The tropical shrub Beauprea was already present in Gondwana when Zealandia drifted away from Antarctica 82 million years ago.
Keywords: Antarctica, New Caledonia, Beauprea, Beaupreaidites, transoceanic dispersal, vicariance, allopatry, Zealandia, Gondwana
Abstract
New Caledonia and New Zealand belong to the now largely submerged continent Zealandia. Their high levels of endemism and species richness are usually considered the result of transoceanic dispersal events followed by diversification after they re-emerged from the Pacific Ocean in the mid-Cenozoic. We explore the origin and evolutionary history of Beauprea (Proteaceae), which is now endemic to New Caledonia but was once spread throughout eastern Gondwana, including New Zealand. We review the extensive Beauprea-type pollen data in the fossil records and analyze the relationship of these fossil taxa to extant genera within Proteaceae. We further reconstruct the phylogenetic relations among nine extant species of Beauprea and estimate the age of the Beauprea clade. By incorporating extinct taxa into the Beauprea phylogenetic tree, we reconstruct the ancient distribution of this genus. Our analysis shows that Beauprea originated c. 88 Ma (million years ago) in Antarctica–Southeastern Australia and spread throughout Gondwana before its complete breakup. We propose that Beauprea, already existing as two lineages, was carried with Zealandia when it separated from the rest of Gondwana c. 82 Ma, thus supporting an autochthonous origin for Beauprea species now in New Caledonia and historically in New Zealand up to 1 Ma. We show that the presence of Beauprea through transoceanic dispersal is implausible. This means that neither New Caledonia nor New Zealand has been entirely submerged since the Upper Cretaceous; thus, possible vicariance and allopatry must be taken into account when considering the high levels of endemism and species richness of these island groups.
INTRODUCTION
Phylogenetic analyses, divergence-time estimation, and ancient distribution reconstruction of extant taxa are increasingly used to test biogeographical hypotheses (1). Extinction has long been acknowledged as a key component of observable diversity and biogeographical patterns, and it is essential to include extinct taxa, wherever possible, when identifying regional diversification patterns (2), particularly when the ranges of fossil species do not overlap those of extant species. Fossil records are crucial in explaining extant biodiversity because phylogenetic analyses of extant organisms cannot replace paleontology as a way of reconstructing the past and cannot reveal the existence of extinct taxa or lineages (3).
The Antarctic continent is the most inhospitable place on Earth because of its freezing climate, blizzards, and ice caps, with 99.7% of the current terrain covered by permanent 4-km-thick ice. Yet as far back as two-and-a-half centuries ago, both Charles Darwin and Joseph Hooker speculated that the Antarctic continent must have supported a flora containing elements now restricted to southern forests. One of the most important breakthroughs in Antarctic geological research over the last three decades has been the elucidation of the continent’s fossil records. A surprising diversity of fossil plants and animals has now been recorded in Antarctica, mainly in the Antarctic Peninsula (nearest South America), supporting the idea that dense vegetation was once able to survive close to the South Pole (4). Francis et al. (5) reconstructed the vegetation of Antarctica over the past 100 My (million years) and suggested that flowering plants thrived under subtropical climates in Antarctica 85 Ma (million years ago; mid–Late Cretaceous). Analysis of leaf and flower fossils indicates that summer temperatures averaged 20°C during this global thermal maximum (5). Our knowledge of the paleontological and paleobotanical history of Antarctica has increased greatly over the last few decades, with detailed studies of many key groups (5–7) that have been important in developing our understanding of the broad patterns of the history of life on Earth. It has become apparent that Antarctica has had a much more significant role than simply acting as a convenient stepping stone for taxa that originated elsewhere (8) and may in fact be the center of the origin and early diversification of some important plant taxa.
