Abstract
BACKGROUND
BRAF mutations occur in 5% to 11% of patients with metastatic colorectal cancer (mCRC) and have been associated with poor prognosis. The current study was undertaken to determine the clinicopathologic characteristics, PIK3CA (phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha) mutation frequency, and outcomes after metastasectomy in patients with BRAF-mutant mCRC.
METHODS
Data from 1941 consecutive patients with mCRC who underwent KRAS/BRAF mutation testing between 2009 and 2012 at a single institution were identified to identify BRAF-mutant mCRC cases (92 cases). BRAF wild-type mCRC cases from 2011 (423 cases) served as a control group.
RESULTS
BRAF-mutated mCRC was found to be significantly associated with older age at diagnosis, female sex, right-sided location, poorly differentiated morphology, and mucinous histology compared with wild-type cases. BRAF-mutant cases more frequently progressed from stage III disease (32% vs 17%; P =.003) and among those patients with stage III disease, T4 disease was more common (48% vs 27%; P =.05). PIK3CA was found to be co-mutated in 5% of BRAF-mutant tumors versus 17% of KRAS-mutant tumors (P <.01) and 4% of BRAF/KRAS wild-type cases. Patients with BRAF-mutated mCRC presented more frequently with peritoneal involvement (26% vs 14%; P <0.01) and less frequently with liver-limited metastases (41% vs 63%; P <.01). Patients with BRAF-mutated mCRC were less likely to undergo metastasectomy (41% vs 26% at 2 years from diagnosis of metastatic disease; P <.01) and were found to have lower overall survival (P <.01) after metastasectomy.
CONCLUSIONS
BRAF-mutant mCRC is associated with worse clinical outcome. Patients with BRAF-mutant tumors more commonly develop peritoneal metastases, less frequently present with disease limited to the liver, and have shorter survival after metastasectomy compared with patients with BRAF wild-type tumors.
Keywords: BRAF; colorectal cancer; liver resection; PIK3CA (phosphatidylinositol-4, 5-bisphosphate 3-kinase, catalytic subunit alpha); prognosis
INTRODUCTION
It has long been noted that outcomes for patients with metastatic colorectal cancer (mCRC) vary widely. Large-scale efforts to sequence the CRC genome1,2 have revealed that colorectal tumors as defined by site of origin exhibit a diversity of genomic alterations. Subsequent attempts to identify genomic predictors of prognosis have had varied results. In the case of KRAS mutations, the most common genetic alteration in CRC, studies have been conflicting, with some series demonstrating worse outcomes whereas others failed to confirm a significant difference.3–6 In contrast, BRAF mutations, which are reported in 5% to 11% of mCRC cases,7,8 have been consistently associated with a worse prognosis in patients with metastatic disease in both retrospective clinical series and therapeutic trials.7,9–12 Although the median survival for patients with mCRC overall has improved to >20 months, the median survival for patients with BRAF-mutant mCRC as reported in small series has been estimated at <1 year.7,12 The basis for this shorter survival and the implications for the clinical care of patients with BRAF-mutant mCRC remain unclear.
BRAF is a protein kinase downstream of RAS in the canonical mitogen-activated protein kinase pathway and its activation leads to cellular proliferation and survival. Mutations in BRAF are nearly always mutually exclusive with mutations in the RAS proteins. BRAF-mutant CRC has been associated with distinct clinical features,13,14 including female sex, right-sided primary tumors, older age, and microsatellite instability, and with a unique gene signature.15
In the current study, we assembled what to our knowledge is the largest series published to date of BRAF-mutant mCRC with the goal of better defining the clinicopathologic characteristics, frequency of PIK3CA co-mutations, and clinical outcomes after metastasectomy in these patients.
MATERIALS AND METHODS
Patient Population
Cases were derived from patients seen at Memorial Sloan-Kettering Cancer Center (MSKCC) with mCRC. Beginning in 2009, genotyping was performed in patients with mCRC as part of the standard of care to guide the use of epidermal growth factor receptor-targeting antibodies. Tumor sequencing was performed in all patients after in-house resection of metastatic disease and by physician request for all other patients. Therefore, the number of cases sequenced in our molecular pathology laboratory closely approximates the population of mCRC patients at MSKCC. A computerized search of electronic medical records was performed to prevent recall bias and to identify tumors with a BRAF mutation among all tumors submitted for testing between 2009 and 2012. Consecutive sequenced cases seen in 2011 with wild-type BRAF (423 cases) were selected as a representative sample of controls because there was no change to standard therapy during this period. An additional group of cases with early-stage BRAF-mutant CRC was identified by a computerized search of electronic medical records and of a large data set of genetically annotated primary tumors collected at MSKCC.8 This group provided a comparison for the frequency of concurrent PIK3CA mutations in early-stage BRAF-mutant CRC and consisted of 19 cases (1 with stage I disease, 15 with stage II disease, and 3 with stage III disease by the TNM staging system).
