A catalytic alkane metathesis provides a mild and selective degradation of polyethylene wastes into valuable fuels and waxes.
Keywords: polymer recycling, polymer degradation, polyethylene, alkane metathesis, iridium catalyst, liquid fuel, wax, feedstock recovery
Abstract
Polyethylene (PE) is the largest-volume synthetic polymer, and its chemical inertness makes its degradation by low-energy processes a challenging problem. We report a tandem catalytic cross alkane metathesis method for highly efficient degradation of polyethylenes under mild conditions. With the use of widely available, low-value, short alkanes (for example, petroleum ethers) as cross metathesis partners, different types of polyethylenes with various molecular weights undergo complete conversion into useful liquid fuels and waxes. This method shows excellent selectivity for linear alkane formation, and the degradation product distribution (liquid fuels versus waxes) can be controlled by the catalyst structure and reaction time. In addition, the catalysts are compatible with various polyolefin additives; therefore, common plastic wastes, such as postconsumer polyethylene bottles, bags, and films could be converted into valuable chemical feedstocks without any pretreatment.
INTRODUCTION
Synthetic plastics play an indispensible role in every aspect of modern life. However, the widespread use of large volumes of plastics has created serious environmental issues, which demand proper end-of-life management of plastic wastes (1, 2). Polyolefins, mainly high-density polyethylene (HDPE), low-density PE (LDPE), linear low-density PE (LLDPE), and polypropylene (PP), constitute more than 60% of the total plastic content of municipal solid waste (3–5). In current technology, the monomers for plastics originate primarily from fossil fuels. In view of the large and still strongly increasing amount of produced plastics and the steadily dwindling global oil reserves, one promising solution to plastic wastes is to convert them into valuable liquid fuels or chemical feedstocks.
PE is the largest-volume plastic in the world, with annual production exceeding 100 million metric tons (6). In contrast to the successful feedstock recovery from specialty plastics, such as poly(ethylene terephthalate) and polystyrene (7), PE is remarkably inert and difficult to degrade without special treatment (4). The chemical inertness arises from all atoms of PE being connected by strong single C−C and C−H bonds. In addition, PE contains predominantly secondary carbons and a few primary carbons, both of which are robust toward oxidation from exposure to heat or ultraviolet radiation. Thermal and catalytic pyrolysis at high temperatures (typically >400°C) has been applied to PE degradation (8, 9). However, these processes suffer from low energy efficiency and lack of product control, often resulting in the formation of complex product compositions, including hydrocarbon gas, oil, wax, and char (10). In addition, branched, cyclic, and aromatic hydrocarbons are formed along with linear alkanes (5, 8, 11). As for the few milder PE recycling methods, some involve highly reactive radical species (12), whereas others form light hydrocarbon gases (methane and ethane) as the predominant products at high conversions (13, 14).
Alkane metathesis is a process in which alkanes are covalently rearranged to give a new distribution of alkane products (15–19). Because PE is essentially composed of ultra-long alkanes, we envisioned that alkane metathesis could be used to cleave PE chains. Here, we report a mild and efficient degradation of PE into liquid fuels and waxes using inexpensive and readily available light alkanes, such as petroleum ether, as the reagents. These alkanes with low carbon number are major constituents of a variety of refinery and petrochemical streams (20), the Fischer-Tropsch process (21), and some biomass conversion pathways (22). We show that the cross metathesis between these low-value light alkanes and PE results in very efficient degradation of various grades of PE. In particular, we demonstrate that the wastes of commercial PE plastics can be selectively degraded into transportation fuels and waxes under mild conditions in a controllable manner.
RESULTS
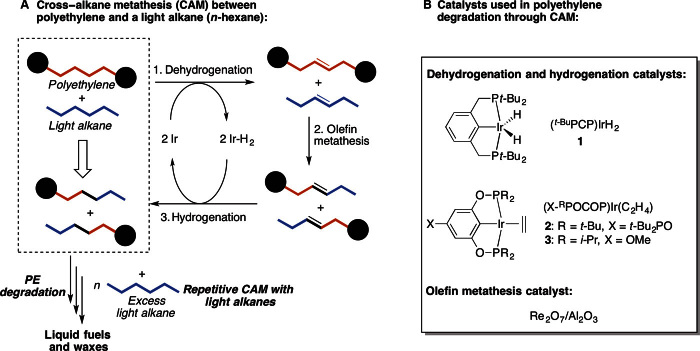
Our strategy for PE degradation is based on a tandem catalytic cross alkane metathesis (CAM) process developed by Goldman et al. (18) and Huang et al. (23), which involves one catalyst for alkane dehydrogenation and another catalyst for olefin metathesis (Fig. 1A). First, the dehydrogenation catalyst Ir removes hydrogen from both PE and a light alkane in a sealed system to form unsaturated species and Ir-H2. Next, the olefin metathesis catalyst scrambles the alkenes, resulting in breakdown of PE chains. Finally, hydrogenation of the newly formed alkenes with Ir-H2 affords saturated alkanes. The metathesis of PE with the light alkane reduces the chain length of PE when an internal double bond of PE scrambles with a double bond of the light alkene. In the presence of a large excess of light alkanes, the initial CAM products should react further with the light alkane to deliver the secondary CAM products with even shorter chain length. After multiple cycles of CAM with light alkanes, PE will be eventually converted to short hydrocarbons suitable for transportation oils. A unique advantage of this process is that the excess light alkane used for the degradation dissolves PE to form a dilute solution with low viscosity, avoiding mass and heat transfer issues encountered in the conventional catalytic pyrolysis processes involving PE melts (8).
