Abstract
Natural products have always been exploited to promote health and served as a valuable source for the discovery of new drugs. In this review, the great potential of natural compounds and medicinal plants for the treatment or prevention of cardiovascular and metabolic disorders, global health problems with rising prevalence, is addressed. Special emphasis is laid on natural products for which efficacy and safety have already been proven and which are in clinical trials, as well as on plants used in traditional medicine. Potential benefits from certain dietary habits and dietary constituents, as well as common molecular targets of natural products, are also briefly discussed. A glimpse at the history of statins and biguanides, two prominent representatives of natural products (or their derivatives) in the fight against metabolic disease, is also included. The present review aims to serve as an “opening” of this special issue of Molecules, presenting key historical developments, recent advances, and future perspectives outlining the potential of natural products for prevention or therapy of cardiovascular and metabolic disease.
Keywords: natural products, cardiovascular disease, metabolic disorders, diabetes mellitus, statins, biguanides, dietary constituents, coffee, molecular targets
1. Introduction
It is well known that natural products have been a valuable source of therapeutic agents for millenia and even today, many medicines are natural products or their derivatives [1]. Although natural products have played an important role in lead discovery [1], nowadays the pharmaceutical industry tends to not prioritize natural product research anymore [2]. Instead, common strategies in industry are high throughput screening (HTS) of synthetic compound databases and structural modifications of existing leads. However, the HTS and combinatorial chemistry approaches followed by many pharmaceutical companies have not been very successful. Furthermore, even stakeholders in industry still see a high potential in natural products as drug leads [3]. In line with this view, the number of scientific studies in the area of natural products research is increasing rapidly [1]. The 2015 Nobel Prize in Physiology or Medicine, which was awarded to Youyou Tu, William C. Campbell, and Satoshi Ōmura for the discovery of natural products for the treatment of tropical parasitic diseases [4,5], might be considered emblematic for the revival of natural product drug discovery. It clearly shows the therapeutic value of natural products and underlines that natural products are an effective source of new drugs.
This review is intended to serve as an “opening” for the Molecules special issue entitled “Effects of Natural Products in the Context of Cardiometabolic Disease”. It presents selected prominent illustrative examples of natural products with effects on cardiovascular and metabolic disorders, and is far from being comprehensive. A focus is set on medicinal plants and terrestrial plant-derived natural products, and readers are referred to other recent reviews for an overview on natural products with relevant activities from seaweeds and other marine organisms [6,7,8,9].
2. Cardiovascular and Metabolic Disorders—A Global Health Problem
The metabolic syndrome is considered to be a progressive pathophysiological state which is clinically manifested by a cluster of interrelated risk factors (abdominal obesity, atherogenic dyslipidemia, increased blood pressure, insulin resistance, pro-inflammatory and pro-thrombotic state) and associated with an increased expectation for developing diabetes mellitus type 2 and atherosclerotic cardiovascular disease [10,11]. Atherosclerosis, alongside with hypertension, is the main cause of cardiovascular disease representing the leading cause of death in the world. A sedentary life-style together with a diet comprising high calorie intake in westernized societies render the disease prevalence high and atherosclerosis is therefore the underlying cause of approximately 50% of all deaths [12]. Moreover, the prevalence of cardiovascular disease in the world is rising globally and according to the World Health Organization (WHO), this increasing tendency is likely to continue in the next years. While in 2012, cardiovascular disease caused 17.5 million deaths, it is projected to be responsible for 22.2 million deaths in 2030 [13].
Diabetes mellitus is considered one of the most common chronic metabolic diseases in nearly all countries. Especially the prevalence of diabetes mellitus type 2, which accounts for around 90% of all diabetes cases worldwide, continues to increase due to the changing lifestyles that involve reduced physical activity and increased incidence of obesity. In 2014, the prevalence of diabetes reported by the WHO was estimated to be 9% among adults aged 18+ years while in 2012, an estimated 1.5 million deaths were directly caused by this disease [14]. According to projections, its prevalence will further increase [15], becoming the 7th leading cause of death by 2030 [16].
3. Increasing Scientific Interest in Natural Products with Potential Application in Cardiovascular and Metabolic Disorders
Considering the huge morbidity and mortality burden related to cardiometabolic disorders with no end in sight, there is a high interest in the discovery of novel compounds as well as novel pharmacological targets that might be effective in the treatment or prevention of cardiovascular and/or metabolic disorders. Although natural product drug discovery often requires more effort compared to HTS and combinatorial chemistry, nature is still considered as the most productive source of potential drug leads for new medicines [3].
In recent decades, herbal remedies and natural products have undisputedly attracted much research attention in the context of prevention or treatment of cardiovascular and metabolic disease [17,18,19]. Thus, when searching Scopus using the keywords “cardiovascular disease” and “natural products” (CVD+NP) or “metabolic disease” and “natural products” (MD+NP) it becomes evident that the scientific interest in these areas increased exponentially in the period 2004–2014 (Figure 1).
Figure 1.
Annual number of publications resulting from the search with the keywords “cardiovascular disease” and “natural products” (CVD + NP) (a) and “metabolic disease” and “natural products” (MD + NP) (b), (Scopus, January 2016).
4. Plants Traditionally Used in the Context of Cardiovascular and Metabolic Disorders
Millenary civilizations rely on plants or other natural resources to sustain or restore health, and in various situations they still represent interesting therapeutic alternatives to synthetic drugs. According to the WHO, over 100 million Europeans and many more people in Africa, Asia, Australia, and North America are users of traditional and complementary medicine. Especially in Africa and some developing countries, traditional medicine is often the primary source of health care [20].
Along with herbal extracts and natural products with validated efficacy and safety proven by randomized controlled clinical trials (further discussed in chapter 5), many other medicinal plants are used world-wide to alleviate cardiovascular and metabolic complaints. Table 1 provides an overview of selected traditionally used plants and their targeted indications.
Table 1.
Medicinal plants targeting indications related to cardiovascular or metabolic disease.
| Scientific Name of the Medicinal Plant | Common Name of the Medicinal Plant | Plant Organ | Indications |
|---|---|---|---|
| Aesculus hippocastanum L. | Horse-chestnut | Seeds | Venous insufficiency, varicose veins [21,22,23,24] |
| Allium sativum L. | Garlic | Bulbs/whole plant | Hypertension, hypercholesterolemia, diabetes mellitus type 2 [25,26,27,28,29,30] |
| Aloe vera (L.) Burm. f. | Aloe vera | Leaves | Diabetes mellitus type 2, hypercholesterolemia [31,32,33,34,35] |
| Ammi visnaga (L.) Lam. | Toothpick weed, bisnaga, khella | Fruits | Angina pectoris [36,37,38] |
| Apocynum venetum L. | Dogbane | Leaves | Hypertension [39,40,41,42] |
| Artemisia dracunculus L. | Tarragon | Leaves, aerial parts | Hyperglycemia [17,43,44,45] |
| Artemisia herba-alba Asso | White wormwood | Aerial parts | Hyperlipidemia, diabetes mellitus [46,47,48,49,50] |
| Aspalathus linearis (Burm. f.) R. Dahlgr. | Rooibos | Leaves | Diabetes mellitus type 2 [51,52,53,54] |
| Astragalus membranaceus Moench | Chinese milk vetch | Roots | Angina pectoris, atherosclerosis, diabetic nephropathy [39,55,56,57,58,59] |
| Carthamus tinctorius L. | Safflower | Flowers | Angina pectoris, hypertension, hyperlipidemia [39,60,61,62,63,64] |
| Centaurium erythraea Rafn | Common centaury | Whole plant, leaves | Diabetes mellitus [46,48,65,66,67] |
| Cinnamomum cassia (L.) J. Presl | Chinese cinnamon | Bark | Diabetes mellitus, diabetic nephropathy [68,69,70] |
| Cinnamomum verum J. Presl | Ceylon cinnamon | Bark | Diabetes mellitus type 2 [69,71,72,73,74] |
| Commiphora mukul (Hook. ex Stocks) Engl. | Gugal, guggul, gugul, Indian bdellium-tree, mukul myrrh tree | Resin | Hypercholesterolemia, hypertriglyceridemia [21,75,76] |
| Coptis chinensis Franch. | Chinese goldthread | Roots, flowers | Hypercholesterolemia, diabetes mellitus, non-alcoholic fatty liver disease [18,77,78,79] |
| Coriandrum sativum L. | Coriander | Seeds | Diabetes mellitus, hypercholesterolemia [80,81,82,83] |
| Crataegus monogyna Jacq./C. oxyacantha Jacq./C. laevigata (Poir.) DC./C. pinnatifida Bunge | Hawthorn | Sprigs with both leaves and flowers, fruits | Angina pectoris, atherosclerosis, hyperlipidemia [84,85,86,87] |
| Cynara scolimus L. | Globe artichoke | Leaves | Hypercholesterolemia [88,89] |
| Fraxinus excelsior L. | European ash | Fruits, seeds | Diabetes mellitus type 2, hepatic steatosis [90,91,92,93,94,95] |
| Galega officinalis L. | French lilac | Aerial parts | Diabetes mellitus [72,96,97,98] |
| Gingko biloba L. | Gingko, maidenhair tree | Leaves | Cerebrovascular disease, peripheral vascular disease, hypertension, diabetes nephropathy [75,85,99,100] |
| Glycine max (L.) Merr. | Soybean | Fruits, seeds | Diabetes mellitus, hyperlipidemia [101,102,103] |
| Glycyrrhiza glabra L. | Licorice | Roots | Atherosclerosis, hypercholesterolemia [85,104] |
| Helianthus tuberosus L. | Jerusalem artichoke | Tubers | Diabetes mellitus type 2, non-alcoholic fatty liver disease [105] |
| Ilex paraguariensis A. St.-Hil. | Yerba mate | Leaves | Obesity, diabetes mellitus [106,107,108,109,110,111] |
| Lycium barbarum L. | Chinese wolfberry | Fruits, roots | Diabetes mellitus, hyperlipidemia, hypertension [112,113,114,115,116,117,118,119] |
| Momordica charantia L. | Bitter melon | Fruits | Diabetes mellitus type 2 [109,120,121] |
| Morus alba L. | White mulberry tree | Root bark, leaves | Hyperglycemia [122,123,124,125,126,127] |
| Nigella sativa L. | Black cumin, black seed | Seeds, seed oil | Diabetes mellitus type 2, dyslipidemia [128,129,130,131,132] |
| Ocimum sanctum L. | Holy basil | Leaves, whole plant | Hypertension, dyslipidemia, diabetes mellitus [133,134] |
| Olea europaea L. | Olive | Leaves, fruit oil | Hypertension, atherosclerosis, diabetes mellitus, hepatic steatosis [135,136,137,138,139,140,141,142] |
| Panax notoginseng (Burkill) F.H. Chen ex C.H. Chow | Notoginseng, pseudoginseng | Roots | Angina pectoris, coronary artery disease [21,75,143] |
| Rauvolfia serpentina (L.) Benth. ex Kurz | Indian snakeroot | Roots | Hypertension [75,144,145] |
| Rhodiola rosea L. | Golden root | Roots | Angina pectoris, ischemic heart disease [39,146,147] |
| Rosmarinus officinalis L. | Rosemary | Leaves | Capillary permeability and fragility disturbances [21,148,149] |
| Ruscus aculeatus L. | Butcher’s broom | Rhizomes | Venous insufficiency, varicose veins [21,150] |
| Sambucus nigra L. | European elder, black elder | Flowers | Diabetes mellitus type 2 [151,152,153] |
| Schisandra chinensis (Turcz.) Baill. | Five-flavor berry | Fruits, seeds | Hypertension, myocardial infarction, hyperlipidemia, diabetic nephropathy, diabetes mellitus [154,155,156,157,158] |
| Silybum marianum (L.) Gaertn. | Milk thistle | Seeds, aerial parts | Diabetes mellitus type 1 and 2 [90,159,160,161,162,163] |
| Stevia rebaudiana (Bertoni) Bertoni | Sweet leaf, candyleaf | Leaves | Diabetes mellitus type 2 [164,165,166,167] |
| Trigonella foenum-graecum L. | Fenugreek | Seeds | Metabolic syndrome, diabetes mellitus type 2 [168,169] |
| Vaccinium spp. | Blueberries | Fruits, leaves | Diabetes mellitus type 2, metabolic syndrome [96,109,170,171,172,173] |
| Veratrum album L./V. nigrum L./V. japonicum (Baker) Loes./V. viride Aiton | False helleborine/black false hellebore | Rhizomes | Hypertension [75,174,175] |
| Viscum album L. | Mistletoe | Aerial parts | Hypertension [176] |
There is no doubt that medicinal plants and natural products are used for the treatment or prevention of cardiovascular and metabolic disorders, also with rising popularity in western societies. However, in most cases the expected health benefits are not scientifically proven by rigorous clinical trials. Hence, it is vital to provide robust scientific evidence for clinical efficacy and safety.
5. Herbal Products in Recruiting Clinical Trials Targeting Indications Related to Cardiovascular and Metabolic Disorders
Many medicinal plants and natural products are considered by the public as a safe, natural, and cost-effective alternative to synthetic drugs without unambiguous proof by randomized controlled clinical trials. On this background, there is an increased interest in the development of products with validated efficacy and safety, similar to the recently FDA-approved botanical drugs Veregen® (sinecatechins; green tea (Camellia sinensis (L.) Kuntze) leaf extract), Fulyzaq® (crofelemer; extract from the red latex of the Dragon’s blood tree (Croton lechleri Müll.Arg.)), and Grastek® (Timothy grass (Phleum pretense L.) pollen allergen extract) [177,178]. Some herbal extracts and pure compounds are currently undergoing clinical trials for cardiometabolic indications; an overview is presented in Table 2.
Table 2.
