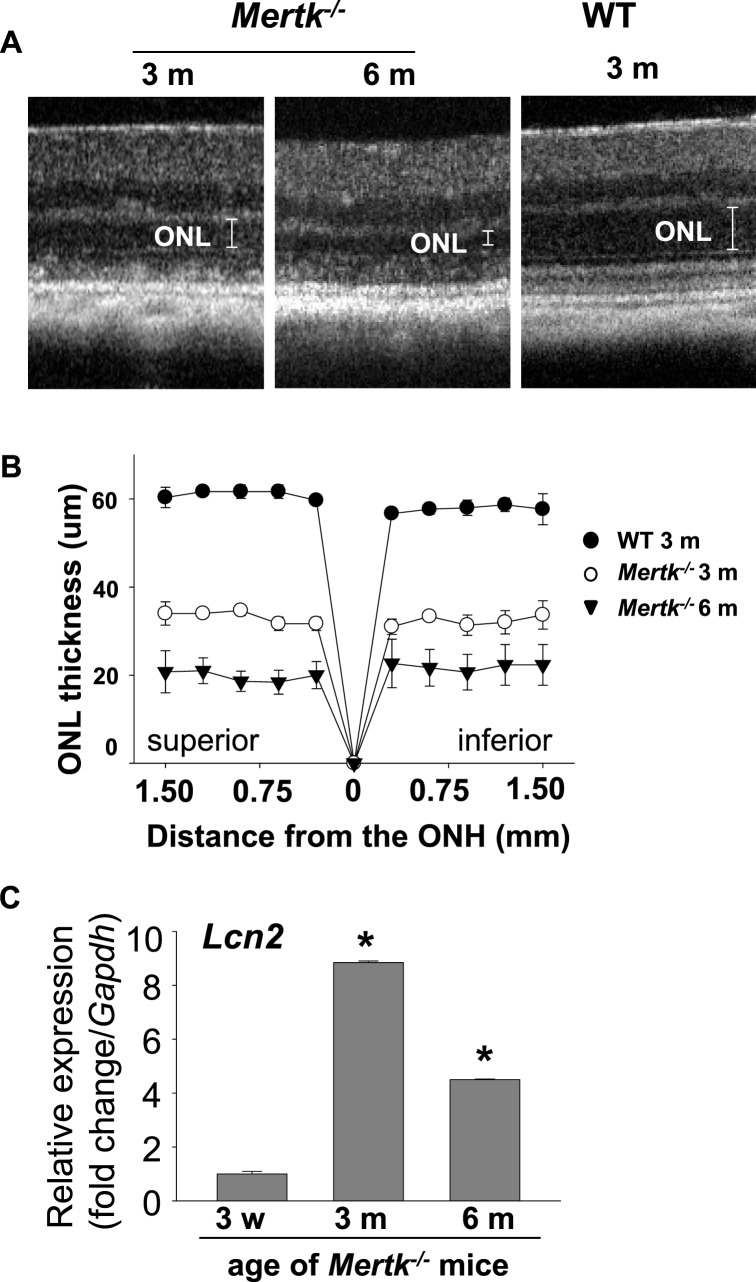
Mutations in the MERTK gene are a cause of retinal dystrophies in humans and animal models.20 MERTK, c-mer proto-oncogene tyrosine kinase, is essential for RPE phagocytosis. In Mertk−/− mice, defective RPE phagocytosis results in accumulation of photoreceptor debris in the subretinal space and is associated closely with photoreceptor degeneration.20,21 With the aim of investigating the role of LCN2 in retinal degeneration, also we investigated LCN2 expression in Mertk−/− mice. In vivo SD-OCT imaging revealed a decrease in ONL thickness by 3 months of age with further reduction of ONL at 6 months (Figs. 5A, 5B). Total RNA was extracted from eyes of these mice at the age of 3 weeks, and 3 and 6 months. Lcn2 expression increased 8.8-fold in 3-month-old Mertk−/− mice compared to 3-week-old Mertk−/− mice (Fig. 5C). Collectively, these observations suggested that increased Lcn2 expression is related to disease progression of the retina, and can be a potential marker for the acute phase before pathology manifests.