New Caledonia is a fragment of the continental crust Zealandia—which also includes New Zealand, Lord Howe Rise, Norfolk Ridge, Campbell Plateau, and Chatham Rise—that rafted away from Antarctica on its southwest side about 82 Ma and reached its present position 50 Ma (9, 10). It had already separated from Australia on its northwest side by 89 to 85 Ma (11). New Caledonia is a global biodiversity hot spot, harboring extraordinary levels of species richness and endemism (77%) among the angiosperms (12). There has been a debate over the origin of New Caledonia’s unique biota, which can be traced to Gondwanan times owing to its long geological history and the presence of phylogenetic relicts (13). However, some geological evidence indicates that New Caledonia may have been submerged for long periods in the Paleocene and Eocene (9, 14), with the main island only becoming available for terrestrial colonization 38 to 33 Ma (15). The origin of New Caledonian biodiversity has therefore been explained by two incompatible hypotheses. Espeland and Murienne (16) studied the evolutionary history of Angustonicus (a neocaledonian cockroach genus) as a general model for the New Caledonian biota and concluded that the high New Caledonian biodiversity is a result of recent dispersal events followed by diversification. Buckley et al. (17) analyzed the diversification patterns of nine New Caledonian groups and found a significant variation in the diversification rates. As a consequence, Espeland and Murienne (16) argued that the original New Caledonian biota was wiped out during an episode of submersion. Other groups have similarly demonstrated diversification in New Caledonia subsequent to recent dispersal (18–20). However, the generalization of this hypothesis to the entire New Caledonian biota seems implausible, as there are unambiguous counterarguments. These center on the existence of many taxa endemic to New Caledonia with poor dispersal ability and the existence of clades that can be traced beyond the time when New Caledonia supposedly re-emerged in the Late Eocene. These include the world’s most ancient extant flowering plant Amborella, the arachnid Troglosironidae (21),Gondwanan moss bugs (22), the beetle family Passalidae (23), and the saw moth Sabatinca (24). Many of these New Caledonian endemics are traditionally viewed as an inheritance from Gondwana (11), which points to a hypothesis of vicariance where New Caledonia is perceived as a Gondwanan refuge for these relictual taxa.
Similarly, New Zealand has a rich, highly endemic flora at the species level [82% (25)], with interpretations of its history varying from emphasizing continuous links to its Gondwanan past, especially among its temperate rainforest species (26), to proposing that the entire flora has a transoceanic origin (27). The latter is supported by geological evidence indicating that the entire subcontinent was submerged in the Late Oligocene to the Early Miocene (28). More recent work indicates that continuous uplift among basement blocks ensured that some land persisted above sea level even then (29). The topic is so controversial that an entire issue of the New Zealand Journal of Geology and Geophysics was devoted to it in 2014 (30).
The proteaceous genus Beauprea now contains 13 species, all endemic to New Caledonia (31). Beauprea species usually occur in mountainous regions bearing diverse rainforests in the interior region of the main island Grande Terre (32). Ten species, except for Beauprea spathulifolia, Beauprea montana, and Beauprea neglecta, have strictly local distributions known only from a few records. For example, the upland Beauprea penariensis was last collected in 1876 as the only known specimen. (Note that T.H. and B.B.L. failed to locate this species at the recorded location during a collecting trip in 2013.) There are no Beauprea-type pollen fossils recorded in New Caledonia. However, Beauprea-like pollen in the fossil records has been described from New Zealand (33, 34), Australia (35, 36), Antarctica (37–39), and southern South America (40) over a continuous 85-My period from the mid–Upper Cretaceous to the mid-Pleistocene. Beauprea mostly became extinct in mainland Australia from the Early Miocene, with final records in the mid-Pleistocene (41), probably initially from the onset of aridity and fire (32) followed by the cold associated with glaciation (41). The genus existed in New Zealand until recently, with extinction in the area undoubtedly resulting from increasing climatic cooling as the Cenozoic progressed (42), supported by the youngest recorded Beauprea fossils (1 Ma) located at the northernmost tip of the North Island (34).
Sauquet et al. (42) estimated that Beauprea originated 69 Ma in the Late Cretaceous, although they did not make use of fossil records known to be older than that. Despite questioning the provenance and identification of the Early Cenozoic, Beauprea-like pollen (Peninsulapollis) in Antarctica (38), Pocknall and Crosbie (32) accepted that the genus arose in the Late Cretaceous and speculated that its center of origin lay where Antarctica, Australia, and New Zealand retained possible connections. However, a phenetic analysis by Sauquet et al. (42) confirmed that both Beaupreaidites and Peninsulapollis are uniquely linked to Beauprea, whereas both have since been recorded even earlier in the Campanian-Maastrichtian sediments of the Antarctic Peninsula (32–48), alluding to a possibly more significant role for Antarctica in the origin of Beauprea. Although not specifically mentioning Beauprea and (mistakenly) treating New Zealand as separating from Australia, Barker et al. (49) invoked “island hopping” to explain the distribution of other proteaceous genera in Zealandia. Also neglecting a possible role for Antarctica, Sauquet et al. (42) were uncertain of Beauprea’s immediate origins but, once linked with the African genus Protea, they posited an Australian origin for its clade.
