Abstract
Calcium (Ca2+) is a universal second messenger in all higher organisms and centrally involved in the launch of responses to environmental stimuli. Ca2+ signals in the cytosol are initiated by the activation of Ca2+ channels in the plasma membrane and/or in endomembranes. Yeast (Saccharomyces cerevisiae) contains a Ca2+-permeable channel of the TRP family, TRPY1, which is localized in the vacuolar membrane and contributes to cytosolic free Ca2+ ([Ca2+]cyt) elevations, for example in response to osmotic upshock. A TRPY1 homologue in the rice blast fungus is known to be important for growth and pathogenicity. To determine the role of the TRP channel family in the maize pathogen Colletotrichum graminicola, proteins homologous to TRPY1 were searched. This identified not one, but four genes in the C. graminicola genome, which had putative orthologs in other fungi, and which we named CgTRPF1 through 4. The topology of the CgTRPF proteins resembled that of TRPY1, albeit with a variable number of transmembrane (TM) domains additional to the six-TM-domain core and a diverse arrangement of putatively Ca2+-binding acidic motifs. All CgTRPF genes were expressed in axenic culture and throughout the infection of maize. Like TRPY1, all TRPF proteins of C. graminicola were localized intracellularly, albeit three of them were found not in large vacuoles, but co-localized in vesicular structures. Deletion strains for the CgTRPF genes were not altered in processes thought to involve Ca2+ release from internal stores, i.e. spore germination, the utilization of complex carbon sources, and the generation of tip-focussed [Ca2+]cyt spikes. Heterologous expression of CgTRPF1 through 4 in a tryp1Δ yeast mutant revealed that none of the channels mediated the release of Ca2+ in response to osmotic upshock. Accordingly, aequorin-based [Ca2+]cyt measurements of C. graminicola showed that in this fungus, osmotic upshock-triggered [Ca2+]cyt elevations were generated entirely by influx of Ca2+ from the extracellular space. Cgtrpf mutants did not show pathogenicity defects in leaf infection assays. In summary, our study reveals major differences between different fungi in the contribution of TRP channels to Ca2+-mediated signal transduction.
Introduction
Like any organism, fungi must perceive and respond to their environment to survive and propagate. For example, spores of plant pathogenic fungi perceive certain features of the host surface, which initiates a developmental programme that may culminate in an appressorium. This highly specialized cell allows for a pressure-mediated penetration of intact host cuticle and epidermal cell wall. This pressure, which may reach values of 5.5 MPa in Colletotrichum graminicola, is generated by the accumulation of osmotically active compounds [1] and needs to be sensed and tightly controlled to ensure successful penetration while preventing a bursting of the appressorium [2]. C. graminicola is a hemibiotrophic pathogen of maize, which, inside its host, passes through a short biotrophic and a longer necrotrophic phase, characterised by the controlled expression of subsets of genes [3, 4]. Again, perception of and response to the environment within the host is important for an effective colonization [5, 6]. One of the fungus' environmental parameters that may change abruptly, within a wide range, and throughout the fungal life cycle is the osmotic potential. Osmotic shock situations occur, for example, during exposure to rainwater or during the lysis of host cells.
The coupling of stimulus perception by a fungus and its responses on transcriptional or post-transcriptional levels involves numerous interacting signalling networks, including, for example, G-proteins, MAP kinases, and cyclic nucleotides [7, 8]. Calcium (Ca2+) is another ubiquitous second messenger in all higher organisms and plays a central role in the initiation of responses to external stimuli, including osmotic shock, and to internal cues [9, 10]. In the cytosol, Ca2+ binds to target proteins, such as calcineurin and calmodulin (CaM), resulting in conformational changes that modulate their activity or their interaction with other proteins. In fungi, the Ca2+- and CaM-activated protein phosphatase calcineurin dephosphorylates the transcription factor Crz1 allowing it to enter the nucleus and triggering transcription [11]. Deletions of either gene in filamentous fungi result in growth retardation and reduced virulence [12, 13].
Ca2+ signals are generated by the passive diffusion of Ca2+ into the cytosol, facilitated by Ca2+-permeable channels. The elevation of cytosolic free Ca2+ ([Ca2+]cyt) is terminated by the activity of Ca2+/H+ antiporters and Ca2+-ATPases which transport Ca2+ out of the cytosol [14, 15, 16]. Hence, Ca2+ channels are actively regulated by a signal transduction pathway, while Ca2+/H+ antiporters and Ca2+-ATPases respond to the increased [Ca2+]cyt. Ca2+-permeable channels may be activated by a number of ligands, such as inositol phosphates, cyclic nucleotides or amino acids, and by physical parameters, such as voltage or stretch of the membrane [17]. They may be either located in the plasma membrane or in membranes of intra-cellular compartments, hence mediating the entry of extracellular Ca2+ into the cytosol or Ca2+ release from internal stores, respectively. Albeit this diversity of Ca2+ conductances suggests a number of underlying genes, in fungi the molecular identity has been resolved for only very few channel systems. Comparative genomic analyses indicated that some fungi bear mitochondrial calcium uniporters, and some basal fungi also have genes encoding putative P2X receptors in their genomes [18, 19]. However, none of these putative fungal Ca2+ channel classes has been functionally analysed so far. The plasma membrane of the yeast Saccharomyces cerevisiae harbours a homologue of animal voltage-gated Ca2+ channels, Cch1, which physically interacts with another membrane protein, Mid1 [20, 21, 22, 23]. The Cch1Mid1 complex forms a high-affinity Ca2+ uptake system (HACS), which is activated by multiple stimuli, such as osmotic, iron, cold, and alkali stress [24, 25, 26]. Deletion of either Cch1 or Mid1 leads to an increased sensitivity of yeast cells to these stresses, in particular if the Ca2+ concentration in the medium is low. In filamentous fungi, deletion of Cch1 or Mid1 homologues causes reduced hyphal growth, albeit fungal species differ in their requirement of this channel system [27, 28, 29]. Next to the HACS, there also exists a low-affinity Ca2+ uptake system (LACS) of unclear genetic identity [22].
The vacuole represents the largest intracellular store for Ca2+ in yeast. The vacuolar membrane harbours a Ca2+-permeable channel, initially named Yeast Vacuolar Channel 1 (Yvc1), which is related to Transient Receptor Potential (TRP) channels of animals [30]. Animal TRP channels group into seven subfamilies (TRPC, TRPV, TRPA, TRPM, TRPP, TRPML, and TRPN), and most of them are permeable for Ca2+ [31]. Animal TRP channels locate to various endomembranes as well as to the plasma membrane. All TRP channels are supposed to contain at least six transmembrane (TM) domains and a pore loop between TM domain 5 and 6. The C- and N-termini of TRP channels are highly diverse. TRP channels are often activated in a polymodal way, i.e. a single channel integrates different stimuli, such as temperature, voltage, and ligands [31]. Fungal TRP channels form a separate subfamily [32]. In analogy to the animal TRP nomenclature, the TRP channel of yeast, Yvc1, was also denominated TRPY1. This channel is activated by cytosolic Ca2+ and by osmotic upshock leading to mechanical force on the vacuolar membrane [30, 32, 33, 34]. Heterologous expression of the TRPY1 homologues of Kluyveromyces lactis and Candida albicans, as well as the filamentous plant pathogenic fungus Fusarium graminearum (teleomorph Gibberella zeae), in S. cerevisiae demonstrated a mechanosensitivity and a responsiveness to osmotic upshock, similar to TRPY1 [35, 36]. In a comparative RNAi knock-down study, Nguyen and co-workers (2008) [37] examined the importance of homologues of the yeast Ca2+ channels Cch1, Mid1, and TRPY1 in the rice blast fungus Magnaporthe oryzae. Interestingly, in this pathogen TRPY1 was clearly more important than Cch1 and Mid1 for growth and virulence [37]. Similarly, hyphal growth and virulence were strongly impaired in a trpy1 mutant of the dimorphic fungus C. albicans [38].
Despite the obvious importance of TRPY1-like channels in filamentous fungi, their functioning has been rarely analysed, with the notable exception of TRPGz from F. graminearum [36]. We therefore searched for TRPY1 homologues in the maize pathogen C. graminicola. Intriguingly, we identified four genes with similarity to TRPY1 in this organism, which were functionally characterised by heterologous expression in yeast and sub-cellular localization. Deletion strains were analysed for [Ca2+]cyt signal generation, germination, growth rates, tolerance to osmotic stress and Ca2+ starvation, as well as virulence. Surprisingly, our results differed considerably from data obtained previously on TRPY1 homologues in other fungal species, indicating that fungi vary largely in their employment of Ca2+ channel types.
Materials and Methods
Bioinformatic analyses
A tBLASTn search of the C. graminicola whole genome sequence hosted at the Broad Institute was carried out using default parameters. This identified four fragments with similarity to the TRPY1 channel (synonym Yvc1; systematic name: YOR087W) of Saccharomyces cerevisiae. The corresponding genes were denominated CgTRPF1 through CgTRPF4. Full-length sequences of the CgTRPF genes were obtained by RACE-PCR as described below. Membrane topology of the CgTRPF proteins was analysed with TOPCONS (accessed at http://topcons.net/) using default settings. The putative pore loop was inferred from previous analyses [18]. Canonical Ca2+-binding sites were searched with PFAM (accessed at http://pfam.xfam.org/) using default settings. TRPY1 is known to contain no classical Ca2+-binding sites, but binds Ca2+ by a tetra-aspartate motif (DDDD) [34]. Therefore, motifs with four or more consecutive acidic amino acid residues (Asp and Glu) were searched in the CgTRPF protein sequences. Homologous sequences of other fungi were obtained by tBLASTn searches of their annotated genomes. Multiple sequence alignments of the full-length proteins, including 43 sequences from 20 fungal species, were performed using ClustalW [39]. Resulting alignments were trimmed with Jalview [40] (S1 File). Subsequent phylogenetic analyses were performed by neighbour joining (10,000 bootstrap replicates) using Phylip hosted at http://www.es.embnet.org/Services/. A consensus tree was created by using plottree (http://www.bioinformatics.nl/tools/plottree.html). Amino acid identity and similarity was calculated with the help of the trimmed alignments that were used for the phylogenetic analysis at http://imed.med.ucm.es/Tools/sias.html with standard settings.