Figure 5.
Gene Lcn2 expression in Mertk−/− murine model of retinitis pigmentosa. (A) Representative SD-OCT shows age-related retinal degeneration in Mertk−/− mice. Images were obtained from the inferior retina at 500 to 750 μm from the ONH. (B) Outer nuclear layer thickness measured by SD-OCT is presented (n = 4 for each group). Error bars: Mean ± SD. (C) Quantitative RT-PCR revealed 8.8- or 4.5-fold increased Lcn2 expression in the eye at 3 or 6 months of age compared to that of 3 weeks coinciding with onset of retinal degeneration in Mertk−/− mice. *P < 0.05 vs. 3-week-old mice (n = 4 for each group).
Human RPE Cells Produce LCN2 in Response to LPS
Our previous study reported that excessive phagocytosis of RPE cells can lead to retinal inflammation with CCL2 production and also provided evidence of TLR4-mediated CCL2 production of RPE cells in response to LPS.19 Increase in Ccl2 and Lcn2 expression in Abca4−/−Rdh8−/− eyes after light exposure (see Table) and LCN2 production from RPE cells when incubated with POS (Fig. 4C) were documented. Because roles of LCN2 in inflammation have been reported,22 we next examined if human RPE cells can produce LCN2 in the presence of LPS. Human RPE cells were differentiated from pluripotent stem cells and cultured according to procedures reported previously.16 Human RPE cells (1 × 106) were stimulated with 1 μg/mL of LPS for 24 hours at 37°C to examine the production of LCN2 in response to inflammatory stress caused by LPS. Coincubation with LPS did not cause morphologic changes in RPE cells (Fig. 6A). Levels of LCN2 examined by qRT-PCR showed 4-fold upregulation after incubation with LPS (Fig. 6B). Similar 2.5-fold upregulation in protein levels was observed in human RPE cells when cocultured with compared to without LPS. A trend of LPS-dependent increase in LCN2 secretion in cell culture medium also was observed (Fig. 6C). Taken together, human RPE cells produce LCN2 under stress conditions, and such production can contribute to the pathogenesis of retinal diseases.
Figure 6.
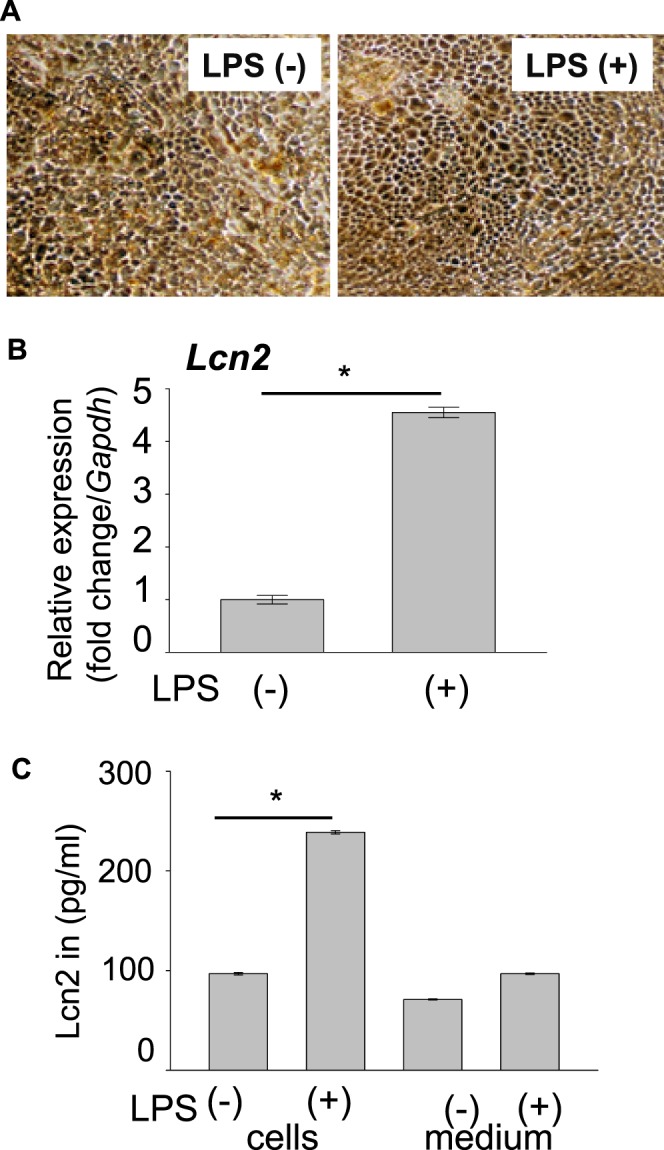
Lipocalin 2 production from human RPE cells. (A) Human RPE cells (1 × 106) differentiated from pluripotent stem cells were stimulated with 1 μg/mL of LPS. Representative image (n = 6) is presented. (B, C) Differentiated human RPE cell extract and culture medium were collected 24 hours after coincubation with 1 μg/mL of LPS (n = 5 for each group). (B) A 4-fold increase in mRNA levels of LCN2 was observed in the cell extract after 24 hours. *P < 0.05 versus no LPS treated (n = 5 for each group). Error bars: Mean ± SD. (C) Lipocalin 2 protein levels were increased by 2.5-fold in cell extract and a similar trend was observed in LCN2 secreted in the medium. *P < 0.05 versus no LPS treated (n = 5 for each group). Error bars: Mean ± SD.
Discussion