Here, we aimed to trace the origin and reconstruct the evolutionary history of Beauprea. Because Beauprea-like pollen was widespread in eastern Gondwana before the severance of Zealandia from Antarctica/Australia in the Upper Cretaceous, our intention was to explore its pathway to New Caledonia and New Zealand. Our working hypothesis was that Beauprea was already present throughout Zealandia at the time of separation, making (recent) transoceanic dispersal, say from Australia,redundant. We first reviewed the presence of Beauprea-type pollen in the fossil records and analyzed the relationship of a selection of fossil taxa with extant genera within the Proteaceae. We further reconstructed the phylogenetic relationships among extant species of Beauprea and estimated the age of the Beauprea clade and its species. Lastly, by incorporating extinct taxa into the Beauprea phylogenetic tree, we were able to reconstruct the ancient geographical distribution of this genus. Together with an understanding of its dispersal biology, we were also able to use the history of Beauprea in New Caledonia and New Zealand to comment on the likelihood that both landforms were completely submerged at some point during the last 82 My.
RESULTS
Our literature search revealed 129 records for Beaupreaidites/Peninsulapollis fossil pollen, which Sauquet et al. (42) assigned with confidence to Beauprea, and these records were spread over much of Australia, New Zealand, Antarctica, and South America for an extended period (83.8 to 1 Ma; Fig. 1 and Table 1). The oldest record was for Beaupreaidites orbiculatus in the Otway Basin, Southeastern Australia (SE Australia), at 83.8 Ma, whereas the youngest record was for the B. orbiculatus “type” in the Antarctic at 51.5 Ma. Peninsulapollis gillii was the second oldest pollen and was recorded in the Antarctic Peninsula at 81.4 Ma. P. gillii was also recorded at 80.3 Ma in the Gippsland Basin, Victoria. The Beaupreaidites elegansiformis type has the longest time span, ranging from 77.6 Ma in the Antarctic Peninsula to 1 Ma in Auckland, New Zealand. B. elegansiformis was recorded from the Campbell Plateau (between Antarctica and New Zealand) in the period 83.5 to 70.6 Ma, and from the Sydney and Gippsland Basins continuously from 55.8 to 9.7 Ma. Pocknall and Crosbie (32) compared B. elegansiformis favorably with extant Beauprea gracilis (in particular), Beauprea filipes, and B. spathulifolia. Beaupreaidites verrucosa (often co-occurring with, but distinct from, B. elegansiformis) was recorded from the Antarctic Peninsula 67.5 Ma, and from the Sydney and Gippsland Basins 57.4 to 30 Ma. For 38 records of Beaupreaidites/Peninsulapollis in the Cretaceous, 92% co-occurred with Nothofagites; for 40 records in the Cenozoic, 98% co-occurred with Nothofagites.
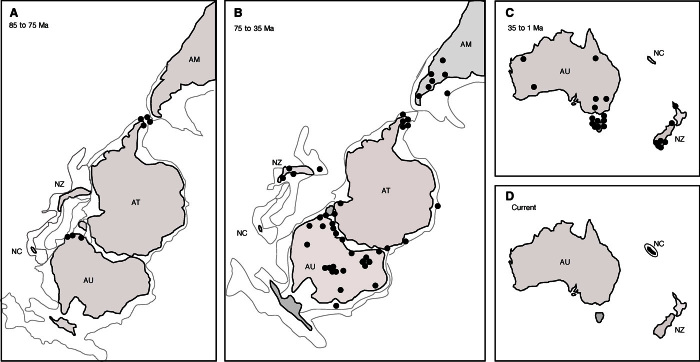
Fig. 1.
Occurrences of Beauprea-type pollen and their geographical location at 85 to 75 Ma (A), 75 to 35 Ma (B), 35 to 1 Ma (C), and present time (D). AT, Antarctica; NZ, New Zealand; NC, New Caledonia; AU, Australia; AM, America. The light gray line indicates the boundary of landmass at that time.
Table 1. Records for the five regions where Beauprea-like fossils have been identified at 15-My intervals through the geological record.
Mean age was used to assign each species observed in a given study, although multiple entries are given if species occurred in successive 15-My intervals.
| Antarctica | SE Australia | Rest of Australia | Zealandia | South America | ||
| Oldest pollen recorded (Ma) | 81.4 | 83.8 | 74.5 | * | 68.0 | |
| Period (Ma) | Approximate epoch | |||||
| 84–70 | Upper Cretaceous | 6 | 8 | 3 | 1* | 0 |
| 69–55 | Paleocene | 9 | 3 | 9 | 4 | 5 |
| 54–35 | Eocene | 12 | 11 | 13 | 4 | 3 |
| 34–20 | Oligocene | 2 | 8 | 4 | 6 | 1 |
| 19–5 | Miocene | 0 | 2 | 3 | 3 | 0 |
| <5 | Pliocene/Quaternary | 0 | 3 | 0 | 6 | 0 |
| Total records | 29 | 34 | 32 | 24 | 9 | |
|
P (Fisher’s exact probability test, two-tailed) for the last three periods combined |
— | 0.0099 | 0.3327 | 0.0001 | 0.3739 | |
|
P (Fisher’s exact probability test, two-tailed) for the last three periods omitted |
— | 0.2651 | 0.7046 | 0.8839 | 0.2778 | |
*“Upper Cretaceous” Couper (67).