Expression analysis
Expression of CgTRPF1 through 4 was examined by amplifying the full-length CDS from cDNA obtained from OMA-grown falcate conidia and hyphae grown on modified Leach's Complete Medium (mLCM) overlaid with a PVDF membrane [41]. Transcript abundance of CgTRPF genes during infection of maize was analysed by qRT-PCR and RNA-Seq. For qRT-PCR experiments, maize (cv. Mikado, KWS Saat AG, Einbeck, Germany) plants were cultivated on compost soil in a greenhouse [42]. Detached segments of the middle of the third leaf of 17-day-old plants were infected with one 10-μL drop per segment containing 104 conidia in a 0.04% Tween 20 (Carl Roth) solution. Leaf segments were incubated in moist chambers at 23°C in darkness. After the indicated time, leaf discs of 8 mm diameter were excised and immediately frozen in liquid nitrogen. Four leaf discs were pooled for each point in time. Infection assays were performed in three biological replicates in consecutive weeks. Leaf discs were ground in liquid nitrogen. The resulting powder was suspended in RLT buffer (Qiagen, Venlo, The Netherlands) and processed in aliquots for RNA extraction using the PeqGold Plant RNA kit with on-column DNase I treatment according to the manual (PeqLab, Erlangen, Germany). qRT-PCR was performed using the Power SYBR Green RNA-to-CT 1-step kit (Applied Biosystems—Thermo Fisher Scientific, Waltham, MA, USA). Reactions comprising volumes of 20 μL were set up according to the manufacturer’s instructions using 0.2 μM of each oligonucleotide and 50 ng RNA and executed in a MyiQ real-time detection system (Bio-Rad Laboratories, Hercules, USA). After reverse transcription at 48°C for 30 min, the resulting cDNA was denaturated for 10 min at 95°C and amplified in 50 cycles (95°C for 15 seconds, 60°C for 1 min). Calculation of the results was done according to Liu and Saint (2002) [43]. The oligonucleotides used are listed in S1 Table. As reference for normalisation, transcript levels of HistonH3, Actin, and ILV5 were used. RNA-Seq data were obtained from the study of Schliebner and co-workers (2014) [44].
Media and culture conditions
To induce the production of falcate conidia, the fungus was grown on oat meal agar (OMA) [45]. Colony growth assays were performed as described before [45]. Colony diameters were recorded daily for 4 to 10 days. Vegetative hyphae of C. graminicola were grown in liquid mLCM or on mLCM solidified with 1.5% agar (Carl Roth, Karlsruhe, Germany) [41]. For osmotic stress treatments, glycerol was added to mLCM agar prior to autoclaving at the indicated final concentrations. For assays testing growth on different carbon sources, mLCM, potato dextrose agar (PDA), and minimal medium supplemented with the respective carbon source were used. PDA was made of 2.4% potato dextrose broth (Formedium, Hunstanton, UK) and 1.5% agar. Minimal medium contained salt solutions as used in mLCM, 1.5% agar, and 2% of the respective carbon source (glucose, sucrose, sorbitol, mannitol, raffinose, cellulose, malate, or pectate). Sodium malate and sodium pectate were obtained by neutralizing DL-malic acid and pectic acid to pH 7.0 with NaOH, respectively. Ca2+-depleted SC medium was prepared as described before [45].
RNA extraction for cloning and RACE-PCR
C. graminicola RNA was extracted from fungal mycelium grown for four days at 23°C and 30 rpm in mLCM medium, and S. cerevisiae RNA was extracted from a log-phase culture using the Spectrum Plant Total RNA Kit (Sigma). An on-column DNase I digest was performed with the RNase-free DNase I set (Omega bio-tek, Norcross, GA, USA) according to the manufacturer's instructions.
RACE-PCR was performed with the BD SMART RACE cDNA amplification kit (BD, Franklin Lakes, NJ, USA) according to the manufacturer’s recommendations. PCR products were purified from agarose gel slices using the Wizard SV Gel and PCR Clean-Up System (Promega, Madison, WI, USA). RACE-PCR products were sequenced using the BigDye Terminator v1.1 Cycle Sequencing Kit (Thermo Fisher Scientific, Waltham, MA, USA) and gene-specific oligonucleotides (S1 Table). All oligonucleotides were purchased from Eurofins MWG Operon (Ebersberg, Germany).
Targeted gene deletion and Southern Blotting
A hygromycin B resistance cassette was amplified from pAN7-1 (GenBank No. Z32698) [46] using the oligonucleotides unihyg-F1 and unihyg-R1 (S1 Table) [47]. Deletion cassettes were generated by double-joint PCR using the oligonucleotides listed in S1 Table [48]. Transformation of the C. graminicola M2 (M1.001) wild type isolate was performed as described previously [49]. Transformants were screened by PCR for the presence of the deletion cassette. For this purpose, DNA was isolated by using a quick extraction protocol [50]. PCR-positive clones were assayed by Southern blotting for homologous integration of the deletion cassette and ectopic integration events. The probe was generated using the oligonucleotides Hph-5'-South-for and Hph-5'-South-rev (S1 Table) as described elsewhere [49]. DNA extraction and blotting were performed as described before [51].
Yeast complementation analysis and [Ca2+]cyt measurements
The Saccharomyces cerevisiae trpy1Δ deletion strain CEN.SR36-3C (Acc. No. B0257A; genotype: CEN.PK; YOR087w/088w::HIS3) was obtained from Euroscarf (Frankfurt, Germany). trpy1Δ cells were transformed with the plasmid pEVP11-AEQ encoding apoaequorin [52] as described elsewhere [53]. Full-length coding sequences of the four CgTRPF genes and the TRPY1 gene were cloned into the NotI-site of the pFL61 plasmid [54]. The pEVP11-AEQ-containing trpy1Δ strain was transformed with these pFL61 descendants. An RT-PCR with RNA from log-phase liquid cultures was performed to confirm the presence of full-length mRNA of the CgTRPF genes in these strains. For luminometric analysis of [Ca2+]cyt, yeast cultures were grown on a rotating shaker in liquid SC-Leu-Ura medium containing 2 μM coelenterazine (Carl Roth) at 30°C to a final density of 1 to 5 x 107 cells per mL and diluted to 1 x 107 cells per mL with fresh coelenterazine-containing medium. For [Ca2+]cyt measurements in a tube luminometer (Sirius-1, Berthold Detection Systems, Pforzheim, Germany), 20 μl of the cell suspension were used per experiment. After 1 min baseline recording, cells were treated with 200 μl of a solution (pH 7.0) containing 1.5 M NaCl, 50 mM MES, and 25 mM EGTA. Total aequorin luminescence was discharged at the end of the experiment by injecting 220 μl of a solution containing 2 M CaCl2 and 20% ethanol. [Ca2+]cyt was calculated as described by Allen and co-workers (1977) [55], which normalized differences in Aequorin expression (i.e., total aequorin luminescence) in different strains, and which is the most commonly used equation in the mycology community. Albeit absolute [Ca2+]cyt values obtained by this procedure may be offset from the calculated values due to the cytosolic aequorin environment, this effect should be similar in all yeast studies employing this formula.
[Ca2+]cyt measurements in Colletotrichum graminicola
To test [Ca2+]cyt responses of C. graminicola wild type and ΔCgtrpf deletion strains to osmotic upshock, the strains were transformed with the pAEQS1-G418 plasmid [45] which encodes a codon-optimized Apoaequorin [56]. Transformants were selected by using G418 (geneticin; 600 μg mL-1 during transformation, 150 μg mL-1 during selection and single-spore isolation). For [Ca2+]cyt measurements, 1 mL mLCM agar supplemented with 10 μM coelenterazine was poured into sterile polystyrene cylinders with slip lid (36 mm diameter x 29 mm height; neoLab, Heidelberg, Germany). 300 washed macroconidia were inoculated onto the solidified medium, and the polystyrene cylinders were covered loosely to allow for gas exchange. The fungal cultures were incubated in darkness for 3 d at 23°C and 65% relative humidity. Prior to [Ca2+]cyt measurements, cultures were overlaid with 3 mL of a solution (pH 7.0) containing 50 mM MES-KOH and 0 or 25 mM EGTA. Cylinders were covered with parafilm and incubated for 30 min in the chamber of the Sirius-1 luminometer. After 1 min baseline recording, 4 mL of a solution (pH 7.0) containing 50 mM MES-KOH and 0 or 3 M NaCl plus 0 or 25 mM EGTA were injected through the parafilm cover. Aequorin luminescence was recorded for 30 min. To discharge total aequorin at the end of the experiment, 8 mL discharge solution containing 2 M CaCl2 and 100 μM digitonin (Applichem, Darmstadt, Germany) were injected, and recording continued for 60 min. Data shown represent three biological repetitions performed on different days. [Ca2+]cyt was calculated as described above.
For [Ca2+]cyt analyses in individual hyphae, wild type and ΔCgtrpf deletion strains were transformed with a pGEM-T-PtrpC-nptII-TtrpC-PtoxB-YC3.6-TtrpC plasmid [45], encoding the FRET-based ratiometric Ca2+ reporter protein Yellow Cameleon 3.6 (YC3.6) [57]. Measurements of tip-focussed [Ca2+]cyt spikes during hyphal growth were performed as described before [45].
Subcellular localization
For subcellular localization and co-localization of the CgTRPF proteins, a dual-tagging plasmid system was employed, following the cloning workflow described by Lange and co-workers (2014) [50]. Oligonucleotides used for the cloning of localization plasmids are listed in S1 Table. Transformation and microscopic analyses were performed as described before [50].
Germination assays
Germination and appressorium formation were assayed on polystyrene and on onion epidermis. Ten-μL droplets of washed macroconidia (see above) containing 100 and 1000 spores were inoculated onto 90-mm polystyrene Petri dishes (Greiner Bio One) and the hydrophobic face of onion epidermal strips, respectively. After incubation for 24 h in a humid chamber at 23°C in darkness, infection structures were counted by phase contrast microscopy using an Axiovert 40 CFL inverted microscope (Carl Zeiss, Jena, Germany) equipped with a 10 x / 0.25 Ph1 objective for germination assays on polystyrene and an Axioskop upright microscope (Zeiss) equipped with a 20 x / 0.45 Ph 2 objective for germination assays on onion epidermis.
Leaf segment infection assays
Segments of the third leaf of 14-day-old maize (Zea mays cv. Golden Jubilee) plants cultivated in an air-conditioned greenhouse were excised and incubated to assess virulence of C. graminicola as published previously [58].