Fig. 1. Degradation of PE through CAM with light alkanes (for example, n-hexane).
(A) Proposed PE degradation pathway through catalytic CAM. Dehydrogenation of both PE and light alkane (n-hexane used as an example) creates unsaturated olefins. Subsequently, cross olefin metathesis followed by hydrogenation causes breakdown of PE chain into shorter chains. Repeating the tandem reaction in multiple cycles results in degradation of PE into short alkanes appropriate for use as transportation oil. (B) Structures of the dehydrogenation and olefin metathesis catalysts used in this study.
A dual catalyst system containing a supported “pincer”-ligated iridium complex, (t-BuPCP)Ir (1) (24, 25) (Fig. 1B) and Re2O7/γ-Al2O3 (26), has proven to be robust and effective for short alkane metathesis (18, 23); thus, we commenced our study by using this catalyst system for PE degradation. For initial proof of the concept, the degradation of 120 mg of laboratory-grade HDPE [HDPE-1, powder; weight-average molecular weight (Mw) = 3350; polydispersity index (PDI), 1.6] with 3 ml of n-hexane (7.7 M) in the presence of 20.1 μmol of iridium catalyst 1, 40.2 μmol of t-butylethylene as the hydrogen acceptor, and Re2O7/γ-Al2O3 (57 μmol of Re2O7) proved to be successful. The mixture was heated at 150°C under argon in a sealed vessel, and the PE sample was completely dissolved under the conditions. For comparison, a control experiment without PE was conducted under otherwise identical conditions. Product distributions were monitored by gas chromatography (GC) with mesitylene as an internal standard. Whereas the product distribution of the control was concentrated in a range of C2-C21 n-alkanes (Table 1, entry 2, and fig. S1B), the reaction with HDPE-1 afforded a significant amount of C22–40 n-alkanes (Table 1, entry 1, and fig. S1A) (almost 30 times more), which is indicative of CAM between HDPE-1 and n-hexane. Only linear alkane products were produced, and no aromatic compounds or alkenes were detected by GC (18). Aside from the oil products that are soluble in n-alkanes and suitable for GC analysis, the degradation process also generates relatively high–molecular-weight wax hydrocarbon products, which are insoluble in n-alkanes at ambient temperature. However, following separation of the solid catalysts from the hydrocarbon solution by simple filtration at 160°C, the wax products precipitated after cooling the solution to room temperature and thus could be further separated from the solution containing the oil products (see the Supplementary Materials for the procedure in details). The reaction starting with 120 mg of HDPE-1 gave 53 mg of wax hydrocarbon products—a 56% PE degradation to oil products (Table 1, entry 1).
Table 1. Cross metathesis of HDPE-1 (120 mg) with n-hexane (7.7 M, 3 ml) using various γ-Al2O3– supported Ir catalysts (20.1 μmol Ir) and Re2O7/γ-Al2O3 (57 μmol Re2O7): The conversion of PE to oil and wax products and the distribution of soluble n-alkane products after heating the mixture for 3 days at 150°C.
The amounts of soluble products were determined by GC analysis with mesitylene as an internal standard. The amounts of n-hexane after the degradation reactions were not included. As the starting material, the intensity of the n-hexane signal was too strong (relative to other n-alkane products) to be precisely measured by GC analysis. Wax products are the isolated solid hydrocarbon products. Aside from wax products, the major products from PE degradation are liquid oil hydrocarbons. Thus, weight percentages (wt %) of oils from PE degradation were estimated with the mass of the starting PE minus the wax products. N/A, not applicable.
