Abstract
Complex, multidirectional interactions between hormones, seizures, and the medications used to control them can present a challenge for clinicians treating patients with epilepsy. Many hormones act as neurosteroids, modulating brain excitability via direct binding sites. Thus, changes in endogenous or exogenous hormone levels can affect the occurrence of seizures directly as well as indirectly through pharmacokinetic effects that alter the concentrations of antiepileptic drugs. The underlying structural and physiological brain abnormalities of epilepsy and the metabolic activity of antiepileptic drugs can adversely affect hypothalamic and gonadal functioning. Knowledge of these complex interactions has increased and can now be incorporated in meaningful treatment approaches for men and women with epilepsy.
Introduction
Neurologists have long accepted that hormones are involved in various ways in the manifestations of epilepsy and in its treatment with antiepileptic drugs. For example, raised concentrations of prolactin in serum after seizure-like events have been used as diagnostic tools to differentiate epileptic seizures from non-epileptic events.1 Further, treatment of infantile spasms with adrenocorticotropic hormone is a standard approach.2 These relatively common scenarios are not routinely thought of as part of neuroendocrinology, but they clearly show the presence of a connection between neurosteroid hormones and epilepsy.
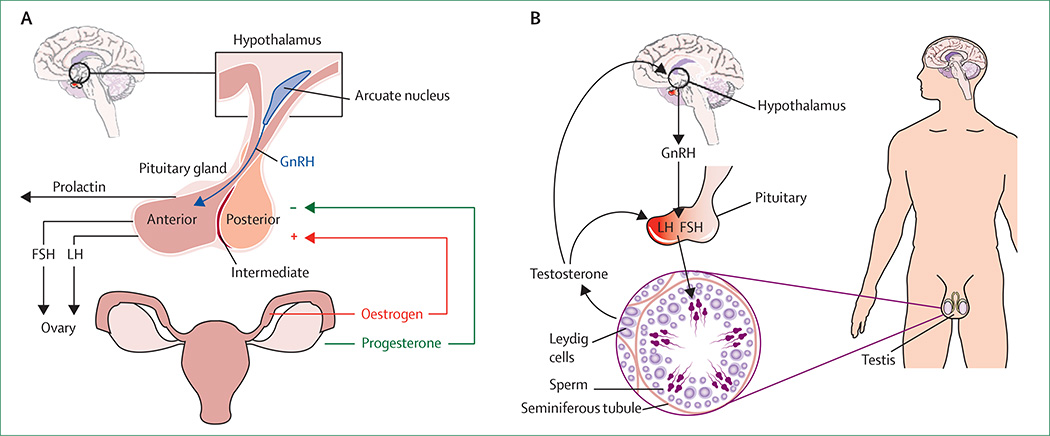
The dynamic role of hormones in epilepsy is multidirectional, involving many factors.3 In this Review we present key research on neurosteroids in the context of epilepsy, hypothalamic hormonal dysregulation in epilepsy, and the effects of antiepileptic drugs on reproductive hormones. Figure 1 shows the hypothalamicpituitary-gonadal axis for women and men, including the hormones relevant to the scope of this article. Relevant clinical scenarios, including catamenial epilepsy (seizures related to the menstrual cycle), polycystic ovary syndrome (PCOS), fertility, and perimenopausal and menopausal concerns are discussed. Management options are suggested in view of the findings presented in these sections.
Figure 1. Hypothalamic-pituitary-gonadal axis in women and men.
Hormones relevant to the scope of this Review are shown for women (A) and men (B). FSH=follicle-stimulating hormone. LH=luteinising hormone. GnRH=gonadotropin-releasing hormone.
Neurosteroids
Neurosteroids are steroid molecules that modulate brain excitability and, therefore, can affect the occurrence of seizures. Oestrogen and progesterone, the primary reproductive hormones for women, both affect neuronal excitability.4,5 The complex actions of oestrogen on neuroexcitability occur via two mechanisms. The first is a short-latency, non-genomic effect that is thought to be mediated by the neuronal membrane. For example, the potentiation of kainite-induced currents has been noted after exposure to 17β-oestradiol in whole-cell, hippocampal patch-clamp recordings. This action is rapid in onset, reversible, and dose-related, which is consistent with a membrane effect.6 The second mechanism, more thoroughly explored, is a long-latency (hours to days) genomic effect. As for other steroid hormones, oestrogen enters passively into the cell, where it binds to and activates the oestrogen receptor, a dimeric nuclear protein that binds to DNA and controls gene expression. After oestrogen binding, the receptor complex forms a transcription factor that binds to hormone-receptor elements on genes to modify cellular responses. An example of this effect is oestradiol-induced increased density of agonist binding sites on the NMDA-receptor complex in hippocampal cells, which can be detected 2 days after the start of oestradiol treatment.7 Oestrogen has long been suggested to have proconvulsant properties, but its effects might be more complex, dependent on dose, route of administration, acute versus chronic administration, natural hormonal environment, and oestrogenic species.8–10 Overall, at physiological concentrations, oestrogen does not seem to be strongly proconvulsant. Women with epilepsy, however, might be susceptible to the neurophysiological proconvulsant effects of this hormone during the rapid oestrogen spike associated with ovulation.
Progesterone, on the other hand, clearly promotes neuroinhibition, primarily through the action of its reduced metabolite, allopregnanolone, which acts as a positive allosteric modulator of GABA conductance.11,12 In experimental models, cyclical surges and decrements in the concentrations of progesterone and allopregnanolone alter susceptibility to seizures in predictable ways,13 although whether these cyclical hormonal effects fully account for catamenial seizure exacerbations is unclear.3
The effect of allopregnanolone on GABA conductance is subject to a feedback mechanism within the GABAA receptor. GABAA-receptor-subunit components undergo compensatory alterations in response to changes in endogenous neurosteroids and the use of pharmacological agents that modulate GABAA receptors, such as benzodiazepines.13,14 For example, sustained exposure to allopregnanolone in rats causes increased expression of the GABAA-receptor α4-subunit in the hippocampus, which leads to decreased sensitivity of GABAA-receptor currents to benzodiazepine.15
The other main reproductive hormone, testosterone, is also a neurosteroid. Testosterone has two major classes of biologically active metabolites, which have opposite neuroexcitatory effects: oestrogens formed through the action of a cytochrome P450 enzyme, aromatase, and the 5α-reduced androgens. Aromatase and 5α-reductase are expressed in several organ systems, including the brain.16 The 5α-reduced androgens have mainly anticonvulsant effects due at least partly to their ability to act as substrates for the biosynthesis of 5α-androstane-3α-diol (3α-DIOL). This molecule is structurally similar to allopregnanolone, is formed through similar reductive metabolic pathways, and modulates the activity of the GABAA receptor in the same way. 3α-DIOL is synthesised peripherally in prostate, liver, and skin, as well as de novo by glial cells in the brain.17 3α-DIOL has been shown to act in a dose-dependent manner as an anticonvulsant against seizures in mice induced by the GABAA-receptor antagonists pentylenetetrazol, picrotoxin, and β-carboline ester. In the pentylenetetrazol-induced seizure model, 3α-DIOL metabolite is strongly anticonvulsant.17 The potential importance of 3α-DIOL for the antiseizure effect of circulating androgens was shown in 5α-reductase-knockout mice, in which testosterone had no protective effect against seizures.18 Two other endogenous testosterone metabolites that are present in fairly high concentrations in men, androsterone and etiocholanolone, also have anti convulsant neurosteroid properties.19 Both metabolites protected male adult mice against seizures to varying degrees in different models, including exposure to 6 Hz electrical stimulation, pentylenetetrazol, pilocarpine, 4-aminopyridine, and maximum electroshock, and in hippocampal slice preparations.19
Causes of reproductive dysfunction in epilepsy
Increased rates of PCOS, decreased libido, infertility, and early menopause have been described in people with epilepsy. Epilepsy itself is implicated as an endocrine disruptor and leads to hypothalamic dysfunction that in turn generally has opposite effects on androgen concentrations in men and women—ie, androgenic activity is reduced in men but increased in women. The effect of epilepsy itself on central reproductive activity is confounded by the metabolic alterations induced by antiepileptic drugs on reproductive hormones, which are the same in men and women.
Hypothalamic dysfunction
Regions of the limbic cortex, particularly the amygdala, have extensive reciprocal connections with the hypothalamus20 and can modulate the hypothalamic-pituitarygonadal axis (figure 1). Disrupted release of pituitary hormones is seen in women and men with epilepsy.21–26 The abnormal release of follicle-stimulating hormone (FSH) and luteinising hormone (LH) follows dysfunction of gonadotropin-releasing hormone (GnRH) cells in the hypothalamus. GnRH, which is produced by a small number of cells located primarily in the preoptic area of the hypothalamus, controls gonadal activity via pulsatile secretion and stimulation of pituitary hormone release.
The GnRH-cell population is vulnerable to injury by seizures. In female rats with seizures induced by local injection of kainic acid into the amygdala, ipsilateral loss of GnRH-staining fibres in the hypothalamus was noted.27,28 Lateralisation of temporal lobe epilepsy seems to be associated with specific types of reproductive dysfunction. In an investigation of 30 women with complex partial seizures who had unilateral temporal lobe epileptiform discharges and evidence of reproductive endocrine disorders, left-sided discharges were associated with PCOS and right-sided discharges with hypo gonadotropic hypogonadism. By definition, the latter is a reproductive endocrinopathy of brain (hypothalamic or pituitary) origin.29
Effects of antiepileptic drugs on reproductive hormones
In general, antiepileptic drugs that induce hepatic metabolic enzymes are known as enzyme-inducing AEDs and directly alter concentrations of reproductive hormones (panel 1). They also induce production of sex-hormone-binding globulin, which reduces concentrations of biologically active (free) reproductive hormones in serum.30–32 Substantial increases in concentrations of sex-hormone-binding globulin have been reported in men with focal epilepsy being treated with carbamazepine and phenytoin compared with those taking lamotrigine or healthy controls.33,34 Decreased levels of free testosterone have been reported in men taking carbamazepine,35 phenytoin,33 oxcarbazepine,34 and valproate,34,36 and in those with untreated partial epilepsy.35
Panel 1. Reported interactions between antiepileptic drugs and endogenous and exogenous reproductive steroid hormones.