Sequence Analysis
Genomic DNA was extracted from formalin-fixed paraffin-embedded tissue obtained from biopsies or surgical resections. Sequencing was performed on a metastatic specimen, in cases in which tissue was available from metastasectomy or diagnostic biopsy, and on the primary tumor in all other cases. Before October 2010, testing was performed by Sanger sequencing of KRAS exon 2 and BRAF exon 15. Subsequently, a mass spectrometry-based assay (Sequenom, San Diego, Calif) was used to detect mutations in KRAS (codons 12, 13, 61, 117, and 146), BRAF (codons 469, 594, and 600), and PIK3CA (codons 88, 345, 420, 542, 545, 1043, and 1047) as previously described.16 Mutations were confirmed either by a separate Sequenom assay or by Sanger sequencing.
Data Collection
Cases were analyzed for specific clinical and pathologic characteristics including age, sex, primary tumor site, stage of disease at diagnosis, tumor histology, microsatellite instability (MSI), sites of metastatic disease, metastasectomy, and survival. The primary tumor site was divided into right-sided and left-sided tumors; right-sided was defined as tumors arising anywhere from the cecum to the transverse colon and left-sided was defined as tumors arising from the splenic flexure to the anorectal junction. MSI was identified by either immunohistochemistry (loss of hMLH1, hMSH2, hMSH6, or PMS2 expression) or DNA testing (using the recommended National Cancer Institute panel of microsatellite markers, ≥2 foci demonstrating instability). Metastatic sites were identified by review of medical records and/or imaging. Complete resection was defined as resection of all known disease. If the patient had oligometastatic disease involving the liver and lungs requiring sequential resections, the date of complete resection was defined as the later surgical date when all macroscopic disease had been resected. Surgery was not limited to procedures performed at MSKCC, although only a small minority of cases involved surgeries performed at outside hospitals. Date of disease recurrence after surgery was either the date of biopsy proof or the date of imaging demonstrating convincing evidence of disease progression. All research was conducted under appropriate Institutional Review Board/Privacy Board protocols and waivers.
Clinical Risk Score Calculation
To calculate the clinical risk score (CRS) for patients undergoing hepatectomy, a single point was given for the presence of each of the following: lymph node-positive primary tumor, disease-free interval of <12 months between resection of the primary tumor and detection of liver metastases, >1 liver metastasis, presence of a liver metastasis measuring >5 cm, and a serum preoperative carcinoembryonic antigen level >200 ng/mL.17
Statistical Analysis
Associations between BRAF-mutant tumors and clinicopathologic characteristics were analyzed using the Fisher exact test for categorical variables and the Wilcoxon rank sum test for continuous factors. Overall survival (OS) was examined from time of diagnosis of metastatic disease until time of death.
The effect of BRAF mutation on OS was further evaluated in a Cox proportional hazards model by adjusting the known clinical factors: age, tumor differentiation, primary tumor location, stage at diagnosis, and occurrence of metastasectomy.18 Occurrence of metastasectomy was treated as time-dependent covariate in the regression model.19,20
Cumulative incidence function was used to estimate the probability of peritoneal and liver metastases from the date of the initial metastasis and separately in the subset of patients who did not have these sites involved at the time of initial presentation. Patients who died or were alive but developed subsequent disease elsewhere were treated as competing events.
Cumulative incidence function was also used to estimate the time from diagnosis of mCRC to time of metastasectomy. Patients who died or those who underwent only partial surgery were treated as competing events. The Gray test was used to compare the cumulative incidence functions by BRAF mutation status.
The effect of BRAF mutation status on OS and recurrence-free survival (RFS) was examined in a subset of patients who had undergone a complete resection from the time of surgical resection until the time of death (for OS) or until the first disease recurrence or death, whichever came first (for RFS). OS and RFS were estimated by the Kaplan-Meier method and the log-rank test was used to compare the survival outcomes by BRAF mutation status. All P values were based on 2-tailed statistical analysis and all analyses were performed with SAS (version 9.2; SAS Institute Inc, Cary, NC) and R (version 2.10.1; R Foundation for Statistical Computing, Vienna, Austria) statistical software.
RESULTS
BRAF Mutation Spectrum and Frequency
Between the years 2009 and 2012, a total of 1941 unique cases of mCRC were genotyped. During this period, BRAF mutation was identified in 92 mCRC cases (5%), with a similar number of cases identified each year (23 in 2009, 21 in 2010, 23 in 2011, and 25 in 2012). Eighty-nine tumors had a BRAF V600E mutation, 2 had a BRAF D594G mutation, and 1 tumor had a BRAF D594N mutation.
Clinicopathologic Features Associated With BRAF Mutation
The clinical characteristics of BRAF-mutant and BRAF wild-type tumors are shown in Table 1. BRAF-mutant mCRC was significantly more likely to occur in older (64 years vs 58 years; P <.01) and female (59% vs 46%; P =.03) patients and to involve the right colon (63% vs 30%; P <.01). The majority of patients with both BRAF wild-type and BRAF-mutant tumors in this mCRC series presented with metastatic disease. However, BRAF-mutant mCRC was more likely to have progressed from stage III disease (32% vs 17%; P =.003) and in those patients with stage III disease, T4 disease was more common in the BRAF-mutant cases (48% vs 27%; P =.05).