To identify early markers of retinal degeneration we examined the entire murine transcriptome for changes in Abca4−/−Rdh8−/− mice exposed to light stress. In our analysis we identified an interrelated group of genes, associated with an early stress response, that display unique upregulation after light-induced retinal damage. We further explored the possible function of one of the genes identified, Lcn2, and found it to be associated with inflammation in the retina.
Acute Stress Response and LCN2
The current study revealed that acute stress responses are the highest enriched biologic processes in Abca4−/−Rdh8−/− mice after light exposure. The acute phase response is part of an early-defense mechanism activated by infection, stress and inflammation. Although nonspecific, it functions as an essential component of the innate immune response, which acts as the first line of defense to combat cell damage.1 RNA-seq analysis of whole eye transcriptome identified Lcn2 as the most upregulated acute stress response gene. LCN2 is a 22.6 kDa secreted glycoprotein coded by 198 or 200 amino acids in humans or mice,23,24 respectively. Lipocalin 2 also is known as 24p3, MSFI, NGAL, SIP24, and AW212229. Lipocalin 2 belongs to a family of small glycoproteins characterized by their ability to bind small hydrophobic molecules25 and functionally typified by retinol binding protein (RBP), which binds all-trans-retinol as its physiologic ligand.26 Notably, we found increased expression of RBP family members, including Rbp2 and Rbp3, after light exposure in the current study, suggesting an association of LCN2, RBPs and retinoids including all-trans-retinol in the eye. These observations could improve our understanding of the stress response protein LCN2 and how it is involved in modulating inflammation and cell death in the retina.
Roles of LCN2 in Murine Retinal Degeneration
Lipocalin 2 is involved in multiple biologic processes, such as the innate immune response, apoptosis, protein endocytosis, and iron transport.27–31 Its role in retinal degeneration remains unclear, although few reports have identified LCN2 localization in the eye in response to retinal degeneration in mice. Studies with Bardet-Biedl Syndrome 4 (BBS4−/−) mice, Bardet-Biedl Syndrome is a ciliopathic human genetic disorder and individuals with this disease have retinal degeneration,32 and have shown that photoreceptor loss is accompanied by a significant increase of stress proteins, such as LCN2, EDN2, SOCS3, and SERPINA3N. Studies of retinal detachment also have revealed that increased expression of early stress response genes enable photoreceptor cells to survive the initial detachment and breakdown of these protective mechanisms could lead to cell death.33 Increased LCN2 expression has been reported in RPE cells of aging mice with deficient crystalline beta A1 (CRYBA1) and this study proposed a role for LCN2 in lysosomal trafficking processes.34 Chen et al.35 reported an increase of LCN2 among other iron binding proteins, such as ceruloplasmin and metallothionin in response to oxidative stress. Rattner and Nathans6 reported an increased expression of LCN2 in retinal Müller cells after light exposure in mice indicating the involvement of Müller-endothelial cell signaling activated by light damage.