Antiepileptic drugs that decrease concentrations of reproductive steroid hormones
Phenobarbital
Phenytoin
Carbamazepine
Primidone
Topiramate
Oxcarbazepine
Rufinamide
Valproate*
Clobazam
Antiepileptic drugs that do not substantially decrease concentrations of reproductive steroid hormones
Ethosuximide
Gabapentin
Valproate
Lamotrigine
Levetiracetam†
Zonisamide
Pregabalin
Lacosamide
Retigabine
*Decreased free testosterone concentrations reported in men on valproate and increased androgen concentrations reported in women taking valproate. †Increased testosterone concentrations reported in men after initiation of levetiracetam.
Valproate is generally associated with increased testosterone concentrations. Importantly for women, this drug has been reported to induce androgen synthesis in the ovaries.36,37 Several inhibitory mechanisms are suggested to contribute to this effect, and could be relevant for men and women: direct inhibition of cytochrome P450 isoenzymes 2C9 and 2C19,38 which metabolise the formation of androstenedione from testosterone;39 inhibition of aromatase, which converts testosterone to oestradiol;40 and inhibition of epoxide hydrolase, which might also enzymatically mediate the conversion of testosterone to oestrogen, as shown in cultured human ovarian granulosa cells.41 For women, the inhibitory activity of valproate has clear adverse reproductive effects. Normal granulosa cells secrete testosterone and surround the ovarian follicle as it matures to ovulation. These cells produce gradually increasing amounts of oestrogen by enzymatic con version of testosterone, until the peak in oestrogen concentration associated with ovulation is reached. The follicle itself is subject to stimulation by FSH, which promotes follicular development. Enzymatic inhibition of normal ovarian hormone production results in an immature, non-ovulatory follicle that secretes testosterone. This mechanism might explain how valproate promotes a hyperandrogenic state as well as anovulation and cystic ovaries. Exposure to valproate delivered orally to male and female rats altered gonadal morphology by inducing ovarian cysts in female rats42 and testicular atrophy in male rats.43 In both genders of animals, valproate was associated with reduced concentrations of oestrogen, unchanged testosterone levels, and increased FSH and LH levels in serum.44 Central and peripheral inhibition of aromatase might account for these effects.
Löfgren and colleagues45 reported low total testosterone concentrations and free-androgen indices (100×testosterone/sex-hormone-binding globulin) in women with epilepsy who were taking carbamazepine or oxcarbazepine. However, antiepileptic drugs that do not induce hepatic metabolic enzymes have little effect on androgens.46 Lossius and colleagues47 showed that the effects of enzyme-inducing antiepileptic drugs are reversible: total testosterone con centrations and free-androgen indices significantly increased after withdrawal from carbamazepine in men and women. Additionally, 17β-oestradiol and progesterone levels increased significantly in men, although not in women, after carbamazepine was stopped.
Levetiracetam, which binds to the SV2A receptor present in brain and gonadal and endocrine tissues, was associated with increased total testosterone levels 1 month after the start of treatment in men.48 Concentrations of LH and FSH did not change, which suggests a direct gonadal effect.48 A cross-sectional study of men and women with epilepsy showed no difference in levels of reproductive hormones between groups taking carbamazepine, lamotrigine, or levetiracetam.49 However, the patients taking carbamazepine had lower free-androgen indices and levels of dehydroepiandrosterone sulphate, and higher levels of steroid-hormone-binding globulin, FSH, and LH than did those taking the other drugs. Nevertheless, the results of these two studies are not as conflicting as they seem: the former study48 measured a change over time after initiation of levetiracetam treatment, and in the latter concentrations were measured only once in each patient.
Endogenous hormones and catamenial epilepsy
Clusters of seizures that correlate with the onset of menstruation are termed catamenial, derived from the Greek katamenios, meaning monthly. Sir Charles Locock first described the seizures associated with the menstrual cycle in 1857.50 Menstrual exacerbations of seizures due to fluctuations in endogenous hormone concentrations have been reported in up to 70% of women with epilepsy, but the relation between menses and seizures can be difficult to establish because seizures are clustered in many patients, both men and women, without an association with the menstrual cycle.51
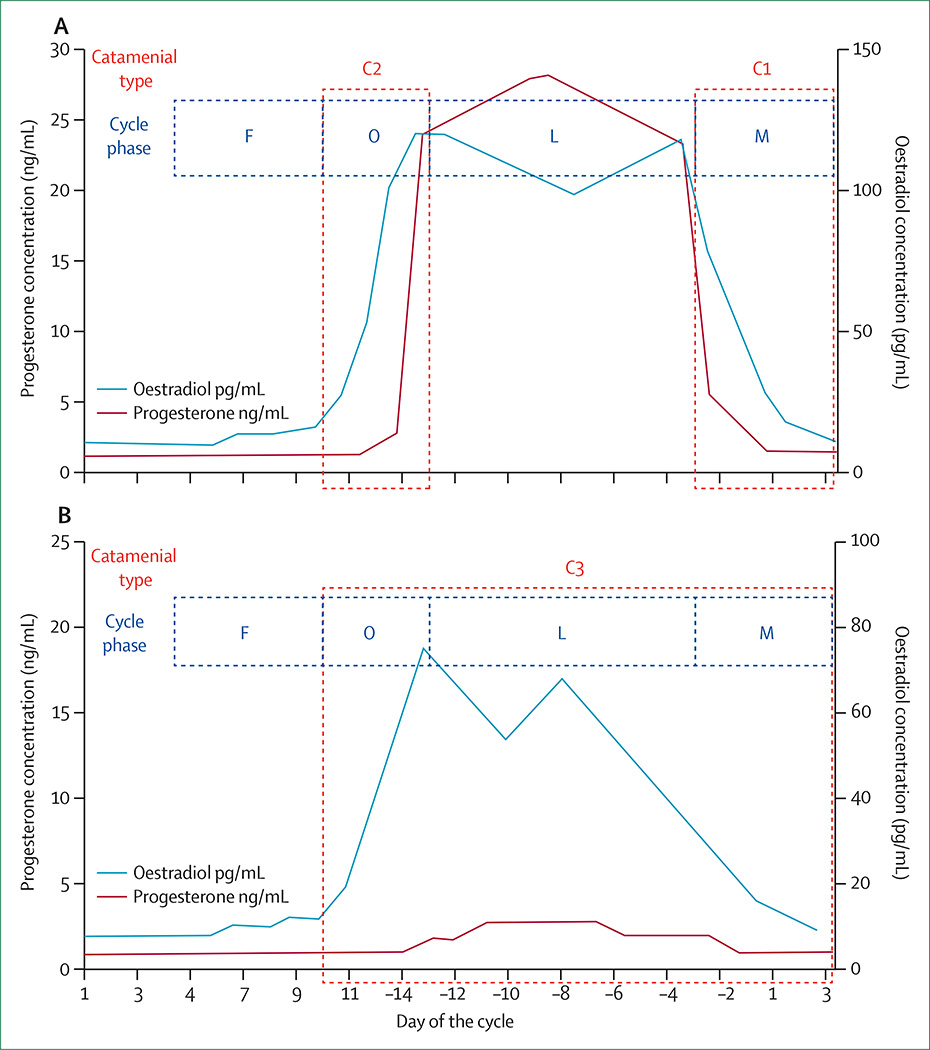
The average menstrual cycle is 28 (range 24–35) days, with day 1 being the first day of menses and ovulation occurring on day 14 (sometimes designated day −14 to adjust for cycle lengths other than 28 days, as ovulation always occurs 14 days prior to menstrual onset). The menstrual cycle has two major phases: the follicular phase (days 1–14) and the luteal phase (days 15–28; sometimes designated days −14 to −1). During the follicular phase, the ovarian follicles grow and the dominant follicle with the most follicular receptors becomes the ovulatory follicle containing the oocyte. On day 14, the oocyte is released (ovulation) and the non-dominant follicles degenerate. In the luteal phase, the dominant follicle forms the progesterone-producing corpus luteum. Figure 2 shows the fluctuation of oestradiol and progesterone in normal menstrual cycling, as well as in anovulatory menstrual cycling with an inadequate luteal phase.53 The hormonal fluctuations most relevant for catamenial seizure exacerbations are the beginning of the rapid surge of oestrogen at day 13, which initiates ovulation, and the rapid decline of progesterone then oestrogen on days 26–28, just before the onset of menstrual bleeding.
Figure 2. Patterns of catamenial epilepsy.
Day 1 is the first day of menstrual flow and day –14 is the day of ovulation. (A) Normal cycle with normal ovulation. C1 pattern is associated with exacerbation of seizures in the perimenstrual phase, and C2 pattern is associated with exacerbation of seizures in the periovulatory phase. (B) Inadequate luteal phase cycle with anovulation. The C3 pattern is associated with exacerbations beginning day 10 of one cycle through day 3 of the next cycle. C=catamenial seizure pattern. F=follicular phase. O=periovulatory. L=luteal phase. M=perimenstrual. Modified and reproduced from Herzog and colleagues,52 by permission of Blackwell Publishing.