TABLE 1.
Clinical Characteristics of BRAF-Mutant and BRAF Wild-Type mCRC Cases
| Characteristic | BRAF MUT mCRC (N=92) | BRAF WT mCRC (n=423) | P |
|---|---|---|---|
| Age, y | <.01 | ||
| Mean (range) | 64 (28–86) | 58.3 (20–91) | |
| Median | 65 | 59 | |
| Sex | .03 | ||
| Male | 38 (41%) | 230 (54%) | |
| Female | 54 (59%) | 193 (46%) | |
| Primary sitea | <.01 | ||
| Right colon | 58 (63%) | 126 (30%) | |
| Left colon | 34 (37%) | 293 (70%) | |
| Stage of disease at time of presentationb | |||
| I | 0 (0%) | 11 (3%) | |
| II | 8 (9%) | 49 (12%) | |
| III | 29 (32%) | 71 (17%) | <.01 |
| IV | 54 (59%) | 289 (68%) | .11 |
Abbreviations: mCRC, metastatic colorectal cancer; MUT, mutant; WT, wild-type.
For 4 cases, the primary tumor site was identified only as the colon.
Tumors staged using the TNM staging system. One sample from the BRAF-mutant mCRC cases did not have any lymph nodes in the primary specimen and therefore could not be staged.
On pathologic evaluation, summarized in Table 2, BRAF-mutant tumors were more likely to display poor differentiation (48% vs 17%; P <.01) and mucinous histology (43% vs 16%; P <.01). MSI status was available for 34 BRAF-mutant and 110 BRAF wild-type mCRC cases. MSI was significantly more common in the BRAF-mutant tumors (29% vs 9%; P <.01).
TABLE 2.
Histologic Features of BRAF-Mutant and BRAF Wild-Type mCRC Cases
| Tumor Histologya | BRAF MUT mCRC (N=91) | BRAF WT mCRC (n=416) | P |
|---|---|---|---|
| Differentiation | <.01 | ||
| Moderate | 48 (53%) | 346 (83%) | |
| Poor | 43 (47%) | 70 (17%) | |
| Mucinous | <.01 | ||
| Colloid/mucinous | 9 (10%) | 21 (5%) | |
| Mucinous features | 30 (33%) | 45 (11%) | |
| Other histologies | |||
| Medullary | 2 (2%) | 0 (0%) | .03 |
| Signet ring | 4 (4%) | 15 (4%) | 1 |
| Combined adenocarcinoma/NEC | 1 (1%) | 5 (1%) | 1 |
Abbreviations: mCRC, metastatic colorectal cancer; MUT, mutant; NEC, neuroendocrine carcinoma; WT, wild-type.
For 8 cases, tumor histology could not be assessed.
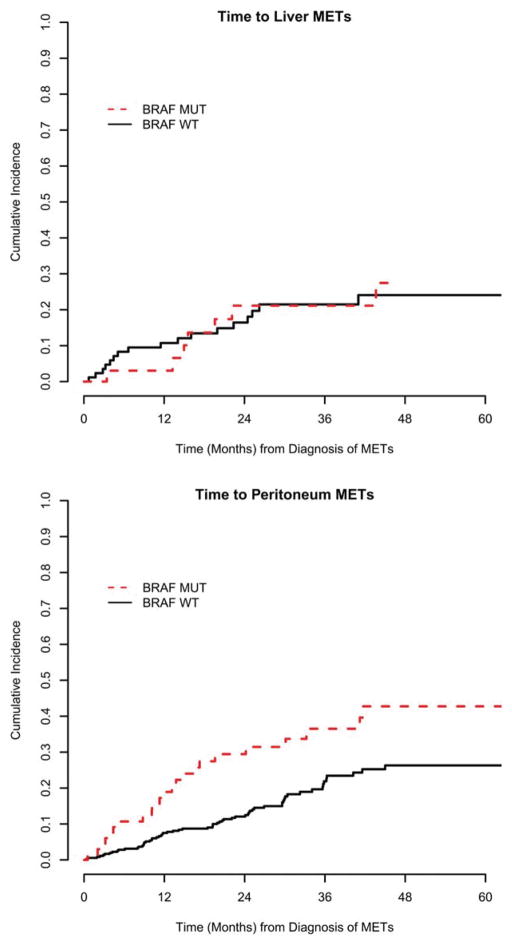
BRAF mutation was associated with a distinct pattern of metastatic spread (Table 3) (Fig. 1). At the time of diagnosis of metastatic disease, BRAF mutation was associated with a lower frequency of liver involvement (60% vs 80%; P <.01) and of disease limited to the liver (41% vs 63%; P <.01). Peritoneal involvement was significantly more common at the time of diagnosis of meta-static disease in the BRAF-mutant mCRC cases (26% vs 14%; P <.01). Furthermore, in patients who did not have peritoneal involvement at time of diagnosis of meta-static disease, the cumulative incidence of peritoneal metastasis was higher among those with BRAF-mutant disease (2-year cumulative incidence of 30% vs 12%; P <.01).
