Abstract
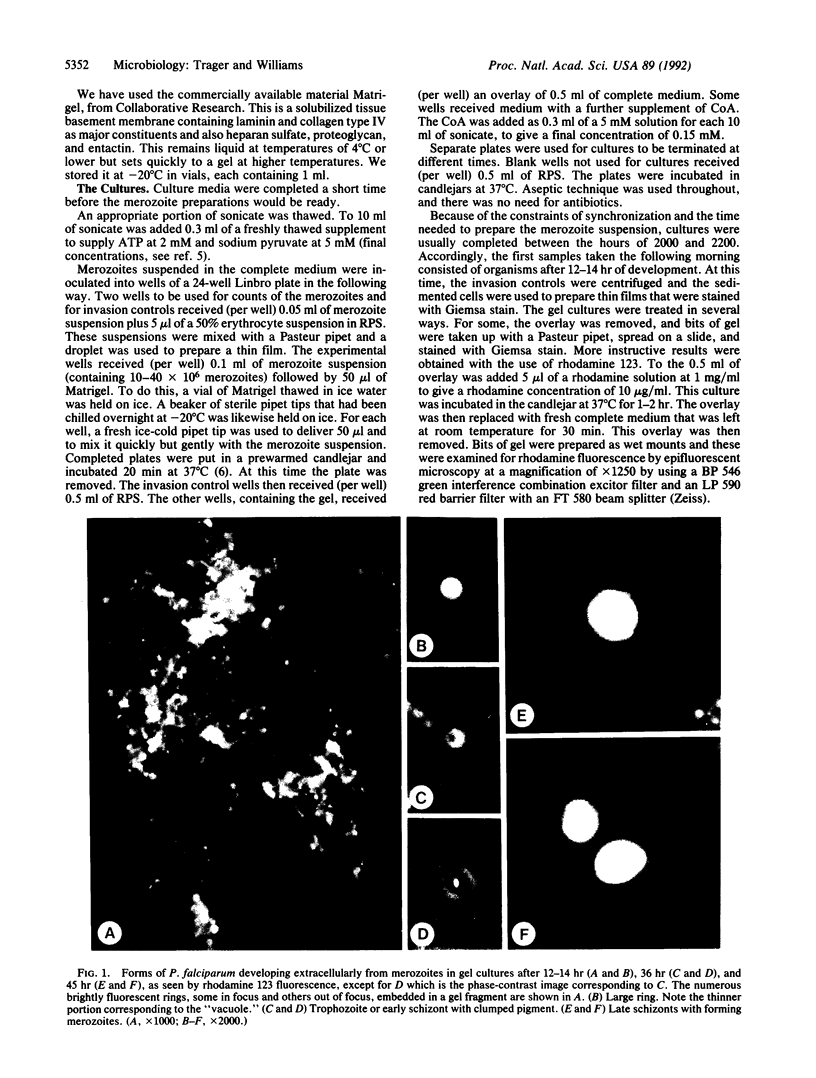
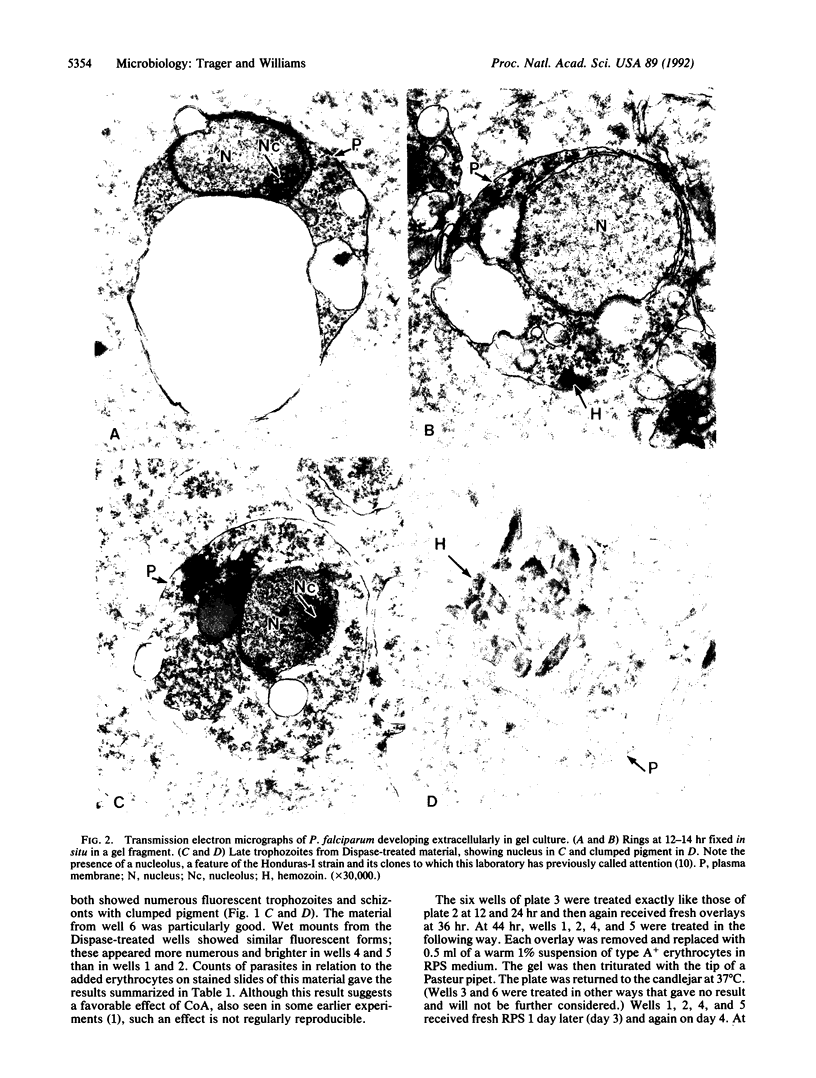
Merozoites of the erythrocytic stage of Plasmodium falciparum were suspended in erythrocyte sonicate medium with ATP and pyruvate and mixed with Matrigel to form a soft gel. The gel was overlaid with complete medium; this was replaced with fresh medium at 12, 24, and 36 hr. At these times and also at 45 hr rhodamine 123 was added to some cultures and gels were sampled. Viable extracellular forms showing rhodamine fluorescence were seen: rings at 12 hr, trophozoites and early schizonts with pigment at 36 hr, and late schizonts with developing merozoites at 45 hr. These merozoites were shown to be infective to erythrocytes added to the cultures at 45 hr. Electron micrographs of 36-hr trophozoites show the organisms to have only their single plasma membrane; no parasitophorous membrane is evident. We conclude that the complex process of entry, the intactness of the host erythrocyte, and the parasitophorous membrane are not essential to the development of a merozoite through its complete asexual cycle.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barcellos-Hoff M. H., Aggeler J., Ram T. G., Bissell M. J. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development. 1989 Feb;105(2):223–235. doi: 10.1242/dev.105.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhasin V. K., Trager W. Gametocyte-forming and non-gametocyte-forming clones of Plasmodium falciparum. Am J Trop Med Hyg. 1984 Jul;33(4):534–537. doi: 10.4269/ajtmh.1984.33.534. [DOI] [PubMed] [Google Scholar]