| Entry | Ir catalyst | HDPE-1(mg) |
Mass (mg) and concentration (mM) of soluble products |
Mass (g) and concentration (M) of total soluble products |
Wax products (mg) | Wt % of oils | ||||
| C3-C5 | C7-C12 | C13-C21 | C22-C30 | ≥C31 | ||||||
| 1 | 1 | 120 | 304/ 1553 |
671/ 1932 |
74/ 115 |
12/ 10 |
10/ 7 |
1.07/ 3.62 |
53 | 56% |
| 2 | 1 | 0 | 374/ 1926 |
788/ 2248 |
57/ 90 |
0.7/ 0.6 |
N/A | 1.22/ 4.27 |
N/A | N/A |
| 3 | 2 | 120 | 202/ 1073 |
416/ 1171 |
93/ 138 |
20/ 18 |
12/ 8 |
0.74/ 2.41 |
2 | 98% |
| 4 | 2 | 0 | 245/ 1290 |
597/ 1662 |
69/ 114 |
2/ 2 |
N/A | 0.91/ 3.07 |
N/A | N/A |
| 5 | 3 | 120 | 225/ 1180 |
523/ 1461 |
116/ 174 |
29/ 24 |
23/ 14 |
0.92/ 2.85 |
6 | 95% |
For significant molecular weight (MW) reduction, olefin metathesis is required to occur at an internal C=C double bond of dehydrogenated PE (see above). Compared to the (t-BuPCP)Ir complex (1), Brookhart’s bis(phosphinite)-ligated (t-BuPOCOP)Ir complex (27) exhibits higher regioselectivity for formation of internal alkenes in catalytic n-alkane dehydrogenation (28). Therefore, we investigated γ-Al2O3–supported (t-Bu2PO-t-BuPOCOP)Ir(C2H4) (2/γ-Al2O3), which was reported to be also very active in alkane dehydrogenation (23, 29). Combining 2/γ-Al2O3 and Re2O7/γ-Al2O3 led to a 98% conversion of PE to oils (Table 1, entry 3). Although 2/γ-Al2O3 is less active than 1/γ-Al2O3 in short-chain alkane metathesis (Table 1, entry 4 versus entry 2), the former is significantly more efficient than the latter for PE degradation to soluble/liquid products (Table 1, entry 3 versus entry 1). Additionally, 2/γ-Al2O3 provided more long-chain alkanes (C22–C40; 26 mM) than did 1/γ-Al2O3 (17 mM) for HDPE-1 degradation. This is consistent with our hypothesis that the dehydrogenation catalyst that favors the formation of internal olefins is more efficient for PE degradation. Furthermore, we synthesized a new iridium complex containing isopropyl substituents on the phosphorus atoms and a methoxy group in the aromatic backbone, (MeO-i-PrPOCOP)Ir(C2H4) (3) (Fig. 1B). A combination of 3/γ-Al2O3 with Re2O7/γ-Al2O3 was also highly effective for PE cleavage, degrading 95% of PE into oils after 3 days at 150°C (Table 1, entry 5).
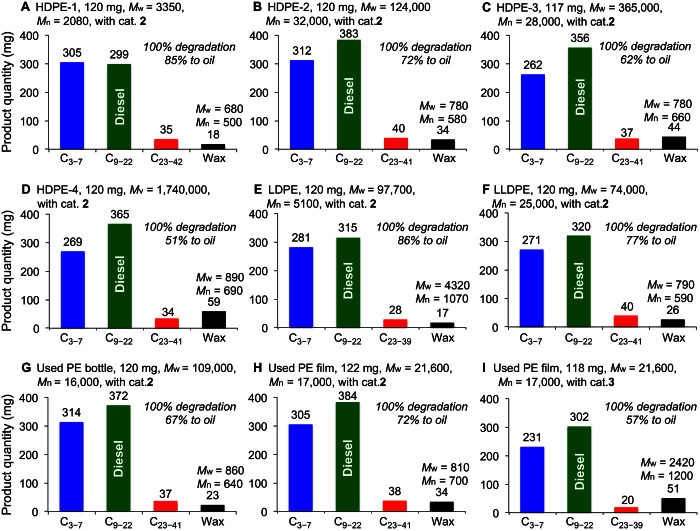
For practical considerations, we then carried out PE degradation at reduced catalyst loading and tested the recyclability of the catalysts. The Re2O7/γ-Al2O3 catalyst and the supported Ir catalyst can be recycled for olefin metathesis and alkane dehydrogenation, respectively, albeit with reduced activity (see the Supplementary Materials). Moreover, the loading of the Ir catalyst can be significantly reduced. With only 4.2 μmol of 2 (3.3 mg), the reaction of HDPE-1 (120 mg) with 2.5 ml of n-octane at 175°C converted 85% of the polymer to oil after 4 days (Fig. 2A). Gel permeation chromatography (GPC) (fig. S4A) analyses of the wax products (18.0 mg) isolated from the PE degradation mixture showed significant reduction in MW compared to the parent PE [Mw = 680 versus 3350; number-average molecular weight (Mn) = 500 versus 2080]. These materials with relatively narrow molecular distribution (PDI, 1.4) can be used as PE waxes, which are valuable slips, dispersants, and resin modifier agents for polymer processing (30). These data indicate that, under catalytic conditions, HDPE-1 was completely degraded to yield oil hydrocarbons as the major products and low-MW waxes as the minor products.
Fig. 2. Degradation of various grades of PEs with n-octane (2.5 ml for HDPE-1 and LLDPE and 4 ml for all other PEs) by 2 (4.2 μmol Ir) and Re2O7/γ-Al2O3 (57 μmol Re2O7): The distribution of degradation products after 4 days at 175°C.
More detailed degradation product distributions are summarized in table S1.