Dysregulation of secretion of FSH leads to poor follicular development and, therefore, poor functioning of the corpus luteum, which is a disorder known as inadequate luteal phase53 and is associated with a lack of ovulation. Inadequate development of the corpus luteum leads to low production of progesterone during the luteal phase, whereas oestrogen production remains robust, which alters the balance between the neuroexcitatory and neuroinhibitory effects of these hormones (figure 2). The neurosteroid effects that promote enhanced seizure susceptibility in catamenial epilepsy therefore include: premenstrual withdrawal of the anticonvulsant effects of neurosteroids mediated through their action on GABAA receptors; alteration in GABAA-receptor sub units and subsequent changes in neuronal inhibition; the sudden oestrogen peak in the day before ovulation; and increased frequency of anovulatory cycles due to hypothalamicpituitary-gonadal axis dysregulation and consequent low-progesterone luteal phases. A recent refinement of a model of catamenial epilepsy in hippocampal-kindled female mice implicates the first two of these mechanisms as key to the occurrence of catamenial seizures: premenstrual progesterone withdrawal combined with insensitivity of the GABAA receptor to neurosteroids owing to alteration in subunit composition at the menstrual phase (premenstrual in humans) led to exacerbation of seizures.13
Criteria for diagnosis
Although the term catamenial epilepsy does not refer to seizure type, localisation, or an epilepsy syndrome, a catamenial pattern has been reported most frequently with focal epilepsy. The apparent incidence of catamenial epilepsy varies widely according to the diagnostic criteria used by investigators. For example, if catamenial epilepsy is defined as 75% of seizures occurring between day 24 of one cycle and day 6 of the next, around 12·5% of women with epilepsy would be affected,54 However, when Herzog and colleagues52 statistically derived the patterns of seizure occurrence throughout the menstrual cycle, a consistent doubling of seizure frequency during three specific portions of the menstrual cycle emerged (panel 2), and around a third of women with focal epilepsy were classified as having catamenial seizures.52 The seizure patterns are described in panel 2 and the biological underpinnings based on menstrual hormonal fluctuations are depicted in figure 2.
Panel 2. Patterns of catamenial seizures.
Perimenstrual pattern
The perimenstrual pattern (C1) is defined as maximum seizure frequency during the menstrual phase (day 25 of one cycle to day 3 of the next), which correlates with progesterone decline, and is the most frequently observed pattern
Periovulatory pattern
The periovulatory pattern (C2) is the second most frequently observed pattern and is characterised by maximum seizure frequency during the ovulatory phase (days 10–15), which correlates with a rapid oestrogen surge
Luteal pattern
The luteal pattern (C3) is characterised by maximum seizure frequency during the ovulatory, midluteal, and menstrual phases (day 10 of one cycle to day 3 of next cycle), compared with frequency in the midfollicular phase (days 3–10) in women with inadequate luteal phase cycles; this is the third most frequently observed pattern and correlates with low progesterone concentrations found in anovulatory cycles
The three predominant patterns of catamenial seizures— perimenstrual (C1), periovulatory (C2), and luteal (C3)—have been borne out in multiple studies. In one report, catamenial seizure patterns that correlate with decreased progesterone levels were described.55 By contrast, another study pointed to variations in oestrogen concentrations as an indicator of a catamenial pattern, with little change in progesterone levels between study groups.56 Several other reports invoke the role of hormones as a contributor to seizure occurrence and cyclic seizure patterns, including the seizure risk imparted by anovulatory cycles, in women with epilepsy.57–59 Therefore, both oestrogen and progesterone cycling have been associated with patterns of seizure occurrence in catamenial epilepsy.
Menstrual cyclic antiepileptic drug levels as a contributor to catamenial epilepsy
In addition to the neurosteroid influence on catamenial seizure occurrence, levels of antiepileptic drugs can fluctuate during the menstrual cycle. A pharmacokinetic mechanism could potentially link hormone concentrations during the menstrual cycle to those of antiepileptic drugs. Specifically, high levels of circulating oestrogen in the luteal phase could induce hepatic isoenzymes used for antiepileptic drug metabolism and thereby lower the levels of circulating antiepileptic drugs premenstrually, potentially permitting breakthrough seizures.60 Rosciszewska and colleagues61 reported that phenytoin levels on day 28 in women with catamenial seizures were significantly lower than those in women without cyclical exacerbations. Phenobarbital concentrations, however, did not change significantly. However, another report of women with catamenial epilepsy showed no relationship between the oestradiol and progesterone levels and serum antiepileptic drug levels.62 The authors suggested that catamenial seizure exacerbations are independent of antiepileptic drug levels and are more closely linked to predictable cyclic hormonal fluctuations. Lamotrigine levels remained unchanged throughout the menstrual cycle in one study,63 but were reported to decline by 31% during the mid-luteal phase in another, although levels of valproate did not change across the cycle.64 Evidence does not, therefore, strongly support alterations in concentrations of antiepileptic drugs as being important to catamenial seizure exacerbations, but an independent effect of neurosteroids is clearly present.
Treatment
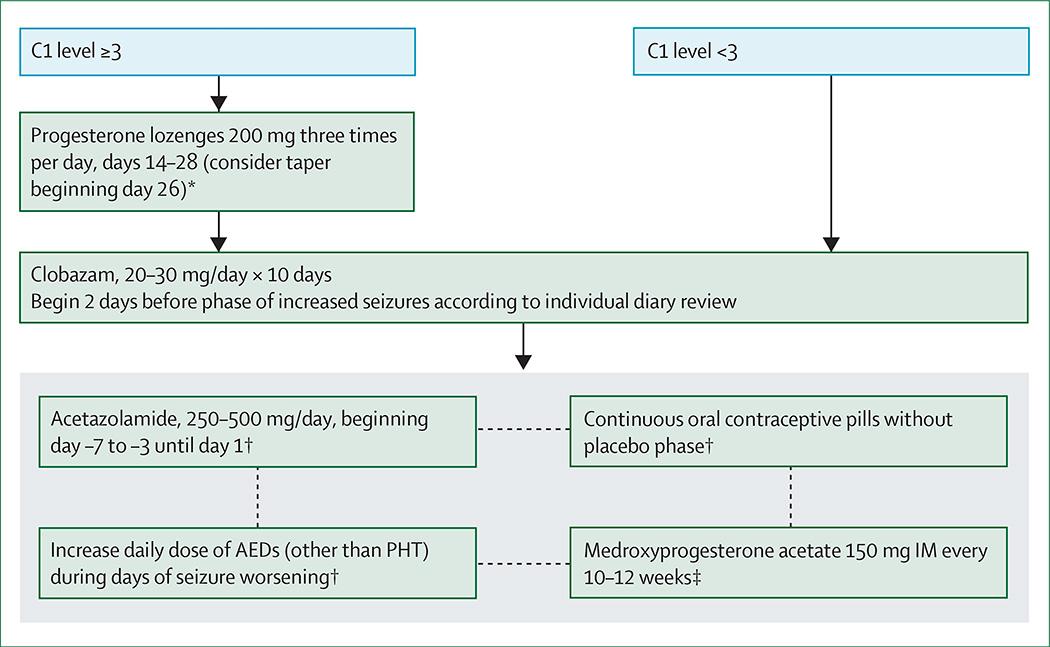
Most interventions have been assessed in the context of the premenstrual C1 seizure pattern (panel 2), the most frequent type of catamenial exacerbation. Women with regular menstrual periods are good candidates for these interventions, since the drugs must be taken a specific number of days after the onset of menstrual bleeding (figure 3). Treatments are generally started at some point during the second half of the cycle (days 14–26), dependent on the individual’s seizure pattern and length of the menstrual cycle, keeping in mind that the luteal phase is 14 days long.
Figure 3. Treatment algorithm for catamenial C1 pattern of seizures.
Most treatments are for focal-onset seizures in women with regular menses. C1 level 3=three times more seizures on days 25–3 compared with other days of the month. AEDs=antiepileptic drugs. PHT=phenytoin. IM=intramuscularly. *If menses start before day 26, start dose tapering on that day according to the same pattern of decreases. †Widely undertaken but not supported by data from randomised, controlled trials. ‡Increased risk of osteoporosis and slow return to normal fertility.
Acetazolamide has been used to treat catamenial epilepsy for 50 years, although it has not been assessed in randomised trials. Effectiveness at doses of 250–500 mg daily administered from 3–7 days before menses has been reported.65,66
Benzodiazepines are used mainly for cessation of seizure clusters but are administered only intermittently because of the risk of habituation and tolerance if used chronically. Clobazam is the only benzodiazepine formally studied for the treatment of catamenial epilepsy. In a double-blind, placebo-controlled, crossover study, clobazam was associated with better seizure control than placebo. Complete control was seen in most patients during a 10-day treatment phase.67
A temporary increase in the dose of the patient’s usual antiepileptic drugs at specific times during the menstrual cycle is another reasonable, empirical approach, although phenytoin should not be increased owing to the risk of toxic effects associated with its non-linear kinetics.
Medroxyprogesterone acetate is a synthetic progestin-only contraceptive agent. Its mechanism of action in reducing seizure frequency is unclear, but is presumed to be related to cessation of cyclical oestrogen and progesterone levels. In patients with catamenial epilepsy, medroxyprogesterone acetate has been associated with seizure frequency reductions of 39% at 1-year follow-up.68,69 Medroxyprogesterone acetate is administered via intramuscular injection and stops the regular menstrual cycle. The standard dose is 150 mg intramuscularly every 12 weeks. Some clinicians advocate shortening the dosing frequency to every 10 weeks to lessen the risk of enzyme-inducing antiepileptic drugs lowering medroxyprogesterone acetate concentrations. However, medroxyprogesterone acetate has been linked to increased risk of osteoporosis, as have many antiepileptic drugs,70 which should be taken into account. Additionally, after cessation of medroxyprogesterone acetate, endogenous hormone concentrations can fluctuate substantially for several months, which could lead to seizure exacerbation as well as a long time to return of normal fertility. Some clinicians use a strategy of inhibiting the normal cyclical release of reproductive hormones by the use of continuous oral contraceptive pills, which suppress ovulation. No data on this strategy from controlled clinical trials have been reported.