- Howard R. J., Lyon J. A., Uni S., Saul A. J., Aley S. B., Klotz F., Panton L. J., Sherwood J. A., Marsh K., Aikawa M. Transport of an Mr approximately 300,000 Plasmodium falciparum protein (Pf EMP 2) from the intraerythrocytic asexual parasite to the cytoplasmic face of the host cell membrane. J Cell Biol. 1987 May;104(5):1269–1280. doi: 10.1083/jcb.104.5.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumo A., Tanabe K., Kato M. A method for monitoring the viability of malaria parasites (Plasmodium yoelii) freed from the host erythrocytes. Trans R Soc Trop Med Hyg. 1987;81(2):264–267. doi: 10.1016/0035-9203(87)90235-5. [DOI] [PubMed] [Google Scholar]
- Jensen J. B., Trager W. Plasmodium falciparum in culture: use of outdated erthrocytes and description of the candle jar method. J Parasitol. 1977 Oct;63(5):883–886. [PubMed] [Google Scholar]
- Langreth S. G., Jensen J. B., Reese R. T., Trager W. Fine structure of human malaria in vitro. J Protozool. 1978 Nov;25(4):443–452. doi: 10.1111/j.1550-7408.1978.tb04167.x. [DOI] [PubMed] [Google Scholar]
- Lanners H. N., Trager W. Intranuclear structures in pyrimethamine-resistant isolates of the malaria parasite Plasmodium falciparum. Cell Biol Int Rep. 1984 Mar;8(3):221–225. doi: 10.1016/0309-1651(84)90034-1. [DOI] [PubMed] [Google Scholar]
- Trager W., Lanners H. N. Initial extracellular development in vitro of merozoites of Plasmodium falciparum. J Protozool. 1984 Nov;31(4):562–567. doi: 10.1111/j.1550-7408.1984.tb05503.x. [DOI] [PubMed] [Google Scholar]
- Trager W., Zung J., Tershakovec M. Initial extracellular development in vitro of erythrocytic stages of malaria parasites (Plasmodium falciparum). Proc Natl Acad Sci U S A. 1990 Aug;87(15):5618–5622. doi: 10.1073/pnas.87.15.5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zung J. M., Trager W., Gubert E. Initial extracellular forms of Plasmodium falciparum: their ultrastructure and their definition with monoclonal antibodies. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):89–92. doi: 10.1073/pnas.88.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]