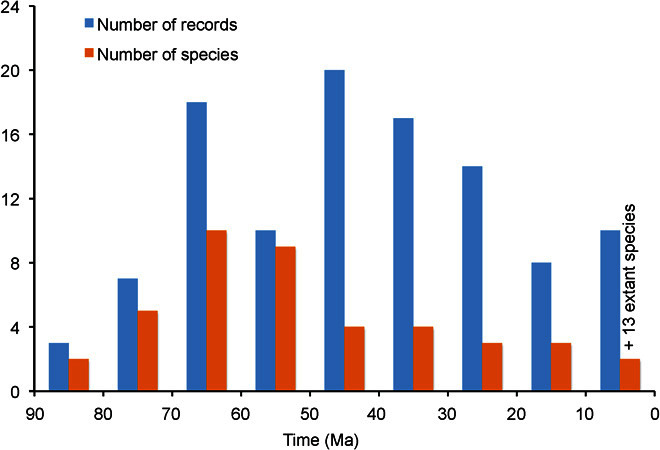
Beauprea-type pollen (Beaupreaidites and Peninsulapollis) was first recorded in the Santonian to Campanian of the Upper Cretaceous in the Antarctic Peninsula and the Otway Basin, when Australia and Antarctica were still joined (Fig. 1A). Antarctica–SE Australia accounted for 80% of records in the period 85 to 70 Ma, dropping to 40% in the period 70 to 55 Ma as Beauprea spread more widely (Table 1). Beauprea fossils peaked in Antarctica and Australia and, overall, during the Eocene (Figs. 1B and 2, and Table 1). The occurrence of Beauprea fossils fell markedly in the Oligocene-Neogene-Quaternary overall, especially in Antarctica and South America, although it peaked in New Zealand (Figs. 1C and 2, and Table 1), eventually becoming extinct everywhere except in New Caledonia (Fig. 1D). The Antarctic Peninsula recorded the highest richness of any region, with seven Beauprea-type species in the Late Cretaceous/Paleocene, finally falling to one species in the Pleistocene at Stony Creek, Victoria [(41); Fig. 2].
Fig. 2. Number of records and number of species with Beauprea-type pollen.
Our phenetic analysis of 11 pollen characters for eight extant Beauprea species and four fossil species with Beauprea-type pollen showed close relationships among all Beauprea, Beaupreaidites, and Peninsulapollis species, but not with other extant genera in the same subfamily such as Protea, Faurea, Franklandia, and Eidothea (Fig. 3). Beaupreaidites is clearly embedded among Beauprea, whereas Peninsulapollis is also well within Beauprea. Maximum likelihood analysis indicated that B. orbiculatus and Beauprea montisfontium have very similar pollen, followed by Beauprea balansae, as Dettermann and Jarzen (33) suggested (Fig. 3). The eight extant Beauprea species and four Beauprea-type fossil species were clustered into two groups. All four fossil species and five extant species formed a group, whereas Beauprea asplenioides and another two extant species formed another group (Fig. 3). Cranwellipollis pollen was grouped with Franklandia (both having almost identical morphology).
Fig. 3. Neighbor-joining tree of fossil species (in red) and extant species.
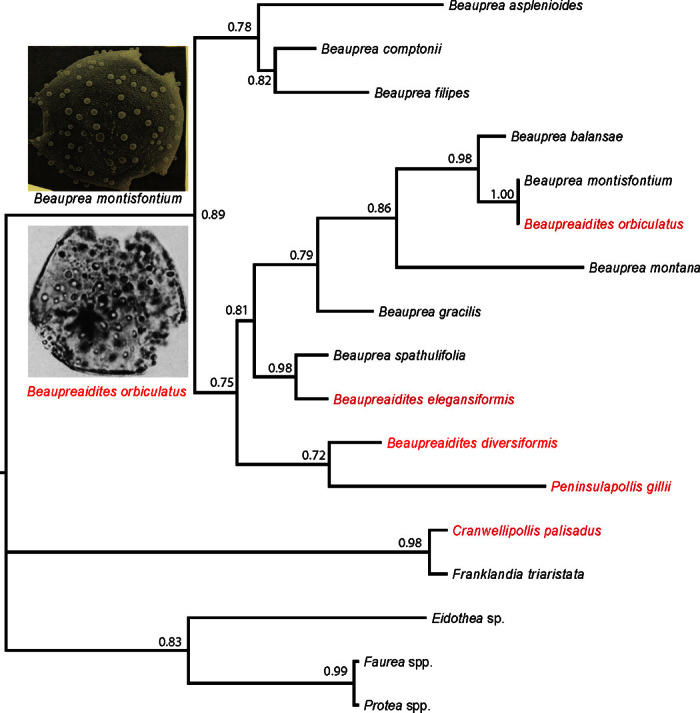
Bootstrapping values are shown on the node. The tree was rooted using Franklandia, Eidothea, Faurea, and Protea as outgroup.