TABLE 3.
Sites Involved With Metastatic Disease in the BRAF-Mutant and Wild-Type mCRC Cases
| BRAF MUT mCRC (N=92) | BRAF WT mCRC (n=423) | P | |
|---|---|---|---|
| Site of first metastasis | <.01 | ||
| Liver only | 38 (41%) | 268 (63%) | |
| Peritoneum only | 16 (17%) | 23 (5%) | |
| Othera | 38 (41%) | 132 (31%) | |
| Liver involved with first metastasis | <.01 | ||
| Yes | 55 (60%) | 337 (80%) | |
| No | 37 (40%) | 86 (20%) | |
| Peritoneum involved with first metastasis | <.01 | ||
| Yes | 24 (26%) | 60 (14%) | |
| No | 68 (74%) | 363 (86%) | |
| Lung involved with first metastasis | .43 | ||
| Yes | 11 (12%) | 64 (15%) | |
| No | 81 (88%) | 359 (85%) |
Abbreviations: mCRC, metastatic colorectal cancer; MUT, mutant; WT, wild-type.
Other sites of first involvement included lymph nodes, lungs, ovaries, small intestine, abdominal wall, pelvic mass, bones, and brain.
Figure 1.
Estimated cumulative incidence of (Top) liver and (Bottom) peritoneal involvement in patients who did not have these sites involved at the time of initial presentation with metastatic disease. METs indicates metastatic disease; MUT, BRAF-mutant disease; WT, BRAF wild-type disease.
Frequency of Concurrent PIK3CA Mutations
Data regarding PIK3CA mutation status were available for 84 BRAF-mutant mCRC cases and for all BRAF wild-type cases included in the current study (Fig. 2). Co-mutation of PIK3CA was identified in 5% of BRAF-mutant cases versus 17% of KRAS-mutant mCRC cases (P <.01) and 4% of BRAF/KRAS wild-type mCRC cases (P =1).
Figure 2.
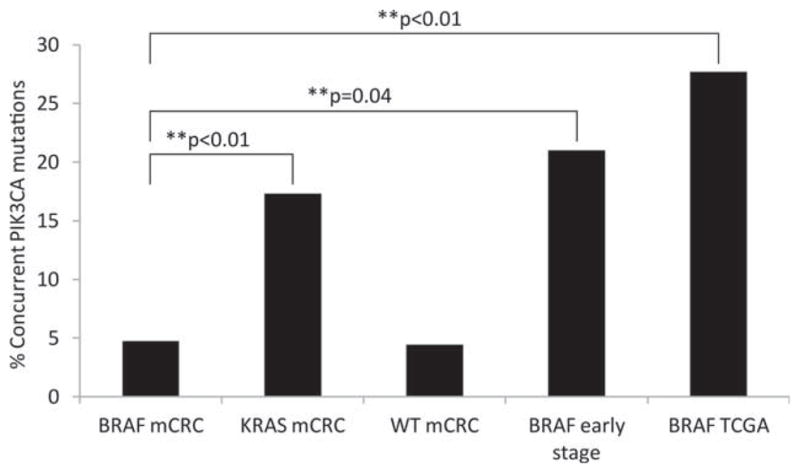
Frequency of concurrent PIK3CA (phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha) mutations are shown in BRAF-mutant metastatic colorectal cancer (mCRC) cases, KRAS-mutant mCRC cases, BRAF/KRAS wild-type (WT) mCRC cases, patients with BRAF-mutant early-stage disease, and BRAF-mutant cases included in The Cancer Genome Atlas (TCGA). P values were determined with the Fisher exact test and significant values are indicated by asterisks.
Finding a low frequency of concurrent PIK3CA mutations in the BRAF-mutant cases, we examined whether limiting our sample to metastatic cases may have affected the frequency of concurrent PIK3CA mutations. Using a cohort of patients with BRAF-mutant early-stage CRC seen at MSKCC, we evaluated the frequency of concurrent PIK3CA mutations in early-stage cases. In this cohort of 19 patients with nonmetastatic tumors, 4 (21%) had concurrent PIK3CA mutations, a frequency similar to that noted in the KRAS-mutant mCRC cases. This frequency was also similar to that found for nonmetastatic BRAF-mutant tumors in the colorectal The Cancer Genome Atlas (TCGA).