Results
The C. graminicola genome contains four genes with similarity to the S. cerevisiae calcium channel TRPY1
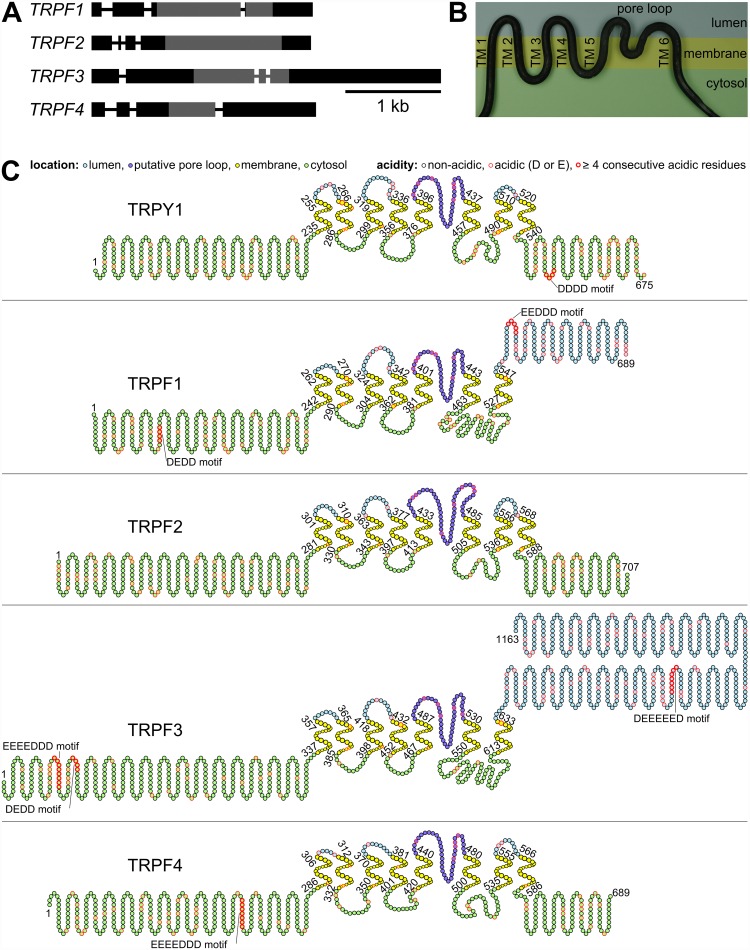
The C. graminicola genome was searched by tBLASTn for an orthologue to TRPY1 of S. cerevisiae. Unexpectedly, not one, but four genes were identified with E-values below 10−3. According to the animal TRP nomenclature, we named these genes CgTRPF1 through 4, standing for C. graminicola TRP of Fungi. Full-length cDNA sequences were obtained by RACE-PCR and verified by cloning and RNA-Seq. In Fig 1A, the regions detected by the tBLASTn search are indicated in grey, the completed sequences in black. Comparison with the genomic regions revealed that each of the genes comprises four exons of varying length (Fig 1A). The CDSs of CgTRPF1, 2, 3 and 4 consist of 2070, 2124, 3492, and 2073 bp from start to stop codon, respectively. The genomic sequences and the gene numbers can be found in S2 File. The amino acid similarities (and identities) of CgTRPF1, 2, 3 and 4 compared to TRPY1 are 60% (46%), 40% (24%), 38% (22%), and 34% (21%), respectively, based on the trimmed alignments. The gene structures that we obtained by tBLASTn searches and RACE-PCR perfectly matched the most recent genomic and transcriptomic data generated by RNA-Seq [44].
Fig 1. Gene structures of C. graminicola TRPF genes and predicted membrane topologies of yeast TRPY1 and C. graminicola TRPF proteins.
(A) Gene structures of CgTRPF genes. Boxes: exons, lines: introns; grey: match of the initial tBLASTn search against TRPY1, black: regions identified by RACE-PCR and verified by cloning PCR. (B) Artistic representation (forged steel) of the TRP channel core structure containing six transmembrane (TM) domains and a pore loop between TM domain 5 and 6. (C) Predicted membrane topology of TRPY1 and CgTRPFs. Cytosolic amino acid residues are indicated in light green, TM domains are shown in yellow, luminal amino acid residues are depicted in light blue, and the predicted pore loop is marked in dark blue. Acidic amino acid residues [Asp (D) or Glu (E)] are indicated by a red edge, which is boldfaced in motifs of 4 or more consecutive acidic amino acid residues. One circle represents one amino acid residue. The first and the last amino acid of the whole protein, as well as of each TM domain, are enumerated.
The membrane topology of yeast TRPY1 shows a Shaker-like core of six TM domains (Fig 1B), a pore loop between TM domain 5 and 6, and, in addition, two predicted additional TM domains after the sixth TM domain (Fig 1C), which were previously described as hydrophobic patches [34]. All predicted TM domains of TRPY1 and CgTRPF1 through 4 are exactly 21 amino acids long (S2 Table). There is also a high degree of conservation in the length of the luminal and cytosolic linkers of the TM domains as well as of the putative pore loop (S2 Table) [18]. CgTRPF2 and CgTRPF4 of C. graminicola have the same predicted topology as TRPY1, i.e. a six-TM-domain core and two additional TM domains. CgTRPF1 and CgTRPF3 also have the six-TM-domain core, but only one additional TM domain. Hence, the C-terminus of those proteins is predicted to reside in the lumen. In coincidence with this luminal C-terminus, CgTRPF1 and CgTRPF3 have a longer cytosolic linker between TM domain 6 and 7, as compared to TRPY1, CgTRPF2, and CgTRPF4, which have a predicted cytosolic C-terminus.
Like TRPY1, all TRPF proteins of C. graminicola have no EF-hands or other canonical Ca2+-binding sites detected by PFAM. However, an acidic DDDD motif in the C-terminus of TRPY1 has been shown to be important for high-affinity Ca2+ binding and channel activation [34]. The luminal C-terminus of CgTRPF1 and CgTRPF3 harbours acidic EEDDD and DEEEEED motifs, respectively, that may bind Ca2+ (Fig 1C). Additionally, acidic motifs, containing at least four consecutive amino acid residues, are present in the cytosolic N-termini of CgTRPF1, CgTRPF3, and CgTRPF4, with CgTRPF3 possessing two such stretches (Fig 1C). In CgTRPF2 there are no areas with least four consecutive acidic amino acid residues. However, the cytosolic N- and C- termini of CgTRPF2 contain many densely clustered acidic amino acid residues that may also confer an ability to bind Ca2+.
The C. graminicola TRPF genes have putative orthologs in other fungi
To examine whether other fungi may also possess multiple predicted proteins with similarities to TRPY1, a phylogenetically diverse set of annotated genomes of Ascomycota and Basidiomycota was searched by tBLASTn (S3 Table). The obtained sequences were aligned, trimmed, and a phylogenetic tree was generated that comprised six major branches (Fig 2). Species belonging to the Saccharomycotina harboured only one putative TRP protein and clustered on a distinct branch. C. graminicola paralogs are present in four of the five remaining branches. The fifth branch comprises only predicted proteins from Basidiomycota. In general, all examined filamentous fungi carry one to four predicted TRPF proteins. Tuber melanosporum contains just one TRPF which is most closely related to CgTRPF1; Puccinia triticina has one TRPF that co-groups with CgTRPF2. Two predicted TRPF proteins, grouping with CgTRPF1 and CgTRPF3, were found in Aspergillus fumigatus, Botrytis cinerea and Penicillium chrysogenum. Fusarium graminearum carries two TRPFs homologous to CgTRPF1 and CgTRPF4. Cryptococcus neoformans and Ustilago maydis also bear two TRPFs; one homologous to CgTRPF2, with the other one not grouping with any of the CgTRPFs. Laccaria bicolor has two homologues to CgTRPF2 and one not grouping with a CgTRPF. Three TRPFs were found in Mycosphaerella graminicola (homologous to CgTRPF1, 2, and 4), Magnaporthe oryzae (homologous to CgTRPF1, 3 and 4), Pyrenophora tritici-repentis (homologous to CgTRPF1, 2 and 3). Colletotrichum higginsianum and Neurospora crassa are the only of the analyzed species containing predicted proteins with similarity to all four CgTRPFs. There is no apparent correlation between nutritional lifestyle or pathogenicity and the number of TRPF proteins per species.
Fig 2. Phylogenetic tree of fungal TRP proteins.
Red: yeast TRPYs; blue, orange, violet and green: TRPF groups 1, 2, 3, and 4, each containing a C. graminicola homologue; yellow: TRPF group not containing a C. graminicola homologue.
The C. graminicola TRPF genes are expressed in axenic culture and throughout infection
To determine whether the four CgTRPF genes may play a role during growth, we first determined their expression in spores and in colonies cultivated on a membrane overlying mLCM agar, as described by Lange and co-workers (2014) [41]. Full-length transcripts for all four genes were detectable in vegetative hyphae and conidial spores (Fig 3A). To determine transcript abundance throughout the infection process on maize, two independent methods were applied on two different cultivars (Mikado, Golden Jubilee). Transcript levels were assessed from 0 to 120 hours post inoculation (hpi). Irrespective of the cultivar, both, qRT-PCR and RNA-Seq experiments indicated that transcripts of all CgTRPF genes were clearly detectable from conidial to necrotrophic stage of infection (Fig 3B and 3C). Compared to 0 hpi, transcript levels of CgTRPF1 were induced from 12 hpi onward by up to 13 fold in both data sets. Transcript abundances of CgTRPF4 were also consistently elevated throughout earlier stages of the infection process, albeit to a lesser extent (Fig 3B and 3C). The transcriptomic data indicate that all four CgTRPF genes may play a role in all stages of growth and infection.
Fig 3. Expression profiles of the CgTRPF genes in axenic culture and during infection of C. graminicola on maize.
(A) Full-length cDNA of CgTRPF genes was amplified from RNA extracted from in vitro grown mycelium and spores. Products were expected at 2098, 2152, 3522, and 2101 bp for CgTRPF1, CgTRPF2, CgTRPF3, and CgTRPF4, respectively. (B, C) Expression during the infection process relative to the expression in spores (0 hours post infection, hpi); black: CgTRPF1, dark grey: CgTRPF2, light grey: CgTRPF3, white: CgTRPF4. (B) Detached third leaves of 2.5-week-old drop-infected plants (cv. Mikado); assayed by qRT-PCR. Data are means ± SE (N = 3). (C) Third leaf of intact two-week-old drop-infected plants (cv. Golden Jubilee); assayed by RNA-Seq. Data are means ± SE (N = 3).