Herbal extracts and natural products in recruiting clinical trials targeting indications related to metabolic or cardiovascular diseases 1.
| Name of the Product | National Clinical Trial (NCT) Identifier | Phase | Studied Condition |
|---|---|---|---|
| BeneFlax® (Flaxseed (Linum usitatissimum L.) lignans) | NCT02391779 | Phase 2 | Hypertension |
| Biscuit containing “Kothala Himbutu” (Salacia reticulata Wight) | NCT02290925 | Phase 3 | Diabetes mellitus type 2 |
| Coleus forskohlii (Willd.) Briq. | NCT02143349 | Phase 3 | Risk factors of metabolic syndrome |
| Combined Rg3-enriched Korean red ginseng and American ginseng | NCT01578837 | Phase 1 and 2 | Diabetes mellitus type 2, hypertension |
| Curcumin | NCT01968564 | - 2 | Vascular aging |
| Curcumin | NCT02529982 | Phase 2 | Non insulin dependent diabetes |
| Curcumin | NCT02529969 | Phase 2 | Non insulin dependent diabetes |
| Dantonic® (T89) | NCT01659580 | Phase 3 | Angina pectoris |
| Euiiyin-tang | NCT01724099 | Phase 2 and 3 | Obesity |
| Fibre grain herb | NCT02553382 | Phase 3 | Diabetes mellitus type 2 |
| “Fu-zheng-qu-zhuo” oral liquid | NCT02044835 | Phase 2 and 3 | Ischemic nephropathy |
| Ginger | NCT02289235 | Phase 0 | Non-alcoholic fatty liver disease |
| Phyllanthus niruri L. and Sida cordifolia L. (Vedicine) | NCT02107469 | - | Diabetic peripheral polyneuropathy |
| Quercetin | NCT00065676 | Phase 2 | Diabetes mellitus, obesity |
| Red grapes polyphenol supplementation | NCT02633150 | - | Obesity, insulin resistance |
| Resveratrol | NCT02245932 | Phase 3 | Overweight |
| Resveratrol | NCT01564381 | Phase 1 and 2 | Cardiovascular disease |
| Resveratrol | NCT01842399 | Phase 1 and 2 | Vascular resistance, hypertension |
| Resveratrol | NCT02246660 | - | Peripheral arterial disease |
| Resveratrol | NCT02137421 | - | Metabolic syndrome, coronary artery disease |
| Resveratrol | NCT02129595 | - | Pre-diabetes |
| Resveratrol | NCT01997762 | Phase 4 | Gestational diabetes |
| Resveratrol | NCT02216552 | Phase 2 and 3 | Non-alcoholic fatty liver disease, diabetes mellitus type 2, metabolic syndrome |
| Resveratrol | NCT02419092 | - | Obesity |
| Resveratrol | NCT01881347 | - | Diabetes mellitus |
| Resveratrol | NCT02549924 | Phase 2 | Diabetes mellitus type 2 |
| Resveratrol | NCT02244879 | Phase 3 | Diabetes mellitus type 2, inflammation, insulin resistance |
1 Information retrieved from www.clinicaltrials.gov on 21 January 2016; 2 “-“ indicates that there is no information for the phase provided on the corresponding trial page at www.clinicaltrials.gov.
6. Dietary Constituents with Potential Benefits in the Context of Cardiovascular and Metabolic Disorders
Ample evidence demonstrates that dietary patterns can affect the development of cardiovascular and metabolic disorders [179,180]. The reduced intake of highly processed foods by replacing them with fruits, nuts, seeds, vegetables, and legumes [181] is considered health promoting. The latter dietary constituents are free of food additives, low in salt content, and rich in phenolics, carotenoids, fibers, minerals, and unsaturated fats. They possess antioxidant effects, lower glycemic indices, and normalize levels of cholesterol in blood. The traditional Mediterranean diet is one example, which is associated with longer life expectancy, lower rates of cardiovascular and metabolic disorders, and even lower rates of certain cancers [182]. This diet is characterized by an abundance of seasonally fresh plant foods (fruits, vegetables, beans, nuts, seeds, etc.), minimal food processing, olive oil, and wine consumed in low to moderate amounts, normally with meals [19,182,183].
Another example for a presumably health promoting dietary constituent is coffee, one of the most popular beverages worldwide. It exhibits a range of bioactivities and potential health benefits. Since coffee drinking is very common in Western societies, its bioactivities and in particular its impact on cardiovascular and metabolic parameters have been widely investigated [184,185,186,187,188,189,190]. Compared to non-drinkers, coffee consumption of one to five cups/day was associated with lower risk of mortality, while coffee consumption of more than five cups/day did not affect mortality risk. Additionally, coffee consumption (with or without caffeine) was associated with significantly lower death risk due to cardiovascular disease, neurological disorders, and suicide [191]. It was also linked to a lower risk of diabetes mellitus type 2, independent of race, geographic distribution and gender of the studied populations [192]. Major bioactive ingredients in coffee include phenolics (chlorogenic acid and its isomers), diterpenes (cafestol and kahweol), and caffeine (Figure 2). Coffee is considered to be a very prominent source of phenolic compounds, and it appears that it is the number one source of dietary antioxidants in the US [193,194]. The total phenolic content per cup of coffee ranges between 200 and 550 mg, with chlorogenic acid being the main phenolic compound [192].
Figure 2.
Chemical structures of bioactive compounds found in coffee.
Chlorogenic acid intake leads to lower blood glucose and insulin concentrations 15 minutes after ingestion [195]. In streptozocin-nicotinamide induced diabetic rats, a dose of 5 mg chlorogenic acid/kg body weight exerts antidiabetic effects [195,196]. Additionally, coffee phenolics can intensify energy metabolism and decrease lipogenesis by down-regulation of SREBP-1c and related molecules [197]. Moreover, coffee phenolics are able to modulate whole-body substrate oxidation by suppressing postprandial hyperglycemia and hyperinsulinemia [198].
Another commonly consumed beverage is tea (Camellia sinensis (L.) Kuntze). Infusions from tea are enormously rich in phenolic substances, and also contain considerable amounts of caffeine [199,200,201]. Consumption of tea was found to correlate with several health benefits including beneficial effects on the cardiovascular system [202]. Several studies showed that regular consumption of this polyphenol-rich beverage may exert cardio-protective effects in humans and reduce the risk of cardiovascular disease [203,204,205]. The phenolics of tea are represented particularly by epicatechin (EC), epigallocatechin (EGC), epicatechin-3-gallate (ECG), and epigallocatechin-3-gallate (EGCG) [206]. The effects of EGCG (Figure 3) are multifaceted and include among others the inhibition of the activator protein 1 (AP-1), the nuclear factor kappa B (NF-κB), the tumor necrosis factor α (TNFα) signaling, the inhibition of the vascular endothelial growth factor (VEGF) signaling, the insulin-like growth factor (IGF-1) signaling, and the activation of peroxisome proliferator-activated receptor (PPAR) [207].
Figure 3.

Chemical structure of epigallocatechin-3-gallate (EGCG).
The significance of dietary constituents in the context of metabolic and cardiovascular diseases is also evident in Table 2, which among herbal extracts also lists several prominent dietary constituents (e.g., curcumin and resveratrol).
Detailed studies on the efficacy of dietary constituents and the mechanisms by which they exert beneficial effects on cardiovascular and metabolic diseases are of critical importance in order to better rationalize dietary recommendations, and might also allow the development of novel effective nutraceuticals and functional foods [208,209,210,211,212]. Metabolism, bioavailability, and interaction with the intestinal microbiome will be important aspects to consider in this endeavor and also need to be taken into account for any natural product which is taken up orally.
7. Common Molecular Targets Affected by Natural Compounds in the Context of Cardiovascular and Metabolic Disorders
Diverse natural compounds have been shown to affect cardiovascular and metabolic disorders via different mechanisms, such as anti-inflammatory activity, improvement of blood lipid profiles, improvement of insulin sensitivity, or normalization of blood glucose levels [72,213,214,215,216,217]. Often the underlying molecular targets mediating these beneficial effects are not well understood. However, there are several molecular targets or pathways that are already well established to mediate the beneficial effects of natural compounds in the context of cardiovascular and metabolic disorders. Of those, selected examples, i.e., the AMP-activated protein kinase (AMPK), cyclooxygenase (COX)-1 and -2, the dipeptidyl peptidase-4 (DPP-4), the endothelial nitric oxide synthase (eNOS), the transcription factors NF-κB, nuclear factor-erythroid 2-related factor 2 (Nrf2), and PPARγ, the protein-tyrosine phosphatase 1B (PTP1B), and 5-lipoxygenase (5-LO), are listed in Table 3, together with their major physiological consequences and some examples of compound classes of interacting natural products.
Table 3.
Selected molecular targets relevant for cardiovascular and metabolic disorders, which are well known to be affected by diverse natural products.
| Molecular Target/Pathway | Major Physiological Consequence | Selected Compound Classes of Interacting Natural Products |
|---|---|---|
| AMPK | Activation leads among others to inhibition of fat and cholesterol synthesis, promotion of fat oxidation, enhancement of mitochondrial biogenesis, and promotion of glucose uptake in skeletal muscle and fat cells | Alkaloids, chalcones, flavonoids and other polyphenols, galegine, salicylate, terpenoids [214,218,219,220,221] |
| COX-1/-2 | Inhibition leads to reduced biosynthesis of pro-inflammatory prostaglandins | Alkaloids, stilbenes, flavonoids and other polyphenols, terpenoids [222,223,224] |
| DPP-4 | Inhibition leads to decreased incretin degradation (and thus increased insulin secretion) | Alkaloids, flavonoids and other polyphenols, polypeptides, terpenoids [225,226,227] |
| eNOS | Activation leads to increased availability of anti-inflammatory nitric oxide (NO), a major antiatherogenic factor in the vasculature | Anthocyanidins, fatty acids, flavonoids and other polyphenols, ginsenosides, triterpenoic acids [228,229,230,231,232,233,234] |
| NF-κB pathway | Inhibition leads to impaired expression of pro-inflammatory mediators | Alkaloids, curcuminoids, chalcones, diterpenes, flavonoids, iridoids, naphtoquinones, salicylates, sesquiterpene lactones, stilbenes, triterpenes [235,236,237,238,239] |
| Nrf2 pathway | Activation leads to increased expression of cytoprotective (e.g., antioxidant) and reduced expression of lipo-and gluconeogenic genes | Carotenoids, chalcones, curcuminoids, diterpenes, flavonoids and other polyphenols, isothiocyanates, phytoprostanes, sesquiterpenes, sesquiterpene lactones, triterpenes [240,241,242,243] |
| PPARγ | Activation leads to insulin sensitization and normalization of blood glucose levels | Amorfrutins, diterpenequinones, flavonoids, neolignans, polyacetylenes, sesquiterpene lactones, stilbenes [244,245,246,247,248,249] |
| PTP1B | Inhibition leads to prolonged and enhanced insulin and leptin signaling (increased insulin sensitivity and reduced food intake) | Alkaloids, bromophenols, chalcones, coumarins, diterpenes, flavonoids, lignans, N- or S-containing compounds, sesquiterpenes, sesterterpenes, steroids, triterpenes [250,251,252,253,254] |
| 5-LO | Inhibition leads to reduced biosynthesis of pro-inflammatory leukotrienes | Alkaloids, coumarins, depsides, quinones, flavonoids and other polyphenols, polyacetylenes, sesquiterpenes, triterpenes [222,255,256,257,258] |
8. Natural Products (or Their Derivatives) Developed as Drugs for the Treatment of Cardiovascular and Metabolic Disorders
Other than providing a direct remedy, natural products also represent an excellent pool of inspiring lead structures for the development of successful pharmaceuticals to combat cardiovascular and metabolic disorders. This could be demonstrated with historical views on the development of the statins and the biguanides.
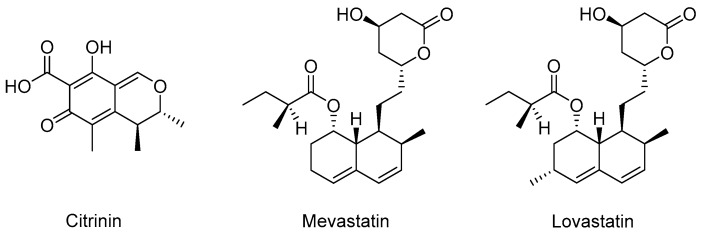
Aberrantly high cholesterol is causally connected to atherosclerosis and coronary heart disease. Therefore, in the 1950s and 1960s, companies were searching for compounds which block one of the 30 enzymatic reactions involved in cholesterol biosynthesis. However, none of the developed synthetic inhibitors of cholesterol biosynthesis had an ideal efficacy and safety profile [259]. In the early 1970s, the natural product citrinin (Figure 4) was isolated from fungi and identified as a potent inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase [260], the rate-controlling enzyme in the cholesterol biosynthesis. Citrinin also displayed serum cholesterol lowering effects in rats [261]. Shortly after that, mevastatin (compactin; Figure 4), the first statin, was isolated from Penicillium citrinum [262]. It was found to be a strong HMG-CoA reductase inhibitor [263] with great structural similarity with HMG-CoA, the substrate of HMG-CoA reductase [259]. Mevastatin potently inhibited cholesterol biosynthesis in vitro and in vivo [262,264]. Clinical studies started in 1978 but it never came on the market due to side effects in dogs at a dosage of about 200 times the dosage used in human patients [259]. In the 1980s, clinical studies and long-term toxicity studies showed that lovastatin (Figure 4), a natural product isolated from Aspergillus terreus [265] and Monascus ruber [266], effectively lowered blood cholesterol levels and was well tolerated [259]. In 1987, lovastatin was approved by the FDA and became the first commercial statin. After lovastatin, several synthetic and semi-synthetic statins were also introduced to the market [259,267]. Today, statins represent the first-line pharmacologic intervention for dyslipidemia patients with failed treatment with diet and exercise alone [268] and are one of the most widely prescribed class of drugs worldwide.
Figure 4.
Chemical structures of natural inhibitors of cholesterol biosynthesis.
Since the Middle Ages, Galega officinalis (also known as French lilac, Italian fitch, goat’s rue) has been known to relieve symptoms (the intense urination) of a disease now described as diabetes mellitus type 2 [98,269]. Galegine (Figure 5), a guanidine derivative which lowers blood glucose levels [270], turned out to be the bioactive constituent in G. officinalis [271,272]. Guanidine itself also decreases blood glucose levels [273], but is too toxic for clinical application. Galegine from G. officinalis is less toxic, nevertheless, clinical trials conducted with diabetic patients in the 1920s and 1930s, were not successful. However, the identification of the antidiabetic natural product galegine led to the development of the biguanide compound metformin, which is now one of the most important therapeutic agents for the treatment of diabetes mellitus type 2 [98,274,275].
Figure 5.

Chemical structure of the natural blood glucose lowering agent galegine.
9. Conclusions and Future Perspectives
The reviewed key examples and recent developments clearly demonstrate the great potential and the future promise of natural products for the treatment or prevention of cardiovascular and metabolic disorders. This work should provide an inspiration for authors who consider preparing further submissions to the special issue “Effects of Natural Products in the Context of Cardiometabolic Disease”. With the present review as well as with the expected valuable contributions to this special issue we do hope to further boost the scientific interest and knowledge on the efficacy of natural products with regard to the prevention and the therapy of cardiovascular and metabolic disease.