Following the successful model study, we further carried out a degradation study of commercial-grade high-MW PEs. In commercial PE plastics, various additives, such as antioxidants, are often added to stabilize and/or modify the properties of PEs. To test the compatibility of the CAM catalyst system, we further carried out a degradation study in the presence of commercial additives. The degradation of commercial HDPE pellets with an Mw of 12.4 × 104 (HDPE-2; PDI, 3.9) that contain antioxidants 1010 and 168 (polyphenol- and phosphite-based stabilizers, respectively) and zinc stearate (~1000 ppm each) occurred smoothly. With a combination of 2 (4.2 μmol Ir) and Re2O7/γ-Al2O3 (57 μmol Re2O7), the reaction of HDPE-2 (120 mg) with 4 ml of n-octane delivered a 72% conversion of PE to oil products. GPC analysis of the isolated wax product (34 mg) revealed only low-MW species (Mw = 780; Mn = 580) (Fig. 2B and fig. S4B). The lack of any high-MW peak in the GPC trace suggests that no PE starting material (HDPE-2) remained intact during the process. HDPE-3 pellets with an Mw of 36.5 × 104 (PDI, 13.0), which contain the same type and similar amount of additives as HDPE-2, also successfully underwent degradation (Fig. 2C and fig. S4E). The degradation of an ultrahigh-MW PE, HDPE-4 powder with a viscosity average molecular weight (Mv) of 1.74 × 106, gave a 51% conversion to oil products. GPC analyses revealed wax products with an Mw of 890 with a narrow PDI (1.3) (Fig. 2D). Again, no high-MW polymer (Mw > 1000) was detected in the residual solid (fig. S4F), indicating a full breakdown of this ultrahigh-MW PE. The wax products could be further degraded into oil products upon addition of more CAM catalysts (table S1, entry 5); the conversion into oil was increased to 72%, accompanied by a lowering of the MW of the resulting wax (Mw = 890 versus 780) (table S1, entry 5). These results demonstrate that the CAM strategy is very effective for degrading commercial-grade HDPEs of different MW in the presence of normal additives into low-MW liquid fuels and wax products (Fig. 2, B to D).
The CAM system is not limited to the degradation of HDPE. The other two major types of PEs, LDPE and LLDPE, also efficiently undergo degradation. The reactions of a commercial LDPE film with an Mw of 9.17 × 104 (120 mg; PDI, 18.0) and of commercial LLDPE pellets with an Mw of 7.4 × 104 (120 mg; PDI, 3.0), both containing the same antioxidant and lubricant as HDPE-2, resulted in 86 and 77% conversion to oils, respectively. The resulting wax products from LDPE and LLDPE have an Mw of 4320 and 790 (Mn of 1070 and 590), respectively (Fig. 2, E and F).
After demonstrating the efficiency and robustness of the CAM catalyst system for the degradation of commercial-grade PEs, we investigated the degradation of postconsumer PE plastic bottles and films, which are common plastic wastes in real life. The plastic bottle used in this study was made of HDPE with an Mw of 10.9 × 104 (PDI, 6.8), as revealed by GPC. The material was simply dried in air and used without any treatment. The degradation of the HDPE waste using n-octane led to a 64% conversion to oils and a 36% conversion to solid waxes having an Mw of 860 (PDI, 1.3) (Fig. 2G). The degradation of a postconsumer plastic food packaging HDPE film with an Mw of 21.6 × 104 (PDI, 12.7) by n-octane afforded a 72% conversion to oils and a 28% conversion to solid waxes with an Mw of 810 (PDI, 1.2) (Fig. 2H).
Because of their low cost and wide availability, petroleum ethers are much more practical metathesis reagents than pure n-hexane or n-octane for PE degradation. The degradation of 0.3 g of HDPE plastic bottle waste with petroleum ether (8 ml; distillation fraction, 35° to 60°C) using our normal protocol afforded 2 mg of PE wax and 1.08 g of isolated alkanes ranging from C7 to C38, among which the diesel fractions of n-alkanes (that is, C9-C22 n-alkanes) are the predominant products (Fig. 3C). The degradation of a food packaging HDPE film with an Mw of 5.42 × 104 (PDI, 17.2) and a grocery shopping bag (a blend of HDPE and LLDPE; the MW of the plastic bag could not be determined by GPC because the materials contain too much inorganic salts) (0.3 g each) resulted in the isolation of 0.77 g of C7-C38 alkanes and 7 and 3 mg of waxes, respectively (Fig. 3A). Because of the presence of branched alkanes in the petroleum ether, small amounts of isoparaffins were also observed by GC (Fig. 3C). The existence of branched alkanes can enhance the cold flow properties of the obtained diesel products (31–33). For comparison, the reaction with the petroleum ether in the absence of the PE plastic waste gave a trace amount of C10-C12 alkanes (fig. S5A). Because the volatile petroleum ether can be easily separated from the products by distillation and reused for further PE degradation, the degradation of PE wastes into diesel fuels using petroleum ether through CAM could become an economically feasible process.
Fig. 3. Degradation of postconsumer PE plastic bottle (HDPE), food packaging film (HDPE), and grocery shopping bag (a blend of HDPE and LLDPE) into oils.