The C. graminicola TRPF proteins localize at intracellular membranes
TRP channels of animals localize either at the plasma membrane or at membranes of intracellular organelles, while the S. cerevisiae TRPY1 channel localizes at the membrane of the central vacuole. To determine the sub-cellular localization of the CgTRPF proteins, fusions of their genes, including native promoters, to the EGFPf gene were created, and the C. graminicola wild type was transformed with the fusion constructs. All CgTRPF-EGFPf proteins localized at intracellular organelles (Fig 4A). However, only CgTRPF4 resided in membranes delineating large vacuoles, similar to TRPY1 in S. cerevisiae, whereas CgTRPF1, CgTRPF2, and CgTRPF3 were found in small vesicular structures. Because CgTRPF1 through 3 exhibited a similar localization pattern, we investigated whether they are present in the same compartment. To this end, the respective genes were fused to mCherry and combined with EGFPf fusion constructs in a dual-tag plasmid system [50]. The mCherry and EGFPf fusion constructs were co-expressed in C. graminicola. Fluorescence microscopy of the resulting transformants strongly suggested that CgTRPF1, CgTRPF2, and CgTRPF3 do indeed co-localize in the same cellular compartment and may thus share a common function (Fig 4B).
Fig 4. Sub-cellular localization of Colletotrichum graminicola TRPF proteins.
(A) Hyphae expressing CgTRPF1 through 4 fused to EGFPf driven by the respective native CgTRPF promoters. (B) Co-localisation of CgTRPF1 through 3 with each other. Oval conidia expressing CgTRPF1 through 3 genetically fused to mCherry and EGFPf driven by the respective native CgTRPF promoters. Each strain was transformed with a mCherry-tagged CgTRPF gene and another EGFPf-tagged CgTRPF gene. Upper panel: CgTRPF1-mCherry and CgTRPF2-EGFPf, middle panel: CgTRPF1-mCherry and CgTRPF3-EGFPf, bottom panel: CgTRPF2-mCherry and CgTRPF3-EGFPf. Bars: 10 μm.
Deletion strains for all four CgTRPF genes were obtained
As all CgTRPF genes were expressed in spores, plate cultures, and throughout the infection process, and since mutants of other fungi for TRPY1 homologues show severe defects [37, 38], we analysed the role of CgTRPF1 through 4 in growth and pathogenicity. To this end, we created deletion strains for each gene by homologous recombination. One strain for ΔCgtrpf1 and ΔCgtrpf3 and two strains for ΔCgtrpf2 with the desired single integration of the deletion cassette were obtained (S1 Fig). For ΔCgtrpf4 only strains with several integrations were obtained. For further analysis of this gene, three individual strains were chosen that showed different Southern blot patterns for one additional integration event (S1 Fig).
As there are four TRPY1 homologs in the genome of C. graminicola, a functional redundancy of the genes is not unlikely. This may be indicated by an increased expression of the remaining CgTRPF genes in the deletion strains. We therefore performed qRT-PCR experiments on wild type and Cgtrpf deletion strains, which are shown in S2 Fig. In the deletion strains, an occasional weak upregulation of other family members was observed. However, this alteration was always well below two-fold, which does not indicate a strong compensatory response.
Spore germination is not altered in Cgtrpf deletion mutants
Germination of spores and appressorium formation are initial steps in the infection process of Colletotrichum species, which have been reported to be dependent on Ca2+ release from internal stores [59]. As all CgTRPF genes are expressed in spores (Fig 3) and as all CgTRPF proteins are localized to endomembranes (Fig 4), a role of those proteins in spore germination appeared likely. Therefore, germination was tested on polystyrene (Fig 5A) and on onion epidermis (Fig 5B). In both types of assay, germination of the deletion strains was not reduced compared to the wild type, indicating either no role of the CgTRPF genes in this process or a functional redundancy of the genes.
Fig 5. Germination of C. graminicola spores on (A) polystyrene and (B) onion epidermis.
Black: germ tubes with appressoria, grey: germ tubes without appressoria, white: ungerminated conidia. On onion epidermis, only appressoria were counted. Data are means ± SE (N = 3; >100 spores per replicate).
Cgtrpf deletion mutants are not defective in the utilization of complex carbon sources
C. graminicola is able to grow on culture media and plant tissues containing complex carbon sources. To utilize those, the fungus has to secrete hydrolytic enzymes by exocytosis, allowing the uptake of low-molecular compounds. Since exocytosis is known to be dependent on locally elevated [Ca2+]cyt, intracellular Ca2+ channels may have a potential impact on the secretion of enzymes that hydrolyse carbohydrates. To test whether CgTRPFs may function in this process or in the utilization of diverse carbon sources, wild type and deletion strains for all of the four genes were assayed for growth on mLCM and PDA, as well as on minimal media supplemented with glucose, sucrose, raffinose, sorbitol, mannitol, malate, pectate, or cellulose. The strains did not show any consistent and reproducible growth differences on any of the tested media (Fig 6). Hence, CgTRPF genes are either not required for those secretion events, or the genes are functionally redundant in this process.
Fig 6. Growth of C. graminicola colonies on different carbon sources.
Growth was assessed on mLCM agar, PDA, and minimal media administered with 2% of the respective carbon source. All values were normalized to the growth of the wild type on the respective medium. Colony diameter was measured 117 hours post inoculation. Data are means ± SE (N = 3).
Cgtrpf deletion mutants are not defective in growth on low-Ca2+ media and in the generation of tip-focussed [Ca2+]cyt spikes
As the CgTRPF proteins have similarities to the Ca2+-permeable TRPY1, which is known to regulate [Ca2+]cyt homeostasis in yeast, we tested if they play a role for growth under Ca2+-limited conditions. To this end, the wild type and deletion strains were cultivated on Ca2+-depleted SC agar medium, containing 1.7 μM total Ca2+, and on Ca2+-replete SC medium supplemented with 900 μM Ca2+ [45]. As previously reported [45], the wild type showed a growth reduction by around 20% on low-Ca2+ medium (Fig 7). This decrease in growth was also apparent, but not exacerbated, in the Cgtrpf mutant strains (Fig 7). Therefore, a role of CgTRPF proteins during Ca2+ starvation is unlikely.
Fig 7. Colony growth of C. graminicola on low-Ca2+ media.
Wild type and deletion strains were grown for 144 h on Ca2+-depleted SC media, and colony diameter was determined. Data are means ± SE (N = 3).
To directly analyse a possible role of the CgTRPF proteins in the generation of [Ca2+]cyt signals, wild type and Cgtrpf deletion mutants were transformed with the Ca2+ reporter Yellow Cameleon 3.6, and individual hyphae were examined by ratiometric fluorescence microscopy. As reported previously [45], the C. graminicola wild type showed tip-focused spikes of high [Ca2+]cyt during hyphal growth in a highly variable manner (Fig 8). It has been suggested that in N. crassa, a tip-focussed [Ca2+]cyt gradient is generated by Ca2+ release from intracellular vesicles [60]. We therefore tested whether any of the CgTRPFs may contribute to the apical [Ca2+]cyt spikes of C. graminicola. However, all mutants still showed [Ca2+]cyt spiking at the hyphal tip (Fig 8), with the spike occurrence being highly variable between individual hyphae.
Fig 8. Yellow Cameleon-based measurements of [Ca2+]cyt in individual hyphae.
Kymographs of individual hyphae of wild type and deletion mutants. Each horizontal pixel line represents the mean [Ca2+]cyt of a 5-pixel-wide ROI in the middle of each hypha. ROIs of subsequent images, acquired every 2 sec, were plotted one below the other. The slope of the kymographs, read from top to bottom, thus indicates the growth rate. Relative [Ca2+]cyt is displayed in false-colour using the RGB rainbow scale. All measurements were performed on hyphae growing at an mLCM agar-glass interface [45].
To observe [Ca2+]cyt spike occurrence in undisturbed whole colonies, we developed a protocol based on aequorin luminometry [45]. Similar to our previous study [45], the wild type generated distinct [Ca2+]cyt spikes during growth, which had a similar duration as the spikes observed in individual tips (not shown). The rate of spike occurrence was not reduced in any of the deletion strains (S3 Fig).
CgTRPF proteins do not function as osmotic stress sensors
TRPY1 is known to act as a sensor for osmotic disturbance in S. cerevisiae. To analyse whether CgTRPFs of C. graminicola may share this function, deletion mutants were analysed for growth under osmotic stress conditions. As expected, growth of the C. graminicola wild type strain on mLCM agar was increasingly inhibited by increasing glycerol concentrations (Fig 9A). All deletion mutants of the four CgTRPF genes were inhibited similar to the wild type on medium containing 0.5 M glycerol (Fig 9B).
Fig 9. Response of mycelial growth of C. graminicola and [Ca2+]cyt of C. graminicola and yeast to hyperosmotic stress.
(A, B) Mycelial growth of C. graminicola osmotically stressed with glycerol. (A) Colony diameter of wild type stressed with varying concentrations of glycerol, normalized to unstressed colonies. (B) Wild type and deletion strains stressed with 0.5 M glycerol, normalized to the wild type. Colony diameters were determined 122 hours post inoculation. Data are means ± SE (N = 3). (C, D) [Ca2+]cyt response of yeast and C. graminicola to NaCl measured by aequorin luminescence. (C) Response of trpy1Δ yeast mutant cells transformed with the indicated vectors to a solution (pH 7.0) containing 1.5 M NaCl, 50 mM MES-KOH, and 25 mM EGTA. Treatment was started at 1 min. Red line: empty pFL61 vector (negative control); green line: pFL61-ScTRPY1 (positive control); blue lines: yeast strains transformed with pFL61 containing CgTRPF1 through 4. Data are means ± SE (N = 3). (D) [Ca2+]cyt measurements on C. graminicola wild type colonies. Whole colonies were pre-treated with 50 mM MES-KOH (pH 7.0) for 30 min prior to recording, followed by treatment with a solution (pH 7.0) containing 50 mM MES-KOH and no NaCl (grey line) or 1.5 M NaCl (final concentration; red line). To abolish the influx of extracellular Ca2+, colonies were pre-treated with a solution (pH 7.0) containing 50 mM MES-KOH and 25 mM EGTA for 30 min prior to measurement, followed by treatment with a solution (pH 7.0) containing 50 mM MES-KOH, 25 mM EGTA, and no NaCl (green line) or 1.5 M NaCl (final concentration) (blue line). Treatment solutions were added after 1 min of recording. Traces show individual measurements in order to demonstrate [Ca2+]cyt spikes in the MES-KOH control treatment. Replicate measurements can be found in S4 Fig.