Lcn2−/− mice are susceptible to bacterial infections, thereby indicating important roles for LCN2 in innate immune defense mechanisms.36,37 Our investigation of the whole eye transcriptome revealed that gene expression levels of Lcn2 increased by 3 hours, peaking at 24 hours and return to levels comparable to no light exposed by 7 days. Sublocalization of LCN2 showed increased expression in the RPE compared to the retina at 24 hours after light damage. An increased expression of LCN2 occurs before age-related decrease of ONL thickness in Mertk−/− mice; therefore, increased LCN2 expression may indicate impaired RPE function preceding retinal degeneration. An earlier study investigating gene expression changes in response to light exposure in BALB/c mice identified a 5-fold upregulation of LCN2 in the RPE compared to expression in the neural retina.35 Taken together, these data propose an important role for the acute stress response mediated by LCN2 in RPE cells during the process of retinal degeneration.
Potential Roles of LCN2 in Human Retinal Degeneration
This study provided evidence that human RPE cells produce LCN2 in response to the endotoxin LPS, which can activate TLR4.17 Our previous study identified that POS proteins are possible endogenous TLR4 ligands and are associated with CCL2 production.19 These observations also suggested a close association between early stress and immune responses in the eye especially when impaired photoreceptor cells are phagocytized by RPE cells during the process of retinal degeneration. Because recent studies suggest that retinal inflammation in chronic retinal diseases aggravates the severity of retinal degeneration,8,38–40 early detection of retinal inflammation and degeneration could contribute to earlier intervention and improved patient care. It is of note that the lipocalin family of proteins has been found elevated in several human diseases, including atherosclerosis, inflammatory bowel diseases, ischemic kidney disease and cerebrovascular diseases.41–43 Additionally, several studies proposed that measurement of serum levels of lipocalin proteins as biomarkers can lead to early detection and intervention of human diseases.44–49 Collectively, LCN2 secreted protein from RPE cells can serve as a biomarker for early detection of the retinal diseases in humans.
Increased Expression of Inflammatory Chemokines in Retinal Degeneration in Abca4−/−Rdh8−/− Mice
Our previous studies have shown that accumulating toxicity of all-trans-retinal causes activation of several immune factors, proinflammatory and anti-inflammatory cytokines after light damage,19 suggesting an interrelationship between cellular stress and the immune response in photoreceptor cell death of Abca4−/−Rdh8−/− mice. Chemokine array analysis in Abca4−/−Rdh8−/− mice previously identified Ccl3 to be the most upregulated chemokine 24 hours after light exposure,8 and our current study found that expression of Ccl3 is lower than that of Ccl2. The above discrepancy in expression could be attributed to difference in the two platforms; an array versus RNA-seq, the latter is able to interrogate a large portion of the transcriptome with more depth, across a wider range of fold changes and is able to assign a larger range of expression values to the genes measured. RNA-seq has revolutionized whole transcriptome analysis as it can identify various novel and low expressing transcripts in the cell21 coupled with higher sensitivity and specificity of transcript detection. Nonetheless, both of our studies showed a strong association between inflammation and the severity of retinal damage.8 In addition to Ccl2 and Ccl3, the current study also identified an increase of several proinflammatory cytokines including Ccl5 and Cxcl5, validating our earlier findings. Though our studies demonstrated contribution of retinal inflammation and acute stress responses to the pathogenesis of retinal degeneration, inflammatory responses often are the result of activation of complex multicomponent innate immune responses against initial cell stress. Considering the complexity of retinal degeneration processes, the involvement of several other factors and the involvement of a cohort of similar early stress proteins cannot be ruled out.