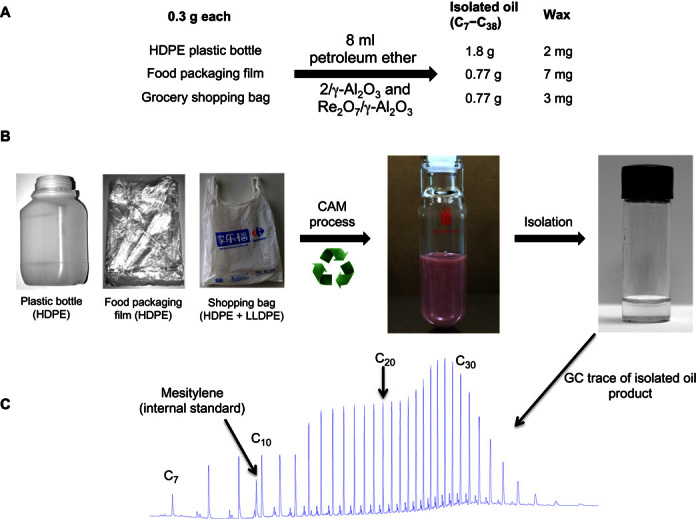
(A) Degradation of PE plastic wastes (0.3 g) with petroleum ether (8 ml) by 2 (10 μmol Ir) and Re2O7/γ-Al2O3 (86 μmol Re2O7) at 175°C after 4 days. (B) PE plastic wastes used in the degradation (left), CAM degradation reaction mixture (middle), and oil products isolated from the degradation mixture (right). (C) GC trace for the oil products from the degradation of the PE plastic bottle.
The distribution of the degradation products (oil versus wax) can be controlled by reaction time and by using different dehydrogenation catalysts. The degradation processes of the commercial HDPE pellets HDPE-2 (Mw = 12.4 × 104; PDI, 3.9) were monitored (Fig. 4 and table S2). Several features should be noted. First, no parent PE materials were detected by GPC analysis at the early stage of the reaction (2 hours), indicating a rather rapid breakdown of PE into oils and PE waxes. Second, the PE waxes were gradually converted into oils over the course of the reaction (Fig. 4A). Finally, the MW and the MW distribution of the PE waxes decreased with degradation time (Fig. 4, B and C). For example, the degradation of the HDPE-2 after 2 hours gave 81% of waxes with relatively high MW (Mw = 4390) and a broad molecular distribution (PDI, 10.1); after 1 day, the process gave 48% of waxes with an Mw of 1060 (PDI, 1.5). In addition, the iridium catalyst plays a role in controlling the yields of oils and waxes and in the MW distribution of the resulting PE waxes. The degradation of various PEs using 3/γ-Al2O3 as the dehydrogenation catalyst consistently gave higher yields of the wax products than that using 2/γ-Al2O3. Furthermore, the MW of the resulting PE waxes in the reaction with 3/γ-Al2O3 is almost double of that obtained with 2/γ-Al2O3 (for example, Fig. 2H versus Fig. 2I and table S1, entry 2 versus entry 3, entry 7 versus entry 8, and entry 9 versus entry 10). Although the mechanism remains to be studied, the formation of linear PE waxes with a controllable MW (Mw = 500 to 2500) and narrow PDI through PE waste degradation is of significant interest because selective synthesis of highly linear PE waxes with narrow molecular distribution is difficult by ethylene polymerization or by other means (34).
Fig. 4. Degradation of HDPE-2 (120 to 135 mg) with n-octane (4 ml) by 2 (4.2 μmol Ir) and Re2O7/γ-Al2O3 (57.0 μmol Re2O7) at 175 °C after 2, 4, 6, 8, 24, 48, and 96 hours.
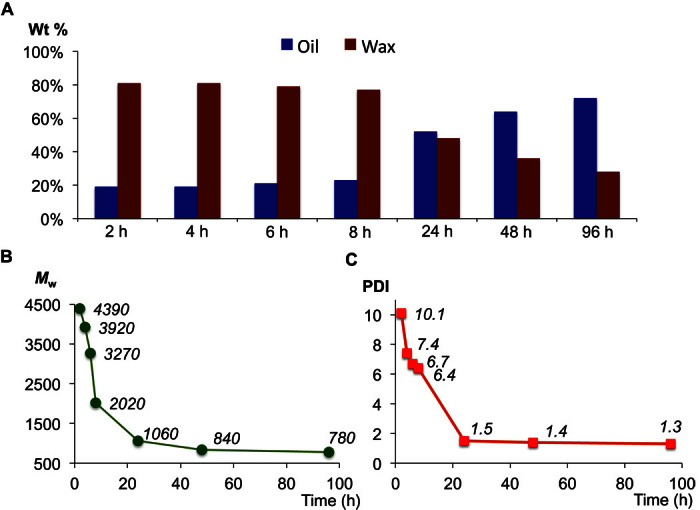
(A) Distributions (wt %) of oil and wax products. (B) Mw of the isolated PE wax products. (C) Molecular weight distributions (PDI) of the isolated PE wax products.
DISCUSSION
Here, we demonstrate an efficient method for degradation of PEs under mild conditions using a tandem catalytic CAM method. With excess, inexpensive light alkanes (such as petroleum ether) as the reagents, various types of PEs, including HDPE, LDPE, and LLDPE with an MW of up to millions, can be completely degraded to low-MW oils and waxes within 1 day at 175°C. The CAM system uses a highly efficient alkane dehydrogenation catalyst, the molecular pincer-type iridium complex, to dehydrogenate the PE and the light alkane. The resulting unsaturated PE and light alkene undergo rhenium-catalyzed cross metathesis to form two new olefins, which are hydrogenated by the iridium catalyst to produce saturated alkanes. Note that only the metathesis of the internal double bonds in PE can lead to the breakdown of the polymer. The closer the double bond is to the middle site of PE, the more efficient the chain length of PE would be reduced. As a result, the bis(phosphinite)-based (POCOP)Ir complex 2 proved to be more efficient for PE degradation than the bis(phosphine)-based (PCP)Ir complex 1 (Table 1, entry 1 versus entry 3) because the latter has a higher tendency for the formation of terminal alkenes in n-alkane dehydrogenation (28). In addition, the use of a large excess of low-cost, recyclable light alkanes relative to the PE (that is, depending on the MW of PE, the molar ratios of light alkane/PE are typically larger than 5000) minimizes the possibility for redundant metathesis between two PE chains, which would not result in effective breakdown of PE.