A possible redundancy of the CgTRPF proteins in osmotic sensing was investigated by heterologous expression in yeast harbouring the [Ca2+]cyt reporter apoaequorin. S. cerevisiae responds to hyperosmotic stress with a Ca2+ influx from the extracellular medium and a concomitant release of Ca2+ from the vacuole mediated by TRPY1 [32]. Chelation of extracellular Ca2+ renders the osmotic upshock-triggered [Ca2+]cyt elevation absolutely dependent on TRPY1. Under those conditions, a trpy1Δ deletion mutant carrying the empty vector pFL61 did not show any [Ca2+]cyt response to an osmotic upshock exerted by 1.5 M NaCl (Fig 9C, red line). As expected, the transient increase in [Ca2+]cyt after application of osmotic stress was restored in transformants complemented with the native TRPY1 from S. cerevisiae (Fig 9C, green line). To test if any of the C. graminicola TRPF proteins mediates a Ca2+ flux in response to osmotic upshock, the full-length cDNAs of CgTRPF1 though 4 were constitutively expressed in the trpy1Δ mutant. Expression of the CgTRPF genes was confirmed by RT-PCR (S5 Fig). Surprisingly, none of the CgTRPF genes complemented the [Ca2+]cyt response of the yeast mutant (Fig 9C, blue lines), suggesting that they may either not be sensitive to osmotic shock or not active, e.g. due to problems with heterologous expression.
Since the CgTRPF genes did not complement the trpy1Δ yeast strain, we asked whether this fungus, like S. cerevisiae, responds to hyperosmotic shock with a [Ca2+]cyt transient that is partially generated by Ca2+ release from internal stores. To this end, we employed the apoaequorin-harbouring wild type strain. Treatment of whole colonies with buffer alone caused a short and small response that phased out entirely after about 3 min (Fig 9D, grey line). This baseline was stable until the end of the measurement at 30 min except for very short [Ca2+]cyt spikes in the range of a few seconds, as described above (S3 Fig). Treatment with a solution containing 1.5 M NaCl evoked a large initial peak in [Ca2+]cyt that was followed by a shoulder and a sustained elevation at a level well above the baseline (Fig 9D, red line). This strong response was nearly completely prevented by the addition of EGTA, which chelates extracellular Ca2+ (Fig 9D, blue line). This indicates that, in contrast to S. cerevisiae, the hyperosmotic stress-triggered [Ca2+]cyt response of C. graminicola is sourced nearly entirely from the external medium. EGTA alone provoked no discernible response (Fig 9D, green line).
Cgtrpf deletion mutants are unaffected in pathogenicity
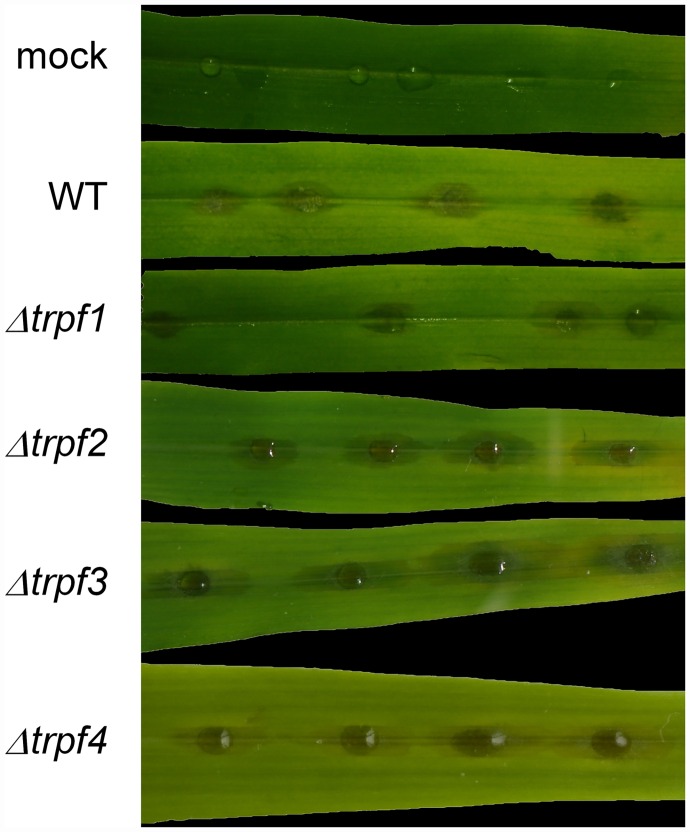
As all assays failed to identify a possible involvement of C. graminicola TRPF proteins in various aspects of growth and environmental responses, and as all CgTRPF genes were expressed during infection of maize (Fig 3), we tested the deletion strains for virulence, which integrates a large array of sensing and signalling mechanisms. In a detached leaf segment assay, none of the mutants exhibited symptoms that differed noticeably from those of the wild type (Fig 10).
Fig 10. Detached-leaf infection assay.
Sections of the third leaf of two-week-old maize plants (cv. Golden Jubilee) were infected with 104 spores of C. graminicola wild type or Cgtrpf1 through 4 deletion mutants per 10-μL drop, or mock-infected with 10 μL of 0.02% Tween 20 in bidistilled water. Representative images of three biological replicates are shown; the experiment was repeated twice with similar results.
Discussion
A number of pharmacological studies have suggested a role of Ca2+ release from internal organelles in the regulation of various developmental processes in fungi, such as germination [59] and hyphal tip elongation [60], as well as in responses of fungi to environmental stimuli, such as changes in osmolarity [24, 32, 56]. In S. cerevisiae, the Transient Receptor Potential channel homologue TRPY1 is a Ca2+-permeable channel in endomembranes that has been demonstrated to contribute to the generation of [Ca2+]cyt signals [30]. In support of a pivotal role of this class of ion channels in fungi, the knock-down of a TRPY1 homologue in M. oryzae resulted in a drastically reduced colony growth [37]. We therefore considered this family as a potential target for plant protection strategies against the devastating maize pathogen C. graminicola, and analysed its involvement in growth, environmental responses, and pathogenicity of this fungus. Our initial database queries revealed that this gene family was expanded in most filamentous fungi, with up to four members in the examined genomes, while the genomes of all examined yeasts contain only a single TRPY1 homologue. This is in good agreement with a comparative genomic analysis pointing to a possible expansion of the TRP family in filamentous fungi [18]. The members in filamentous fungi, which we denominate TRPFs, cluster in five subgroups. In the genome of C. graminicola, four TRPF genes were found. All were expressed throughout development and infection, and all encoded proteins were localized in intracellular organelles. Further on, the proteins display a membrane topology similar to the yeast TRPY1 channel, albeit with a variable number of TM domains additional to the core of six TM domains. CgTRPF1, 3, and 4 also have acidic motifs that are similar to the Ca2+-binding tetra-aspartate motif of TRPY1 [34]. These motifs are predicted to reside in cytosolic or luminal termini. Interestingly, a luminal Ca2+-binding site and regulation of channel activity by luminal Ca2+ have been demonstrated for the Two Pore Channel 1 (TPC1) [61], a vacuolar cation channel in plants [62]. CgTRPF1 and CgTRPF3 may thus sense and be regulated by luminal [Ca2+]. Collectively, an important role of these genes in Ca2+-related processes, in particular those involving Ca2+ release from internal stores, seemed likely, but could not be unveiled in our experiments.
C. graminicola TRPFs are not activated by osmotic upshock
In S. cerevisiae, the only TRP member, TRPY1 (Yvc1), forms a stretch-activated Ca2+-permeable cation channel in the vacuolar membrane [30, 33] that contributes to the generation of the hyperosmotic-shock-triggered [Ca2+]cyt signal [32]. By complementation analysis of the trpy1Δ mutant, mechanosensitivity and osmotic response were also shown for the TRPY1 homologues of the yeasts K. lactis and C. albicans [35], as well as of the filamentous fungus Fusarium graminearum [36]. In contrast, none of the CgTRPF genes from C. graminicola complemented the defective [Ca2+]cyt response of the S. cerevisiae trpy1Δ mutant to osmotic upshock. This might indicate that technical problems of the heterologous expression system (e.g. incorrect folding, missing interaction partners, insufficient stabilization against protein degradation, or mislocalization of the CgTRPF proteins) have prevented yeast complementation. However, we consider this as not very likely because other TRP channels are functional in this system. Alternatively, the failure to complement the trpy1Δ response to osmotic upshock may indicate that not all TRPF proteins act in the perception of osmotic stress. To further test this presumption, the response of C. graminicola to hyperosmotic shock was analysed. In C. graminicola, 1.5 M NaCl triggered a massive Ca2+ influx via the plasma membrane, but, unlike in yeast, a negligible release of Ca2+ from internal stores. Since TRPF channels of C. graminicola were localized to intracellular membranes, these results further substantiate the idea that in C. graminicola TRP proteins are not involved in Ca2+ release into the cytosol upon hyperosmotic stress.
C. graminicola TRPFs are dispensable for hyphal growth in axenic culture
A further process that we considered likely to be regulated by TRPF proteins is the utilization of complex carbon sources, which relies on the exocytosis of hydrolytic enzymes. In yeast, membrane fusion, a prerequisite of exocytosis, is dependent on the activation of calmodulin by Ca2+ release from the fusing vesicles [63]. Furthermore, tricalbins, which contain three Ca2+-binding C2 domains, and which are homologous to Ca2+-activated synaptotagmin in animals, have been linked to membrane fusion in yeast [64]. However, despite the presumed [Ca2+]cyt dependence of enzyme secretion, the Cgtrpf deletion strains did not differ from the wild type in their growth on complex carbon sources.