In conclusion, a comprehensive analysis using RNA-seq identified important roles of the acute stress response in the degenerating retina of Abca4−/−Rdh8−/− mice that are predisposed to retinal degeneration under light stress. The acute stress protein LCN2, produced from RPE cells and the neural retina, could modulate retinal inflammation and retinal homeostasis; therefore, such families of proteins could contribute to cell survival during retinal insults.
Supplementary Material
Acknowledgments
The authors thank Krzysztof Palczewski, Debarshi Mustafi, Brian Kevany, Zhiqian Dong, and Tividar Orban (Case Western Reserve University) for helpful comments on the manuscript, and Simone Edelheit and Ernest Chan (Case Western Reserve University Genomics Core) for providing help with RNA-sequencing, and Visual Sciences Research Center Core Facility.
Supported by funding from the National Institutes of Health (NIH; Bethesda, MD, USA; EY022658 and EY11373), Research to Prevent Blindness Foundation, Foundation Fighting Blindness, and Ohio Lions Eye Research Foundation.
Disclosure: T. Parmar, None; V.M. Parmar, None; E. Arai, None; B. Sahu, None; L. Perusek, None; A. Maeda, None
References
- 1. Cray C,, Zaias J,, Altman NH. Acute phase response in animals: a review. Comp Med. 2009; 59: 517–526. [PMC free article] [PubMed] [Google Scholar]
- 2. Beatty S,, Koh H,, Phil M,, Henson D,, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000; 45: 115–134. [DOI] [PubMed] [Google Scholar]
- 3. Muralidharan S,, Mandrekar P. Cellular stress response and innate immune signaling: integrating pathways in host defense and inflammation. J Leukocyte Biol. 2013; 94: 1167–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maeda A,, Maeda T,, Golczak M,, Palczewski K. Retinopathy in mice induced by disrupted all-trans-retinal clearance. J Biol Chem. 2008; 283: 26684–26693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Quazi F,, Lenevich S,, Molday RS. ABCA4 is an N-retinylidene-phosphatidylethanolamine and phosphatidylethanolamine importer. Nat Comm. 2012; 3: 925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rattner A,, Smallwood PM,, Nathans J. Identification and characterization of all-trans-retinol dehydrogenase from photoreceptor outer segments the visual cycle enzyme that reduces all-trans-retinal to all-trans-retinol. J Biol Chem. 2000; 275: 11034–11043. [DOI] [PubMed] [Google Scholar]
- 7. Allikmets R,, Shroyer NF,, Singh N,, et al. Mutation of the Stargardt disease gene (ABCR) in age-related macular degeneration. Science. 1997; 277: 1805–1807. [DOI] [PubMed] [Google Scholar]
- 8. Kohno H,, Maeda T,, Perusek L,, Pearlman E,, Maeda A. CCL3 production by microglial cells modulates disease severity in murine models of retinal degeneration. J Immunol. 2014; 192: 3816–3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maeda A,, Maeda T,, Golczak M,, et al. Involvement of all-trans-retinal in acute light-induced retinopathy of mice. J Biol Chem. 2009; 284: 15173–15183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maeda A,, Golczak M,, Chen Y,, et al. Primary amines protect against retinal degeneration in mouse models of retinopathies. Nat Chem Biol. 2012; 8: 170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sawada O,, Perusek L,, Kohno H,, et al. All-trans-retinal induces Bax activation via DNA damage to mediate retinal cell apoptosis. Exp Eye Res. 2014; 123: 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Samardzija M,, Wenzel A,, Naash M,, Reme CE,, Grimm C. Rpe65 as a modifier gene for inherited retinal degeneration. Eur J Neurosci. 2006; 23: 1028–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mattapallil MJ,, Wawrousek EF,, Chan CC,, et al. The Rd8 mutation of the Crb1 gene is present in vendor lines of C57BL/6N mice and embryonic stem cells, and confounds ocular induced mutant phenotypes. Invest Ophthalmol Vis Sci. 2012; 53: 2921–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maeda T,, Golczak M,, Maeda A. Retinal photodamage mediated by all-trans-retinal. Photochem Photobiol. 