Acknowledgments
The work was supported by the Austrian Science Fund (FWF) project P25971-B23, by the Vienna Anniversary Foundation for Higher Education (Hochschuljubiläumsstiftung der Stadt Wien) project H-297332/2014, and by the European Social Found (Human Resources Development Operational Programme 2007–2013) project No. POSDRU/159/1.5/S/136893.
Abbreviations
The following abbreviations are used in this manuscript:
| 5-LO | 5-Lipoxygenase |
| AMPK | AMP-Activated Protein Kinase |
| AP-1 | Activator Protein 1 |
| COX-1/2 | Cyclooxygenase-1/2 |
| DPP-4 | Dipeptidyl Peptidase-4 |
| eNOS | Endothelial Nitric Oxide Synthase |
| FDA | US Food and Drug Administration |
| HMG-CoA | 3-Hydroxy-3-Methylglutaryl Coenzyme A |
| HTS | High Throughput Screening |
| IGF-1 | Insulin-Like Growth Factor |
| LDL | Low-Density Lipoprotein |
| NCT | National Clinical Trial |
| NF-κB | Nuclear Factor Kappa B |
| Nrf2 | Nuclear Factor-Erythroid 2-Related Factor 2 |
| NO | Nitric Oxide |
| PPAR | Peroxisome Proliferator-Activated Receptor |
| PTP1B | Protein-Tyrosine Phosphatase 1B |
| TNFα | Tumor Necrosis Factor α |
| VEGF | Vascular Endothelial Growth Factor |
| WHO | World Health Organization |
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Atanasov A.G., Waltenberger B., Pferschy-Wenzig E.M., Linder T., Wawrosch C., Uhrin P., Temml V., Wang L., Schwaiger S., Heiss E.H., et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015;33:1582–1614. doi: 10.1016/j.biotechadv.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.David B., Wolfender J.-L., Dias D.A. The pharmaceutical industry and natural products: Historical status and new trends. Phytochem. Rev. 2015;14:299–315. doi: 10.1007/s11101-014-9367-z. [DOI] [Google Scholar]
- 3.Amirkia V., Heinrich M. Natural products and drug discovery: A survey of stakeholders in industry and academia. Front. Pharmacol. 2015;6 doi: 10.3389/fphar.2015.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Efferth T., Zacchino S., Georgiev M.I., Liu L., Wagner H., Panossian A. Nobel Prize for artemisinin brings phytotherapy into the spotlight. Phytomedicine. 2015;22:A1–A3. doi: 10.1016/j.phymed.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Hertweck C. Natural products as source of therapeutics against parasitic diseases. Angew. Chem. Int. Ed. Engl. 2015;54:14622–14624. doi: 10.1002/anie.201509828. [DOI] [PubMed] [Google Scholar]
- 6.Cardoso S.M., Pereira O.R., Seca A.M., Pinto D.C., Silva A.M. Seaweeds as preventive agents for cardiovascular diseases: From nutrients to functional foods. Mar. Drugs. 2015;13:6838–6865. doi: 10.3390/md13116838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cornish M.L., Critchley A.T., Mouritsen O.G. A role for dietary macroalgae in the amelioration of certain risk factors associated with cardiovascular disease. Phycologia. 2015;54:649–666. doi: 10.2216/15-77.1. [DOI] [Google Scholar]
- 8.Tierney M.S., Croft A.K., Hayes M. A review of antihypertensive and antioxidant activities in macroalgae. Bot. Mar. 2010;53:387–408. doi: 10.1515/bot.2010.044. [DOI] [Google Scholar]
- 9.Wijesekara I., Kim S.K. Angiotensin-I-converting enzyme (ACE) inhibitors from marine resources: Prospects in the pharmaceutical industry. Mar. Drugs. 2010;8:1080–1093. doi: 10.3390/md8041080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sperling L.S., Mechanick J.I., Neeland I.J., Herrick C.J., Després J.-P., Ndumele C.E., Vijayaraghavan K., Handelsman Y., Puckrein G.A., Araneta M.R.G., et al. The CardioMetabolic Health Alliance: Working toward a new care model for the metabolic syndrome. J. Am. Coll. Cardiol. 2015;66:1050–1067. doi: 10.1016/j.jacc.2015.06.1328. [DOI] [PubMed] [Google Scholar]
- 11.Huang T.H.-W., Teoh A.W., Lin B.-L., Lin D.S.-H., Roufogalis B. The role of herbal PPAR modulators in the treatment of cardiometabolic syndrome. Pharmacol. Res. 2009;60:195–206. doi: 10.1016/j.phrs.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 12.Lusis A.J. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization (WHO) Global Status Report on Noncommunicable Diseases 2014. WHO; Geneva, Switzerland: 2014. [Google Scholar]
- 14.World Health Organization (WHO) Global Report on Diabetes 2016. WHO; Geneva, Switzerland: 2016. [Google Scholar]
- 15.Shaw J.E., Sicree R.A., Zimmet P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Xiao J. Natural polyphenols and diabetes: Understanding their mechanism of action. Curr. Med. Chem. 2015;22:2–3. doi: 10.2174/0929867321666141012173816. [DOI] [PubMed] [Google Scholar]
- 17.Cefalu W.T., Ye J., Wang Z.Q. Efficacy of dietary supplementation with botanicals on carbohydrate metabolism in humans. Endocr. Metab. Immune Disord. Drug Targets. 2008;8:78–81. doi: 10.2174/187153008784534376. [DOI] [PubMed] [Google Scholar]
- 18.Dong H., Lu F.-E., Zhao L. Chinese herbal medicine in the treatment of nonalcoholic fatty liver disease. Chin. J. Integr. Med. 2012;18:152–160. doi: 10.1007/s11655-012-0993-2. [DOI] [PubMed] [Google Scholar]
- 19.Heber D. Herbs and atherosclerosis. Curr. Atheroscler. Rep. 2001;3:93–96. doi: 10.1007/s11883-001-0016-9. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization (WHO) Traditional Medicine Strategy 2014–2023. WHO; Geneva, Switzerland: 2013. [Google Scholar]
- 21.Mashour N.H., Lin G.I., Frishman W.H. Herbal medicine for the treatment of cardiovascular disease: Clinical considerations. Arch. Intern. Med. 1998;158:2225–2234. doi: 10.1001/archinte.158.20.2225. [DOI] [PubMed] [Google Scholar]
- 22.Pittler M.H., Ernst E. Horse chestnut seed extract for chronic venous insufficiency. Cochrane Database Syst. Rev. 2012;11:CD003230. doi: 10.1002/14651858.CD003230.pub4. [DOI] [PubMed] [Google Scholar]
- 23.Suter A., Bommer S., Rechner J. Treatment of patients with venous insufficiency with fresh plant horse chestnut seed extract: A review of 5 clinical studies. Adv. Ther. 2006;23:179–190. doi: 10.1007/BF02850359. [DOI] [PubMed] [Google Scholar]
- 24.Siebert U., Brach M., Sroczynski G., Berla K. Efficacy, routine effectiveness, and safety of horsechestnut seed extract in the treatment of chronic venous insufficiency. A meta-analysis of randomized controlled trials and large observational studies. Int. Angiol. 2002;21:305–315. [PubMed] [Google Scholar]
- 25.Rahman K., Lowe G.M. Garlic and cardiovascular disease: A critical review. J. Nutr. 2006;136:736S–740S. doi: 10.1093/jn/136.3.736S. [DOI] [PubMed] [Google Scholar]
- 26.Sobenin I.A., Nedosugova L.V., Filatova L.V., Balabolkin M.I., Gorchakova T.V., Orekhov A.N. Metabolic effects of time-released garlic powder tablets in type 2 diabetes mellitus: The results of double-blinded placebo-controlled study. Acta Diabetol. 2008;45:1–6. doi: 10.1007/s00592-007-0011-x. [DOI] [PubMed] [Google Scholar]
- 27.Al Disi S.S., Anwar M.A., Eid A.H. Anti-hypertensive herbs and their mechanism of action: Part I. Front. Pharmacol. 2016;6 doi: 10.3389/fphar.2015.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwak J.S., Kim J.Y., Paek J.E., Lee Y.J., Kim H.R., Park D.S., Kwon O. Garlic powder intake and cardiovascular risk factors: A meta-analysis of randomized controlled clinical trials. Nutr. Res. Pract. 2014;8:644–654. doi: 10.4162/nrp.2014.8.6.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stabler S.N., Tejani A.M., Huynh F., Fowkes C. Garlic for the prevention of cardiovascular morbidity and mortality in hypertensive patients. Cochrane Database Syst. Rev. 2012;8:CD007653. doi: 10.1002/14651858.CD007653.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeng T., Guo F.F., Zhang C.L., Song F.Y., Zhao X.L., Xie K.Q. A meta-analysis of randomized, double-blind, placebo-controlled trials for the effects of garlic on serum lipid profiles. J. Sci. Food Agric. 2012;92:1892–1902. doi: 10.1002/jsfa.5557. [DOI] [PubMed] [Google Scholar]
- 31.Okyar A., Can A., Akev N., Baktir G., Sütlüpinar N. Effect of Aloe vera leaves on blood glucose level in type I and type II diabetic rat models. Phytother. Res. 2001;15:157–161. doi: 10.1002/ptr.719. [DOI] [PubMed] [Google Scholar]
- 32.Rajasekaran S., Ravi K., Sivagnanam K., Subramanian S. Beneficial effects of Aloe vera leaf gel extract on lipid profile status in rats with streptozotocin diabetes. Clin. Exp. Pharmacol. Physiol. 2006;33:232–237. doi: 10.1111/j.1440-1681.2006.04351.x. [DOI] [PubMed] [Google Scholar]
- 33.Alinejad-Mofrad S., Foadoddini M., Saadatjoo S.A., Shayesteh M. Improvement of glucose and lipid profile status with Aloe vera in pre-diabetic subjects: A randomized controlled-trial. J. Diabetes Metab. Disord. 2015;14 doi: 10.1186/s40200-015-0137-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dick W.R., Fletcher E.A., Shah S.A. Reduction of fasting blood glucose and hemoglobin A1c using oral Aloe vera: A meta-analysis. J. Altern. Complement. Med. 2016 doi: 10.1089/acm.2015.0122. [DOI] [PubMed] [Google Scholar]
- 35.Devaraj S., Yimam M., Brownell L.A., Jialal I., Singh S., Jia Q. Effects of Aloe vera supplementation in subjects with prediabetes/metabolic syndrome. Metab. Syndr. Relat. Disord. 2013;11:35–40. doi: 10.1089/met.2012.0066. [DOI] [PubMed] [Google Scholar]
- 36.Hashim S., Jan A., Marwat K.B., Khan M.A. Phytochemistry and medicinal properties of Ammi visnaga (Apiacae) Pak. J. Bot. 2014;46:861–867. [Google Scholar]
- 37.Durate J., Vallejo I., Perez-Vizcaino F., Jimenez R., Zarzuelo A., Tamargo J. Effects of visnadine on rat isolated vascular smooth muscles. Planta Med. 1997;63:233–236. doi: 10.1055/s-2006-957660. [DOI] [PubMed] [Google Scholar]
- 38.Duarte J., Perez-Vizcaino F., Torres A.I., Zarzuelo A., Jimenez J., Tamargo J. Vasodilator effects of visnagin in isolated rat vascular smooth muscle. Eur. J. Pharmacol. 1995;286:115–122. doi: 10.1016/0014-2999(95)00418-K. [DOI] [PubMed] [Google Scholar]
- 39.Hao P.-P., Jiang F., Chen Y.-G., Yang J., Zhang K., Zhang M.-X., Zhang C., Zhao Y.-X., Zhang Y. Traditional Chinese medication for cardiovascular disease. Nat. Rev. Cardiol. 2015;12:115–122. doi: 10.1038/nrcardio.2014.177. [DOI] [PubMed] [Google Scholar]
- 40.Kim D.-W., Yokozawa T., Hattori M., Kadota S., Namba T. Effects of aqueous extracts of Apocynum venetum leaves on spontaneously hypertensive, renal hypertensive and NaCl-fed-hypertensive rats. J. Ethnopharmacol. 2000;72:53–59. doi: 10.1016/S0378-8741(00)00197-5. [DOI] [PubMed] [Google Scholar]
- 41.Xie W., Zhang X., Wang T., Hu J. Botany, traditional uses, phytochemistry and pharmacology of Apocynum venetum L. (Luobuma): A review. J. Ethnopharmacol. 2012;141:1–8. doi: 10.1016/j.jep.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 42.Wang W., Liang X., Fu D., Tie R., Xing W., Ji L., Liu F., Zhang H., Li R. Apocynum venetum leaf attenuates myocardial ischemia/reperfusion injury by inhibiting oxidative stress. Am. J. Chin. Med. 2015;43:71–85. doi: 10.1142/S0192415X15500056. [DOI] [PubMed] [Google Scholar]
- 43.Aggarwal S., Shailendra G., Ribnicky D.M., Burk D., Karki N., Qingxia Wang M.S. An extract of Artemisia dracunculus L. stimulates insulin secretion from β cells, activates AMPK and suppresses inflammation. J. Ethnopharmacol. 2015;170:98–105. doi: 10.1016/j.jep.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Watcho P., Stavniichuk R., Tane P., Shevalye H., Maksimchyk Y., Pacher P., Obrosova I.G. Evaluation of PMI-5011, an ethanolic extract of Artemisia dracunculus L., on peripheral neuropathy in streptozotocin-diabetic mice. Int. J. Mol. Med. 2011;27:299–307. doi: 10.3892/ijmm.2011.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watcho P., Stavniichuk R., Ribnicky D.M., Raskin I., Obrosova I.G. High-fat diet-induced neuropathy of prediabetes and obesity: Effect of PMI-5011, an ethanolic extract of Artemisia dracunculus L. Mediat. Inflamm. 2010;2010 doi: 10.1155/2010/268547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hamza N., Berke B., Cheze C., Marais S., Lorrain S., Abdouelfath A., Lassalle R., Carles D., Gin H., Moore N. Effect of Centaurium erythraea Rafn, Artemisia herba-alba Asso and Trigonella foenum-graecum L. on liver fat accumulation in C57BL/6J mice with high-fat diet-induced type 2 diabetes. J. Ethnopharmacol. 2015;171:4–11. doi: 10.1016/j.jep.2015.05.027. [DOI] [PubMed] [Google Scholar]
- 47.Boudjelal A., Siracusa L., Henchiri C., Sarri M., Abderrahim B., Baali F., Ruberto G. Antidiabetic effects of aqueous infusions of Artemisia herba-alba and Ajuga iva in alloxan-induced diabetic rats. Planta Med. 2015;81:696–704. doi: 10.1055/s-0035-1546006. [DOI] [PubMed] [Google Scholar]