Effect of BRAF Mutations on OS
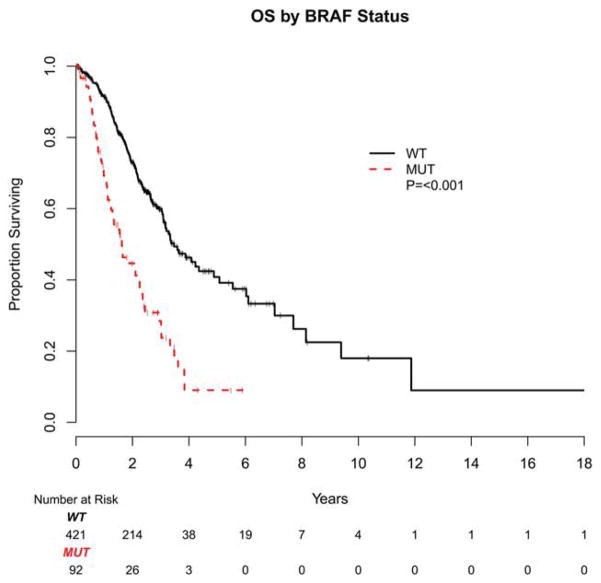
In the current series, patients with BRAF-mutant mCRC were found to have a poor prognosis. With a median follow-up of 25 months for survivors, the median OS from the time of diagnosis of metastatic disease was 20 months for patients with BRAF-mutant mCRC versus 47 months for those with BRAF wild-type tumors (P <.001) (Fig. 3). Multivariate analysis, correcting for patient age, tumor differentiation, primary tumor location, stage at diagnosis, and occurrence of metastasectomy treated as a time-dependent covariate, assigned a hazards ratio of 2.0 for OS to BRAF mutation (95% confidence interval [95% CI], 1.4–2.8; P <.01).
Figure 3.
Kaplan-Meier estimates of overall survival (OS) are shown based on diagnosis of metastatic disease by BRAF mutation status. WT indicates wild-type; MUT, mutant.
Frequency and Outcomes After Metastasectomy by BRAF Mutation Status
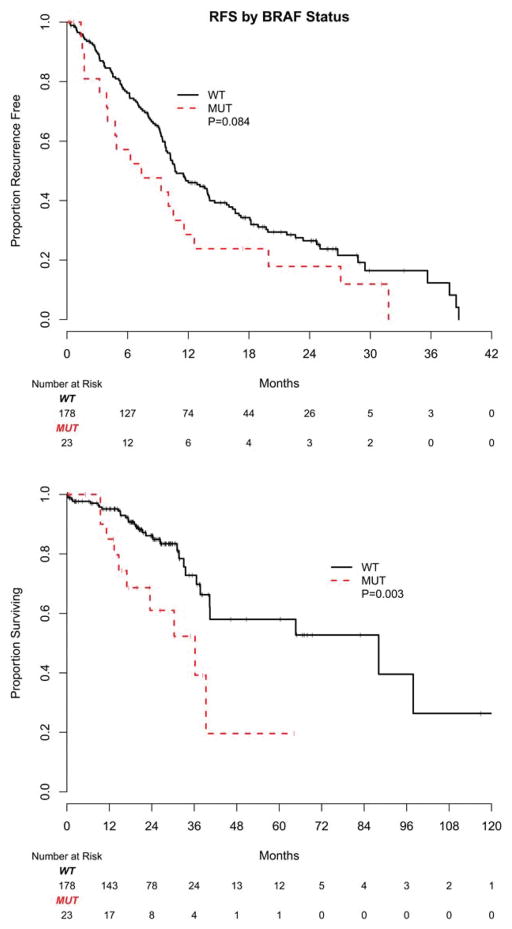
Complete resection of metastases was performed in 201 patients (23 with BRAF-mutant and 178 with BRAF wild-type tumors). Complete resection was defined as resection of all metastatic disease with intention of cure and included hepatectomy, pulmonary wedge resection, and optimal debulking surgery. Patients with BRAF-mutated mCRC were less likely to undergo metastasectomy (41% vs 26% at 2 years from the time of diagnosis of metastatic disease; P <.01). Patients with BRAF-mutated tumors demonstrated a trend toward shorter RFS after metastasectomy, with a median RFS of 7 months compared with 11 months in patients with BRAF wild-type tumors (P =.084) (Fig. 4 Top). With a median follow-up of 21 months for survivors and 43 deaths, OS after metastasectomy was found to be significantly shorter in patients with BRAF-mutant mCRC with 61% of these patients alive at 2 years (95% CI, 34%–80%) compared with 86% of patients with BRAF wild-type mCRC (95% CI, 78%–91%) (P =.003) (Fig. 4 Bottom).
Figure 4.
Kaplan-Meier estimates of (Top) recurrence-free survival (RFS) and (Bottom) overall survival from metastasectomy by BRAF mutation status are shown. WT indicates wild-type; MUT, mutant.
A total of 23 patients with BRAF-mutant mCRC underwent complete resection (18 hepatectomies, 4 pulmonary lobectomies, and 1 oophorectomy). All patients had single-organ–limited metastatic disease. The median RFS was 7.4 months (95% CI, 4.0 months-11.6 months). RFS rates at 1 year and 2 years were 28% (95% CI, 12%–48%) and 18% (95% CI, 5%–37%), respectively.