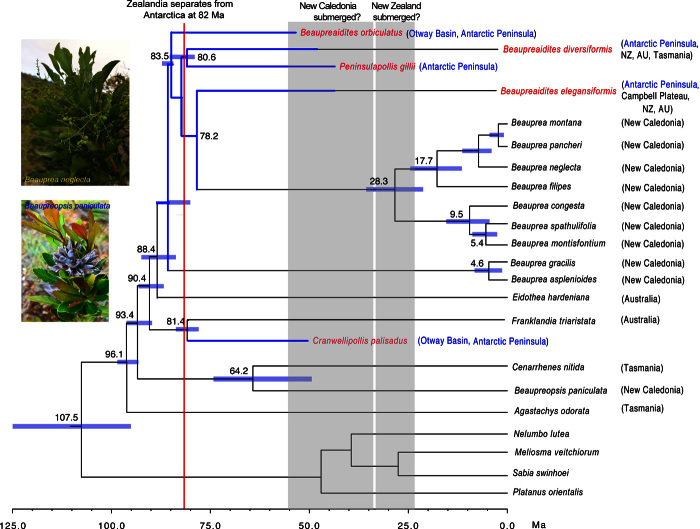
Our molecular phylogenetic reconstruction of extant Beauprea species revealed two major lineages within the genus. Age estimates using Monte Carlo Markov chain (MCMC) procedures suggested that the two lineages diverged at 82.5 Ma [79.5 to 84.7 Ma; 95% highest posterior density (HPD)], a few million years after the genus originated 88.4 Ma (83.7 to 92.1 Ma; 95% HPD). This early divergence was strongly supported in the phylogenetic reconstruction with a posterior probability of 1.0. Both lineages have a typical “broom-and-handle” shape, with a number of lineages arising in a relatively short period at the end of a long stem. Beaupreopsis, another New Caledonian endemic proteaceous genus, was dated at 64.2 Ma (50.1 to 74.8 Ma; 95% HPD) from an immediate ancestor at 93.4 Ma.
All four fossil species incorporated into the phylogeny were grouped with the lineage containing B. montisfontium and B. spathulifolia, which have a pollen structure similar to that of B. orbiculatus and B. elegansiformis, respectively. Beaupreaidites diversiformis and P. gillii were positioned at 80.6 Ma, and the two species share a similar pollen structure, as revealed by the neighbor-joining tree (Fig. 3). Because the actual age of B. orbiculatus (83.5 Ma) exceeded the median crown age, as estimated from MCMC (82.5 Ma), the age of the lineage was pulled back to 83.5 Ma, which was still within the 95% HPD of the MCMC age estimate of the lineage (Fig. 4). In the supertree incorporating known fossil and extant species of Beauprea (Fig. 4), taxa in the earliest branches were first recorded in Antarctica and/or SE Australia, suggesting a possible Antarctica–SE Australian origin for the genus.
Fig. 4. Supertree of Beauprea with fossil (in red) and extant taxa, and the closest phylogenetically related outgroup species.
Blue lines indicate the known or estimated duration of fossil species in Antarctica and SE Australia (AU); blue bars are 95% HPD of the divergence age. Note that Beauprea arose at a mean age of 88.4 Ma. Gray areas indicate the hypothesized time period when New Caledonia and New Zealand (NZ) were submerged according to some studies (but shown here to be unlikely).
DISCUSSION
A central Gondwanan origin for Beauprea
Beauprea-type pollen (Beaupreaidites and Peninsulapollis) were first recorded in the Santonian to Campanian of the Upper Cretaceous in the Antarctic Peninsula and the Otway Basin—the rift valley that developed between Antarctica and Australia before they finally separated 45 Ma. Time-based molecular phylogenetic analysis, in conjunction with the fossil records, indicates that Beauprea diverged from the rest of Proteaceae 88.4 Ma (92.1 to 83.7 Ma; 95% HPD), well before the post-African/Indian landmasses of Gondwana (Antarctica, Australia, Zealandia, and South America) began to separate 82 Ma (50). This means that, despite the unavailability of most of Antarctica for fossil sampling, the origins of Beauprea can be traced to the central core of Gondwana in the Early Upper Cretaceous. Initiation of the two extant lineages occurred 82.5 to 83.5 Ma, when Zealandia was still connected to Antarctica. Although direct records for Zealandia are limited (earliest Beauprea fossils for New Zealand and the Campbell Plateau are described vaguely as “Upper Cretaceous”; Table 1), it is thus likely that Beauprea, including its two basal lineages, was already widespread across Antarctica and Zealandia before they separated 82 Ma.