Metabolism of natural progesterone to allopregnanolone, a neurosteroid anticonvulsant, makes it an option for seizure control. Two small, open-label studies that assessed natural progesterone as a treatment for complex partial seizures,71,72 one of which included long-term follow-up,73 suggested that natural progesterone administered as a vaginal suppository or an oral lozenge from ovulation (day 14) until the onset of menses was efficacious for the treatment of catamenial epilepsy. A randomised, double-blind, placebo-controlled multicentre clinical trial indicated that the degree of perimenstrual seizure exacerbation (C1 pattern) was a significant predictor of response to progesterone treatment when progesterone was given as 200 mg oral lozenges twice daily on days 14–28.74 Responder rates did not differ for progesterone and placebo when all patients were combined, or for all catamenial versus non-catamenial patients. Nevertheless, a secondary analysis showed that the level of perimenstrual seizure exacerbation (C1 pattern) was a predictor of response to progesterone. If women had a three-times or greater increase in seizures during the C1 period (days −3 to +3) compared with the other days of the month, then 37·8% were responders to progesterone (50% or more reduction in seizure frequency; percentage in the placebo group was 11·1%). For women who had a C1 increase in seizure frequency of eight times or more, the percentage reduction in seizure frequency was around 70%. In follow-up open-label extension phases, progesterone could be tapered to 100 mg twice daily on days 26–27, 50 mg twice on day 28, and then stopped until day 14 of the next cycle in most women. If menses began before day 26, tapering would begin on that day. Occasional side-effects of natural progesterone are breast tenderness, fatigue, depressed mood, and vaginal spotting.
The lack of overall reduction in seizure frequency in the study by Herzog and colleagues,74 except in those with specifically premenstrual seizures, indicates that progesterone supplementation suppresses progesterone-withdrawal seizures, in accordance with molecular and in-vivo models.13–15 However, this hypothesis would imply that catamenial seizures at ovulation could not be treated effectively with progesterone. Therefore, when patients with different patterns of catamenial seizures are grouped together such as in this trial, there might be no overall benefit of natural progesterone. Supporting evidence for the effectiveness of progesterone in patients with the C1 pattern of seizure exacerbation is that the benefit increased as the increased seizure phase shortened and became confined to the perimenstrual days.
Panel 3 provides a proposed algorithm for the evaluation and treatment of catamenial seizures. When a catamenial C1 pattern is present, a proposed treatment algorithm is presented in figure 3.
Panel 3. Suggested algorithm for evaluation and treatment of catamenial seizures.
These are suggestions by the authors in the order of consideration based on clinical experience and limited available supporting data
Consider a catamenial pattern in women with epilepsy who are in reproductive years, are not on any reproductive hormones (eg, oral contraceptive pills), and are continuing to have seizures despite traditional antiepileptic drug therapies
- Evaluate seizure diaries, preferably for at least three menstrual cycles
- To assess relationship of seizure occurrence to menstrual onset
- To determine if menses are regular (occurring every 26–32 days) in order that a scheduled intervention can be undertaken
- If menses occur regularly (see figure 3), and if seizures are focal-onset, consider natural progesterone treatment
- Calculate if a threefold or greater increase in seizures during the C1 period (days −3 to +3) exists compared with the other days of the month
- If present, consider treating with progesterone lozenges orally 200 mg three times per day on days 14–25, then decrease to 100 mg three times per day for days 26–27, 50 mg three times per day on day 28, then stop (if menses begin prior to day 26, the tapering would begin on that day)
- If menses occur regularly (see figure 3), and a catamenial seizure pattern is apparent in C1, C2, or C3 pattern
- Consider clobazam at 20–30 mg each evening, for 10 days starting 2 days before the day on which seizures usually occur
- Consider a small increase in the patient’s usual medicine 2 days before the day on which seizures usually occur
- Consider acetazolamide at 250–500 mg daily from 3 to 7 days prior to menses
- If menses do not occur regularly
- Consider suppressing menstrual cycling with an oral contraceptive that suppresses menses for multiple cycles, if there are no significant interactions with the patient’s antiepileptic drugs (note: although this approach has been undertaken by multiple practitioners, there are no published reports of the outcomes)
- If catamenial exacerbations are severe and intractable to other interventions, consider medroxyprogesterone acetate, with adequate discussion of adverse effects
Effects of antiepileptic drugs and seizures on reproductive function
Polycystic ovary syndrome
PCOS is a major cause of infertility and appears to occur at a higher rate in women with epilepsy than in the general population. What exactly constitutes PCOS remains a moving target; a task force report from The Androgen Excess and PCOS Society defined the criteria for PCOS as the presence of hyperandrogenism (clinical, biochemical, or both), ovarian dysfunction (oligoanovulation, polycystic ovaries, or both), and the exclusion of related disorders.75 PCOS is thought to be multigenic and susceptible to a variety of environmental triggers. Hypothalamic and pituitary dysregulation are likely to occur in PCOS, as evidenced by elevated LH secretion and an increased ratio of LH to FSH. FSH stimulates ovarian steroidogenesis, and elevated LH/FSH will produce follicles that do not fully mature, but become numerous and cystic. Immature follicles are deficient in aromatase, the enzyme that produces oestrogen in the ovary from its precursor, testosterone. In this manner, the PCOS ovarian follicle produces primarily androgens. This abnormal system is disrupted further by the conversion of androgen to oestrogen by aromatase in the periphery, producing elevated circulating oestrogen, which feeds back to the pituitary and deregulates normal LH secretion.76
For women with epilepsy, hypothalamic dysfunction could contribute to the increased rate of PCOS, as well as a wasting of ovarian follicles, and early menopause. An increased ratio of LH to FSH was described in women with localisation-related epilepsy and idiopathic generalised epilepsy, with the ratio being highest in the latter group. Many of the women with idiopathic generalised epilepsy, however, were using valproate, which might confound this finding.25
Isojärvi and colleagues,77 in 1993, first reported the association between valproate and cystic ovaries. They reported that nearly half of the 13 women with epilepsy treated with valproate monotherapy had ovarian cysts (without full-blown PCOS), amenorrhoea, oligomenorrhoea, or prolonged menstrual cycles, compared with 19% of the women taking carbamazepine monotherapy. In a follow-up study the same investigative group reported that 70% of 37 valproate-treated women with epilepsy had polycystic ovaries or hyperandrogenism or both, compared with 19% of the 52 control participants.78 They also showed that metabolic effects of centripetal obesity, hyperinsulinaemia, lipid abnormalities, and polycystic ovaries or hyperandrogenism in women with epilepsy were reduced by discontinuing valproate.79 Obesity, another common feature of PCOS, may also be contributed to by valproate; a recent study suggested that valproate inhibits insulin metabolism, causing increased circulating insulin levels, thus exacerbating insulin resistance.80
Another recent study81 in 102 women with epilepsy of reproductive age found that 12% had PCOS, defined as having two or more of the following components: polycystic ovaries, hyperandrogenism, and amenorrhoea or oligomenorrhoea (a/oligomenorrhoea). The women with PCOS had an onset of epilepsy at 13·8 (SD 6·5) years, significantly younger than that of patients without these disorders (16·9 [8·6] years, p<0·05). In this study as well, valproate therapy was associated with an increased incidence of PCOS.
The rates of occurrence of PCOS have not been completely consistent in the literature, which is probably because of research definitions used: if components of PCOS are assessed, the findings may be different than when evaluating for the complete syndrome. From another study evaluating specifically for PCOS, no differences were found in the rates between women taking carbamazepine (10·0%), valproate (11·1%), or no antiepileptic drugs (10·5%).82 This rate is still slightly greater than the rate of PCOS in the general population of women, which is approximately 7%.83
Taken together, these reports suggest direct association between epilepsy and PCOS due to hypothalamic dysfunction as well as an increased risk of developing features consistent with PCOS with valproate use. It is possible that epilepsy and valproate are both risk factors for the development of PCOS.
Clinical implications for reproductive function
In women with epilepsy, the clinical visit should include assessment for abnormal menstrual cycles, hirsutism, acne, male pattern balding, and body mass index. The index of suspicion should be higher in women treated with valproate and in women with onset of epilepsy at a young age. A simple screening evaluation in such patients would include obtaining testosterone levels, and prospectively keeping track of menstrual cycle length; a normal cycle length is likely to be associated with ovulation. If PCOS features are present, referral to an endocrinologist or gynaecologist, or both, is warranted for further evaluation and consideration of treatment options.
For women of childbearing potential, valproate should be avoided, owing to the high structural teratogenic and neurodevelopmental risks,84 and other anti epileptic drugs should be considered. In men with epilepsy, adverse effects such as weight gain and metabolic syndrome might be an appropriate reason to change from or avoid valproate.
Sexual dysfunction
Many reports suggest a physiological sexual dysfunction in men and women with epilepsy.33,49,85–94 However, the roles of enzyme-inducing antiepileptic drugs decreasing androgen levels49,90,95–97 and the adverse influences of mood98,99 and perceived stigma100 on sexuality for people with epilepsy dictate that the management approaches must be multidimensional. Assessing testosterone levels, and considering the effect of enzyme-inducing antiepileptic drugs on these levels, assessing depression, anxiety, and social factors are reasonable strategies.
