Abstract
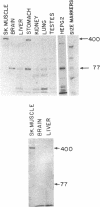
The known Duchenne muscular dystrophy (DMD) gene products, the muscle- and brain-type dystrophin isoforms, are 427-kDa proteins translated from 14-kilobase (kb) mRNAs. Recently we described a 6.5-kb mRNA that also is transcribed from the DMD gene. Cloning and in vitro transcription and translation of the entire coding region show that the 6.5-kb mRNA encodes a 70.8-kDa protein that is a major product of the DMD gene. It contains the C-terminal and the cysteine-rich domains of dystrophin, seven additional amino acids at the N terminus, and some modifications formed by alternative splicing in the C-terminal domain. It lacks the entire large domain of spectrin-like repeats and the actin-binding N-terminal domain of dystrophin. This protein is the major DMD gene product in brain and other nonmuscle tissues but is undetectable in skeletal muscle extracts.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arahata K., Ishiura S., Ishiguro T., Tsukahara T., Suhara Y., Eguchi C., Ishihara T., Nonaka I., Ozawa E., Sugita H. Immunostaining of skeletal and cardiac muscle surface membrane with antibody against Duchenne muscular dystrophy peptide. Nature. 1988 Jun 30;333(6176):861–863. doi: 10.1038/333861a0. [DOI] [PubMed] [Google Scholar]
- Bar S., Barnea E., Levy Z., Neuman S., Yaffe D., Nudel U. A novel product of the Duchenne muscular dystrophy gene which greatly differs from the known isoforms in its structure and tissue distribution. Biochem J. 1990 Dec 1;272(2):557–560. doi: 10.1042/bj2720557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea E., Zuk D., Simantov R., Nudel U., Yaffe D. Specificity of expression of the muscle and brain dystrophin gene promoters in muscle and brain cells. Neuron. 1990 Dec;5(6):881–888. doi: 10.1016/0896-6273(90)90348-j. [DOI] [PubMed] [Google Scholar]
- Boyce F. M., Beggs A. H., Feener C., Kunkel L. M. Dystrophin is transcribed in brain from a distant upstream promoter. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1276–1280. doi: 10.1073/pnas.88.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J. M., Man N. T., Morris G. E., Ginjaar I. B., Moorman A. F., van Ommen G. J. Specificity of dystrophin analysis improved with monoclonal antibodies. Lancet. 1990 Oct 6;336(8719):881–882. doi: 10.1016/0140-6736(90)92392-u. [DOI] [PubMed] [Google Scholar]
- Ervasti J. M., Campbell K. P. Membrane organization of the dystrophin-glycoprotein complex. Cell. 1991 Sep 20;66(6):1121–1131. doi: 10.1016/0092-8674(91)90035-w. [DOI] [PubMed] [Google Scholar]
- Feener C. A., Koenig M., Kunkel L. M. Alternative splicing of human dystrophin mRNA generates isoforms at the carboxy terminus. Nature. 1989 Apr 6;338(6215):509–511. doi: 10.1038/338509a0. [DOI] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig M., Monaco A. P., Kunkel L. M. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell. 1988 Apr 22;53(2):219–228. doi: 10.1016/0092-8674(88)90383-2. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lemaire C., Heilig R., Mandel J. L. The chicken dystrophin cDNA: striking conservation of the C-terminal coding and 3' untranslated regions between man and chicken. EMBO J. 1988 Dec 20;7(13):4157–4162. doi: 10.1002/j.1460-2075.1988.tb03311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love D. R., Hill D. F., Dickson G., Spurr N. K., Byth B. C., Marsden R. F., Walsh F. S., Edwards Y. H., Davies K. E. An autosomal transcript in skeletal muscle with homology to dystrophin. Nature. 1989 May 4;339(6219):55–58. doi: 10.1038/339055a0. [DOI] [PubMed] [Google Scholar]
- Makover A., Zuk D., Breakstone J., Yaffe D., Nudel U. Brain-type and muscle-type promoters of the dystrophin gene differ greatly in structure. Neuromuscul Disord. 1991;1(1):39–45. doi: 10.1016/0960-8966(91)90041-p. [DOI] [PubMed] [Google Scholar]
- Moser H. Duchenne muscular dystrophy: pathogenetic aspects and genetic prevention. Hum Genet. 1984;66(1):17–40. doi: 10.1007/BF00275183. [DOI] [PubMed] [Google Scholar]
- Nguyen thi Man, Cartwright A. J., Morris G. E., Love D. R., Bloomfield J. F., Davies K. E. Monoclonal antibodies against defined regions of the muscular dystrophy protein, dystrophin. FEBS Lett. 1990 Mar 26;262(2):237–240. doi: 10.1016/0014-5793(90)80199-s. [DOI] [PubMed] [Google Scholar]
- Nudel U., Robzyk K., Yaffe D. Expression of the putative Duchenne muscular dystrophy gene in differentiated myogenic cell cultures and in the brain. Nature. 1988 Feb 18;331(6157):635–638. doi: 10.1038/331635a0. [DOI] [PubMed] [Google Scholar]
- Nudel U., Zuk D., Einat P., Zeelon E., Levy Z., Neuman S., Yaffe D. Duchenne muscular dystrophy gene product is not identical in muscle and brain. Nature. 1989 Jan 5;337(6202):76–78. doi: 10.1038/337076a0. [DOI] [PubMed] [Google Scholar]
- Pons F., Augier N., Léger J. O., Robert A., Tomé F. M., Fardeau M., Voit T., Nicholson L. V., Mornet D., Léger J. J. A homologue of dystrophin is expressed at the neuromuscular junctions of normal individuals and DMD patients, and of normal and mdx mice. Immunological evidence. FEBS Lett. 1991 Apr 22;282(1):161–165. doi: 10.1016/0014-5793(91)80468-i. [DOI] [PubMed] [Google Scholar]
- Rapaport D., Lederfein D., den Dunnen J. T., Grootscholten P. M., Van Ommen G. J., Fuchs O., Nudel U., Yaffe D. Characterization and cell type distribution of a novel, major transcript of the Duchenne muscular dystrophy gene. Differentiation. 1992 Apr;49(3):187–193. doi: 10.1111/j.1432-0436.1992.tb00666.x. [DOI] [PubMed] [Google Scholar]
- Sicinski P., Geng Y., Ryder-Cook A. S., Barnard E. A., Darlison M. G., Barnard P. J. The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science. 1989 Jun 30;244(4912):1578–1580. doi: 10.1126/science.2662404. [DOI] [PubMed] [Google Scholar]
- Watkins S. C., Hoffman E. P., Slayter H. S., Kunkel L. M. Immunoelectron microscopic localization of dystrophin in myofibres. Nature. 1988 Jun 30;333(6176):863–866. doi: 10.1038/333863a0. [DOI] [PubMed] [Google Scholar]
- Zubrzycka-Gaarn E. E., Bulman D. E., Karpati G., Burghes A. H., Belfall B., Klamut H. J., Talbot J., Hodges R. S., Ray P. N., Worton R. G. The Duchenne muscular dystrophy gene product is localized in sarcolemma of human skeletal muscle. Nature. 1988 Jun 2;333(6172):466–469. doi: 10.1038/333466a0. [DOI] [PubMed] [Google Scholar]