Follow-up data were available for 20 patients (Table 4). Fifteen patients with BRAF-mutant mCRC underwent hepatectomy with curative intent. Seven patients had a solitary liver metastasis, 4 patients had 2 metastases, 2 patients had 3 metastases, and 1 patient each had 4 and 5 metastases. Despite the presumed favorable prognosis of patients with this small number of metastases, actual curative outcome from resection was rare, with 13 of 15 patients developing disease recurrence. Two patients, each of whom had undergone resection of a solitary liver metastasis, were free of disease at the time of last follow-up at 18 months and 30 months, respectively. Four patients had undergone resection of lung metastases. None were cured, with disease recurrences occurring between 4 and 20 months. One patient developed an ovarian metastasis with invasion into the small bowel that was resected en bloc, but peritoneal metastases occurred within 3 months. Thus, of 20 patients who underwent surgery with curative intent, at least 18 failed to achieve a cure.
TABLE 4.
Metastasectomies and Outcomes for Patients With BRAF-Mutant mCRC Who Underwent Resection With Curative Intent
| Surgery | CRS (For Hepatectomy) | No. of Metastases Resected | Microscopic Clear (R0) or Involved (R1) Margins | First Site of Disease Recurrence |
|---|---|---|---|---|
| Partial hepatectomy | 2 | 1 | R0 | None |
| Partial hepatectomy | 2 | 1 | R0 | None |
| Partial hepatectomy | 3 | 1 | R0 | Liver |
| Partial hepatectomy | 1 | 1 | R0 | Liver, peritoneum |
| Partial hepatectomy | 3 | 1 | R0 | Kidney, peritoneum |
| Partial hepatectomy | 2 | 1 | R0 | Liver |
| Partial hepatectomy | 2 | 1 | R0 | Lung, retroperitoneal lymph nodes |
| Partial hepatectomy | 3 | 2 | R0 | Liver |
| Partial hepatectomy | 3 | 2 | R0 | Abdominal and retroperitoneal lymph nodes |
| Partial hepatectomy | NAa | 2 | R0 | Liver |
| Partial hepatectomy | 3 | 3 | R0 | Liver |
| Partial hepatectomy | 3 | 3 | R1 | Liver |
| Partial hepatectomy | 2 | 4 | R0 | Liver |
| Partial hepatectomy | 2 | 5 | R0 | Abdominal wall |
| Left upper lobe wedge resection | 1 | R0 | Lung | |
| Left upper lobe wedge resection | 1 | R0 | Lung | |
| Left upper lobe and left lower lobe wedge resections | 2 | R0 | Lung | |
| Left and right lung wedge resections | >10 | R1 | Lung, peritoneum | |
| Partial hepatectomy, excision of ileocolic mass | 2 | R0 | Lung, mediastinal lymph nodes | |
| Hysterectomy, salpingectomy, resection of small intestine | 4 | R0 | Peritoneum |
Abbreviations: CRS, clinical risk score; mCRC, metastatic colorectal cancer; NA, not available.
Hepatectomy was not performed at Memorial Sloan-Kettering Cancer Center. Presurgical carcinoembryonic antigen level was not available with which to calculate the CRS.
Table 4 also shows sites of first disease recurrence after metastasectomy. In patients with BRAF-mutant mCRC who underwent liver resection and later developed disease recurrence, approximately two-thirds (8 of 13 patients) developed recurrence first in the liver, whereas one-third developed disease recurrence in other sites, most commonly the peritoneum and lymph nodes. All patients with BRAF-mutant mCRC who underwent resection of pulmonary metastases first developed disease recurrence in the lungs.
To determine whether the poor outcomes observed after metastasectomy in the BRAF-mutant cases resulted from higher-risk disease, we evaluated the CRS for patients undergoing hepatectomy. The CRS uses preoperative features to predict risk of disease recurrence after hepatectomy and has been found to be associated with long-term outcomes.17 Patients with BRAF-mutant mCRC had a low CRS, ranging from 1 to 3 (Table 4). Thus, in the current series, BRAF mutation was found to be a poor prognostic marker for potentially curable disease, even in the presence of a low CRS.
DISCUSSION
The majority of the literature regarding BRAF mutations, including TCGA data, is based primarily on studies of patients with early-stage disease. The study of 92 patients with BRAF-mutant CRC reported herein is therefore to our knowledge the largest analysis of BRAF mutations in the metastatic setting published to date.
A potential bias of the current series, which identified mCRC cases by standard-of-care molecular pathology testing, is a possible enrichment for patients undergoing metastasectomy. In order for tumors to be genotyped, tissue must be available at the study institution or patients must provide unstained slides from an outside surgical specimen for DNA extraction. Genotyping occurs automatically for all metastasectomies performed at MSKCC or after physician request for other cases (generally from a medical oncologist in preparation for treatment with epidermal growth factor receptor-targeted agents). This potential bias may have resulted in the relatively low frequency of 5% for BRAF-mutant cases and may have led to improved outcomes for patients in the current series.
In the current series, we found that the previously identified association between BRAF mutations in early-stage CRC with older age, female sex, and right-sided primary tumors was also present in the metastatic setting. We also confirmed that these tumors demonstrate a high frequency of mucinous histology and poor differentiation.