Analysis of the liquid products reveals the formation of a range of n-alkanes. However, regardless of the structures and MWs of the parent PEs, all reactions afforded alkanes in the diesel range as the major products (Fig. 2). With the use of the iridium catalyst 2, the degradations of various PEs (except for LDPE), with MWs ranging from thousands to millions and with PDI values of up to 13, all gave PE wax products with narrow MW distributions (PDI, ~1.3) and an Mw of less than 1000 (Fig. 2, A to D and F to H). A simple change of the iridium catalyst from 2 to 3 resulted in the consistent formation of PE wax products with broader MW distributions (PDI, ~2.0) and an Mw of ~2000 (Fig. 2I and table S1, entries 3, 8, and 10). The iridium catalysts appear to play an important role in controlling the distribution of the degradation products.
Both the pincer iridium dehydrogenation catalyst and the rhenium metathesis catalyst tolerate the polyphenol- and phosphite-based stabilizers and zinc stearate that are present in the commercial-grade PEs. The robust catalyst systems allow full degradation of waste PE bottles, films, and bags into valuable liquid fuels (diesel) and waxes. To the best of our knowledge, the degradation of real-world postconsumer PEs under such mild reaction conditions is unprecedented.
Featuring high efficiency, mild reaction conditions, and fine control of degradation products, this method shows distinct advantages over traditional pyrolysis processes. For further studies, we are developing more practical alkane metathesis catalyst systems that could provide new approaches for feedstock recovery from PE plastic wastes.
MATERIALS AND METHODS
A 10-ml thick-wall Kontes flask was charged with iridium complex (4.2 μmol), n-octane (2.5 or 4.0 ml), 5 wt % Re2O7/Al2O3 (546 mg), PE (120 mg), and mesitylene (20 μl) as an internal standard. The flask was sealed with a Teflon plug and then heated at 175°C for 4 days. After that, an aliquot was removed from the flask and analyzed by GC. The distributions of soluble products were calculated for each aliquot. The residual solution was filtered at 160°C and washed with n-octane. The filtrates were combined and cooled to room temperature. The wax products precipitated from the solution and were separated by centrifugation. The detailed methods and characterization are available in the Supplementary Materials.
Supplementary Material
Acknowledgments
We acknowledge K. Ding, Y. Tang, and L. Li for helpful suggestions and comments, and X. Ge and J. Li for GPC analysis. Funding: We acknowledge financial support from the National Basic Research Program of China (2015CB856600) and the National Natural Science Foundation of China (21422209, 21432011, and 21421091 to Z.H.), from the U.S. National Science Foundation (CHE-1012422 and DMR-1217651) and the U.S. Department of Energy Division of Materials Sciences (DE-FG02-04ER46162 to Z.G.), and from the CAS/SAFEA International Partnership Program for Creative Research Teams (to Z.H. and Z.G.). Author contributions: Z.H. and Z.G. directed the project and wrote the manuscript. X.J. carried out complex synthesis and most of the PE degradation reactions. C.Q. carried out some PE degradation reactions. T.F. carried out GPC analysis. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/2/6/e1501591/DC1
Supplementary Materials and Methods
fig. S1. GC traces for the reactions of HDPE-1 with Ir complex 1 or 2, and the control experiments without HDPE-1.
fig. S2. GC trace for the reaction of 1-octene with recycled Re2O7/Al2O3.
fig. S3. GC traces for the control experiments in the absence of one of the dual catalysts or in the absence of both catalysts.
fig. S4. GPC traces for the wax products.
fig. S5. GC traces for the oil products from the degradation of the PE plastic bottle, film, and bag.
fig. S6. GPC traces for the wax products obtained from the reaction with HDPE-2 after 2, 4, 6, 8, 24, 48, and 96 hours.
table S1. Degradation of various grades of PE with n-octane (2.5 or 4.0 ml) by [Ir] (4.2 μmol) and Re2O7/Al2O3 (57 μmol Re2O7): The distribution of n-alkane products and conversion of PE to oil products after 4 days at 175°C.
table S2. Degradation of HDPE-2 at different time intervals: The distribution of n-alkane products and conversion of PE to oil products.