Growth of C. graminicola colonies is very sensitive to the inhibitors 2-APB and capsazepine, which block a range of TRP channels in animals [45]. However, none of the Cgtrpf deletion stains showed a diminished growth potential on various standard media, or on media with complex carbon sources, all containing high amounts of Ca2+. Since release of Ca2+ from intracellular stores might become important for growth under Ca2+-limiting conditions, we cultivated the strains on Ca2+-depleted medium, which causes a moderate growth depression in the wild type. However, growth depression in Cgtrpf deletion strains was not more severe than in the wild type. Hence, individual CgTRPF proteins are dispensable for growth in Ca2+-limiting environments.
During undisturbed growth, filamentous fungi, including C. graminicola, generate short tip-focussed [Ca2+]cyt pulses that can be visualized by fluorescence ratio imaging microscopy of the Yellow Cameleon (YC) reporter protein [45, 65, 66]. Unlike in other tip-growing systems, such as pollen tubes [67], these [Ca2+]cyt pulses are apparently not related to growth kinetics, but may rather be involved in environmental sensing [45]. [Ca2+]cyt spikes of short duration are also detectable on whole-colony level as luminescence of the Ca2+ reporter aequorin [45]. In N. crassa, a tip-focussed Ca2+ gradient has been suggested to be maintained by Ca2+ release from intracellular vesicles [60]. However, in Cgtrpf deletion mutants, [Ca2+]cyt spike generation was affected neither on single-hypha level nor on whole-colony level. This corresponds well to the fact that on whole-colony level, spike occurrence was nearly completely abolished by chelation of extracellular Ca2+ [45], suggesting that those [Ca2+]cyt spikes depend on Ca2+ influx rather than Ca2+ release from internal stores.
Deletion of C. graminicola TRPFs does not impede pathogenicity
The CgTRPF genes were expressed throughout the infection process of C. graminicola on maize plants. Interestingly, there was a transcriptional regulation of the genes by up to 13-fold during the course of infection. This is unexpected for ion channels, which are primarily regulated on post-translational level. However, regulation of TRP channels on mRNA level is also known for a number of mammalian family members, such as TRPC1, TRPC3, TRPV4, and TRPV6 [68, 69, 70].
Leaf segment infection assays were performed to integrate all pathogenic processes from spore germination to leaf necrosis [71]. These assays did not indicate pathogenicity defects in any of the Cgtrpf deletion strains. This is in stark contrast to the phenotype of a trpf1 RNAi knockdown mutant of M. oryzae, which showed a severely repressed virulence [37]. However, the M. oryzae genome bears only two TRPF genes, so that in C. graminicola, there may be higher degree of functional redundancy between the family members, albeit their topologies and structures vary. To resolve this question, we attempted a quadruple-RNAi knockdown approach, employing a vector system that has previously been successfully used to diminish expression of a C. graminicola β-1,3-Glucan Synthase-encoding gene [5]. Unfortunately, RNAi-based knockdown of CgTRPF genes was not very efficient, with their expression being decreased by not more than 50% (data not shown). Quadruple-RNAi strains did not show any phenotypic differences to the wild type during the strain selection process and were still pathogenic in leaf segment assays (data not shown). Unfortunately, it was therefore not possible to analyse a functional redundancy of the four CgTRPF genes, which might be the cause of the absence of phenotypical alterations in any of the single Cgtrpf mutants. Albeit a redundancy was not obvious on transcriptional level in the deletion strains, a redundancy may also occur on the level of protein activity. It is also possible that the TRPF genes of C. graminicola are important under conditions not tested in this study, but occurring in its native habitat, such as extreme temperatures, high light intensities, or interaction with other microorganisms.
Evidence for a functional diversification of orthologous Ca2+ channels in fungi
In contrast to M. oryzae, TRPF proteins in C. graminicola are not essential for colony growth and virulence. Furthermore, these channels do not mediate a [Ca2+]cyt elevation after osmotic upshock, as shown for homologues from other fungal species. This indicates a functional diversification of this ion channel family in fungi. A change in functionality can be similarly observed in an animal TRP family member, TRPV1, the receptor for capsaicin (the spicy component of hot chilli pepper): Rabbits are about 100 times less sensitive to capsaicin than humans and rats, and birds are completely insensitive. A molecular basis for these drastic differences lies in just two point mutations in the TRPV1 gene [31]. Hence, even small variations may also render the TRPF orthologs functionally highly diverse in different fungal species.
Species-specific differences in the regulation of fungal growth have also become apparent for the Cch1/Mid1 complex that mediates Ca2+ uptake across the plasma membrane. In Aspergillus nidulans a deletion of Cch1 and/or Mid1 results in drastically reduced colony growth even on complete media [27], whereas in Cryptococcus neoformans and Botrytis cinerea the deletion of Cch1 and/or Mid1 affects growth only under severe Ca2+ limitation, and deletion strains of B. cinera are phenotypically indifferent from the wild type inside their native host [28, 29]. These examples support the notion that components of the Ca2+ signalling toolbox may be employed differently in different fungi and that results may not always be simply extrapolated from one species to another.
Supporting Information
Genomic DNA was digested using the indicated restriction endonucleases and probed with a digoxigenin-labelled probe binding to the 5’ region of the hygromycinB phosphotransferase gene. Clones used in this study are indicated in red.
(TIF)
Strains were cultivated for 3.5 days on mLCM agar using the PAAP protocol [41] and assayed by qRT-PCR. Black: CgTRPF1, dark grey: CgTRPF2, middle grey: CgTRPF3, light grey: CgTRPF4. Data are means ± SE (N = 3).
(TIF)
Colonies of C. graminicola wild type and Cgtrpf1 through 4 deletion strains expressing apoaequorin were grown for 80 h in 35-mm Petri dishes on mLCM agar supplemented with 10 μM coelenterazine. [Ca2+]cyt-dependent luminescence was detected for 20 min. Data are the means ± SE (N = 4).
(TIF)
Whole colonies were pre-treated with 50 mM MES-KOH (pH 7.0) for 30 min prior to recording, followed by treatment with a solution (pH 7.0) containing 50 mM MES-KOH and no NaCl (grey line) or 1.5 M NaCl (final concentration; red line). To abolish the influx of extracellular Ca2+, colonies were pre-treated with a solution (pH 7.0) containing 50 mM MES-KOH and 25 mM EGTA for 30 min prior to measurement, followed by treatment with a solution (pH 7.0) containing 50 mM MES-KOH, 25 mM EGTA, and no NaCl (green line) or 1.5 M NaCl (final concentration; blue line). Treatment solutions were added after 1 min of measurement. Traces show single measurements in order to demonstrate [Ca2+]cyt spikes in the MES-KOH control treatment.
(TIF)
Full-length cDNAs of the TRPF genes were amplified from RNA extracted from log-phase cultures of S. cerevisiae trpy1Δ transformed with pFL61-CgTRPF1 through pFL61-CgTRPF4. Products were expected at 2098, 2152, 3522, and 2101 bp for CgTRPF1, CgTRPF2, CgTRPF3, and CgTRPF4, respectively. RT: + reverse transcriptase added in cDNA synthesis, − reverse transcriptase omitted in cDNA synthesis.
(TIF)
(TXT)
Exons are indicated in uppercase letters, introns are indicated in lowercase letters.
(TXT)
Cytosolic amino acid residues are highlighted in yellow, luminal amino acid residues are highlighted in light blue, amino acid residues in the putative pore loop are highlighted in dark blue. Acidic amino acid residues are indicated in red.
(PDF)
(XLSX)
(PDF)
(PDF)
Topology prediction was performed with TOPCONS (http://topcons.net/) using standard settings and the full-length protein sequences of S. cerevisiae TRPY1, and CgTRPF1, CgTRPF2, CgTRPF3, and CgTRPF4.
(PDF)
Organisms were ordered by phylum and class. The sequenced strain and the NCBI Taxid of each species are given in the indicated columns. To link the proteins of this table with the tree, see column TRPY1 Homologue No. For the distinct identification of the proteins the locus tag may be used. E-values were calculated on NCBI.