Birth rates and fertility
Reduced birth rates have been reported in large cohorts of people with epilepsy. A population-based study showed that men with epilepsy had a 40% lower birth rate than men without epilepsy, and women with epilepsy had a 10% lower birth rate than women without epilepsy.101 A later study from the same population showed that adults with active epilepsy had decreased birth rates compared with those who went into remission prior to adulthood.102 While birth rates encompass psycho social factors such as choosing not to bear children, fertility refers to a couple not using contraceptive methods for 1 year and not conceiving. An important contribution to the discussion of fertility in epilepsy thus far is from the Kerala Registry of Epilepsy and Pregnancy.103 In this registry, young women with epilepsy are followed prospectively and generally try to become pregnant following marriage; a 38% infertility rate was found, and this increased rate of infertility was associated with antiepileptic drug polytherapy versus monotherapy. Several biological reasons could cause decreased fertility in women with epilepsy, including premature ovarian failure, increased rates of anovulatory cycles, and a frequent occurrence of PCOS. Anovulatory cycles occur in women with epilepsy more frequently than in control women; in one report, over a 3 month observation period anovulatory cycles were documented to occur in 11% of cycles in control women, 14% of cycles in women with focal epilepsy, and 27% of cycles in women with idiopathic generalized epilepsy.25 For men with epilepsy, abnormal spermatogenesis may increase the risk of infertility.34
In a study of 60 men with epilepsy and 41 controls, the frequency of morphologically abnormal sperm was significantly higher among men with epilepsy treated with carbamazepine, oxcarbazepine, and valproate compared with controls. Both carbamazepine and valproate were associated with poor motility of sperm and the frequency of abnormally low sperm concentration was high in men taking carbamazepine. The valproate-treated men with abnormal sperm had smaller testicular volumes than the control men,34 consistent with the laboratory findings by Sveberg Røste and colleagues.44 Oxcarbazepine in general had the least adverse effect in this report.34 Other investigators have reported higher rates of abnormal sperm motility and morphology in men with epilepsy compared with controls, which were associated with antiepileptic drug use including valproate and carbamazepine,104 as well as phenytoin.105 However, an independent adverse effect of temporal lobe epilepsy itself has been put forth in the work of Bauer and colleagues,35 who documented testicular failure in men not using antiepileptic drugs, with an additional adverse influence of enzyme-inducing antiepileptic drugs.
No assessment of birth rates has adjusted for confounders such as frequency of intercourse and personal choice to have children or not, which are important considerations for people with epilepsy. Again, several biological factors such as menstrual cycle abnormalities in women due to central dysregulation of menses and components of PCOS could contribute to infertility. There is evidence for polytherapy with antiepileptic drugs contributing to infertility in women, and for enzyme-inducing antiepileptic drugs adversely affecting sperm quality in men.
Clinical implications for infertility
There are no clear strategies known for preventing infertility in people with epilepsy. However, if conception is not occurring after 6 months of unprotected sexual inter course, epilepsy-related factors, including antiepileptic drugs, should be considered.
For women, this would include an evaluation of menstrual cycling including for the occurrence of ovulatory cycles, the presence of PCOS, or early onset of perimenopause (see next section).
For men, this would include a sperm analysis and consideration of changing antiepileptic drug if the results are clearly abnormal, particularly if the drug used is phenytoin, carbamazepine, or valproate.
Perimenopause and menopause in epilepsy
Premature ovarian failure
Women with epilepsy are at risk of early onset of the menopausal transition. The mechanism is likely related to hypothalamic-pituitary-gonadal axis dysfunction, producing dysregulation of maturation of ovarian follicles and early loss of follicles available for ovulation. One of the first scientific reports of early perimenopause was put forth by Klein and colleagues,106 in which 14% of women with epilepsy had premature ovarian failure, compared with 4% of healthy control women (p=0·04). Additionally, women with premature ovarian failure were more likely to have had catamenial exacerbation of their seizures during earlier reproductive years. The risk for earlier menopause appears to be related to seizure frequency. Harden and colleagues107 reported a negative correlation between the age at menopause and seizure frequency (p=0·014). For example, the women with only rare seizures had a normal age of menopause of 50–51 years, while women with frequent seizures experienced earlier menopause at 46–47 years. In this study, there was no relationship between early menopause and specific antiepileptic drug treatments.
Changes in seizures
Although no prospective information is available on the course of epilepsy as women with the disease progress through the menopausal transition, data are available from a cross-sectional evaluation using mailed questionnaires, in which women were asked to recall the course of their epilepsy from their reproductive years through perimenopause and menopause.108 Almost two-thirds of perimenopausal women with epilepsy reported an increase in seizures during perimenopause, and a history of catamenial seizure pattern was significantly associated with an increase in seizures at perimenopause. These findings are consistent with postulated mechanisms for women with hormonally sensitive seizures; during perimenopause, oestrogen levels remain unchanged, may rise steadily, or become erratic with surges until the onset of menopause in response to the elevated FSH levels. However, the cyclic progesterone elevation during the luteal phase of the menstrual cycle gradually becomes less frequent throughout perimenopause, with increased anovulatory cycles.109 The elevation of the oestrogen-to-progesterone ratio may contribute to the increase in seizure frequency at perimenopause. A history of a catamenial seizure pattern (seizures in the week prior to menses) was associated with a decrease in seizures at menopause. A high percentage of women in the perimenopausal group took synthetic hormone-replacement therapy, which was significantly associated with an increase in seizures (p=0·001).108
Hormone-replacement therapy and clinical implications
In a randomised, double-blind, placebo-controlled trial of Prempro (0·625 mg of conjugated equine oestrogens plus 2·5 mg of medroxyprogesterone acetate [CEE/MPA] daily, or double-dose CEE/MPA daily for 3 months; Wyeth, Collegeville, PA, USA), seizure frequency increased significantly with the use of Prempro in a dose-related manner. These findings demonstrate an adverse clinical effect of exogenous reproductive hormones on menopausal women with epilepsy.110
Women with epilepsy may be counselled that there should be extra vigilance for seizure occurrence at perimenopause, particularly if there has been a catamenial seizure pattern. Women with epilepsy should not use CEE/MPA for postmenopausal hormone replacement, and perhaps another regimen such as oestradiol with natural progesterone may be considered.
Conclusions
Much has been elucidated in the field of epilepsy and neuroendocrinology, importantly neurosteroid effects on brain excitability, reciprocal pharmacokinetic interactions involving endogenous and exogenous hormones and antiepileptic drugs, and epilepsy itself as an endocrine disruptor. Clinicians may begin to use this information as tools to improve the treatment options for men and women with epilepsy, such as preferential use of antiepileptic drugs with a hormone-neutral effect and specific treatment strategies for catamenial epilepsy.
The research priorities in this field should continue to include treatments for catamenial epilepsy; the results of the best trial performed to date using a hormone, natural progesterone, to treat a hormonally influenced seizure pattern are both promising and limiting. The population for which natural progesterone appears to be effective is a very specific one—ie, the patients with premenstrual seizure exacerbations. This result fits nicely with the neurosteroid theory of augmenting progesterone during the premenstrual progesterone withdrawal, but is far from a comprehensive solution for cyclical seizures in women. Given the neurophysiological complexity of oestrogen, progesterone, and testosterone, exploration of the use of hormonal stabilisation in women with epilepsy, such as with the use of stable-dose contraceptives, should be studied.
Other research avenues are to quantify and characterise the effect of epilepsy in its various forms on reproductive function. This could have implications outside the reproductive realm, and may be associated with cognitive abilities, as well as affective and anxiety states in people with epilepsy. The effect of long-term antiepileptic drug exposure on reproductive status with the newer generation of antiepileptic drugs is not known.
Recent clinical research, as it becomes widely appreciated, will influence the awareness that catamenial epilepsy is potentially treatable, and this will in turn increase the insight into trying practical treatment approaches. Valproate should by now be appreciated as a powerful endocrine disruptor, and when appropriate, alternatives to its use should be sought. Current and ongoing research will further characterise the risks of infertility in people with epilepsy, which will help to guide family planning.
On the near horizon, patients with epilepsy will benefit from a better understanding of these issues and from a more ready application of our current knowledge.
Acknowledgments
The writing of this Review was supported by the Epilepsy Therapy Project.
CLH has been a speaker for GlaxoSmithKline, Lundbeck, and UCB, and is a consultant for Upsher-Smith.
Footnotes
Contributors
CLH and PBP contributed equally to the literature search, the creation of figures and panels, the interpretation of published data, and the writing of this Review.
Conflicts of interest
PBP declares that she has no conflicts of interest.