REFERENCES AND NOTES
- 1.Jambeck J. R., Geyer R., Wilcox C., Siegler T. R., Perryman M., Andrady A., Narayan R., Law K. L., Plastic waste inputs from land into the ocean. Science 347, 768–771 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Moore C. J., Synthetic polymers in the marine environment: A rapidly increasing, long-term threat. Environ. Res. 108, 131–139 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Butler E., Devlin G., McDonnell K., Waste polyolefins to liquid fuels via pyrolysis: Review of commercial state-of-the-art and recent laboratory research. Waste Biomass Valorization 2, 227–255 (2011). [Google Scholar]
- 4.J. Scheirs, W. Kaminsky, Feedstock Recycling and Pyrolysis of Waste Plastics (John Wiley & Sons Ltd., Chichester, UK, 2006), pp. 709–728. [Google Scholar]
- 5.Kaminsky W., Zorriqueta I.-J. N., Catalytical and thermal pyrolysis of polyolefins. J. Anal. Appl. Pyrolysis 79, 368–374 (2007). [Google Scholar]
- 6.D. Mehmet, 100+ Years of Plastics: Leo Baekeland and Beyond (American Chemical Society, Washington, DC, 2011), vol. 1080, pp. 115–145. [Google Scholar]
- 7.Kaminsky W., Mennerich C., Zhang Z., Feedstock recycling of synthetic and natural rubber by pyrolysis in a fluidized bed. J. Anal. Appl. Pyrolysis 85, 334–337 (2009). [Google Scholar]
- 8.Serrano D. P., Aguado J., Escola J. M., Developing advanced catalysts for the conversion of polyolefinic waste plastics into fuels and chemicals. ACS Catal. 2, 1924–1941 (2012). [Google Scholar]
- 9.Aguado J., Serrano D. P., Escola J. M., Fuels from waste plastics by thermal and catalytic processes: A review. Ind. Eng. Chem. Res. 47, 7982–7992 (2008). [Google Scholar]
- 10.Onwudili J. A., Insura N., Williams P. T., Composition of products from the pyrolysis of polyethylene and polystyrene in a closed batch reactor: Effects of temperature and residence time. J. Anal. Appl. Pyrolysis 86, 293–303 (2009). [Google Scholar]
- 11.Uemichi Y., Takuma K., Ayame A., Chemical recycling of poly(ethylene) by catalytic degradation into aromatic hydrocarbons using H-Ga-silicate. Chem. Commun. 1998, 1975–1976 (1998). [Google Scholar]
- 12.Pifer A., Sen A., Chemical recycling of plastics to useful organic compounds by oxidative degradation. Angew. Chem. Int. Ed. 37, 3306–3308 (1998). [DOI] [PubMed] [Google Scholar]
- 13.Dufaud V., Basset J.-M., Catalytic hydrogenolysis at low temperature and pressure of polyethylene and polypropylene to diesels or lower alkanes by a zirconium hydride supported on silica-alumina: A step toward polyolefin degradation by the microscopic reverse of ziegler–natta polymerization. Angew. Chem. Int. Ed. 37, 806–810 (1998). [DOI] [PubMed] [Google Scholar]
- 14.Tosin G., Santini C. C., Basset J.-M., Polymerization of α-olefins and hydrogenolysis of the resulting polyolefins with hafnium hydrides supported on silica. Top. Catal. 52, 1203–1210 (2009). [Google Scholar]
- 15.Burnett R. L., Hughes T. R., Mechanism and poisoning of the molecular redistribution reaction of alkanes with a dual-functional catalyst system. J. Catal. 31, 55–64 (1973). [Google Scholar]
- 16.Vidal V., Théolier A., Thivolle-Cazat J., Basset J.-M., Metathesis of alkanes catalyzed by silica-supported transition metal hydrides. Science 276, 99–102 (1997). [DOI] [PubMed] [Google Scholar]
- 17.Basset J. M., Copéret C., Lefort L., Maunders B. M., Maury O., Le Roux E., Saggio G., Soignier S., Soulivong D., Sunley G. J., Taoufik M., Thivolle-Cazat J., Primary products and mechanistic considerations in alkane metathesis. J. Am. Chem. Soc. 127, 8604–8605 (2005). [DOI] [PubMed] [Google Scholar]
- 18.Goldman A. S., Roy A. H., Huang Z., Ahuja R., Schinski W., Brookhart M., Catalytic alkane metathesis by tandem alkane dehydrogenation-olefin metathesis. Science 312, 257–261 (2006). [DOI] [PubMed] [Google Scholar]
- 19.Haibach M. C., Kundu S., Brookhart M., Goldman A. S., Alkane metathesis by tandem alkane-dehydrogenation–olefin-metathesis catalysis and related chemistry. Acc. Chem. Res. 45, 947–958 (2012). [DOI] [PubMed] [Google Scholar]
- 20.J. G. Speight, The Chemistry and Technology of Petroleum (CRC Press, Boca Raton, ed. 5, 2014), pp. 591–630. [Google Scholar]
- 21.Khodakov A. Y., Chu W., Fongarland P., Advances in the development of novel cobalt fischer−tropsch catalysts for synthesis of long-chain hydrocarbons and clean fuels. Chem. Rev. 107, 1692–1744 (2007). [DOI] [PubMed] [Google Scholar]
- 22.Huber G. W., Cortright R. D., Dumesic J. A., Renewable alkanes by aqueous-phase reforming of biomass-derived oxygenates. Angew. Chem. Int. Ed. 43, 1549–1551 (2004). [DOI] [PubMed] [Google Scholar]