- 48.Hamza N., Berke B., Cheze C., Le Garrec R., Lassalle R., Agli A.N., Robinson P., Gin H., Moore N. Treatment of high fat diet induced type 2 diabetes in C57BL/6J mice by two medicinal plants used in traditional treatment of diabetes in the east of Algeria. J. Ethnopharmacol. 2011;133:931–933. doi: 10.1016/j.jep.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 49.Al-Shamaony L., al-Khazraji S.M., Twaij H.A. Hypoglycaemic effect of Artemisia herba alba. II. Effect of a valuable extract on some blood parameters in diabetic animals. J. Ethnopharmacol. 1994;43:167–171. doi: 10.1016/0378-8741(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 50.Hamza N., Berke B., Cheze C., Agli A.N., Robinson P., Gin H., Moore N. Prevention of type 2 diabetes induced by high fat diet in the C57BL/6J mouse by two medicinal plants used in traditional treatment of diabetes in the east of Algeria. J. Ethnopharmacol. 2010;128:513–518. doi: 10.1016/j.jep.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 51.Kawano A., Nakamura H., Hata S.-I., Minakawa M., Miura Y., Yagasaki K. Hypoglycemic effect of aspalathin, a rooibos tea component from Aspalathus linearis, in type 2 diabetic model db/db mice. Phytomedicine. 2009;16:437–443. doi: 10.1016/j.phymed.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 52.Son M.J., Minakawa M., Miura Y., Yagasaki K. Aspalathin improves hyperglycemia and glucose intolerance in obese diabetic ob/ob mice. Eur. J. Nutr. 2013;52:1607–1619. doi: 10.1007/s00394-012-0466-6. [DOI] [PubMed] [Google Scholar]
- 53.Mazibuko S.E., Joubert E., Johnson R., Louw J., Opoku A.R., Muller C.J. Aspalathin improves glucose and lipid metabolism in 3T3-L1 adipocytes exposed to palmitate. Mol. Nutr. Food Res. 2015;59:2199–2208. doi: 10.1002/mnfr.201500258. [DOI] [PubMed] [Google Scholar]
- 54.Ku S.K., Kwak S., Kim Y., Bae J.S. Aspalathin and nothofagin from rooibos (Aspalathus linearis) inhibits high glucose-induced inflammation in vitro and in vivo. Inflammation. 2015;38:445–455. doi: 10.1007/s10753-014-0049-1. [DOI] [PubMed] [Google Scholar]
- 55.Chen W., Lai Y., Wang L., Xia Y., Chen W., Zhao X., Yu M., Li Y., Zhang Y., Ye H. Astragalus polysaccharides repress myocardial lipotoxicity in a PPARalpha-dependent manner in vitro and in vivo in mice. J. Diabetes Complicat. 2015;29:164–175. doi: 10.1016/j.jdiacomp.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 56.Kim J., Moon E., Kwon S. Effect of Astragalus membranaceus extract on diabetic nephropathy. Endocrinol. Diabetes Metab. Case Rep. 2014;2014 doi: 10.1530/EDM-14-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qin H., Liu P., Lin S. Effects of astragaloside IV on the SDF-1/CXCR4 expression in atherosclerosis of apoE−/− mice induced by hyperlipaemia. Evid. Based Complement. Altern. Med. 2015;2015 doi: 10.1155/2015/385154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao P., Wang Y., Zeng S., Lu J., Jiang T.-M., Li Y.-M. Protective effect of astragaloside IV on lipopolysaccharide-induced cardiac dysfunction via downregulation of inflammatory signaling in mice. Immunopharmacol. Immunotoxicol. 2015;37:428–433. doi: 10.3109/08923973.2015.1080266. [DOI] [PubMed] [Google Scholar]
- 59.Lu Y., Li S., Wu H., Bian Z., Xu J., Gu C., Chen X., Yang D. Beneficial effects of astragaloside IV against angiotensin II-induced mitochondrial dysfunction in rat vascular smooth muscle cells. Int. J. Mol. Med. 2015;36:1223–1232. doi: 10.3892/ijmm.2015.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bai Y., Lu P., Han C., Yu C., Chen M., He F., Yi D., Wu L. Hydroxysafflor yellow A (HSYA) from flowers of Carthamus tinctorius L. and its vasodilatation effects on pulmonary artery. Molecules. 2012;17:14918–14927. doi: 10.3390/molecules171214918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nie P.H., Zhang L., Zhang W.H., Rong W.F., Zhi J.M. The effects of hydroxysafflor yellow A on blood pressure and cardiac function. J. Ethnopharmacol. 2012;139:746–750. doi: 10.1016/j.jep.2011.11.054. [DOI] [PubMed] [Google Scholar]
- 62.Chen J., Deng J., Zhang Y., Yang J., He Y., Fu W., Xing P., Wan H.T. Lipid-lowering effects of Danhong injection on hyperlipidemia rats. J. Ethnopharmacol. 2014;154:437–442. doi: 10.1016/j.jep.2014.04.023. [DOI] [PubMed] [Google Scholar]
- 63.Li L., Dong P., Hou C., Cao F., Sun S., He F., Song Y., Li S., Bai Y., Zhu D. Hydroxysafflor yellow A (HSYA) attenuates hypoxic pulmonary arterial remodelling and reverses right ventricular hypertrophy in rats. J. Ethnopharmacol. 2016;186:224–233. doi: 10.1016/j.jep.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 64.Maneesai P., Prasarttong P., Bunbupha S., Kukongviriyapan U., Kukongviriyapan V., Tangsucharit P., Prachaney P., Pakdeechote P. Synergistic antihypertensive effect of Carthamus tinctorius L. extract and captopril in l-NAME-induced hypertensive rats via restoration of eNOS and AT1R expression. Nutrients. 2016;8 doi: 10.3390/nu8030122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sefi M., Fetoui H., Lachkar N., Tahraoui A., Lyoussi B., Boudawara T., Zeghal N. Centaurium erythrea (Gentianaceae) leaf extract alleviates streptozotocin-induced oxidative stress and β-cell damage in rat pancreas. J. Ethnopharmacol. 2011;135:243–250. doi: 10.1016/j.jep.2011.02.029. [DOI] [PubMed] [Google Scholar]
- 66.Stefkov G., Miova B., Dinevska-Kjovkarovska S., Stanoeva J.P., Stefova M., Petrusevska G., Kulevanova S. Chemical characterization of Centaurium erythrea L. and its effects on carbohydrate and lipid metabolism in experimental diabetes. J. Ethnopharmacol. 2014;152:71–77. doi: 10.1016/j.jep.2013.11.047. [DOI] [PubMed] [Google Scholar]
- 67.Eddouks M., Bidi A., El Bouhali B., Hajji L., Zeggwagh N.A. Antidiabetic plants improving insulin sensitivity. J. Pharm. Pharmacol. 2014;66:1197–1214. doi: 10.1111/jphp.12243. [DOI] [PubMed] [Google Scholar]
- 68.Yan Y.-M., Fang P., Yang M.-T., Li N., Lu Q., Cheng Y.-X. Anti-diabetic nephropathy compounds from Cinnamomum cassia. J. Ethnopharmacol. 2015;165:141–147. doi: 10.1016/j.jep.2015.01.049. [DOI] [PubMed] [Google Scholar]
- 69.Medagama A.B. The glycaemic outcomes of cinnamon, a review of the experimental evidence and clinical trials. Nutr. J. 2015;14 doi: 10.1186/s12937-015-0098-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Crawford P. Effectiveness of cinnamon for lowering hemoglobin A1C in patients with type 2 diabetes: A randomized, controlled trial. J. Am. Board Fam. Med. 2009;22:507–512. doi: 10.3122/jabfm.2009.05.080093. [DOI] [PubMed] [Google Scholar]
- 71.Mirfeizi M., Mehdizadeh Tourzani Z., Mirfeizi S.Z., Asghari Jafarabadi M., Rezvani H.R., Afzali M., Gholami M.J. Controlling diabetes mellitus type 2 with herbal medicines: A triple blind, randomized clinical trial of efficacy and safety. J. Diabetes. 2015 doi: 10.1111/1753-0407.12342. [DOI] [PubMed] [Google Scholar]
- 72.Ríos J.L., Francini F., Schinella G.R. Natural products for the treatment of type 2 diabetes mellitus. Planta Med. 2015;81:975–994. doi: 10.1055/s-0035-1546131. [DOI] [PubMed] [Google Scholar]
- 73.Whitfield P., Parry-Strong A., Walsh E., Weatherall M., Krebs J.D. The effect of a cinnamon-, chromium- and magnesium-formulated honey on glycaemic control, weight loss and lipid parameters in type 2 diabetes: An open-label cross-over randomised controlled trial. Eur. J. Nutr. 2016;55:1123–1131. doi: 10.1007/s00394-015-0926-x. [DOI] [PubMed] [Google Scholar]
- 74.Beejmohun V., Peytavy-Izard M., Mignon C., Muscente-Paque D., Deplanque X., Ripoll C., Chapal N. Acute effect of Ceylon cinnamon extract on postprandial glycemia: Alpha-amylase inhibition, starch tolerance test in rats, and randomized crossover clinical trial in healthy volunteers. BMC Complement. Altern. Med. 2014;14 doi: 10.1186/1472-6882-14-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Frishman W.H., Beravol P., Carosella C. Alternative and complementary medicine for preventing and treating cardiovascular disease. Dis. Mon. 2009;55:121–192. doi: 10.1016/j.disamonth.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 76.Ojha S.K., Nandave M., Arora S., Mehra R.D., Joshi S., Narang R., Arya D.S. Effect of Commiphora mukul extract on cardiac dysfunction and ventricular function in isoproterenol-induced myocardial infarction. Indian J. Exp. Biol. 2008;46:646–652. [PubMed] [Google Scholar]
- 77.Yuan L., Tu D., Ye X., Wu J. Hypoglycemic and hypocholesterolemic effects of Coptis chinensis Franch inflorescence. Plant Foods Hum. Nutr. 2006;61:139–144. doi: 10.1007/s11130-006-0023-7. [DOI] [PubMed] [Google Scholar]
- 78.Dong H., Wang J.-H., Lu F.-E., Xu L.-J., Gong Y.-L., Zou X. Jiaotai Pill enhances insulin signaling through phosphatidylinositol 3-kinase pathway in skeletal muscle of diabetic rats. Chin. J. Integr. Med. 2013;19:668–674. doi: 10.1007/s11655-013-1560-1. [DOI] [PubMed] [Google Scholar]
- 79.Yang Z., Wang L., Zhang F., Li Z. Evaluating the antidiabetic effects of Chinese herbal medicine: Xiao-Ke-An in 3T3-L1 cells and KKAy mice using both conventional and holistic omics approaches. BMC Complement. Altern. Med. 2015;15 doi: 10.1186/s12906-015-0785-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Eidi M., Eidi A., Saeidi A., Molanaei S., Sadeghipour A., Bahar M., Bahar K. Effect of coriander seed (Coriandrum sativum L.) ethanol extract on insulin release from pancreatic beta cells in streptozotocin-induced diabetic rats. Phytother. Res. 2009;23:404–406. doi: 10.1002/ptr.2642. [DOI] [PubMed] [Google Scholar]
- 81.Dhanapakiam P., Joseph J.M., Ramaswamy V.K., Moorthi M., Kumar A.S. The cholesterol lowering properties of coriander seeds (Coriandrum sativum): Mechanism of action. J. Environ. Biol. 2008;29:53–56. [PubMed] [Google Scholar]
- 82.Sreelatha S., Inbavalli R. Antioxidant, antihyperglycemic, and antihyperlipidemic effects of Coriandrum sativum leaf and stem in alloxan-induced diabetic rats. J. Food Sci. 2012;77:T119–T123. doi: 10.1111/j.1750-3841.2012.02755.x. [DOI] [PubMed] [Google Scholar]
- 83.Aissaoui A., Zizi S., Israili Z.H., Lyoussi B. Hypoglycemic and hypolipidemic effects of Coriandrum sativum L. in Meriones shawi rats. J. Ethnopharmacol. 2011;137:652–661. doi: 10.1016/j.jep.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 84.Chang W.-T., Dao J., Shao Z.-H. Hawthorn: Potential roles in cardiovascular disease. Am. J. Chin. Med. 2005;33:1–10. doi: 10.1142/S0192415X05002606. [DOI] [PubMed] [Google Scholar]
- 85.Chrysant S.G. The clinical significance and costs of herbs and food supplements used by complementary and alternative medicine for the treatment of cardiovascular diseases and hypertension. J. Hum. Hypertens. 2016;30:1–6. doi: 10.1038/jhh.2015.42. [DOI] [PubMed] [Google Scholar]
- 86.Asher G.N., Viera A.J., Weaver M.A., Dominik R., Caughey M., Hinderliter A.L. Effect of hawthorn standardized extract on flow mediated dilation in prehypertensive and mildly hypertensive adults: A randomized, controlled cross-over trial. BMC Complement. Altern. Med. 2012;12 doi: 10.1186/1472-6882-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pittler M.H., Guo R., Ernst E. Hawthorn extract for treating chronic heart failure. Cochrane Database Syst. Rev. 2008;1:CD005312. doi: 10.1002/14651858.CD005312.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bundy R., Walker A.F., Middleton R.W., Wallis C., Simpson H.C.R. Artichoke leaf extract (Cynara scolymus) reduces plasma cholesterol in otherwise healthy hypercholesterolemic adults: A randomized, double blind placebo controlled trial. Phytomedicine. 2008;15:668–675. doi: 10.1016/j.phymed.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 89.Ben Salem M., Affes H., Ksouda K., Dhouibi R., Sahnoun Z., Hammami S., Zeghal K.M. Pharmacological studies of artichoke leaf extract and their health benefits. Plant Foods Hum. Nutr. 2015;70:441–453. doi: 10.1007/s11130-015-0503-8. [DOI] [PubMed] [Google Scholar]
- 90.Maghrani M., Zeggwagh N.-A., Lemhadri A., El Amraoui M., Michel J.-B., Eddouks M. Study of the hypoglycaemic activity of Fraxinus excelsior and Silybum marianum in an animal model of type 1 diabetes mellitus. J. Ethnopharmacol. 2004;91:309–316. doi: 10.1016/j.jep.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 91.Eddouks M., Maghrani M. Phlorizin-like effect of Fraxinus excelsior in normal and diabetic rats. J. Ethnopharmacol. 2004;94:149–154. doi: 10.1016/j.jep.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 92.Gomez-Garcia F., Flanagan J., García-Molina O., Vilaplana-Vivo V., García-Carrillo N., Berthon P.F., Bily A., Roller M., Ortega V.V., Issaly N. Preventive effect of a Fraxinus excelsior L seeds/fruits extract on hepatic steatosis in obese type 2 diabetic mice. J. Diabetes Metab. 2015;6 doi: 10.4172/2155-6156.1000527. [DOI] [Google Scholar]