References
- 1.Chen DK, So YT, Fisher RS. Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Use of serum prolactin in diagnosing epileptic seizures: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2005;65:668–675. doi: 10.1212/01.wnl.0000178391.96957.d0. [DOI] [PubMed] [Google Scholar]
- 2.Mytinger JR, Joshi S. The current evaluation and treatment of infantile spasms among members of the Child Neurology Society. J Child Neurol. 2012;27:1289–1294. doi: 10.1177/0883073812455692. [DOI] [PubMed] [Google Scholar]
- 3.Pack M, Reddy DS, Duncan S, Herzog A. Neuroendocrinological aspects of epilepsy: important issues and trends in future research. Epilepsy Behav. 2011;22:94–102. doi: 10.1016/j.yebeh.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Finocchi C, Ferrari M. Female reproductive steroids and neuronal excitability. Neurol Sci. 2011;32(suppl 1):S31–S35. doi: 10.1007/s10072-011-0532-5. [DOI] [PubMed] [Google Scholar]
- 5.Frye CA. Effects and mechanisms of progestogens and androgens in ictal activity. Epilepsia. 2010;51(suppl 3):135–140. doi: 10.1111/j.1528-1167.2010.02628.x. [DOI] [PubMed] [Google Scholar]
- 6.Gu Q, Moss RL. 17β-estradiol potentiates kainate-induced currents via activation of the cAMP cascade. J Neurosci. 1996;16:3620–3629. doi: 10.1523/JNEUROSCI.16-11-03620.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiland NG. Estradiol selectively regulates agonist binding sites on the N-methyl-D-aspartate receptor complex in the CA-1 region of the hippocampus. Endocrinology. 1992;131:662–668. doi: 10.1210/endo.131.2.1353442. [DOI] [PubMed] [Google Scholar]
- 8.Hoffman GE, Moore N, Fiskum G, Murphy AZ. Ovarian steroid modulation of seizure severity and hippocampal cell death after kainic acid treatment. Exp Neurol. 2003;182:124–134. doi: 10.1016/s0014-4886(03)00104-3. [DOI] [PubMed] [Google Scholar]
- 9.Reibel S, Andre V, Chassagnon S, et al. Neuroprotective effects of chronic estradiol benzoate treatment on hippocampal cell loss induced by status epilepticus in the female rat. Neurosci Lett. 2000;281:79–82. doi: 10.1016/s0304-3940(00)00784-9. [DOI] [PubMed] [Google Scholar]
- 10.Veliškova J, Velišek L. β-Estradiol increases dentate gyrus inhibition in female rats via augmentation of hilar neuropeptide Y. J Neurosci. 2007;27:6054–6063. doi: 10.1523/JNEUROSCI.0366-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reddy DS. Neurosteroids: endogenous role in the human brain and therapeutic potentials. Prog Brain Res. 2010;186:113–137. doi: 10.1016/B978-0-444-53630-3.00008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- 13.Reddy DS, Gould J, Gangisetty O. A mouse kindling model of perimenstrual catamenial epilepsy. J Pharmacol Exp Ther. 2012;341:784–793. doi: 10.1124/jpet.112.192377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith SS, Shen H, Gong QH, Zhou X. Neurosteroid regulation of GABAA receptors: focus on the α4 and δ subunits. Pharmacol Ther. 2007;116:58–76. doi: 10.1016/j.pharmthera.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gulinello M, Gong QH, Li X, Smith SS. Short-term exposure to a neuroactive steroid increases α4 GABAA receptor subunit levels in association with increased anxiety in the female rat. Brain Res. 2001;910:55–66. doi: 10.1016/s0006-8993(01)02565-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stoffel-Wagner B. Neurosteroid biosynthesis in the human brain and its clinical implications. Ann N Y Acad Sci. 2003;1007:64–78. doi: 10.1196/annals.1286.007. [DOI] [PubMed] [Google Scholar]
- 17.Reddy DS. Anticonvulsant activity of the testosterone-derived neurosteroid 3α-androstanediol. Neuroreport. 2004;15:515–518. doi: 10.1097/00001756-200403010-00026. [DOI] [PubMed] [Google Scholar]
- 18.Frye CA, Rhodes ME, Walf AA, Harney JP. Testosterone reduces pentylenetetrazole-induced ictal activity of wildtype mice but not those deficient in type I 5α-reductase. Brain Res. 2001;918:182–186. doi: 10.1016/s0006-8993(01)02967-5. [DOI] [PubMed] [Google Scholar]
- 19.Kaminski RM, Marini H, Kim W, Rogawski MA. Anticonvulsant activity of androsterone and etiocholanolone. Epilepsia. 2005;46:819–827. doi: 10.1111/j.1528-1167.2005.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin JB, Reichlin S. Clinical neuroendocrinology. 2nd. Philadelphia: FA Davis; 1987. [Google Scholar]
- 21.Meo R, Bilo L, Nappi C, et al. Derangement of the hypothalamic GnRH pulse generator in women with epilepsy. Seizure. 1993;2:241–252. doi: 10.1016/s1059-1311(05)80134-7. [DOI] [PubMed] [Google Scholar]
- 22.Herzog AG, Drislane FW, Schomer DL, et al. Abnormal pulsatile secretion of luteinizing hormone in men with epilepsy: relationship to laterality and nature of paroxysmal discharges. Neurology. 1990;40:1557–1561. doi: 10.1212/wnl.40.10.1557. [DOI] [PubMed] [Google Scholar]
- 23.Drislane FW, Coleman AE, Schomer DL, et al. Altered pulsatile secretion of luteinizing hormone in women with epilepsy. Neurology. 1994;44:306–310. doi: 10.1212/wnl.44.2.306. [DOI] [PubMed] [Google Scholar]
- 24.Quigg M, Kiely JM, Shneker B, et al. Interictal and postictal alterations of pulsatile secretions of luteinizing hormone in temporal lobe epilepsy in men. Ann Neurol. 2002;51:559–566. doi: 10.1002/ana.10188. [DOI] [PubMed] [Google Scholar]
- 25.Morrell MJ, Giudice L, Flynn KL, et al. Predictors of ovulatory failure in women with epilepsy. Ann Neurol. 2002;52:704–711. doi: 10.1002/ana.10391. [DOI] [PubMed] [Google Scholar]
- 26.Quigg M, Kiely JM, Johnson ML, Straume M, Bertram EH, Evans WS. Interictal and postictal circadian and ultradian luteinizing hormone secretion in men with temporal lobe epilepsy. Epilepsia. 2006;47:1452–1459. doi: 10.1111/j.1528-1167.2006.00617.x. [DOI] [PubMed] [Google Scholar]
- 27.Silveira DC, Klein P, Ransil BJ, et al. Lateral asymmetry in activation of hypothalamic neurons with unilateral amygdaloid seizures. Epilepsia. 2000;41:34–41. doi: 10.1111/j.1528-1157.2000.tb01502.x. [DOI] [PubMed] [Google Scholar]
- 28.Friedman MN, Geula C, Holmes GL, Herzog AG. GnRH-immunoreactive fiber changes with unilateral amygdala-kindled seizures. Epilepsy Res. 2002;52:73–77. doi: 10.1016/s0920-1211(02)00196-1. [DOI] [PubMed] [Google Scholar]
- 29.Herzog AG. A relationship between particular reproductive endocrine disorders and the laterality of epileptiform discharges in women with epilepsy. Neurology. 1993;43:1907–1910. doi: 10.1212/wnl.43.10.1907. [DOI] [PubMed] [Google Scholar]
- 30.Beastall GH, Cowan RA, Gray JM, Fogelman I. Hormone binding globulins and anticonvulsant therapy. Scott Med J. 1985;30:101–105. doi: 10.1177/003693308503000206. [DOI] [PubMed] [Google Scholar]
- 31.Murialdo G, Galimberti CA, Gianelli MV, et al. Effects of valproate, phenobarbital and carbamazepine on sex steroid setup in women with epilepsy. Clin Neuropharmacol. 1988;21:52–58. [PubMed] [Google Scholar]
- 32.Stoffel-Wagner B, Bauer J, Flugel D, Brennemann W, Klingmuller D, Elger CE. Serum sex hormones are altered in patients with chronic temporal lobe epilepsy receiving anticonvulsant medication. Epilepsia. 1998;39:1164–1173. doi: 10.1111/j.1528-1157.1998.tb01307.x. [DOI] [PubMed] [Google Scholar]
- 33.Herzog AG, Drislane FW, Schomer DL, et al. Differential effects of antiepileptic drugs on sexual function and reproductive hormones in men with epilepsy. Neurology. 2005;65:1016–1020. doi: 10.1212/01.wnl.0000178988.78039.40. [DOI] [PubMed] [Google Scholar]
- 34.Isojärvi JIT, Löfgren E, Juntunen KST, et al. Effect of epilepsy and antiepileptic drugs on male reproductive health. Neurology. 2004;62:247–253. doi: 10.1212/01.wnl.0000098936.46730.64. [DOI] [PubMed] [Google Scholar]
- 35.Bauer J, Dierkes H, Burr W, Reuber M, Stoffel-Wagner B. Disease- and treatment-related effects on the pituitary-gonadal functional axis: a study in men with epilepsy. J Neurol. 2011;258:1080–1084. doi: 10.1007/s00415-010-5888-6. [DOI] [PubMed] [Google Scholar]
- 36.Nelson-De Grave VL, Wickenheisser JK, Cockrell JE, et al. Valproate potentiates androgen biosyntheses in human ovarian theca cells. Endocrinology. 2004;145:799–808. doi: 10.1210/en.2003-0940. [DOI] [PubMed] [Google Scholar]
- 37.Taubøll E, Gregoaszczuk EL, Kolodziej A, Katja M, Ropstad E. Valproate inhibits the conversion of testosterone to estradiol and acts as an apoptotic agent in growing porcine ovarian follicular cells. Epilepsia. 2003;44:1014–1021. doi: 10.1046/j.1528-1157.2003.60702.x. [DOI] [PubMed] [Google Scholar]