The idea of Antarctica being the center of origin of certain taxa is not new. Charles Darwin and Joseph Hooker were the first to recognize the possible importance of the Antarctic region in the origins of southern floras. Furthermore, Wardle (51) proposed that preglacial Antarctica must have been a major source of New Zealand’s mountain plants. Truswell (43) summarized the available fossil records and proposed an Antarctic origin for the fern Lophosia, for the podocarp gymnosperms Lagarostrobus and Dacrydium, and for Nothofagus, Ilex, and several lineages of Proteaceae, including Beauprea. Recently, Barreda et al. (52) proposed that Asteraceae, the most diverse angiosperm family, might have originated in Antarctica during the Cretaceous. To date, our analysis provides the best evidence to support a pivotal role for Antarctica in the possible origin and early diversification of a Southern Hemisphere genus.
An autochthonous origin for Beauprea in New Caledonia and New Zealand
Our analysis supports an autochthonous origin for Beauprea in New Caledonia and New Zealand: (i) by the time Zealandia split from Antarctica, Beauprea had been present in Antarctica–SE Australia for >6 My, giving it ample time to spread overland to, or even diversify in, Zealandia, and (ii) the fossil/extant record indicates continuous occupation of Zealandia from the Upper Cretaceous to present (Table 1). An alternative explanation would require early transoceanic dispersal from Antarctica/Australia (and/or islands associated with the Lord Howe Rise, Campbell Plateau, or Chatham Rise) to New Zealand/New Caledonia and/or later dispersal from New Zealand (and/or islands associated with the Challenger Plateau or Norfolk Ridge) to New Caledonia. Furthermore, were New Caledonia and New Zealand to have had periods of complete submergence in the Cenozoic, as proposed by some (9, 14, 28, 30), then not only would transoceanic transport be required but suitable sources/pathways would need to be demonstrated. Finally, since Beauprea bifurcated c. 83 Ma, two independent migration events to New Caledonia would be required. We show below that these alternative scenarios are both implausible and redundant.
Low supply of propagules for dispersal
Records show that both Beaupreaidites and Peninsulapollis are of low abundance throughout their entire geological history (32), suggesting that Beauprea was never a prominent element in its plant communities. We have observed that this is true even today in New Caledonia. Such rarity would become even more extreme following its peak abundance in the Eocene (Fig. 1). Indeed, our literature survey showed a decline in fossil records in mainland Australia since 35 Ma (Table 1). Even if New Zealand was not totally drowned in the Oligocene, it is accepted that its land area was greatly reduced at that time (29). The diversification of Beauprea began 28.5 Ma (36.3 to 22 Ma; 95% HPD), at about the time New Caledonia supposedly re-emerged (14). Beauprea would then have had to (re)colonize New Caledonia between 33 Ma (when the island became re-inhabitable) and 28.5 Ma (when Beauprea began diversifying). However, populations of Beauprea in either Australia or New Zealand may not have been large enough or may not have existed long enough to produce a propagule source pool sufficient to colonize a new landmass in that small “window of opportunity,” as high propagule pressure is one of the key mechanisms facilitating successful invasion (53). Similarly, if New Zealand was also submerged in the Oligocene but supposedly had re-emerged c. 22 Ma (28), both New Caledonia (itself just recovering from inundation) and distant Australia (with Beauprea on a rapid decline) would be poor propagule sources.
Low dispersibility
Transoceanic dispersal of Beauprea via ocean currents is unlikely for four reasons. First, the distributions of modern Beauprea species are mostly in the mountainous regions of New Caledonia, away from watercourses and coastal environments where water dispersal would be possible. Beauprea-type pollen fossils are rarely found in coastal environments, which led Pocknall and Crosbie (32) to suggest that Beauprea in the past only occupied habitats similar to where it now occurs in New Caledonia, that is, highlands some distance from the sea. Second, ocean currents run in the “wrong” direction—the South Equatorial Current travels east to west, bypassing New Caledonia, runs north to south along the east Australian coast, and then moves west to east as the South Pacific Current passing north and south over New Zealand toward South America (54). Thus, Australia could be a source of propagules to New Zealand, but exchanges between New Caledonia and New Zealand are not possible. Third, Beauprea fruits do not float in seawater. In a simple experiment, we (B.B.L. and T.H.) collected 50 mature fruits from B. neglecta in Kone, New Caledonia, in 2013, placed them in a bowl of seawater, and found that 37 fruits sank immediately, whereas the remaining 13 sank within 24 hours. Fourth, any attraction of the slightly fleshy fruits to birds is unknown, but this would be confined to forest dwellers and not seabirds that might on occasion be swept vast distances over the ocean (again, in the wrong direction).