- 93.Visen P., Saraswat B., Visen A., Roller M., Bily A., Mermet C., He K., Bai N., Lemaire B., Lafay S., et al. Acute effects of Fraxinus excelsior L. seed extract on postprandial glycemia and insulin secretion on healthy volunteers. J. Ethnopharmacol. 2009;126:226–232. doi: 10.1016/j.jep.2009.08.039. [DOI] [PubMed] [Google Scholar]
- 94.Bai N., He K., Ibarra A., Bily A., Roller M., Chen X., Rülh R. Iridoids from Fraxinus excelsior with adipocyte differentiation-inhibitory and PPARα activation activity. J. Nat. Prod. 2010;73:2–6. doi: 10.1021/np9003118. [DOI] [PubMed] [Google Scholar]
- 95.Zulet M.A., Navas-Carretero S., Lara y Sanchez D., Abete I., Flanagan J., Issaly N., Fanca-Berthon P., Bily A., Roller M., Martinez J.A. A Fraxinus excelsior L. seeds/fruits extract benefits glucose homeostasis and adiposity related markers in elderly overweight/obese subjects: A longitudinal, randomized, crossover, double-blind, placebo-controlled nutritional intervention study. Phytomedicine. 2014;21:1162–1169. doi: 10.1016/j.phymed.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 96.Bedekar A., Shah K., Koffas M. Natural products for type II diabetes treatment. Adv. Appl. Microbiol. 2010;71:21–73. doi: 10.1016/S0065-2164(10)71002-9. [DOI] [PubMed] [Google Scholar]
- 97.Perla V., Jayanty S.S. Biguanide related compounds in traditional antidiabetic functional foods. Food Chem. 2013;138:1574–1580. doi: 10.1016/j.foodchem.2012.09.125. [DOI] [PubMed] [Google Scholar]
- 98.Witters L.A. The blooming of the French lilac. J. Clin. Investig. 2001;108:1105–1107. doi: 10.1172/JCI14178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gardner C.D., Zehnder J.L., Rigby A.J., Nicholus J.R., Farquhar J.W. Effect of Ginkgo biloba (EGb 761) and aspirin on platelet aggregation and platelet function analysis among older adults at risk of cardiovascular disease: A randomized clinical trial. Blood Coagul. Fibrinolysis. 2007;18:787–793. doi: 10.1097/MBC.0b013e3282f102b1. [DOI] [PubMed] [Google Scholar]
- 100.Lu Q., Zuo W.-Z., Ji X.-J., Zhou Y.-X., Liu Y.-Q., Yao X.-Q., Zhou X.-Y., Liu Y.-W., Zhang F., Yin X.-X. Ethanolic Ginkgo biloba leaf extract prevents renal fibrosis through Akt/mTOR signaling in diabetic nephropathy. Phytomedicine. 2015;22:1071–1078. doi: 10.1016/j.phymed.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 101.Erukainure O.L., Ajiboye J.A., Lawal B.A., Obode O.C., Okoro E.E., Amisu-Tugbobo A.O., Zaruwa M.Z. Alterations in atherogenic indices and hypolipidemic effect of soybean oil in normocholesteremic rats. Comp. Clin. Pathol. 2016;25:75–78. doi: 10.1007/s00580-015-2142-8. [DOI] [Google Scholar]
- 102.Kwon D.Y., Daily J.W., Kim H.J., Park S. Antidiabetic effects of fermented soybean products on type 2 diabetes. Nutr. Res. 2010;30:1–13. doi: 10.1016/j.nutres.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 103.Jiang H., Tong Y., Yan D., Jia S., Ostenson C.G., Chen Z. The soybean peptide vglycin preserves the diabetic beta-cells through improvement of proliferation and inhibition of apoptosis. Sci. Rep. 2015;5 doi: 10.1038/srep15599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fuhrman B., Volkova N., Kaplan M., Presser D., Attias J., Hayek T., Aviram M. Antiatherosclerotic effects of licorice extract supplementation on hypercholesterolemic patients: Increased resistance of LDL to atherogenic modifications, reduced plasma lipid levels, and decreased systolic blood pressure. Nutrition. 2002;18:268–273. doi: 10.1016/S0899-9007(01)00753-5. [DOI] [PubMed] [Google Scholar]
- 105.Chang W.-C., Jia H., Aw W., Saito K., Hasegawa S., Kato H. Beneficial effects of soluble dietary Jerusalem artichoke (Helianthus tuberosus) in the prevention of the onset of type 2 diabetes and non-alcoholic fatty liver disease in high-fructose diet-fed rats. Br. J. Nutr. 2014;112:709–717. doi: 10.1017/S0007114514001421. [DOI] [PubMed] [Google Scholar]
- 106.Gambero A., Ribeiro M.L. The positive effects of yerba maté (Ilex paraguariensis) in obesity. Nutrients. 2015;7:730–750. doi: 10.3390/nu7020730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.De Moraes Pontilho P., Nunes da Costa Teixeira A.M., Yuan C., Alves Luzia L., Markowicz Bastos D.H., Rondó P.H. Yerba mate (Ilex paraguariensis A. St. Hil) and risk factors for cardiovascular diseases. J. Food Nutr. Res. 2015;3:182–190. doi: 10.12691/jfnr-3-3-9. [DOI] [Google Scholar]
- 108.Cardozo E.L., Jr., Morand C. Interest of mate (Ilex paraguariensis A. St.-Hil.) as a new natural functional food to preserve human cardiovascular health—A review. J. Funct. Foods. 2016;21:440–454. doi: 10.1016/j.jff.2015.12.010. [DOI] [Google Scholar]
- 109.Chang C.L.T., Lin Y., Bartolome A.P., Chen Y.-C., Chiu S.-C., Yang W.-C. Herbal therapies for type 2 diabetes mellitus: Chemistry, biology, and potential application of selected plants and compounds. Evid. Based Complement. Alternat. Med. 2013;2013 doi: 10.1155/2013/378657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim S.-Y., Oh M.-R., Kim M.-G., Chae H.-J., Chae S.-W. Anti-obesity effects of yerba mate (Ilex Paraguariensis): A randomized, double-blind, placebo-controlled clinical trial. BMC Complement. Altern. Med. 2015;15 doi: 10.1186/s12906-015-0859-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yu S., Yue S.w., Liu Z., Zhang T., Xiang N., Fu H. Yerba mate (Ilex paraguariensis) improves microcirculation of volunteers with high blood viscosity: A randomized, double-blind, placebo-controlled trial. Exp. Gerontol. 2015;62:14–22. doi: 10.1016/j.exger.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 112.Luo Q., Cai Y., Yan J., Sun M., Corke H. Hypoglycemic and hypolipidemic effects and antioxidant activity of fruit extracts from Lycium barbarum. Life Sci. 2004;76:137–149. doi: 10.1016/j.lfs.2004.04.056. [DOI] [PubMed] [Google Scholar]
- 113.Zhao R., Jin R., Chen Y., Han F.-m. Hypoglycemic and hypolipidemic effects of Lycium barbarum polysaccharide in diabetic rats. Chin. Herb. Med. 2015;7:310–315. doi: 10.1016/S1674-6384(15)60057-0. [DOI] [Google Scholar]
- 114.Amagase H., Nance D.M. A randomized, double-blind, placebo-controlled, clinical study of the general effects of a standardized Lycium barbarum (Goji) juice, GoChi™. J. Altern. Complement. Med. 2008;14:403–412. doi: 10.1089/acm.2008.0004. [DOI] [PubMed] [Google Scholar]
- 115.Zhang X., Yang X., Lin Y., Suo M., Gong L., Chen J., Hui R. Anti-hypertensive effect of Lycium barbarum L. with down-regulated expression of renal endothelial lncRNA sONE in a rat model of salt-sensitive hypertension. Int. J. Clin. Exp. Pathol. 2015;8:6981–6987. [PMC free article] [PubMed] [Google Scholar]
- 116.Lu S.-P., Zhao P.-T. Chemical characterization of Lycium barbarum polysaccharides and their reducing myocardial injury in ischemia/reperfusion of rat heart. Int. J. Biol. Macromol. 2010;47:681–684. doi: 10.1016/j.ijbiomac.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 117.Ming M., Guanhua L., Zhanhai Y., Guang C., Xuan Z. Effect of the Lycium barbarum polysaccharides administration on blood lipid metabolism and oxidative stress of mice fed high-fat diet in vivo. Food Chem. 2009;113:872–877. doi: 10.1016/j.foodchem.2008.03.064. [DOI] [Google Scholar]
- 118.Cai H., Liu F., Zuo P., Huang G., Song Z., Wang T., Lu H., Guo F., Han C., Sun G. Practical application of antidiabetic efficacy of Lycium barbarum polysaccharide in patients with type 2 diabetes. Med. Chem. 2015;11:383–390. doi: 10.2174/1573406410666141110153858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhu X., Hu S., Zhu L., Ding J., Zhou Y., Li G. Effects of Lycium barbarum polysaccharides on oxidative stress in hyperlipidemic mice following chronic composite psychological stress intervention. Mol. Med. Rep. 2015;11:3445–3450. doi: 10.3892/mmr.2014.3128. [DOI] [PubMed] [Google Scholar]
- 120.Mishra A., Gautam S., Pal S., Mishra A., Rawat A.K., Maurya R., Srivastava A.K. Effect of Momordica charantia fruits on streptozotocin-induced diabetes mellitus and its associated complications. Int. J. Pharm. Pharm. Sci. 2015;7:356–363. [Google Scholar]
- 121.Yang S.J., Choi J.M., Park S.E., Rhee E.J., Lee W.Y., Oh K.W., Park S.W., Park C.-Y. Preventive effects of bitter melon (Momordica charantia) against insulin resistance and diabetes are associated with the inhibition of NF-κB and JNK pathways in high-fat-fed OLETF rats. J. Nutr. Biochem. 2015;26:234–240. doi: 10.1016/j.jnutbio.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 122.Singab A.N.B., El-Beshbishy H.A., Yonekawa M., Nomura T., Fukai T. Hypoglycemic effect of Egyptian Morus alba root bark extract: Effect on diabetes and lipid peroxidation of streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2005;100:333–338. doi: 10.1016/j.jep.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 123.Hunyadi A., Martins A., Hsieh T.-J., Seres A., Zupkó I. Chlorogenic acid and rutin play a major role in the in vivo anti-diabetic activity of Morus alba leaf extract on type II diabetic rats. PLoS ONE. 2012;7:e50619. doi: 10.1371/journal.pone.0050619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Butt M.S., Nazir A., Sultan M.T., Schroën K. Morus alba L. nature’s functional tonic. Trends Food Sci. Technol. 2008;19:505–512. doi: 10.1016/j.tifs.2008.06.002. [DOI] [Google Scholar]
- 125.Cai S., Sun W., Fan Y., Guo X., Xu G., Xu T., Hou Y., Zhao B., Feng X., Liu T. Effect of mulberry leaf (Folium mori) on insulin resistance via IRS-1/PI3K/Glut-4 signalling pathway in type 2 diabetes mellitus rats. Pharm. Biol. 2016 doi: 10.1080/13880209.2016.1178779. [DOI] [PubMed] [Google Scholar]
- 126.Mahmoud A.M., Abd El-Twab S.M., Abdel-Reheim E.S. Consumption of polyphenol-rich Morus alba leaves extract attenuates early diabetic retinopathy: The underlying mechanism. Eur. J. Nutr. 2016 doi: 10.1007/s00394-016-1214-0. [DOI] [PubMed] [Google Scholar]
- 127.Phimarn W., Wichaiyo K., Silpsavikul K., Sungthong B., Saramunee K. A meta-analysis of efficacy of Morus alba Linn. to improve blood glucose and lipid profile. Eur. J. Nutr. 2016 doi: 10.1007/s00394-016-1197-x. [DOI] [PubMed] [Google Scholar]
- 128.Heshmati J., Namazi N. Effects of black seed (Nigella sativa) on metabolic parameters in diabetes mellitus: A systematic review. Complement. Ther. Med. 2015;23:275–282. doi: 10.1016/j.ctim.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 129.Heshmati J., Namazi N., Memarzadeh M.-R., Taghizadeh M., Kolahdooz F. Nigella sativa oil affects glucose metabolism and lipid concentrations in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Food Res. Int. 2015;70:87–93. doi: 10.1016/j.foodres.2015.01.030. [DOI] [Google Scholar]
- 130.Kaatabi H., Bamosa A.O., Badar A., Al-Elq A., Abou-Hozaifa B., Lebda F., Al-Khadra A., Al-Almaie S. Nigella sativa improves glycemic control and ameliorates oxidative stress in patients with type 2 diabetes mellitus: Placebo controlled participant blinded clinical trial. PLoS ONE. 2015;10:e0113486. doi: 10.1371/journal.pone.0113486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mahdavi R., Namazi N., Alizadeh M., Farajnia S. Effects of Nigella sativa oil with a low-calorie diet on cardiometabolic risk factors in obese women: A randomized controlled clinical trial. Food Funct. 2015;6:2041–2048. doi: 10.1039/C5FO00316D. [DOI] [PubMed] [Google Scholar]
- 132.Asgary S., Sahebkar A., Goli-Malekabadi N. Ameliorative effects of Nigella sativa on dyslipidemia. J. Endocrinol. Investig. 2015;38:1039–1046. doi: 10.1007/s40618-015-0337-0. [DOI] [PubMed] [Google Scholar]
- 133.Husain I., Chander R., Saxena J.K., Mahdi A.A., Mahdi F. Antidyslipidemic effect of Ocimum sanctum leaf extract in streptozotocin induced diabetic rats. Indian J. Clin. Biochem. 2015;30:72–77. doi: 10.1007/s12291-013-0404-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Thadani S., Salman M.T., Tewari S., Singh S., Bhagchandani D., Ahmad A. Renoprotective effect of Ocimum sanctum in comparison with olmesartan medoxomil and pitavastatin in metformin treated diabetic rats. Int. J. Pharm. Sci. Res. 2015;6:4433–4441. [Google Scholar]
- 135.El S.N., Karakaya S. Olive tree (Olea europaea) leaves: Potential beneficial effects on human health. Nutr. Rev. 2009;67:632–638. doi: 10.1111/j.1753-4887.2009.00248.x. [DOI] [PubMed] [Google Scholar]