2012; 88: 1309–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ashburner M,, Ball CA,, Blake JA,, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000; 25: 25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maeda T,, Lee MJ,, Palczewska G,, et al. Retinal pigmented epithelial cells obtained from human induced pluripotent stem cells possess functional visual cycle enzymes in vitro and in vivo. J Biol Chem. 2013; 288: 34484–34493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Andreakos E,, Sacre SM,, Smith C,, et al. Distinct pathways of LPS-induced NF-kappa B activation and cytokine production in human myeloid and nonmyeloid cells defined by selective utilization of MyD88 and Mal/TIRAP. Blood. 2004; 103: 2229–2237. [DOI] [PubMed] [Google Scholar]
- 18. Subramanian A,, Tamayo P,, Mootha VK,, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005; 102: 15545–15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kohno H,, Chen Y,, Kevany BM,, et al. Photoreceptor proteins initiate microglial activation via Toll-like receptor 4 in retinal degeneration mediated by all-trans-retinal. J Biol Chem. 2013; 288: 15326–15341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Duncan JL,, LaVail MM,, Yasumura D,, et al. An RCS-like retinal dystrophy phenotype in mer knockout mice. Invest Ophthalmol Vis Sci. 2003; 44: 826–838. [DOI] [PubMed] [Google Scholar]
- 21. Wang Z,, Gerstein M,, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet. 2009; 10: 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang J,, Wu Y,, Zhang Y,, Leroith D,, Bernlohr DA,, Chen X. The role of lipocalin 2 in the regulation of inflammation in adipocytes and macrophages. Molec Endocr. 2008; 22: 1416–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Strausberg RL,, Feingold EA,, Grouse LH,, et al. Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences. Proc Natl Acad Sci U S A. 2002; 99: 16899–16903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mural RJ,, Adams MD,, Myers EW,, et al. A comparison of whole-genome shotgun-derived mouse chromosome 16 and the human genome. Science. 2002; 296: 1661–1671. [DOI] [PubMed] [Google Scholar]
- 25. Flower DR. The lipocalin protein family: structure and function. Biochem J. 1996; 318: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hodam JR,, Creek KE. Uptake and metabolism of [3H]retinoic acid delivered to human foreskin keratinocytes either bound to serum albumin or added directly to the culture medium. Biochim Biophys Acta. 1996; 1311: 102–110. [DOI] [PubMed] [Google Scholar]
- 27. Yang J,, Goetz D,, Li JY,, et al. An iron delivery pathway mediated by a lipocalin. Molec Cell. 2002; 10: 1045–1056. [DOI] [PubMed] [Google Scholar]
- 28. Kjeldsen L,, Bainton DF,, Sengelov H,, Borregaard N. Identification of neutrophil gelatinase-associated lipocalin as a novel matrix protein of specific granules in human neutrophils. Blood. 1994; 83: 799–807. [PubMed] [Google Scholar]
- 29. Mishra J,, Ma Q,, Prada A,, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003; 14: 2534–2543. [DOI] [PubMed] [Google Scholar]
- 30. Borregaard N,, Cowland JB. Neutrophil gelatinase-associated lipocalin a siderophore-binding eukaryotic protein. Biometals. 2006; 19: 211–215. [DOI] [PubMed] [Google Scholar]
- 31. Devireddy LR,, Gazin C,, Zhu X,, Green MR. A cell-surface receptor for lipocalin 24p3 selectively mediates apoptosis and iron uptake. Cell. 2005; 123: 1293–1305. [DOI] [PubMed] [Google Scholar]
- 32. Swiderski RE,, Nishimura DY,, Mullins RF,, et al. Gene expression analysis of photoreceptor cell loss in bbs4-knockout mice reveals an early stress gene response and photoreceptor cell damage. Invest Ophthalmol Vis Sci. 2007; 48: 3329–3340. [DOI] [PubMed] [Google Scholar]