Biological evidence for a lack of complete submergence in the Cenozoic
Although evidence from molecular systematics has pointed to the need for transoceanic dispersals in understanding the evolution of the New Caledonian biota (14, 15, 55), Beauprea is an important anomaly whose evolutionary history does not support the hypothesis that New Caledonia and/or New Zealand was completely submerged during the Eocene-Oligocene or Oligocene-Miocene, respectively. The fossil records indicate continuous occupation of New Zealand by Beauprea, most notably at the Oligocene-Miocene boundary (Table 1), including the pollen species B. elegansiformis that can be traced from the Upper Cretaceous to the Late Pleistocene. The final extinction of Beauprea, with its subtropical affinities, in New Zealand was attributable to climaxing climate deterioration (14), not submergence. Similarly, New Caledonia could never have been completely submerged, because it is not possible for Beauprea to have reached its shores from elsewhere subsequent to emergence, as we have shown above. Instead, our analysis indicates that Beauprea, which arose in Antarctica–SE Australia 88 Ma, must have already been present in Zealandia when it separated from Antarctica 82 Ma.
Hopper (56) predicts that organisms in very old, climatically buffered, infertile landscapes—of which New Caledonia is an example—should exhibit reduced dispersibility, high numbers of localized rare endemics, and strongly differentiated population systems, with elevated persistence of old lineages. Beauprea and a number of other relict taxa in New Caledonia, such as Nothofagus, Beaupreopsis (which we show here as having a history similar to that of Beauprea), and Amborella, fit this description well. Although it is unlikely that New Caledonia and New Zealand have existed intact over 82 My, we provide biological support for the idea that some emergent land has existed continuously in each island complex—sufficient for the survival and moderate speciation of an ancient genus—during that time. This complements the fluctuating, but increasing, geological support for a continuous terrestrial presence there (29, 57).
Relationship with Nothofagus and other ancient lineages
From the fossil records, Beaupreaidites and Peninsulapollis are invariably (95%) associated with Nothofagidites rainforests. Nothofagus has been considered a key group for understanding Southern Hemisphere biogeography because it has a low dispersal capacity (58) and its fossil pollen record dates back to 84 Ma (59). Dettmann (44) suggested that Nothofagus likely originated in Antarctica. There are five endemic species of Nothofagus in New Caledonia. Swenson et al. (60) speculated that the presence of Nothofagus in New Caledonia was the result of a single migration from New Zealand along a possible past land link. It is likely that several species of Beauprea and Nothofagus, plus many other species, spread from Antarctica into, or arose within, Zealandia while they remained connected up to 82 Ma, eventually reaching New Caledonia overland. The details of how Beauprea moved within Zealandia remain unknown, but it may have involved land bridges associated with the Campbell Plateau and Chatham Rise (from Antarctica to New Zealand) and the Challenger Plateau and Norfolk Ridge (from New Zealand to New Caledonia). Much of Gondwana, including New Caledonia and the Lord Howe Rise, was above sea level at 90 Ma (11), that is, at about the time Beauprea originated. Because there are no fossil records for Beauprea in New Caledonia, it is not clear when the ancient stock of extant Beauprea reached New Caledonia from Antarctica, but it cannot be later than the severance of the land connection between New Caledonia and New Zealand that occurred 50 Ma.
For the Late Cretaceous vegetation in the narrow rift valley separating Antarctica from southern Australia, Hill and Scriven (61) recorded pollen of Beauprea, diverse conifers (Podocarpus, Dacrydium, Dacrycarpus, and Araucariaceae), Nothofagus, Ilex, Gunnera, Ascarina, Winteraceae, and Trimeniaceae. Most of these groups have extant members endemic to New Caledonia and New Zealand. Given the recognition of historical connections between New Zealand and New Caledonia via Antarctica, it is now redundant to invoke hypotheses of dispersal across essentially insurmountable oceans as the origin of some of the floras of these island complexes. Instead, vicariance and allopatry must be considered when interpreting the high levels of endemism and diversity of their floras. Refuge spots must have existed in the mountainous uplands from which these relicts would have spread and speciated as the full islands re-emerged, and this can explain the spurt in speciation of Beauprea from 28 Ma. Complete submergence would not have allowed recovery because we have shown that the likelihood of the transoceanic dispersal of Beauprea from another region is negligible.