- 23.Huang Z., Rolfe E., Carson E. C., Brookhart M., Goldman A. S., El-Khalafy S. H., MacArthur A. H. R., Efficient heterogeneous dual catalyst systems for alkane metathesis. Adv. Synth. Catal. 352, 125–135 (2010). [Google Scholar]
- 24.Liu F., Pak E. B., Singh B., Jensen C. M., Goldman A. S., Dehydrogenation of n-alkanes catalyzed by iridium “pincer” complexes: Regioselective formation of α-olefins. J. Am. Chem. Soc. 121, 4086–4087 (1999). [Google Scholar]
- 25.Gupta M., Hagen C., Flesher R. J., Kaska W. C., Jensen C. M., A highly active alkane dehydrogenation catalyst: Stabilization of dihydrido rhodium and iridium complexes by a P-C-P pincer ligand. Chem. Commun. 23, 2687 (1996). [Google Scholar]
- 26.Pariya C., Jayaprakash K. N., Sarkar A., Alkene metathesis: New developments in catalyst design and application. Coord. Chem. Rev. 168, 1–48 (1998). [Google Scholar]
- 27.Göttker-Schnetmann I., White P., Brookhart M., Iridium bis(phosphinite) p-XPCP pincer complexes: Highly active catalysts for the transfer dehydrogenation of alkanes. J. Am. Chem. Soc. 126, 1804–1811 (2004). [DOI] [PubMed] [Google Scholar]
- 28.Yao W., Zhang Y., Jia X., Huang Z., Selective catalytic transfer dehydrogenation of alkanes and heterocycles by an iridium pincer complex. Angew. Chem. Int. Ed. 53, 1390–1394 (2014). [DOI] [PubMed] [Google Scholar]
- 29.Huang Z., Brookhart M., Goldman S., Kundu S., Ray A., Scott S. L., Vicente B. C., Highly active and recyclable heterogeneous iridium pincer catalysts for transfer dehydrogenation of alkanes. Adv. Synth. Catal. 351, 188–206 (2009). [Google Scholar]
- 30.Jia Y.-y., Zhang L., A process for preparing polyethylene wax microspheres and optimization of their dissolution precipitation by response surface methodology. J. Appl. Polym. Sci. 129, 1476–1483 (2013). [Google Scholar]
- 31.Escola J. M., Aguado J., Serrano D. P., Garcia A., Peral A., Briones L., Calvo R., Fernandez E.. Catalytic hydroreforming of the polyethylene thermal cracking oil over Ni supported hierarchical zeolites and mesostructured aluminosilicates. Appl. Catal. B 106, 405–415 (2011). [Google Scholar]
- 32.Escola J. M., Aguado J., Serrano D. P., Briones L., Díaz de Tuesta J. L., Calvo R., Fernandez E., Conversion of polyethylene into transportation fuels by the combination of thermal cracking and catalytic hydroreforming over Ni-supported hierarchical β zeolite. Energy Fuel 26, 3187 (2012). [Google Scholar]
- 33.Escola J. M., Aguado J., Serrano D. P., Briones L., Transportation fuel production by combination of LDPE thermal cracking and catalytic hydroreforming. Waste Manag. 34, 2176–2184 (2014). [DOI] [PubMed] [Google Scholar]
- 34.Cui X.-M., Production and application of polyethylene wax. Fine Spec. Chem. 22, 33–35 (2014). [Google Scholar]
- 35.Basson S. S., Leipoldt J. G., Purcell W., Schoeman J. B., Bromide catalysis in the oxidative addition of iodomethane to iridium(I) complexes. Inorg. Chim. Acta 173, 155–158 (1990). [Google Scholar]
- 36.J. L. Herde, J. C. Lambert, C. V. Senoff, M. A. Cushing, Inorganic Syntheses, G. W. Parshall, Ed. (John Wiley & Sons Inc., New York, 2007), pp. 18–20. [Google Scholar]
- 37.A. S. Goldman, R. Ghosh, Handbook of C-H Transformations: Applications in Organic Synthesis, G. Dyker, Ed. (Wiley-VCH, New York, 2005), pp. 616–621. [Google Scholar]
- 38.Vabre B., Spasyuk D. M., Zargarian D., Impact of backbone substituents on POCOP-Ni pincer complexes: A structural, spectroscopic, and electrochemical study. Organometallics 31, 8561–8570 (2012). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/2/6/e1501591/DC1
Supplementary Materials and Methods
fig. S1. GC traces for the reactions of HDPE-1 with Ir complex 1 or 2, and the control experiments without HDPE-1.
fig. S2. GC trace for the reaction of 1-octene with recycled Re2O7/Al2O3.
fig. S3. GC traces for the control experiments in the absence of one of the dual catalysts or in the absence of both catalysts.
fig. S4. GPC traces for the wax products.
fig. S5. GC traces for the oil products from the degradation of the PE plastic bottle, film, and bag.
fig. S6. GPC traces for the wax products obtained from the reaction with HDPE-2 after 2, 4, 6, 8, 24, 48, and 96 hours.
table S1. Degradation of various grades of PE with n-octane (2.5 or 4.0 ml) by [Ir] (4.2 μmol) and Re2O7/Al2O3 (57 μmol Re2O7): The distribution of n-alkane products and conversion of PE to oil products after 4 days at 175°C.
table S2. Degradation of HDPE-2 at different time intervals: The distribution of n-alkane products and conversion of PE to oil products.