- 33. Zacks DN,, Zheng QD,, Han Y,, Bakhru R,, Miller JW. FAS-mediated apoptosis and its relation to intrinsic pathway activation in an experimental model of retinal detachment. Invest Ophthalmol Vis Sci. 2004; 45: 4563–4569. [DOI] [PubMed] [Google Scholar]
- 34. Valapala M,, Edwards M,, Hose S,, et al. Increased lipocalin-2 in the retinal pigment epithelium of Cryba1 cKO mice is associated with a chronic inflammatory response. Aging Cell. 2014; 13: 1091–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen L,, Wu W,, Dentchev T,, et al. Light damage induced changes in mouse retinal gene expression. Exp Eye Res. 2004; 79: 239–247. [DOI] [PubMed] [Google Scholar]
- 36. Flo TH,, Smith KD,, Sato S,, et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004; 432: 917–921. [DOI] [PubMed] [Google Scholar]
- 37. Goetz DH,, Holmes MA,, Borregaard N,, Bluhm ME,, Raymond KN,, Strong RK. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Molec Cell. 2002; 10: 1033–1043. [DOI] [PubMed] [Google Scholar]
- 38. Hu SJ,, Calippe B,, Lavalette S,, et al. Upregulation of P2RX7 in Cx3cr1-deficient mononuclear phagocytes leads to increased interleukin-1beta secretion and photoreceptor neurodegeneration. J Neurosci. 2015; 35: 6987–6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Luhmann UF,, Robbie SJ,, Bainbridge JW,, Ali RR. The relevance of chemokine signalling in modulating inherited and age-related retinal degenerations. Adv Exp Med Biol. 2014; 801: 427–433. [DOI] [PubMed] [Google Scholar]
- 40. Sennlaub F,, Auvynet C,, Calippe B,, et al. CCR2(+) monocytes infiltrate atrophic lesions in age-related macular disease and mediate photoreceptor degeneration in experimental subretinal inflammation in Cx3cr1 deficient mice. EMBO Mol Med. 2013; 5: 1775–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nielsen BS,, Borregaard N,, Bundgaard JR,, Timshel S,, Sehested M,, Kjeldsen L. Induction of NGAL synthesis in epithelial cells of human colorectal neoplasia and inflammatory bowel diseases. Gut. 1996; 38: 414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cowland JB,, Borregaard N. Molecular characterization and pattern of tissue expression of the gene for neutrophil gelatinase-associated lipocalin from humans. Genomics. 1997; 45: 17–23. [DOI] [PubMed] [Google Scholar]
- 43. Xu S,, Venge P. Lipocalins as biochemical markers of disease. Biochim Biophys Acta. 2000; 1482: 298–307. [DOI] [PubMed] [Google Scholar]
- 44. Lacquaniti A,, Chirico V,, Donato V,, et al. NGAL as an early biomarker of kidney disease in Joubert syndrome: three brothers compared. Ren Fail. 2012; 34: 495–498. [DOI] [PubMed] [Google Scholar]
- 45. Mishra J,, Mori K,, Ma Q,, et al. Amelioration of ischemic acute renal injury by neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol. 2004; 15: 3073–3082. [DOI] [PubMed] [Google Scholar]
- 46. Guiddir T,, Deghmane AE,, Giorgini D,, Taha MK. Lipocalin 2 in cerebrospinal fluid as a marker of acute bacterial meningitis. BMC Infect Dis. 2014; 14: 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wu G,, Li H,, Fang Q,, et al. Elevated circulating lipocalin-2 levels independently predict incident cardiovascular events in men in a population-based cohort. Arterioscler Thromb Vasc Biol. 2014; 34: 2457–2464. [DOI] [PubMed] [Google Scholar]
- 48. Nielsen OH,, Gionchetti P,, Ainsworth M,, et al. Rectal dialysate and fecal concentrations of neutrophil gelatinase-associated lipocalin, interleukin-8, and tumor necrosis factor-alpha in ulcerative colitis. Am J Gastroenterol. 1999; 94: 2923–2928. [DOI] [PubMed] [Google Scholar]
- 49. Lin CW,, Tseng SW,, Yang SF,, et al. Role of lipocalin 2 and its complex with matrix metalloproteinase-9 in oral cancer. Oral Diseases. 2012; 18: 734–740. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.