(PDF)
Acknowledgments
We thank Liane Freitag and Tina Peiter-Volk for excellent technical assistance and Elke Vollmer for skilful plant husbandry.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by a grant (PE1500/2-1 within the Research Unit FOR 666) from the Deutsche Forschungsgemeinschaft (DFG) to EP and by a grant from the Ministry of Agriculture and the Environment of the Federal State of Sachsen-Anhalt to EP. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bechinger C, Giebel K-F, Schnell M, Leiderer P, Deising HB, Bastmeyer M. Optical measurements of invasive forces exerted by appressoria of a plant pathogenic fungus. Science 1999; 285: 1896–1899. [DOI] [PubMed] [Google Scholar]
- 2.Bergstrom GC, Nicholson RL. The biology of corn anthracnose. Knowledge to exploit for improved management. Plant Dis. 1999; 83: 596–608. [DOI] [PubMed] [Google Scholar]
- 3.Krijger J-J, Horbach R, Behr M, Schweizer P, Deising HB, Wirsel SGR. The yeast signal sequence trap identifies secreted proteins of the hemibiotrophic corn pathogen Colletotrichum graminicola. Mol. Plant Microbe Interact. 2008; 21: 1325–1336. 10.1094/MPMI-21-10-1325 [DOI] [PubMed] [Google Scholar]
- 4.O'Connell RJ, Thon MR, Hacquard S, Amyotte SG, Kleemann J, Torres MF et al. Lifestyle transitions in plant pathogenic Colletotrichum fungi deciphered by genome and transcriptome analyses. Nature Genetics 2012; 44: 1060–1065. 10.1038/ng.2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oliveira-Garcia E, Deising HB. Infection structure-specific expression of β-1,3-glucan synthase is essential for pathogenicity of Colletotrichum graminicola and evasion of β-glucan-triggered immunity in maize. Plant Cell 2013; 25: 2356–2378. 10.1105/tpc.112.103499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albarouki E, Schafferer L, Ye F, von Wirén N, Haas H, Deising HB. Biotrophy-specific downregulation of siderophore biosynthesis in Colletotrichum graminicola is required for modulation of immune responses of maize. Mol. Microbiol. 2014; 92: 338–355. 10.1111/mmi.12561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li L, Wright SJ, Krystofova S, Park G, Borkovich KA. Heterotrimeric G protein signaling in filamentous fungi. Annu. Rev. Microbiol. 2007; 61: 423–452. [DOI] [PubMed] [Google Scholar]
- 8.Xue C, Hsueh Y-P, Heitman J. Magnificent seven: roles of G protein-coupled receptors in extracellular sensing in fungi. FEMS Microbiol. Rev. 2008; 32: 1010–1032. 10.1111/j.1574-6976.2008.00131.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nature Rev. Mol. Cell Biol. 2003; 4: 517–529. [DOI] [PubMed] [Google Scholar]
- 10.Zelter A, Bencina M, Bowman BJ, Yarden O, Read ND. A comparative genomic analysis of the calcium signaling machinery in Neurospora crassa, Magnaporthe grisea, and Saccharomyces cerevisiae. Fungal Genet. Biol. 2004; 41: 827–841. [DOI] [PubMed] [Google Scholar]
- 11.Stathopoulos-Gerontides A, Guo JJ, Cyert MS. Yeast calcineurin regulates nuclear localization of the Crz1p transcription factor through dephosphorylation. Genes & Development 1999; 13: 798–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.da Silva Ferreira ME, Heinekamp T, Härtl A, Brakhage AA, Semighini CP, Harris SD et al. Functional characterization of the Aspergillus fumigatus calcineurin. Fungal Genet. Biol. 2007; 44: 219–230. [DOI] [PubMed] [Google Scholar]
- 13.Schumacher J, De Larrinoa IF, Tudzynski B. Calcineurin-responsive zinc finger transcription factor CRZ1 of Botrytis cinerea is required for growth, development, and full virulence on bean plants. Eukaryot. Cell 2008; 7: 584–601. 10.1128/EC.00426-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bowman BJ, Abreu S, Margolles-Clark E, Draskovic M, Bowman EJ. Role of four calcium transport proteins, encoded by nca-1, nca-2, nca-3, and cax, in maintaining intracellular calcium levels in Neurospora crassa. Eukaryot. Cell 2011; 10: 654–661. 10.1128/EC.00239-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dinamarco TM, Freitas FZ, Almeida RS, Brown NA, dos Reis TF, Ramalho LNZ et al. Functional characterization of an Aspergillus fumigatus calcium transporter (PmcA) that is essential for fungal infection. PLoS ONE 2012; 7: e37951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu Y, Wang J, Ying S-H, Feng M-G. Five vacuolar Ca2+ exchangers play different roles in calcineurin-dependent Ca2+/Mn2+ tolerance, multistress responses and virulence of a filamentous entomopathogen. Fungal Genet. Biol. 2014; 73: 12–19. 10.1016/j.fgb.2014.09.005 [DOI] [PubMed] [Google Scholar]
- 17.Peiter E. The plant vacuole: Emitter and receiver of calcium signals. Cell Calcium 2011; 50: 120–128. 10.1016/j.ceca.2011.02.002 [DOI] [PubMed] [Google Scholar]
- 18.Prole DL, Taylor CW. Identification and analysis of cation channel homologues in human pathogenic fungi. PLoS ONE 2012; 7: e42404 10.1371/journal.pone.0042404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cai X. P2X receptor homologs in basal fungi. Purinergic Signal. 2012; 8: 11–13. 10.1007/s11302-011-9261-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paidhungat M, Garret S. A homolog of mammalian, voltage-gated calcium channels mediates yeast pheromone-stimulated Ca2+ uptake and exacerbates the cdc1 (Ts) growth defect. Mol. Cell. Biol. 1997; 17: 6339–6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fischer M, Schnell N, Chattaway J, Davies P, Dixon G, Sanders D. The Saccharomyces cerevisiae CCH1 gene is involved in calcium influx and mating. FEBS Lett. 1997; 419: 259–262. [DOI] [PubMed] [Google Scholar]
- 22.Locke EG, Bonilla M, Liang L, Takita Y, Cunningham KW. A homolog of voltage-gated Ca2+ channels stimulated by depletion of secretory Ca2+ in yeast. Mol. Cell. Biol. 2000; 20: 6686–6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iida H, Nakamura H, Ono T, Okumara MS, Anraku Y. MID1, a novel Saccharomyces cerevisiae gene encoding a plasma membrane protein, is required for Ca2+ influx and mating. Mol. Cell. Biol. 1994; 14: 8259–8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsumoto TK, Ellsmore AJ, Cessna SG, Low PS, Pardo JM, Bressnan RA et al. An osmotically induced cytosolic Ca2+ transient activates calcineurin signaling to mediate ion homeostasis and salt tolerance of Saccharomyces cerevisiae. J. Biol. Chem. 2002; 277: 33075–33080. [DOI] [PubMed] [Google Scholar]
- 25.Peiter E, Fischer M, Sidaway K, Roberts SK, Sanders D. The Saccharomyces cerevisiae Ca2+ channel Cch1pMid1p is essential for tolerance to cold stress and iron toxicity. FEBS Lett. 2005; 579: 5697–5703. [DOI] [PubMed] [Google Scholar]
- 26.Viladevall L, Serrano R, Ruiz A, Domenech G, Giraldo J, Barceló A et al. Characterization of the calcium-mediated response to alkaline stress in Saccharomyces cerevisiae. J. Biol. Chem. 2004; 279: 43614–43624. [DOI] [PubMed] [Google Scholar]
- 27.Wang S, Cao J, Liu X, Hu H, Shi J, Zhang S et al. Putative calcium channels CchA and MidA play the important roles in conidiation, hyphal polarity and cell wall components in Aspergillus nidulans. PLoS ONE 2012; 7: e46564 10.1371/journal.pone.0046564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu M, Du P, Heinrich G, Cox GM, Gelli A. Cch1 mediates calcium entry in Cryptococcus neoformans and is essential in low-calcium environments. Eukaryot. Cell 2006; 5: 1788–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harren K, Tudzynski B. Cch1 and Mid1 are functionally required for vegetative growth under low-calcium conditions in the phytopathogenic ascomycete Botrytis cinerea. Eukaryot. Cell 2013; 12: 712–724. 10.1128/EC.00338-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palmer CP, Zhou X-L, Lin J, Loukin SH, Kung C, Saimi Y. A TRP homolog in Saccharomyces cerevisiae forms an intracellular Ca2+-permeable channel in the yeast vacuolar membrane. Proc. Natl. Acad. Sci. USA 2001; 98: 7801–7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nilius B, Szallasi A. Transient Receptor Potential channels as drug targets: from the science of basic research to the art of medicine. Pharmacol. Rev. 2014; 66: 676–814. 10.1124/pr.113.008268 [DOI] [PubMed] [Google Scholar]
- 32.Denis V, Cyert MS. Internal Ca2+ release in yeast is triggered by hypertonic shock and mediated by a TRP channel homologue. J. Cell Biol. 2002; 156: 29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou X-L, Batiza AF, Loukin SH, Palmer CP, Kung C, Saimi Y. The transient receptor potential channel on the yeast vacuole is mechanosensitive. Proc. Natl. Acad. Sci. USA 2003; 100: 7105–7110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su Z, Zhou X, Loukin SH, Saimi Y, Kung C. Mechanical force and cytoplasmic Ca2+ activate yeast TRPY1 in parallel. J. Membr. Biol. 2009; 227: 141–150. 10.1007/s00232-009-9153-9 [DOI] [PubMed] [Google Scholar]
- 35.Zhou X-L, Loukin SH, Coria R, Kung C, Saimi Y. Heterologously expressed fungal transient receptor potential channels retain mechanosensitivity in vitro and osmotic response in vivo. Eur. Biophys. J. 2005; 34: 413–422. [DOI] [PubMed] [Google Scholar]
- 36.Ihara M, Hamamoto S, Miyanoiri Y, Takeda M, Kainosho M, Yabe I et al. Molecular bases of multimodal regulation of a fungal Transient Receptor Potential (TRP) channel. J. Biol. Chem. 2013; 288: 15303–15317. 10.1074/jbc.M112.434795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nguyen QB, Kadotani N, Kasahara S, Tosa Y, Mayama S, Nakayahiki H. Systematic functional analysis of calcium-signalling proteins in the genome of the rice-blast fungus, Magnaporthe oryzae, using a high-throughput RNA-silencing system. Mol. Microbiol. 2008; 68: 1348–1365. 10.1111/j.1365-2958.2008.06242.x [DOI] [PubMed] [Google Scholar]
- 38.Yu Q, Wang F, Zhao Q, Chen J, Zhang B, Ding X et al. A novel role of the vacuolar calcium channel Yvc1 in stress response, morphogenesis and pathogenicity of Candida albicans. Int. J. Med. Microbiol. 2014; 304: 339–350. 10.1016/j.ijmm.2013.11.022 [DOI] [PubMed] [Google Scholar]
- 39.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H et al. Clustal W and clustal X version 2.0. Bioinformatics 2007; 23: 2947–2948. [DOI] [PubMed] [Google Scholar]