- 38.Wen X, Wang J, Kivisto KT, Neuvonen PJ, Backman JT. In vitro evaluation of valproic acid as an inhibitor of human cytochrome P450 isoforms: preferential inhibition of cytochrome P450 2C9 (CYP2C9) Br J Clin Pharmacol. 2001;52:547–553. doi: 10.1046/j.0306-5251.2001.01474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi MH, Skipper PL, Wishnok JS, Tannenbaum SR. Characterization of testosterone 11β-hydroxylation catalyzed by human liver microsomal cytochromes P450. Drug Metab Dispos. 2005;33:714–718. doi: 10.1124/dmd.104.003327. [DOI] [PubMed] [Google Scholar]
- 40.Jacobsen NW, Halling-Sørensen B, Birkved FK. Inhibition of human aromatase complex (CYP19) by antiepileptic drugs. Toxicol In Vitro. 2008;22:146–153. doi: 10.1016/j.tiv.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 41.Hattori N, Fujiwara H, Maeda M, Fujii S, Ueda M. Epoxide hydrolase affects estrogen production in the human ovary. Endocrinology. 2000;141:3353–3365. doi: 10.1210/endo.141.9.7682. [DOI] [PubMed] [Google Scholar]
- 42.Røste LS, Taubøll E, Berner A, Isojärvi JI, Gjerstad L. Valproate, but not lamotrigine, induces ovarian morphological changes in Wistar rats. Exp Toxicol Pathol. 2001;52:545–552. doi: 10.1016/S0940-2993(01)80014-2. [DOI] [PubMed] [Google Scholar]
- 43.Sveberg Røste L, Taubøll E, Berner A, Berg KA, Aleksandersen M, Gjerstad L. Morphological changes in the testis after long-term valproate treatment in male Wistar rats. Seizure. 2001;10:559–565. doi: 10.1053/seiz.2001.0545. [DOI] [PubMed] [Google Scholar]
- 44.Sveberg Røste L, Taubøll E, Isojärvi JI, et al. Effects of chronic valproate treatment on reproductive endocrine hormones in female and male Wistar rats. Reprod Toxicol. 2002;16:767–773. doi: 10.1016/s0890-6238(02)00054-0. [DOI] [PubMed] [Google Scholar]
- 45.Löfgren E, Tapanainen JS, Koivunen R, Pakarinen A, Isojärvi JI. Effects of carbamazepine and oxcarbazepine on the reproductive endocrine function in women with epilepsy. Epilepsia. 2006;47:1441–1446. doi: 10.1111/j.1528-1167.2006.00506.x. [DOI] [PubMed] [Google Scholar]
- 46.Stephen LJ, Sills GJ, Leach JP, et al. Sodium valproate versus lamotrigine: a randomised comparison of efficacy, tolerability and effects on circulating androgenic hormones in newly diagnosed epilepsy. Epilepsy Res. 2007;75:122–129. doi: 10.1016/j.eplepsyres.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 47.Lossius MI, Taubøll E, Mowinckel P, Mørkrid L, Gjerstad L. Reversible effects of antiepileptic drugs on reproductive endocrine function in men and women with epilepsy—a prospective randomized double-blind withdrawal study. Epilepsia. 2007;48:1875–1882. doi: 10.1111/j.1528-1167.2007.01147.x. [DOI] [PubMed] [Google Scholar]
- 48.Harden CL, Nikolov BG, Kandula P, Labar DR, Pannullo S. Effect of levetiracetam on testosterone levels in male patients. Epilepsia. 2010;51:2348–2351. doi: 10.1111/j.1528-1167.2010.02732.x. [DOI] [PubMed] [Google Scholar]
- 49.Svalheim S, Taubøll E, Luef G, et al. Differential effects of levetiracetam, carbamazepine, and lamotrigine on reproductive endocrine function in adults. Epilepsy Behav. 2009;16:281–287. doi: 10.1016/j.yebeh.2009.07.033. [DOI] [PubMed] [Google Scholar]
- 50.Locock C. Discussion. Analysis of fifty-two cases of epilepsy observed by the author. In: Sieveking EH, editor. Med Times Gaz. Vol. 14. 1857. pp. 524–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haut SR, Lipton RB, LeValley AJ, Hall CB, Shinnar S. Identifying seizure clusters in patients with epilepsy. Neurology. 2005;65:1313–1315. doi: 10.1212/01.wnl.0000180685.84547.7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Herzog AG, Klein P, Ransil BJ. Three patterns of catamenial epilepsy. Epilepsia. 1997;38:1082–1088. doi: 10.1111/j.1528-1157.1997.tb01197.x. [DOI] [PubMed] [Google Scholar]
- 53.Jones GS. The luteal phase defect. Fertil Steril. 1976;27:351–356. doi: 10.1016/s0015-0282(16)41769-3. [DOI] [PubMed] [Google Scholar]
- 54.Duncan S, Read CL, Brodie MJ. How common is catamenial epilepsy? Epilepsia. 1993;34:827–831. doi: 10.1111/j.1528-1157.1993.tb02097.x. [DOI] [PubMed] [Google Scholar]
- 55.El-Khayat HA, Soliman NA, Tomoum HY, Omran MA, El-Wakad AS, Shatla RH. Reproductive hormonal changes and catamenial pattern in adolescent females with epilepsy. Epilepsia. 2008;49:1619–1626. doi: 10.1111/j.1528-1167.2008.01622.x. [DOI] [PubMed] [Google Scholar]
- 56.Hussain Z, Qureshi MA, Hasan KZ, Aziz H. Influence of steroid hormones in women with mild catamenial epilepsy. J Ayub Med Coll Abbottabad. 2006;18:17–20. [PubMed] [Google Scholar]
- 57.Murialdo G, Magri F, Tamagno G, et al. Seizure frequency and sex steroids in women with partial epilepsy on antiepileptic therapy. Epilepsia. 2009;50:1920–1926. doi: 10.1111/j.1528-1167.2009.02178.x. [DOI] [PubMed] [Google Scholar]
- 58.Quigg M, Fowler KM, Herzog AG, et al. Circalunar and ultralunar periodicities in women with partial seizures. Epilepsia. 2008;49:1081–1085. doi: 10.1111/j.1528-1167.2008.01537.x. [DOI] [PubMed] [Google Scholar]
- 59.Herzog AG, Fowler KM, Sperling MR. Variation of seizure frequency with ovulatory status of menstrual cycles. Epilepsia. 2011;52:1843–1848. doi: 10.1111/j.1528-1167.2011.03194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kumar N, Behari M, Aruja GK, Jailhani BL. Phenytoin levels in catamenial epilepsy. Epilepsia. 1988;29:155–158. doi: 10.1111/j.1528-1157.1988.tb04412.x. [DOI] [PubMed] [Google Scholar]
- 61.Rosciszewska D, Buntner B, Guz I, Zawisza L. Ovarian hormones, anticonvulsant drugs, and seizures during the menstrual cycle in women with epilepsy. J Neurol Neurosurg Psychiatry. 1986;49:A47–A51. doi: 10.1136/jnnp.49.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bäckström T, Jorpes P. Serum phenytoin, phenobarbital, carbamazepine, albumin; and plasma estradiol, progesterone concentrations during the menstrual cycle in women with epilepsy. Acta Neurol Scand. 1979;59:63–71. doi: 10.1111/j.1600-0404.1979.tb02912.x. [DOI] [PubMed] [Google Scholar]
- 63.Wegner I, Edelbroek PM, Bulk S, Lindhout D. Lamotrigine kinetics within the menstrual cycle, after menopause, and with oral contraceptives. Neurology. 2009;73:1388–1393. doi: 10.1212/WNL.0b013e3181bd8295. [DOI] [PubMed] [Google Scholar]
- 64.Herzog AG, Blum AS, Farina EL, et al. Valproate and lamotrigine level variation with menstrual cycle phase and oral contraceptive use. Neurology. 2009;72:911–914. doi: 10.1212/01.wnl.0000344167.78102.f0. [DOI] [PubMed] [Google Scholar]
- 65.Poser CM. Modification of therapy for exacerbation of seizures during menstruation. J Pediatr. 1974;84:779–780. doi: 10.1016/s0022-3476(74)80037-5. [DOI] [PubMed] [Google Scholar]
- 66.Ansell B, Clarke E. Acetazolamide in treatment of epilepsy. BMJ. 1956;1:650–661. doi: 10.1136/bmj.1.4968.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Feely M, Calvert R, Gibson J. Clobazam in catamenial epilepsy: A model for evaluating anticonvulsants. Lancet. 1982;2:71–73. doi: 10.1016/s0140-6736(82)91691-9. [DOI] [PubMed] [Google Scholar]
- 68.Zimmerman AW, Holden KR, Reiter EO, Dekaban AS. Medroxyprogesterone acetate in the treatment of seizures associated with menstruation. J Pediatr. 1973;83:959–963. doi: 10.1016/s0022-3476(73)80529-3. [DOI] [PubMed] [Google Scholar]
- 69.Mattson RH, Cramer JA, Caldwell BV, Siconolfi BC. Treatment of seizures with medroxyprogesterone acetate: preliminary report. Neurology. 1984;34:1255–1258. doi: 10.1212/wnl.34.9.1255. [DOI] [PubMed] [Google Scholar]
- 70.Pitts CJ, Kearns AE. Update on medications with adverse skeletal effects. Mayo Clin Proc. 2011;86:338–343. doi: 10.4065/mcp.2010.0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Herzog AG. Intermittent progesterone therapy of partial complex seizures in women with menstrual disorders. Neurology. 1986;36:1607–1610. doi: 10.1212/wnl.36.12.1607. [DOI] [PubMed] [Google Scholar]
- 72.Herzog AG. Progesterone therapy in women with complex partial and secondary generalized seizures. Neurology. 1995;45:1660–1662. doi: 10.1212/wnl.45.9.1660. [DOI] [PubMed] [Google Scholar]
- 73.Herzog A. Progesterone therapy in women with epilepsy: a 3-year follow-up. Neurology. 1999;52:1917–1918. doi: 10.1212/wnl.52.9.1917-a. [DOI] [PubMed] [Google Scholar]