In the current series, MSI status was available for 34 of the BRAF-mutant mCRC cases. We observed that BRAF mutation was coassociated with MSI, with a frequency of 29% MSI in the current series. Historical series, consisting of all stages of CRC, have suggested a much higher frequency of MSI in BRAF-mutant CRC.13,21 However, series of BRAF-mutant mCRC report a similar frequency, with an MSI rate of 29% in a series of 42 BRAF-mutant mCRC cases by Tran et al.7 These data suggest that BRAF-mutant CRC that does not exhibit MSI is more likely to proceed to metastatic disease, leading to the higher frequency of microsatellite-stable cases noted among patients with BRAF-mutant mCRC.
In the current series, we found that concurrent PIK3CA mutations are present in 5% of patients with BRAF-mutant mCRC. This is in contrast to patients with early-stage BRAF-mutant CRC, in whom >20% of cases have co-mutation of PIK3CA. PI3K pathway activation has been associated with favorable outcomes in some series, although to the best of our knowledge this has not been a consistent finding in all studies.22–24 Genomic characterization of a large series of patients with mCRC and analysis of TCGA data have indicated a tendency toward a nonoverlapping pattern of PIK3CA and TP53 mutations,8 suggesting that wild-type PIK3CA may be associated with genetic alterations that contribute to more aggressive tumor biology.
The results of the current study demonstrate that BRAF mutation is associated with a distinct pattern of metastatic spread. We found significantly greater peritoneal and less liver involvement in the patients with BRAF-mutated tumors. These findings corroborate and extend the results of Tran et al, who reported that peritoneal involvement during the disease course is more common in patients with BRAF-mutant mCRC compared with those with wild-type tumors.7 Because peritoneal metastases have been identified as a poor prognostic factor in patients with mCRC,25,26 this pattern of spread may partly explain the poor outcomes of patients with mCRC whose tumors harbor a BRAF mutation.
The results of the current series confirm the poor prognosis of BRAF-mutant mCRC in what to our knowledge is the largest data set reported to date. We found the median OS from the time of diagnosis of metastasis in patients with BRAF-mutant disease was less than one-half that for patients with wild-type tumors (20 months for BRAF-mutant cases compared with 47 months for wild-type cases). In addition, patients with BRAF-mutant mCRC had a 2-fold increased risk of death after adjusting for metastasectomy and other known confounders. In the current study, significantly fewer patients with BRAF-mutant mCRC underwent metastasectomy compared with patients with wild-type disease, which is most likely the result of the higher incidence of peritoneal disease noted among patients with BRAF-mutant mCRC. More importantly, those who did undergo metastasectomy appeared to have a substantially lower cure rate and a higher recurrence rate than patients with BRAF wild-type disease. BRAF mutation was found to be a poor prognostic indicator in patients with resectable disease, even among those patients with a good CRS for surgery. Consistent with the findings of the current study, an evaluation by Teng et al of a series of patients with CRC with liver-only metastases who were undergoing hepatectomy and that included 6 patients with BRAF-mutant mCRC reported significantly worse survival for patients with BRAF-mutant CRC.27
The data from the current study suggest that BRAF mutation testing has prognostic value in patients with mCRC, including those undergoing complete resection for curative intent. Given the poor prognosis of this subset of patients with mCRC, the results of the current study support the routine testing of patients with mCRC for BRAF mutations and highlight the need to develop targeted therapies for these patients.
Acknowledgments
We thank Jonathan Wills for assistance with computerized searches of the electronic medical record. We thank Dr. Laetitia Borsu for assistance with Sequenom assays.
FUNDING SUPPORT
Supported by an American Association for Cancer Research-Colorectal Cancer Coalition Fellows Grant, in memory of Lisa Dubow and an American Cancer Society Postdoctoral Fellowship (to Dr. Yaeger). The Memorial Sloan-Kettering Cancer Center clinical Sequenom facility is supported by the Anbinder Fund.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
Dr. Ladanyi has received fees from Puma Biotechnology and Novartis for work conducted outside of the current study.