- 136.Poudyal H., Campbell F., Brown L. Olive leaf extract attenuates cardiac, hepatic, and metabolic changes in high carbohydrate-, high fat-fed rats. J. Nutr. 2010;140:946–953. doi: 10.3945/jn.109.117812. [DOI] [PubMed] [Google Scholar]
- 137.Susalit E., Agus N., Effendi I., Tjandrawinata R.R., Nofiarny D., Perrinjaquet-Moccetti T., Verbruggen M. Olive (Olea europaea) leaf extract effective in patients with stage-1 hypertension: Comparison with captopril. Phytomedicine. 2011;18:251–258. doi: 10.1016/j.phymed.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 138.Efentakis P., Iliodromitis E.K., Mikros E., Papachristodoulou A., Dagres N., Skaltsounis A.-L., Andreadou I. Effects of the olive tree leaf constituents on myocardial oxidative damage and atherosclerosis. Planta Med. 2015;81:648–654. doi: 10.1055/s-0035-1546017. [DOI] [PubMed] [Google Scholar]
- 139.Wainstein J., Ganz T., Boaz M., Bar Dayan Y., Dolev E., Kerem Z., Madar Z. Olive leaf extract as a hypoglycemic agent in both human diabetic subjects and in rats. J. Med. Food. 2012;15:605–610. doi: 10.1089/jmf.2011.0243. [DOI] [PubMed] [Google Scholar]
- 140.Lepore S.M., Morittu V.M., Celano M., Trimboli F., Oliverio M., Procopio A., Di Loreto C., Damante G., Britti D., Bulotta S., et al. Oral administration of oleuropein and its semisynthetic peracetylated derivative prevents hepatic steatosis, hyperinsulinemia, and weight gain in mice fed with high fat cafeteria diet. Int. J. Endocrinol. 2015;2015 doi: 10.1155/2015/431453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Perona J.S., Cañizares J., Montero E., Sánchez-Domínguez J.M., Catalá A., Ruiz-Gutiérrez V. Virgin olive oil reduces blood pressure in hypertensive elderly subjects. Clin. Nutr. 2004;23:1113–1121. doi: 10.1016/j.clnu.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 142.Quintieri A.M., Filice E., Amelio D., Pasqua T., Lupi F.R., Scavello F., Cantafio P., Rocca C., Lauria A., Penna C., et al. The innovative “Bio-Oil Spread” prevents metabolic disorders and mediates preconditioning-like cardioprotection in rats. Nutr. Metab. Cardiovasc. Dis. 2016 doi: 10.1016/j.numecd.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 143.Zhang Y.-G., Zhang H.-G., Zhang G.-Y., Fan J.-S., Li X.-H., Liu Y.-H., Li S.-H., Lian X.-M., Tang Z. Panax notoginseng saponins attenuate atherosclerosis in rats by regulating the blood lipid profile and an anti-inflammatory action. Clin. Exp. Pharmacol. Physiol. 2008;35:1238–1244. doi: 10.1111/j.1440-1681.2008.04997.x. [DOI] [PubMed] [Google Scholar]
- 144.Bello C.T., Turner L.W. Reserpine as an antihypertensive in the outpatient clinic: A double-blind clinical study. Am. J. Med. Sci. 1956;232:194–197. doi: 10.1097/00000441-195608000-00010. [DOI] [PubMed] [Google Scholar]
- 145.Shamon S.D., Perez M.I. Blood pressure lowering efficacy of reserpine for primary hypertension. Cochrane Database Syst. Rev. 2009;4:CD007655. doi: 10.1002/14651858.CD007655.pub2. [DOI] [PubMed] [Google Scholar]
- 146.Yu L., Qin Y., Wang Q., Zhang L., Liu Y., Wang T., Huang L., Wu L., Xiong H. The efficacy and safety of Chinese herbal medicine, Rhodiola formulation in treating ischemic heart disease: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2014;22:814–825. doi: 10.1016/j.ctim.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 147.Wu T., Zhou H., Jin Z., Bi S., Yang X., Yi D., Liu W. Cardioprotection of salidroside from ischemia/reperfusion injury by increasing N-acetylglucosamine linkage to cellular proteins. Eur. J. Pharmacol. 2009;613:93–99. doi: 10.1016/j.ejphar.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 148.Sinkovic A., Suran D., Lokar L., Fliser E., Skerget M., Novak Z., Knez Z. Rosemary extracts improve flow-mediated dilatation of the brachial artery and plasma PAI-1 activity in healthy young volunteers. Phytother. Res. 2011;25:402–407. doi: 10.1002/ptr.3276. [DOI] [PubMed] [Google Scholar]
- 149.Posadas S.J., Caz V., Largo C., de la Gándara B., Matallanas B., Reglero G., de Miguel E. Protective effect of supercritical fluid rosemary extract, Rosmarinus officinalis, on antioxidants of major organs of aged rats. Exp. Gerontol. 2009;44:383–389. doi: 10.1016/j.exger.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 150.Vanscheidt W., Jost V., Wolna P., Lücker P.W., Müller A., Theurer C., Patz B., Grützner K.I. Efficacy and safety of a butcher’s broom preparation (Ruscus aculeatus L. extract) compared to placebo in patients suffering from chronic venous insufficiency. Arzneimittelforschung. 2002;52:243–250. doi: 10.1055/s-0031-1299887. [DOI] [PubMed] [Google Scholar]
- 151.Ciocoiu M., Mirón A., Mares L., Tutunaru D., Pohaci C., Groza M., Badescu M. The effects of Sambucus nigra polyphenols on oxidative stress and metabolic disorders in experimental diabetes mellitus. J. Physiol. Biochem. 2009;65:297–304. doi: 10.1007/BF03180582. [DOI] [PubMed] [Google Scholar]
- 152.Bhattacharya S., Christensen K.B., Olsen L.C.B., Christensen L.P., Grevsen K., Færgeman N.J., Kristiansen K., Young J.F., Oksbjerg N. Bioactive components from flowers of Sambucus nigra L. increase glucose uptake in primary porcine myotube cultures and reduce fat accumulation in Caenorhabditis elegans. J. Agric. Food Chem. 2013;61:11033–11040. doi: 10.1021/jf402838a. [DOI] [PubMed] [Google Scholar]
- 153.Christensen K.B., Petersen R.K., Kristiansen K., Christensen L.P. Identification of bioactive compounds from flowers of black elder (Sambucus nigra L.) that activate the human peroxisome proliferator-activated receptor (PPAR) gamma. Phytother. Res. 2010;24:S129–S132. doi: 10.1002/ptr.3005. [DOI] [PubMed] [Google Scholar]
- 154.Li L., Zhou X., Li N., Sun M., Lv J., Xu Z. Herbal drugs against cardiovascular disease: Traditional medicine and modern development. Drug Discov. Today. 2015;20:1074–1086. doi: 10.1016/j.drudis.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 155.Chun J.N., Cho M., So I., Jeon J.-H. The protective effects of Schisandra chinensis fruit extract and its lignans against cardiovascular disease: A review of the molecular mechanisms. Fitoterapia. 2014;97:224–233. doi: 10.1016/j.fitote.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 156.Liu H., Wu C., Wang S., Gao S., Liu J., Dong Z., Zhang B., Liu M., Sun X., Guo P. Extracts and lignans of Schisandra chinensis fruit alter lipid and glucose metabolism in vivo and in vitro. J. Funct. Foods. 2015;19:296–307. doi: 10.1016/j.jff.2015.09.049. [DOI] [Google Scholar]
- 157.Zhang M., Liu M., Xiong M., Gong J., Tan X. Schisandra chinensis fruit extract attenuates albuminuria and protects podocyte integrity in a mouse model of streptozotocin-induced diabetic nephropathy. J. Ethnopharmacol. 2012;141:111–118. doi: 10.1016/j.jep.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 158.Li J., Wang J., Shao J.-Q., Du H., Wang Y.-T., Peng L. Effect of Schisandra chinensis on interleukins, glucose metabolism, and pituitary-adrenal and gonadal axis in rats under strenuous swimming exercise. Chin. J. Integr. Med. 2015;21:43–48. doi: 10.1007/s11655-014-1765-y. [DOI] [PubMed] [Google Scholar]
- 159.Huseini H.F., Larijani B., Heshmat R., Fakhrzadeh H., Radjabipour B., Toliat T., Raza M. The efficacy of Silybum marianum (L.) Gaertn. (silymarin) in the treatment of type II diabetes: A randomized, double-blind, placebo-controlled, clinical trial. Phytother. Res. 2006;20:1036–1039. doi: 10.1002/ptr.1988. [DOI] [PubMed] [Google Scholar]
- 160.Tamayo C., Diamond S. Review of clinical trials evaluating safety and efficacy of milk thistle (Silybum marianum [L.] Gaertn.) Integr. Cancer Ther. 2007;6:146–157. doi: 10.1177/1534735407301942. [DOI] [PubMed] [Google Scholar]
- 161.Derosa G., D’Angelo A., Maffioli P. The role of a fixed Berberis aristata/Silybum marianum combination in the treatment of type 1 diabetes mellitus. Clin. Nutr. 2015 doi: 10.1016/j.clnu.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 162.Ebrahimpour Koujan S., Gargari B.P., Mobasseri M., Valizadeh H., Asghari-Jafarabadi M. Effects of Silybum marianum (L.) Gaertn. (silymarin) extract supplementation on antioxidant status and hs-CRP in patients with type 2 diabetes mellitus: A randomized, triple-blind, placebo-controlled clinical trial. Phytomedicine. 2015;22:290–296. doi: 10.1016/j.phymed.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 163.Di Pierro F., Bellone I., Rapacioli G., Putignano P. Clinical role of a fixed combination of standardized Berberis aristata and Silybum marianum extracts in diabetic and hypercholesterolemic patients intolerant to statins. Diabetes Metab. Syndr. Obes. 2015;8:89–96. doi: 10.2147/DMSO.S78877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bhasker S., Madhav H., Chinnamma M. Molecular evidence of insulinomimetic property exhibited by steviol and stevioside in diabetes induced L6 and 3T3L1 cells. Phytomedicine. 2015;22:1037–1044. doi: 10.1016/j.phymed.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 165.Ritu M., Nandini J. Nutritional composition of Stevia rebaudiana—A sweet herb and its hypoglycaemic and hypolipidaemic effect on patients with non insulin dependent diabetes mellitus. J. Sci. Food Agric. 2016 doi: 10.1002/jsfa.7627. [DOI] [PubMed] [Google Scholar]
- 166.Asemi Z., Khorrami-Rad A., Alizadeh S.A., Shakeri H., Esmaillzadeh A. Effects of synbiotic food consumption on metabolic status of diabetic patients: A double-blind randomized cross-over controlled clinical trial. Clin. Nutr. 2014;33:198–203. doi: 10.1016/j.clnu.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 167.Saravanan R., Vengatash babu K., Ramachandran V. Effect of rebaudioside A, a diterpenoid on glucose homeostasis in STZ-induced diabetic rats. J. Physiol. Biochem. 2012;68:421–431. doi: 10.1007/s13105-012-0156-0. [DOI] [PubMed] [Google Scholar]
- 168.Fuller S., Stephens J.M. Diosgenin, 4-hydroxyisoleucine, and fiber from fenugreek: Mechanisms of actions and potential effects on metabolic syndrome. Adv. Nutr. 2015;6:189–197. doi: 10.3945/an.114.007807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gaddam A., Galla C., Thummisetti S., Marikanty R.K., Palanisamy U.D., Rao P.V. Role of fenugreek in the prevention of type 2 diabetes mellitus in prediabetes. J. Diabetes Metab. Disord. 2015;14 doi: 10.1186/s40200-015-0208-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Basu A., Du M., Leyva M.J., Sanchez K., Betts N.M., Wu M., Aston C.E., Lyons T.J. Blueberries decrease cardiovascular risk factors in obese men and women with metabolic syndrome. J. Nutr. 2010;140:1582–1587. doi: 10.3945/jn.110.124701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Johnson M.H., de Mejia E.G. Phenolic compounds from fermented berry beverages modulated gene and protein expression to increase insulin secretion from pancreatic beta-cells in vitro. J. Agric. Food Chem. 2016;64:2569–2581. doi: 10.1021/acs.jafc.6b00239. [DOI] [PubMed] [Google Scholar]
- 172.Johnson M.H., de Mejia E.G., Fan J., Lila M.A., Yousef G.G. Anthocyanins and proanthocyanidins from blueberry-blackberry fermented beverages inhibit markers of inflammation in macrophages and carbohydrate-utilizing enzymes in vitro. Mol. Nutr. Food Res. 2013;57:1182–1197. doi: 10.1002/mnfr.201200678. [DOI] [PubMed] [Google Scholar]
- 173.Vendrame S., Daugherty A., Kristo A.S., Riso P., Klimis-Zacas D. Wild blueberry (Vaccinium angustifolium) consumption improves inflammatory status in the obese Zucker rat model of the metabolic syndrome. J. Nutr. Biochem. 2013;24:1508–1512. doi: 10.1016/j.jnutbio.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 174.Elek S.R., McNair J.D., Griffith G.C. Veratrum viride: Hypotensive and cardiac effects of intravenous use. Calif. Med. 1953;79:300–305. [PMC free article] [PubMed] [Google Scholar]
- 175.Nand V., Doggrell S.A., Barnett C.W. Effects of veratridine on the action potentials and contractility of right and left ventricles from normo- and hypertensive rats. Clin. Exp. Pharmacol. Physiol. 1997;24:570–576. doi: 10.1111/j.1440-1681.1997.tb02092.x. [DOI] [PubMed] [Google Scholar]
- 176.Singh B.N., Saha C., Galun D., Upreti D.K., Bayry J., Kaveri S.V. European Viscum album: A potent phytotherapeutic agent with multifarious phytochemicals, pharmacological properties and clinical evidence. RSC Adv. 2016;6:23837–23857. doi: 10.1039/C5RA27381A. [DOI] [Google Scholar]