- 74.Herzog AF, Fowler KM, Smithson SD, et al. Progesterone vs placebo therapy for women with epilepsy: a randomized clinical trial. Neurology. 2012;78:1959–1966. doi: 10.1212/WNL.0b013e318259e1f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Azziz R, Carmina E, Dewailly D, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456–488. doi: 10.1016/j.fertnstert.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 76.Rasgon N. The relationship between polycystic ovary syndrome and antiepileptic drugs. J Clin Psychopharmacol. 2004;24:322–334. doi: 10.1097/01.jcp.0000125745.60149.c6. [DOI] [PubMed] [Google Scholar]
- 77.Isojärvi JIT, Laatikainen TJ, Pakarinen AJ, Juntunen KTS, Myllyla VV. Polycystic ovaries and hyperandrogenism in women taking valproate for epilepsy. N Engl J Med. 1993;329:1383–1381. doi: 10.1056/NEJM199311043291904. [DOI] [PubMed] [Google Scholar]
- 78.Isojärvi JI, Taubøll E, Pakarinen AJ, et al. Altered ovarian function and cardiovascular risk factors in valproate-treated women. Am J Med. 2001;111:290–296. doi: 10.1016/s0002-9343(01)00806-3. [DOI] [PubMed] [Google Scholar]
- 79.Pylvänen V, Pakarinen A, Knip M, Isojärvi J. Characterization of insulin secretion in valproate-treated patients with epilepsy. Epilepsia. 2006;47:1460–1464. doi: 10.1111/j.1528-1167.2006.00546.x. [DOI] [PubMed] [Google Scholar]
- 80.Isojärvi JI, Rättyä J, Myllylä VV, et al. Valproate, lamotrigine, and insulin-mediated risks in women with epilepsy. Ann Neurol. 1998;43:446–451. doi: 10.1002/ana.410430406. [DOI] [PubMed] [Google Scholar]
- 81.Zhou JQ, Zhou LM, Chen LJ, et al. Polycystic ovary syndrome in patients with epilepsy: a study in 102 Chinese women. Seizure. 2012;21:729–733. doi: 10.1016/j.seizure.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 82.Bauer J, Jarre A, Klingmuller M, et al. Polycystic ovary syndrome in patients with focal epilepsy: a study in 93 women. Epilepsy Res. 2000;41:163–167. doi: 10.1016/s0920-1211(00)00139-x. [DOI] [PubMed] [Google Scholar]
- 83.Azziz R, Woods KS, Reyna R, Key TJ, Knockenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–2749. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 84.Harden CL, Meador KJ, Pennell PB, et al. Practice parameter update: management issues for women with epilepsy—focus on pregnancy (an evidence-based review): teratogenesis and perinatal outcomes. Report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology. 2009;73:133–141. doi: 10.1212/WNL.0b013e3181a6b312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morrell MJ, Sperling MR, Stecker M, Dichter MA. Sexual dysfunction in partial epilepsy: a deficit in physiologic sexual arousal. Neurology. 1994;44:243–247. doi: 10.1212/wnl.44.2.243. [DOI] [PubMed] [Google Scholar]
- 86.Bergen D, Daugherty S, Eckenfels E. Reduction of sexual activities in females taking antiepileptic drugs. Psychopathology. 1992;25:1–4. doi: 10.1159/000284746. [DOI] [PubMed] [Google Scholar]
- 87.Morrell MJ, Guldner GT. Self-reported sexual function and sexual arousability in women with epilepsy. Epilepsy Behav. 2003;4:407–413. doi: 10.1111/j.1528-1157.1996.tb00554.x. [DOI] [PubMed] [Google Scholar]
- 88.Herzog AG, Coleman AE, Jacobs AR, et al. Relationship of sexual dysfunction to epilepsy laterality and reproductive hormone levels in women. Epilepsia. 1996;37:1204–1210. doi: 10.1016/s1525-5050(03)00121-5. [DOI] [PubMed] [Google Scholar]
- 89.Daniele A, Azzoni A, Bizza A, Rossi A, Gainotti G, Mazza S. Sexual behavior and hemispheric laterality of the focus in the patients with temporal lobe epilepsy. Biol Psychiatry. 1997;42:617–624. doi: 10.1016/S0006-3223(96)00411-8. [DOI] [PubMed] [Google Scholar]
- 90.Mattson RH, Cramer JA, Collins JF, et al. Comparison of carbamazepine, phenobarbital, phenytoin, and primidone in partial and secondarily generalized tonic-clonic seizures. N Engl J Med. 1985;313:145–151. doi: 10.1056/NEJM198507183130303. [DOI] [PubMed] [Google Scholar]
- 91.Keller J, Chen YK, Lin HC. Association between epilepsy and erectile dysfunction: evidence from a population-based study. J Sex Med. 2012;9:2248–2255. doi: 10.1111/j.1743-6109.2012.02670.x. [DOI] [PubMed] [Google Scholar]
- 92.Bauer J, Blumenthal S, Reuber M, Stoffel-Wagner B. Epilepsy syndrome, focus localization, and treatment choice affect testicular function in men with epilepsy. Neurology. 2004;62:243–246. doi: 10.1212/01.wnl.0000091866.48962.79. [DOI] [PubMed] [Google Scholar]
- 93.El-Khayat HA, Shatla HM, Ali GK, Abdulgani MO, Tomoum HY, Attya HA. Physical and hormonal profile of male sexual development in epilepsy. Epilepsia. 2003;44:447–452. doi: 10.1046/j.1528-1157.2003.26502.x. [DOI] [PubMed] [Google Scholar]
- 94.Herzog AG, Farina EL, Drislane FW, et al. A comparison of anastrozole and testosterone versus placebo and testosterone for treatment of sexual dysfunction in men with epilepsy and hypogonadism. Epilepsy Behav. 2010;17:264–271. doi: 10.1016/j.yebeh.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 95.Gil-Nagel A, Lopez-Munoz F, Serratosa JM, Moncada I, Garcia-Garcia P, Alamo C. Effect of lamotrigine on sexual function in patients with epilepsy. Seizure. 2006;15:142–149. doi: 10.1016/j.seizure.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 96.Duncan S, Blacklaw J, Beastall GH, Brodie MJ. Sexual function in women with epilepsy. Epilepsia. 1997;38:1074–1081. doi: 10.1111/j.1528-1157.1997.tb01196.x. [DOI] [PubMed] [Google Scholar]
- 97.Morrell MJ, Flynn KL, Done S, Flaster E, Kalayjian L, Pack AM. Sexual dysfunction, sex steroid hormone abnormalities, and depression in women with epilepsy treated with antiepileptic drugs. Epilepsy Behav. 2005;6:360–365. doi: 10.1016/j.yebeh.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 98.Zelená V, Kuba R, Soška V, Rektor I. Depression as a prominent cause of sexual dysfunction in women with epilepsy. Epilepsy Behav. 2011;20:539–544. doi: 10.1016/j.yebeh.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 99.Duncan S, Talbot A, Sheldrick R, Caswell H. Erectile function, sexual desire, and psychological well-being in men with epilepsy. Epilepsy Behav. 2009;15:351–357. doi: 10.1016/j.yebeh.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 100.Baker GA, Nashef L, Van Hout BA. Current issues in the management of epilepsy: the impact of frequent seizures on cost of illness, quality of life, and mortality. Epilepsia. 1997;38(suppl 1):S1–S8. doi: 10.1111/j.1528-1157.1997.tb04511.x. [DOI] [PubMed] [Google Scholar]
- 101.Artama M, Isojärvi JI, Raitanen J, Auvinen A. Birth rate among patients with epilepsy: a nationwide population-based cohort study in Finland. Am J Epidemiol. 2004;159:1057–1063. doi: 10.1093/aje/kwh140. [DOI] [PubMed] [Google Scholar]
- 102.Löfgren E, Pouta A, von Wendt L, Tapanainen J, Isojärvi JI, Järvelin MR. Epilepsy in the northern Finland birth cohort 1966 with special reference to fertility. Epilepsy Behav. 2009;14:102–107. doi: 10.1016/j.yebeh.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 103.Sukumaran SC, Sarma PS, Thomas SV. Polytherapy increases the risk of infertility in women with epilepsy. Neurology. 2010;75:1351–1355. doi: 10.1212/WNL.0b013e3181f73673. [DOI] [PubMed] [Google Scholar]
- 104.Røste LS, Taubøll E, Haugen TB, Bjørnenak T, Saetre ER, Gjerstad L. Alterations in semen parameters in men with epilepsy treated with valproate or carbamazepine monotherapy. Eur J Neurol. 2003;10:501–506. doi: 10.1046/j.1468-1331.2003.00615.x. [DOI] [PubMed] [Google Scholar]
- 105.Taneja N, Kucheria K, Jain S, Maheshwari MC. Effect of phenytoin on semen. Epilepsia. 1994;35:136–140. doi: 10.1111/j.1528-1157.1994.tb02923.x. [DOI] [PubMed] [Google Scholar]
- 106.Klein P, Serje A, Pezzullo JC. Premature ovarian failure in women with epilepsy. Epilepsia. 2001;42:1584–1589. doi: 10.1046/j.1528-1157.2001.13701r.x. [DOI] [PubMed] [Google Scholar]
- 107.Harden CL, Koppel BS, Herzog AG, Nikolov BG, Hauser WA. Seizure frequency is associated with age of menopause in women with epilepsy. Neurology. 2003;61:451–455. doi: 10.1212/01.wnl.0000081228.48016.44. [DOI] [PubMed] [Google Scholar]
- 108.Harden CL, Pulver MC, Jacobs AR. The effect of menopause and perimenopause on the course of epilepsy. Epilepsia. 1999;40:1402–1407. doi: 10.1111/j.1528-1157.1999.tb02012.x. [DOI] [PubMed] [Google Scholar]
- 109.Burger HG, Dudley EC, Robertson DM, Dennerstein L. Hormonal changes in the menopause transition. Recent Prog Horm Res. 2002;57:257–275. doi: 10.1210/rp.57.1.257. [DOI] [PubMed] [Google Scholar]
- 110.Harden CL, Herzog AG, Nikolov BG, et al. Hormone replacement therapy in women with epilepsy: a randomized, double-blind, placebo-controlled study. Epilepsia. 2006;47:1447–1451. doi: 10.1111/j.1528-1167.2006.00507.x. [DOI] [PubMed] [Google Scholar]