References
- 1.Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wood LD, Parsons DW, Jones S, et al. The genomic landscapes of human breast and colorectal cancers. Science. 2007;318:1108–1113. doi: 10.1126/science.1145720. [DOI] [PubMed] [Google Scholar]
- 3.Andreyev HJ, Norman AR, Cunningham D, et al. Kirsten ras mutations in patients with colorectal cancer: the “RASCAL II” study. Br J Cancer. 2001;85:692–696. doi: 10.1054/bjoc.2001.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imamura Y, Morikawa T, Liao X, et al. Specific mutations in KRAS codons 12 and 13, and patient prognosis in 1075 BRAF wild-type colorectal cancers. Clin Cancer Res. 2012;18:4753–4763. doi: 10.1158/1078-0432.CCR-11-3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peeters M, Douillard JY, Van Cutsem E, et al. Mutant KRAS codon 12 and 13 alleles in patients with metastatic colorectal cancer: assessment as prognostic and predictive biomarkers of response to panitumumab. J Clin Oncol. 2013;31:759–765. doi: 10.1200/JCO.2012.45.1492. [DOI] [PubMed] [Google Scholar]
- 6.Samowitz WS, Curtin K, Schaffer D, Robertson M, Leppert M, Slattery ML. Relationship of Ki-ras mutations in colon cancers to tumor location, stage, and survival: a population-based study. Cancer Epidemiol Biomarkers Prev. 2000;9:1193–1197. [PubMed] [Google Scholar]
- 7.Tran B, Kopetz S, Tie J, et al. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer. 2011;117:4623–4632. doi: 10.1002/cncr.26086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vakiani E, Janakiraman M, Shen R, et al. Comparative genomic analysis of primary versus metastatic colorectal carcinomas. J Clin Oncol. 2012;30:2956–2962. doi: 10.1200/JCO.2011.38.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogino S, Shima K, Meyerhardt JA, et al. Predictive and prognostic roles of BRAF mutation in stage III colon cancer: results from intergroup trial CALGB 89803. Clin Cancer Res. 2012;18:890–900. doi: 10.1158/1078-0432.CCR-11-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roth AD, Tejpar S, Delorenzi M, et al. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60–00 trial. J Clin Oncol. 2010;28:466–474. doi: 10.1200/JCO.2009.23.3452. [DOI] [PubMed] [Google Scholar]
- 11.Van Cutsem EL, Folprecht G, Nowacki M, et al. Cetuximab plus FOLFIRI: final data from the CRYSTAL study on the association of KRAS and BRAF biomarker status with treatment outcome [abstract] J Clin Oncol. 2010;28:15s. Abstract 3570. [Google Scholar]
- 12.Yokota T, Ura T, Shibata N, et al. BRAF mutation is a powerful prognostic factor in advanced and recurrent colorectal cancer. Br J Cancer. 2011;104:856–862. doi: 10.1038/bjc.2011.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weisenberger DJ, Siegmund KD, Campan M, et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet. 2006;38:787–793. doi: 10.1038/ng1834. [DOI] [PubMed] [Google Scholar]
- 14.Zlobec I, Bihl MP, Schwarb H, Terracciano L, Lugli A. Clinicopathological and protein characterization of BRAF- and K-RAS-mutated colorectal cancer and implications for prognosis. Int J Cancer. 2010;127:367–380. doi: 10.1002/ijc.25042. [DOI] [PubMed] [Google Scholar]
- 15.Popovici V, Budinska E, Tejpar S, et al. Identification of a poor-prognosis BRAF-mutant-like population of patients with colon cancer. J Clin Oncol. 2012;30:1288–1295. doi: 10.1200/JCO.2011.39.5814. [DOI] [PubMed] [Google Scholar]
- 16.Arcila M, Lau C, Nafa K, Ladanyi M. Detection of KRAS and BRAF mutations in colorectal carcinoma roles for high-sensitivity locked nucleic acid-PCR sequencing and broad-spectrum mass spectrometry genotyping. J Mol Diagn. 2011;13:64–73. doi: 10.1016/j.jmoldx.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. discussion 318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cox DR. Regression models and life tables. J R Stat Soc B. 1972;20:187–220. [Google Scholar]
- 19.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 20.Satagopan JM, Ben-Porat L, Berwick M, Robson M, Kutler D, Auerbach AD. A note on competing risks in survival data analysis. Br J Cancer. 2004;91:1229–1235. doi: 10.1038/sj.bjc.6602102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogino S, Nosho K, Kirkner GJ, et al. CpG island methylator phenotype, microsatellite instability, BRAF mutation and clinical outcome in colon cancer. Gut. 2009;58:90–96. doi: 10.1136/gut.2008.155473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baba Y, Nosho K, Shima K, et al. Phosphorylated AKT expression is associated with PIK3CA mutation, low stage, and favorable outcome in 717 colorectal cancers. Cancer. 2011;117:1399–1408. doi: 10.1002/cncr.25630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kato S, Iida S, Higuchi T, et al. PIK3CA mutation is predictive of poor survival in patients with colorectal cancer. Int J Cancer. 2007;121:1771–1778. doi: 10.1002/ijc.22890. [DOI] [PubMed] [Google Scholar]
- 24.Liao X, Morikawa T, Lochhead P, et al. Prognostic role of PIK3CA mutation in colorectal cancer: cohort study and literature review. Clin Cancer Res. 2012;18:2257–2268. doi: 10.1158/1078-0432.CCR-11-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Assersohn L, Norman A, Cunningham D, Benepal T, Ross PJ, Oates J. Influence of metastatic site as an additional predictor for response and outcome in advanced colorectal carcinoma. Br J Cancer. 1999;79:1800–1805. doi: 10.1038/sj.bjc.6990287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Catalano V, Loupakis F, Graziano F, et al. Mucinous histology predicts for poor response rate and overall survival of patients with colorectal cancer and treated with first-line oxaliplatin- and/or irinotecan-based chemotherapy. Br J Cancer. 2009;100:881–887. doi: 10.1038/sj.bjc.6604955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teng HW, Huang YC, Lin JK, et al. BRAF mutation is a prognostic biomarker for colorectal liver metastasectomy. J Surg Oncol. 2012;106:123–129. doi: 10.1002/jso.23063. [DOI] [PubMed] [Google Scholar]