- 177.Tang T.Y., Li F.-Z., Afseth J. Review of the regulations for clinical research in herbal medicines in USA. Chin. J. Integr. Med. 2014;20:883–893. doi: 10.1007/s11655-014-2024-y. [DOI] [PubMed] [Google Scholar]
- 178.Nelson H.S. Oral/sublingual Phleum pretense grass tablet (Grazax/Grastek) to treat allergic rhinitis in the USA. Expert Rev. Clin. Immunol. 2014;10:1437–1451. doi: 10.1586/1744666X.2014.963556. [DOI] [PubMed] [Google Scholar]
- 179.Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: A comprehensive review. Circulation. 2016;133:187–225. doi: 10.1161/CIRCULATIONAHA.115.018585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Xiao J.B., Högger P. Dietary polyphenols and type 2 diabetes: Current insights and future perspectives. Curr. Med. Chem. 2015;22:23–38. doi: 10.2174/0929867321666140706130807. [DOI] [PubMed] [Google Scholar]
- 181.Rohn S., van Griensven L. Grain legumes and further gluten free legumes—Science, technology and impacts on human health. Food Res. Int. 2015;76:1–2. doi: 10.1016/j.foodres.2015.03.010. [DOI] [Google Scholar]
- 182.Barringer T.A. Mediterranean diets and cardiovascular disease. Curr. Atheroscler. Rep. 2001;3:437–445. doi: 10.1007/s11883-001-0033-8. [DOI] [PubMed] [Google Scholar]
- 183.Estruch R., Ros E., Salas-Salvadó J., Covas M.-I., Corella D., Arós F., Gómez-Gracia E., Ruiz-Gutiérrez V., Fiol M., Lapetra J., et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013;368:1279–1290. doi: 10.1056/NEJMoa1200303. [DOI] [PubMed] [Google Scholar]
- 184.Naismith D.J., Akinyanju P.A., Szanto S., Yudkin J. The effect, in volunteers, of coffee and decaffeinated coffee on blood glucose, insulin, plasma lipids and some factors involved in blood clotting. Nutr. Metabol. 1970;12:144–151. doi: 10.1159/000175287. [DOI] [PubMed] [Google Scholar]
- 185.Barone J.J., Roberts H.R. Caffeine consumption. Food Chem. Toxicol. 1996;34:119–129. doi: 10.1016/0278-6915(95)00093-3. [DOI] [PubMed] [Google Scholar]
- 186.Santos R.M., Lima D.R. Coffee consumption, obesity and type 2 diabetes: A mini-review. Eur. J. Nutr. 2016;55:1345–1358. doi: 10.1007/s00394-016-1206-0. [DOI] [PubMed] [Google Scholar]
- 187.Morisco F., Lembo V., Mazzone G., Camera S., Caporaso N. Coffee and liver health. J. Clin. Gastroenterol. 2014;48(Suppl. 1):S87–S90. doi: 10.1097/MCG.0000000000000240. [DOI] [PubMed] [Google Scholar]
- 188.Akash M.S., Rehman K., Chen S. Effects of coffee on type 2 diabetes mellitus. Nutrition. 2014;30:755–763. doi: 10.1016/j.nut.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 189.Zulli A., Smith R.M., Kubatka P., Novak J., Uehara Y., Loftus H., Qaradakhi T., Pohanka M., Kobyliak N., Zagatina A., et al. Caffeine and cardiovascular diseases: Critical review of current research. Eur. J. Nutr. 2016;55:1331–1343. doi: 10.1007/s00394-016-1179-z. [DOI] [PubMed] [Google Scholar]
- 190.Ding M., Bhupathiraju S.N., Satija A., van Dam R.M., Hu F.B. Long-term coffee consumption and risk of cardiovascular disease: A systematic review and a dose-response meta-analysis of prospective cohort studies. Circulation. 2014;129:643–659. doi: 10.1161/CIRCULATIONAHA.113.005925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ding M., Satija A., Bhupathiraju S.N., Hu Y., Sun Q., Han J., Lopez-Garcia E., Willett W., van Dam R.M., Hu F.B. Association of coffee consumption with total and cause-specific mortality in three large prospective cohorts. Circulation. 2015;132:2305–2315. doi: 10.1161/CIRCULATIONAHA.115.017341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Natella F., Scaccini C. Role of coffee in modulation of diabetes risk. Nutr. Rev. 2012;70:207–217. doi: 10.1111/j.1753-4887.2012.00470.x. [DOI] [PubMed] [Google Scholar]
- 193.Vinson J.A., Proch J., Bose P., Muchler S., Taffera P., Shuta D., Samman N., Agbor G.A. Chocolate is a powerful ex vivo and in vivo antioxidant, an antiatherosclerotic agent in an animal model, and a significant contributor to antioxidants in the European and American diets. J. Agric. Food Chem. 2006;54:8071–8076. doi: 10.1021/jf062175j. [DOI] [PubMed] [Google Scholar]
- 194.Bonita J.S., Mandarano M., Shuta D., Vinson J. Coffee and cardiovascular disease: In vitro, cellular, animal, and human studies. Pharmacol. Res. 2007;55:187–198. doi: 10.1016/j.phrs.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 195.Van Dijk A.E., Olthof M.R., Meeuse J.C., Seebus E., Heine R.J., van Dam R.M. Acute effects of decaffeinated coffee and the major coffee components chlorogenic acid and trigonelline on glucose tolerance. Diabetes Care. 2009;32:1023–1025. doi: 10.2337/dc09-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Meng S., Cao J., Feng Q., Peng J., Hu Y. Roles of chlorogenic acid on regulating glucose and lipids metabolism: A review. Evid. Based Complement. Altern. Med. 2013;2013 doi: 10.1155/2013/801457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Murase T., Misawa K., Minegishi Y., Aoki M., Ominami H., Suzuki Y., Shibuya Y., Hase T. Coffee polyphenols suppress diet-induced body fat accumulation by downregulating SREBP-1c and related molecules in C57BL/6J mice. Am. J. Physiol. Endocrinol. Metab. 2011;300:E122–E133. doi: 10.1152/ajpendo.00441.2010. [DOI] [PubMed] [Google Scholar]
- 198.Murase T., Yokoi Y., Misawa K., Ominami H., Suzuki Y., Shibuya Y., Hase T. Coffee polyphenols modulate whole-body substrate oxidation and suppress postprandial hyperglycaemia, hyperinsulinaemia and hyperlipidaemia. Br. J. Nutr. 2012;107:1757–1765. doi: 10.1017/S0007114511005083. [DOI] [PubMed] [Google Scholar]
- 199.Gramza-Michalowska A. Caffeine in tea Camellia sinensis—Content, absorption, benefits and risks of consumption. J. Nutr. Health Aging. 2014;18:143–149. doi: 10.1007/s12603-013-0404-1. [DOI] [PubMed] [Google Scholar]
- 200.Beecher G.R., Warden B.A., Merken H. Analysis of tea polyphenols. Proc. Soc. Exp. Biol. Med. 1999;220:267–270. doi: 10.3181/00379727-220-44377A. [DOI] [PubMed] [Google Scholar]
- 201.Li S., Lo C.Y., Pan M.H., Lai C.S., Ho C.T. Black tea: Chemical analysis and stability. Food Funct. 2013;4:10–18. doi: 10.1039/C2FO30093A. [DOI] [PubMed] [Google Scholar]
- 202.Gormaz J.G., Valls N., Sotomayor C., Turner T., Rodrigo R. Potential role of polyphenols in the prevention of cardiovascular diseases: Molecular bases. Curr. Med. Chem. 2016;23:115–128. doi: 10.2174/0929867323666151127201732. [DOI] [PubMed] [Google Scholar]
- 203.Peters U., Poole C., Arab L. Does tea affect cardiovascular disease? A meta-analysis. Am. J. Epidemiol. 2001;154:495–503. doi: 10.1093/aje/154.6.495. [DOI] [PubMed] [Google Scholar]
- 204.Zhang C., Qin Y.Y., Wei X., Yu F.F., Zhou Y.H., He J. Tea consumption and risk of cardiovascular outcomes and total mortality: A systematic review and meta-analysis of prospective observational studies. Eur. J. Epidemiol. 2015;30:103–113. doi: 10.1007/s10654-014-9960-x. [DOI] [PubMed] [Google Scholar]
- 205.Basu A., Lucas E.A. Mechanisms and effects of green tea on cardiovascular health. Nutr. Rev. 2007;65:361–375. doi: 10.1111/j.1753-4887.2007.tb00314.x. [DOI] [PubMed] [Google Scholar]
- 206.Sano M., Tabata M., Suzuki M., Degawa M., Miyase T., Maeda-Yamamoto M. Simultaneous determination of twelve tea catechins by high-performance liquid chromatography with electrochemical detection. Analyst. 2001;126:816–820. doi: 10.1039/b102541b. [DOI] [PubMed] [Google Scholar]
- 207.Chowdhury A., Sarkar J., Chakraborti T., Pramanik P.K., Chakraborti S. Protective role of epigallocatechin-3-gallate in health and disease: A perspective. Biomed. Pharmacother. 2016;78:50–59. doi: 10.1016/j.biopha.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 208.Chen G., Wang H., Zhang X., Yang S.-T. Nutraceuticals and functional foods in the management of hyperlipidemia. Crit. Rev. Food Sci. Nutr. 2014;54:1180–1201. doi: 10.1080/10408398.2011.629354. [DOI] [PubMed] [Google Scholar]
- 209.Magrone T., Perez De Heredia F., Jirillo E., Morabito G., Marcos A., Serafini M. Functional foods and nutraceuticals as therapeutic tools for the treatment of diet-related diseases. Can. J. Physiol. Pharmacol. 2013;91:387–396. doi: 10.1139/cjpp-2012-0307. [DOI] [PubMed] [Google Scholar]
- 210.Liu R.H. Dietary bioactive compounds and their health implications. J. Food Sci. 2013;78:A18–A25. doi: 10.1111/1750-3841.12101. [DOI] [PubMed] [Google Scholar]
- 211.Badimon L., Vilahur G., Padro T. Nutraceuticals and atherosclerosis: Human trials. Cardiovasc. Ther. 2010;28:202–215. doi: 10.1111/j.1755-5922.2010.00189.x. [DOI] [PubMed] [Google Scholar]
- 212.Zuchi C., Ambrosio G., Lüscher T.F., Landmesser U. Nutraceuticals in cardiovascular prevention: Lessons from studies on endothelial function. Cardiovasc. Ther. 2010;28:187–201. doi: 10.1111/j.1755-5922.2010.00165.x. [DOI] [PubMed] [Google Scholar]
- 213.Lacroix I.M., Li-Chan E.C. Overview of food products and dietary constituents with antidiabetic properties and their putative mechanisms of action: A natural approach to complement pharmacotherapy in the management of diabetes. Mol. Nutr. Food Res. 2014;58:61–78. doi: 10.1002/mnfr.201300223. [DOI] [PubMed] [Google Scholar]
- 214.Hung H.Y., Qian K., Morris-Natschke S.L., Hsu C.S., Lee K.H. Recent discovery of plant-derived anti-diabetic natural products. Nat. Prod. Rep. 2012;29:580–606. doi: 10.1039/c2np00074a. [DOI] [PubMed] [Google Scholar]
- 215.Gautam R., Jachak S.M. Recent developments in anti-inflammatory natural products. Med. Res. Rev. 2009;29:767–820. doi: 10.1002/med.20156. [DOI] [PubMed] [Google Scholar]
- 216.Hermansen K., Dinesen B., Hoie L.H., Morgenstern E., Gruenwald J. Effects of soy and other natural products on LDL:HDL ratio and other lipid parameters: A literature review. Adv. Ther. 2003;20:50–78. doi: 10.1007/BF02850119. [DOI] [PubMed] [Google Scholar]
- 217.Vasanthi H.R., ShriShriMal N., Das D.K. Phytochemicals from plants to combat cardiovascular disease. Curr. Med. Chem. 2012;19:2242–2251. doi: 10.2174/092986712800229078. [DOI] [PubMed] [Google Scholar]
- 218.Hardie D.G. AMPK: A target for drugs and natural products with effects on both diabetes and cancer. Diabetes. 2013;62:2164–2172. doi: 10.2337/db13-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Yuan T., Nahar P., Sharma M., Liu K., Slitt A., Aisa H.A., Seeram N.P. Indazole-type alkaloids from Nigella sativa seeds exhibit antihyperglycemic effects via AMPK activation in vitro. J. Nat. Prod. 2014;77:2316–2320. doi: 10.1021/np500398m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Nguyen P.H., Nguyen T.N., Dao T.T., Kang H.W., Ndinteh D.T., Mbafor J.T., Oh W.K. AMP-activated protein kinase (AMPK) activation by benzofurans and coumestans isolated from Erythrina abyssinica. J. Nat. Prod. 2010;73:598–602. doi: 10.1021/np900745g. [DOI] [PubMed] [Google Scholar]
- 221.Zimmermann K., Baldinger J., Mayerhofer B., Atanasov A.G., Dirsch V.M., Heiss E.H. Activated AMPK boosts the Nrf2/HO-1 signaling axis—A role for the unfolded protein response. Free Radic. Biol. Med. 2015;88:417–426. doi: 10.1016/j.freeradbiomed.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Chen S. Natural products triggering biological targets—A review of the anti-inflammatory phytochemicals targeting the arachidonic acid pathway in allergy asthma and rheumatoid arthritis. Curr. Drug Targets. 2011;12:288–301. doi: 10.2174/138945011794815347. [DOI] [PubMed] [Google Scholar]
- 223.Jachak S.M. Cyclooxygenase inhibitory natural products: Current status. Curr. Med. Chem. 2006;13:659–678. doi: 10.2174/092986706776055698. [DOI] [PubMed] [Google Scholar]
- 224.Chi Y.S., Jong H.G., Son K.H., Chang H.W., Kang S.S., Kim H.P. Effects of naturally occurring prenylated flavonoids on enzymes metabolizing arachidonic acid: Cyclooxygenases and lipoxygenases. Biochem. Pharmacol. 2001;62:1185–1191. doi: 10.1016/S0006-2952(01)00773-0. [DOI] [PubMed] [Google Scholar]
- 225.Gao Y., Zhang Y., Zhu J., Li B., Li Z., Zhu W., Shi J., Jia Q., Li Y. Recent progress in natural products as DPP-4 inhibitors. Future Med. Chem. 2015;7:1079–1089. doi: 10.4155/fmc.15.49. [DOI] [PubMed] [Google Scholar]
- 226.Abe M., Akiyama T., Nakamura H., Kojima F., Harada S., Muraoka Y. First synthesis and determination of the absolute configuration of sulphostin, a novel inhibitor of dipeptidyl peptidase IV. J. Nat. Prod. 2004;67:999–1004. doi: 10.1021/np030491b. [DOI] [PubMed] [Google Scholar]