- 40.Waterhouse AM, Procter JB, Martin DMA, Clamp M, Barton GJ. Jalview Version 2—a multiple sequence alignment editor and analysis workbench. Bioinformatics 2009; 25: 1189–1191. 10.1093/bioinformatics/btp033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lange M, Müller C, Peiter E. Membrane-assisted culture of fungal mycelium on agar plates for RNA extraction and pharmacological analyses. Anal. Biochem. 2014; 453: 58–60. 10.1016/j.ab.2014.02.023 [DOI] [PubMed] [Google Scholar]
- 42.Ludwig N, Löhrer M, Hempel M, Mathea S, Schliebner I, Menzel M et al. Melanin is not required for turgor generation but enhances cell-wall rigidity in appressoria of the corn pathogen Colletotrichum graminicola. Mol. Plant Microbe Interact. 2014; 27: 315–327. 10.1094/MPMI-09-13-0267-R [DOI] [PubMed] [Google Scholar]
- 43.Liu W, Saint DA. Validation of a quantitative method for real time PCR kinetics. Biochem. Biophys. Res. Communic. 2002; 294: 347–353. [DOI] [PubMed] [Google Scholar]
- 44.Schliebner I, Becher R, Hempel M, Deising HB, Horbach R. New gene models and alternative splicing in the maize pathogen Colletotrichum graminicola revealed by RNA-Seq analysis. BMC Genomics 2014; 15: 842 10.1186/1471-2164-15-842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lange M, Peiter E. Cytosolic free calcium dynamics as related to hyphal and colony growth in the filamentous fungal pathogen Colletotrichum graminicola. Fungal Genet. Biol. 2016; 91: 55–65. 10.1016/j.fgb.2016.04.001 [DOI] [PubMed] [Google Scholar]
- 46.Punt PJ, Oliver RP, Dingemanse MA, Pouwels PH, van den Hondel CAMJJ. Transformation of Aspergillus based on the hygromycin B resistance marker from Escherichia coli. Gene 1987; 56: 117–124. [DOI] [PubMed] [Google Scholar]
- 47.Lingner U, Münch S, Sode B, Deising HB, Sauer N. Functional characterization of a eukaryotic melibiose transporter. Plant Physiol. 2011; 156: 1565–1576. 10.1104/pp.111.178624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu J-H, Hamari Z, Han K-H, Seo J-A, Reyes-Dominguez Y, Scazzocchio C. Double-joint PCR: a PCR-based molecular tool for gene manipulations in filamentous fungi. Fungal Genet. Biol. 2004; 41: 973–981. [DOI] [PubMed] [Google Scholar]
- 49.Werner S, Sugui JA, Steinberg G, Deising HB. A chitin synthase with a myosin-like motor domain is essential for hyphal growth, appressorium differentiation, and pathogenicity of the maize anthracnose fungus Colletotrichum graminicola. Mol. Plant Microbe Interact. 2007; 20: 1555–1567. [DOI] [PubMed] [Google Scholar]
- 50.Lange M, Oliveira-Garcia E, Deising HB, Peiter E. A modular plasmid system for protein co-localization and bimolecular fluorescence complementation in filamentous fungi. Current Genetics 2014; 60: 343–350. 10.1007/s00294-014-0429-y [DOI] [PubMed] [Google Scholar]
- 51.Horbach R, Graf A, Weihmann F, Antelo L, Mathea S, Liermann JC et al. Sfp-type 4'-phosphopantetheinyl transferase is indispensable for fungal pathogenicity. Plant Cell 2009; 21: 3379–3396. 10.1105/tpc.108.064188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Batiza AF, Schulz T, Masson PH. Yeast respond to hypotonic shock with a calcium pulse. J. Biol. Chem. 1996; 271: 23357–23362. [DOI] [PubMed] [Google Scholar]
- 53.Elble R. A simple and efficient procedure for transformation of yeasts. BioTechniques 1992; 13: 18–20. [PubMed] [Google Scholar]
- 54.Minet M, Dufour M-E, Lacroute F. Complementation of Saccharomyces cerevisiae auxotrophic mutants by Arabidopsis thaliana cDNAs. Plant J. 1992; 2: 417–422. [DOI] [PubMed] [Google Scholar]
- 55.Allen DG, Blinks JR, Prendergast FG. Aequorin luminescence: Relation of light emission to calcium concentration—a calcium-independent component. Science 1977; 195: 996–998. [DOI] [PubMed] [Google Scholar]
- 56.Nelson G, Kozlova-Zwinderman O, Collis AJ, Knight MR, Fincham JRS, Stanger CP et al. Calcium measurement in living filamentous fungi expressing codon-optimized aequorin. Mol. Microbiol. 2004; 52: 1437–1450. [DOI] [PubMed] [Google Scholar]
- 57.Nagai T, Yamada S, Tominaga T, Ichikawa M, Miyawaki A. Expanded dynamic range of fluorescent indicators for Ca2+ by circularly permuted yellow fluorescent proteins. Proc. Natl. Acad. Sci. USA 2004; 101: 10554–10559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Behr M, Humbeck K, Hause G, Deising HB, Wirsel SGR. The hemibiotroph Colletotrichum graminicola locally induces photosynthetically active green islands but globally accelerates senescence on aging maize leaves. Mol. Plant Microbe Interact. 2010; 23: 879–892. 10.1094/MPMI-23-7-0879 [DOI] [PubMed] [Google Scholar]
- 59.Warwar V, Dickman MB. Effects of calcium and calmodulin on spore germination and appressorium development in Colletotrichum trifolii. Appl. Environ. Microbiol. 1996; 62: 74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Silverman-Gavrila LB, Lew RR. An IP3-activated Ca2+ channel regulates fungal tip growth. J. Cell Sci. 2002; 115: 5013–5025. [DOI] [PubMed] [Google Scholar]
- 61.Dadacz-Narloch B, Beyhl D, Larisch C, López-Sanjurjo EJ, Reski R, Kuchitsu K et al. A novel calcium binding site in the slow vacuolar cation channel TPC1 senses luminal calcium levels. Plant Cell 2011; 23: 2696–2707. 10.1105/tpc.111.086751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peiter E, Maathuis FJM, Mills LN, Knight H, Pelloux J, Hetherington AM et al. The vacuolar Ca2+-activated channel TPC1 regulates germination and stomatal movement. Nature 2005; 434: 404–408. [DOI] [PubMed] [Google Scholar]
- 63.Peters C, Mayer A. Ca2+/calmodulin signals the completion docking and triggers a late step of vacuole fusion. Nature 1998; 396: 575–580. [DOI] [PubMed] [Google Scholar]
- 64.Schulz TA, Creutz CE. The tricalbin C2 domains: Lipid-binding properties of a novel, synaptotagmin-like yeast protein family. Biochemistry 2004; 43: 3987–3995. [DOI] [PubMed] [Google Scholar]
- 65.Kim H-S, Czymmek KJ, Patel A, Modla S, Nohe A, Duncan R et al. Expression of the Cameleon calcium biosensor in fungi reveals distinct Ca2+ signatures associated with polarized growth, development, and pathogenesis. Fungal Genet. Biol. 2012; 49: 589–601. 10.1016/j.fgb.2012.05.011 [DOI] [PubMed] [Google Scholar]
- 66.Kim H-S, Kim J-E, Frailey D, Nohe A, Duncan R, Czymmek KJ et al. Roles of three Fusarium oxysporum calcium ion (Ca2+) channels in generating Ca2+ signatures and controlling growth. Fungal Genet. Biol. 2015; 82: 145–157. 10.1016/j.fgb.2015.07.003 [DOI] [PubMed] [Google Scholar]
- 67.Steinhorst L, Kudla J. Calcium—a central regulator of pollen germination and tube growth. Biochim. Biophys. Acta—Mol. Cell Res. 2013; 1833: 1573–1581. [DOI] [PubMed] [Google Scholar]
- 68.Pigozzi D, Ducret T, Tajeddine N, Gala J-L, Tombal B, Gailly P. Calcium store contents control the expression of TRPC1, TRPC3 and TRPV6 proteins in LNCaP prostate cancer cell line. Cell Calcium 2006; 39: 401–415. [DOI] [PubMed] [Google Scholar]
- 69.Jung C, Fandos C, Lorenzo IM, Plata C, Fernandes J, Gene GG et al. The progesterone receptor regulates the expression of TRPV4 channel. Pflügers Arch. 2009; 459: 105–113. 10.1007/s00424-009-0706-7 [DOI] [PubMed] [Google Scholar]
- 70.Walters JRF, Balesaria S, Chavele K-M, Taylor V, Berry JL, Khair U et al. Calcium channel TRPV6 expression in human duodenum: Different relationships to the vitamin D system and aging in men and women. J. Bone Miner. Res. 2006; 21: 1770–1777. [DOI] [PubMed] [Google Scholar]
- 71.Münch S, Ludwig N, Floss DS, Sugui JA, Koszucka AM, Voll LM et al. Identification of virulence genes in the corn pathogen Colletotrichum graminicola by Agrobacterium tumefaciens-mediated transformation. Mol. Plant Pathol. 2011; 12: 43–55. 10.1111/j.1364-3703.2010.00651.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Genomic DNA was digested using the indicated restriction endonucleases and probed with a digoxigenin-labelled probe binding to the 5’ region of the hygromycinB phosphotransferase gene. Clones used in this study are indicated in red.
(TIF)
Strains were cultivated for 3.5 days on mLCM agar using the PAAP protocol [41] and assayed by qRT-PCR. Black: CgTRPF1, dark grey: CgTRPF2, middle grey: CgTRPF3, light grey: CgTRPF4. Data are means ± SE (N = 3).
(TIF)
Colonies of C. graminicola wild type and Cgtrpf1 through 4 deletion strains expressing apoaequorin were grown for 80 h in 35-mm Petri dishes on mLCM agar supplemented with 10 μM coelenterazine. [Ca2+]cyt-dependent luminescence was detected for 20 min. Data are the means ± SE (N = 4).
(TIF)
Whole colonies were pre-treated with 50 mM MES-KOH (pH 7.0) for 30 min prior to recording, followed by treatment with a solution (pH 7.0) containing 50 mM MES-KOH and no NaCl (grey line) or 1.5 M NaCl (final concentration; red line). To abolish the influx of extracellular Ca2+, colonies were pre-treated with a solution (pH 7.0) containing 50 mM MES-KOH and 25 mM EGTA for 30 min prior to measurement, followed by treatment with a solution (pH 7.0) containing 50 mM MES-KOH, 25 mM EGTA, and no NaCl (green line) or 1.5 M NaCl (final concentration; blue line). Treatment solutions were added after 1 min of measurement. Traces show single measurements in order to demonstrate [Ca2+]cyt spikes in the MES-KOH control treatment.
(TIF)
Full-length cDNAs of the TRPF genes were amplified from RNA extracted from log-phase cultures of S. cerevisiae trpy1Δ transformed with pFL61-CgTRPF1 through pFL61-CgTRPF4. Products were expected at 2098, 2152, 3522, and 2101 bp for CgTRPF1, CgTRPF2, CgTRPF3, and CgTRPF4, respectively. RT: + reverse transcriptase added in cDNA synthesis, − reverse transcriptase omitted in cDNA synthesis.
(TIF)
(TXT)
Exons are indicated in uppercase letters, introns are indicated in lowercase letters.
(TXT)
Cytosolic amino acid residues are highlighted in yellow, luminal amino acid residues are highlighted in light blue, amino acid residues in the putative pore loop are highlighted in dark blue. Acidic amino acid residues are indicated in red.
(PDF)
(XLSX)
(PDF)
(PDF)
Topology prediction was performed with TOPCONS (http://topcons.net/) using standard settings and the full-length protein sequences of S. cerevisiae TRPY1, and CgTRPF1, CgTRPF2, CgTRPF3, and CgTRPF4.
(PDF)
Organisms were ordered by phylum and class. The sequenced strain and the NCBI Taxid of each species are given in the indicated columns. To link the proteins of this table with the tree, see column TRPY1 Homologue No. For the distinct identification of the proteins the locus tag may be used. E-values were calculated on NCBI.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.