- 227.Saleem S., Jafri L., ul Haq I., Chang L.C., Calderwood D., Green B.D., Mirza B. Plants Fagonia cretica L. and Hedera nepalensis K. Koch contain natural compounds with potent dipeptidyl peptidase-4 (DPP-4) inhibitory activity. J. Ethnopharmacol. 2014;156:26–32. doi: 10.1016/j.jep.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 228.Schmitt C.A., Dirsch V.M. Modulation of endothelial nitric oxide by plant-derived products. Nitric Oxide. 2009;21:77–91. doi: 10.1016/j.niox.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 229.Waldbauer K., Seiringer G., Nguyen D.L., Winkler J., Blaschke M., McKinnon R., Urban E., Ladurner A., Dirsch V.M., Zehl M., et al. Triterpenoic acids from apple pomace enhance the activity of the endothelial nitric oxide synthase (eNOS) J. Agric. Food Chem. 2016;64:185–194. doi: 10.1021/acs.jafc.5b05061. [DOI] [PubMed] [Google Scholar]
- 230.Xia N., Pautz A., Wollscheid U., Reifenberg G., Förstermann U., Li H. Artichoke, cynarin and cyanidin downregulate the expression of inducible nitric oxide synthase in human coronary smooth muscle cells. Molecules. 2014;19:3654–3668. doi: 10.3390/molecules19033654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Shen K., Leung S.W., Ji L., Huang Y., Hou M., Xu A., Wang Z., Vanhoutte P.M. Notoginsenoside Ft1 activates both glucocorticoid and estrogen receptors to induce endothelium-dependent, nitric oxide-mediated relaxations in rat mesenteric arteries. Biochem. Pharmacol. 2014;88:66–74. doi: 10.1016/j.bcp.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 232.Auger C., Chaabi M., Anselm E., Lobstein A., Schini-Kerth V.B. The red wine extract-induced activation of endothelial nitric oxide synthase is mediated by a great variety of polyphenolic compounds. Mol. Nutr. Food Res. 2010;54(Suppl. 2):S171–S183. doi: 10.1002/mnfr.200900602. [DOI] [PubMed] [Google Scholar]
- 233.Leung K.W., Cheng Y.K., Mak N.K., Chan K.K., Fan T.P., Wong R.N. Signaling pathway of ginsenoside-Rg1 leading to nitric oxide production in endothelial cells. FEBS Lett. 2006;580:3211–3216. doi: 10.1016/j.febslet.2006.04.080. [DOI] [PubMed] [Google Scholar]
- 234.Ndiaye M., Chataigneau M., Lobysheva I., Chataigneau T., Schini-Kerth V.B. Red wine polyphenol-induced, endothelium-dependent NO-mediated relaxation is due to the redox-sensitive PI3-kinase/Akt-dependent phosphorylation of endothelial NO-synthase in the isolated porcine coronary artery. FASEB J. 2005;19:455–457. doi: 10.1096/fj.04-2146fje. [DOI] [PubMed] [Google Scholar]
- 235.Golan-Goldhirsh A., Gopas J. Plant derived inhibitors of NF-κB. Phytochem. Rev. 2014;13:107–121. doi: 10.1007/s11101-013-9293-5. [DOI] [Google Scholar]
- 236.Chan M.M. Inhibition of tumor necrosis factor by curcumin, a phytochemical. Biochem. Pharmacol. 1995;49:1551–1556. doi: 10.1016/0006-2952(95)00171-U. [DOI] [PubMed] [Google Scholar]
- 237.Siedle B., Garcia-Pineres A.J., Murillo R., Schulte-Mönting J., Castro V., Rüngeler P., Klaas C.A., da Costa F.B., Kisiel W., Merfort I. Quantitative structure-activity relationship of sesquiterpene lactones as inhibitors of the transcription factor NF-κB. J. Med. Chem. 2004;47:6042–6054. doi: 10.1021/jm049937r. [DOI] [PubMed] [Google Scholar]
- 238.Fakhrudin N., Waltenberger B., Cabaravdic M., Atanasov A.G., Malainer C., Schachner D., Heiss E.H., Liu R., Noha S.M., Grzywacz A.M., et al. Identification of plumericin as a potent new inhibitor of the NF-κB pathway with anti-inflammatory activity in vitro and in vivo. Br. J. Pharmacol. 2014;171:1676–1686. doi: 10.1111/bph.12558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Kopp E., Ghosh S. Inhibition of NF-κB by sodium salicylate and aspirin. Science. 1994;265:956–959. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- 240.Kumar H., Kim I.S., More S.V., Kim B.W., Choi D.K. Natural product-derived pharmacological modulators of Nrf2/ARE pathway for chronic diseases. Nat. Prod. Rep. 2014;31:109–139. doi: 10.1039/C3NP70065H. [DOI] [PubMed] [Google Scholar]
- 241.Balogun E., Hoque M., Gong P., Killeen E., Green C.J., Foresti R., Alam J., Motterlini R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem. J. 2003;371:887–895. doi: 10.1042/bj20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Heiss E.H., Tran T.V., Zimmermann K., Schwaiger S., Vouk C., Mayerhofer B., Malainer C., Atanasov A.G., Stuppner H., Dirsch V.M. Identification of chromomoric acid C-I as an Nrf2 activator in Chromolaena odorata. J. Nat. Prod. 2014;77:503–508. doi: 10.1021/np400778m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Ahn Y.H., Hwang Y., Liu H., Wang X.J., Zhang Y., Stephenson K.K., Boronina T.N., Cole R.N., Dinkova-Kostova A.T., Talalay P., et al. Electrophilic tuning of the chemoprotective natural product sulforaphane. Proc. Natl. Acad. Sci. USA. 2010;107:9590–9595. doi: 10.1073/pnas.1004104107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Wang L., Waltenberger B., Pferschy-Wenzig E.M., Blunder M., Liu X., Malainer C., Blazevic T., Schwaiger S., Rollinger J.M., Heiss E.H., et al. Natural product agonists of peroxisome proliferator-activated receptor gamma (PPARgamma): A review. Biochem. Pharmacol. 2014;92:73–89. doi: 10.1016/j.bcp.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Atanasov A.G., Blunder M., Fakhrudin N., Liu X., Noha S.M., Malainer C., Kramer M.P., Cocic A., Kunert O., Schinkovitz A., et al. Polyacetylenes from Notopterygium incisum—New selective partial agonists of peroxisome proliferator-activated receptor-gamma. PLoS ONE. 2013;8:e61755. doi: 10.1371/journal.pone.0061755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Atanasov A.G., Wang J.N., Gu S.P., Bu J., Kramer M.P., Baumgartner L., Fakhrudin N., Ladurner A., Malainer C., Vuorinen A., et al. Honokiol: A non-adipogenic PPARgamma agonist from nature. Biochim. Biophys. Acta. 2013;1830:4813–4819. doi: 10.1016/j.bbagen.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Fakhrudin N., Ladurner A., Atanasov A.G., Heiss E.H., Baumgartner L., Markt P., Schuster D., Ellmerer E.P., Wolber G., Rollinger J.M., et al. Computer-aided discovery, validation, and mechanistic characterization of novel neolignan activators of peroxisome proliferator-activated receptor gamma. Mol. Pharmacol. 2010;77:559–566. doi: 10.1124/mol.109.062141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Puhl A.C., Bernardes A., Silveira R.L., Yuan J., Campos J.L., Saidemberg D.M., Palma M.S., Cvoro A., Ayers S.D., Webb P., et al. Mode of peroxisome proliferator-activated receptor gamma activation by luteolin. Mol. Pharmacol. 2012;81:788–799. doi: 10.1124/mol.111.076216. [DOI] [PubMed] [Google Scholar]
- 249.Weidner C., de Groot J.C., Prasad A., Freiwald A., Quedenau C., Kliem M., Witzke A., Kodelja V., Han C.T., Giegold S., et al. Amorfrutins are potent antidiabetic dietary natural products. Proc. Natl. Acad. Sci. USA. 2012;109:7257–7262. doi: 10.1073/pnas.1116971109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Wang L.-J., Jiang B., Wu N., Wang S.-Y., Shi D.-Y. Natural and semisynthetic protein tyrosine phosphatase 1B (PTP1B) inhibitors as anti-diabetic agents. RSC Adv. 2015;5:48822–48834. doi: 10.1039/C5RA01754H. [DOI] [Google Scholar]
- 251.Jiang C.S., Liang L.F., Guo Y.W. Natural products possessing protein tyrosine phosphatase 1B (PTP1B) inhibitory activity found in the last decades. Acta Pharmacol. Sin. 2012;33:1217–1245. doi: 10.1038/aps.2012.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Heiss E.H., Baumgartner L., Schwaiger S., Heredia R.J., Atanasov A.G., Rollinger J.M., Stuppner H., Dirsch V.M. Ratanhiaphenol III from Ratanhiae radix is a PTP1B inhibitor. Planta Med. 2012;78:678–681. doi: 10.1055/s-0031-1298242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Feng Y., Carroll A.R., Addepalli R., Fechner G.A., Avery V.M., Quinn R.J. Vanillic acid derivatives from the green algae Cladophora socialis as potent protein tyrosine phosphatase 1B inhibitors. J. Nat. Prod. 2007;70:1790–1792. doi: 10.1021/np070225o. [DOI] [PubMed] [Google Scholar]
- 254.Yoon G., Lee W., Kim S.N., Cheon S.H. Inhibitory effect of chalcones and their derivatives from Glycyrrhiza inflata on protein tyrosine phosphatase 1B. Bioorg. Med. Chem. Lett. 2009;19:5155–5157. doi: 10.1016/j.bmcl.2009.07.054. [DOI] [PubMed] [Google Scholar]
- 255.Werz O. Inhibition of 5-lipoxygenase product synthesis by natural compounds of plant origin. Planta Med. 2007;73:1331–1357. doi: 10.1055/s-2007-990242. [DOI] [PubMed] [Google Scholar]
- 256.Oettl S.K., Gerstmeier J., Khan S.Y., Wiechmann K., Bauer J., Atanasov A.G., Malainer C., Awad E.M., Uhrin P., Heiss E.H., et al. Imbricaric acid and perlatolic acid: Multi-targeting anti-inflammatory depsides from Cetrelia monachorum. PLoS ONE. 2013;8:e76929. doi: 10.1371/journal.pone.0076929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.De la Puerta R., Ruiz Gutierrez V., Hoult J.R. Inhibition of leukocyte 5-lipoxygenase by phenolics from virgin olive oil. Biochem. Pharmacol. 1999;57:445–449. doi: 10.1016/S0006-2952(98)00320-7. [DOI] [PubMed] [Google Scholar]
- 258.Winekenstädde D., Angelis A., Waltenberger B., Schwaiger S., Tchoumtchoua J., König S., Werz O., Aligiannis N., Skaltsounis A.-L., Stuppner H. Phytochemical profile of the aerial parts of Sedum sediforme and anti-inflammatory activity of myricitrin. Nat. Prod. Commun. 2015;10:83–88. [PubMed] [Google Scholar]
- 259.Endo A. A historical perspective on the discovery of statins. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2010;86:484–493. doi: 10.2183/pjab.86.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Tanzawa K., Kuroda M., Endo A. Time-dependent, irreversible inhibition of 3-hydroxy-3-methylglutaryl-coenzyme A reductase by the antibiotic citrinin. Biochim. Biophys. Acta. 1977;488:97–101. [PubMed] [Google Scholar]
- 261.Endo A., Kuroda M. Citrinin, an inhibitor of cholesterol synthesis. J. Antibiot. Tokyo. 1976;29:841–843. doi: 10.7164/antibiotics.29.841. [DOI] [PubMed] [Google Scholar]
- 262.Endo A., Kuroda M., Tsujita Y. ML-236A, ML-236B, and ML-236C, new inhibitors of cholesterogenesis produced by Penicillium citrinium. J. Antibiot. Tokyo. 1976;29:1346–1348. doi: 10.7164/antibiotics.29.1346. [DOI] [PubMed] [Google Scholar]
- 263.Endo A., Kuroda M., Tanzawa K. Competitive inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase by ML-236A and ML-236B fungal metabolites, having hypocholesterolemic activity. FEBS Lett. 1976;72:323–326. doi: 10.1016/0014-5793(76)80996-9. [DOI] [PubMed] [Google Scholar]
- 264.Endo A., Tsujita Y., Kuroda M., Tanzawa K. Inhibition of cholesterol synthesis in vitro and in vivo by ML-236A and ML-236B, competitive inhibitors of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. Eur. J. Biochem. 1977;77:31–36. doi: 10.1111/j.1432-1033.1977.tb11637.x. [DOI] [PubMed] [Google Scholar]
- 265.Alberts A.W., Chen J., Kuron G., Hunt V., Huff J., Hoffman C., Rothrock J., Lopez M., Joshua H., Harris E., et al. Mevinolin: A highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and a cholesterol-lowering agent. Proc. Natl. Acad. Sci. USA. 1980;77:3957–3961. doi: 10.1073/pnas.77.7.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Endo A. Monacolin K, a new hypocholesterolemic agent produced by a Monascus species. J. Antibiot. Tokyo. 1979;32:852–854. doi: 10.7164/antibiotics.32.852. [DOI] [PubMed] [Google Scholar]
- 267.Endo A. A gift from nature: The birth of the statins. Nat. Med. 2008;14:1050–1052. doi: 10.1038/nm1008-1050. [DOI] [PubMed] [Google Scholar]
- 268.Grundy S.M. Dyslipidaemia in 2015: Advances in treatment of dyslipidaemia. Nat. Rev. Cardiol. 2016;13:74–75. doi: 10.1038/nrcardio.2015.208. [DOI] [PubMed] [Google Scholar]
- 269.Bailey C., Day C. Metformin: Its botanical background. Pract. Diab. Int. 2004;21:115–117. doi: 10.1002/pdi.606. [DOI] [Google Scholar]
- 270.Müller H., Reinwein H. Pharmacology of galegin. Arch. Exp. Pathol. Pharmakol. 1927;125:212–228. doi: 10.1007/BF01862957. [DOI] [Google Scholar]
- 271.Tanret G. An alkaloid extracted from Galega officinalis. Compt. Rend. 1914;158:1182–1184. [Google Scholar]
- 272.Barger G., White F.D. The constitution of galegine. Biochem. J. 1923;17:827–835. doi: 10.1042/bj0170827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Watanabe C.K. Studies in the metabolic changes induced by administration of guanidine bases. I. The influence of injected guanidine hydrochloride upon blood sugar content. J. Biol. Chem. 1918;33:253–265. [Google Scholar]
- 274.Bailey C., Campbell I., Chan J., Davidson J., Howlett H., Ritz P. Metformin: The Gold Standard. A Scientific Handbook. Wiley; Chichester, UK: 2007. [Google Scholar]
- 275.Cusi K., DeFronzo R.A. Metformin: A review of its metabolic effects. Diabetes Rev. 1998;6:89–131. [Google Scholar]