MATERIALS AND METHODS
Fossil Beauprea-type pollen records
The key words Beauprea, Beaupreaidites, and Peninsulapollis were fed into Google Scholar for the period 2013–2015, and all scholarly papers where they were mentioned were obtained. These were supplemented with records in volume 11 (Proteaceae issue) of Australian Systematic Botany. The identity of each Beauprea-type pollen species—rarely leaves—as assigned was noted (“aff.” was treated as a separate species and “cf.” was treated as the same species), as well as its geographical location, estimated geological age, and whether it co-occurred (same sample) with Nothofagidites pollen. The age of the pollen was taken as the oldest recorded in actual stratigraphy diagrams (including multiple records in the same paper) or the exact midpoint if only part of a geological stage was noted; for example, if given as Late Santonian (that is, 85.9 to 83.5 Ma), late was taken as the youngest quarter (that is, 84.1 to 83.5 Ma), and the midpoint of this is 83.8 Ma [for Beaupreaidites (syn. Proteacidites) orbiculatus]. Records with a range of ≥20 My were ignored as too vague. Records were plotted onto recreated maps for the periods 85 to 70, 70 to 35, and ≤35 Ma, and collated at the level of records and species for all 10-My intervals. Records were also collated at 15-My intervals by region (Antarctica, SE Australia and the rest of Australia, Zealandia, and South America), although records for the same site were repeated if they spanned >15 My and also added to the next interval.
Fossil pollen structure and phenetic analysis
We assembled a matrix of pollen characters for four fossil species with Beauprea-type pollen, Cranwellipollis palisadus, eight extant Beauprea species, and species from apparently close genera (Franklandia, Eidothea, Faurea, and Protea) based on the study of Sauquet et al. (42) (table S1). This included Beaupreaidites (syn. Proteacidites) orbiculatus, which Sauquet et al. (42) were unable to link to Beauprea. However, Dettmann and Jarzen (33) considered that “Beauprea montisfontium shows a remarkable similarity to the fossil specimens [of Beaupreaidites orbiculatus] in size, aperture structure, exine stratification, and surface details” and justified our inclusion of this fossil in our analysis. Carpenter et al. (62) have recently accepted B. orbiculatus as being synonymous with Beauprea. We independently assessed all characters for Beauprea, Beaupreaidites, and Peninsulapollis but only supplemented characters for the other taxa from those listed by Sauquet et al. (42) to ensure that all characters used were in common. Data or pollen photos were obtained from the studies of Pocknall and Crosbie (32), Dettmann and Jarzen (33), and Sauquet et al. (42) and from other relevant papers. Characters that were unchanged among all species or for which complete data were unavailable were omitted. The consequent pollen morphological matrix comprised 11 binary and multistate characters (ordered for analysis). A neighbor-joining tree was constructed using PAUP software (63). Estimates of the statistical significance of phylogenies were calculated by performing 1000 neighbor-joining bootstrap replicates.
Divergence-time estimates
We collected fresh leaf material from nine extant Beauprea species, and the single species of Beaupreopsis paniculata, from the wild in New Caledonia. For each species, we sequenced eight DNA fragments: nuclear ribosomal internal transcribed spacers, the chloroplast matK, rbcL, a trnL intron, a trnL-trnF intergenic spacer, atpB, an atpB-rbcL intergenic spacer, and an rpl16 intron, following the protocols in Sauquet et al. (42). Outgroup species included species from closely related genera in the Proteaceae, Eidothea, Cenarrhenes, Franklandia, and Agastachys, as well as species from families close to Proteaceae. Their sequences were either generated with the same protocol as for Beauprea or extracted from the National Center for Biotechnology Information (NCBI). Species and voucher specimens and NCBI sequence numbers are given in table S2. The sequences were edited and aligned, and the alignments were joined using the computer software CLC Bio Genomics Workbench (CLC Bio-Qiagen). We used BEAST v2.1.0 to estimate phylogeny and divergence times under a relaxed clock model (64). The data set partition for chloroplast DNA was unlinked and set to a general time-reversible model with γ-distributed rate heterogeneity. We used a Yule prior for rates of cladogenesis and ran 10,000,000 analyses with a 10% burn-in and sampling every 2000 generations.
Two fossil species were used to calibrate the tree. A minimum age for crown Beauprea was based on B. orbiculatus (38), the oldest pollen with Beauprea affinities according to our literature search, set at 83.8 Ma (exact midpoint of the Upper Santonian, 84.1 to 83.5 Ma, in which the fossil was located). The crown of Proteaceae was set at 95.1 Ma, the midpoint of the Upper Cenomanian (96.6 to 93.6 Ma) in which the oldest pollen (Triorites africaensis) with Proteaceae affinities is recorded (48), as also supported by Sauquet et al. (42). We used the lognormal distribution prior for age reconstruction in BEAST. The Bayesian framework, as implemented in BEAST, allows modeling of calibration uncertainties (65), and 95% HPD intervals of age estimates are given as output data. Five separate runs were implemented, and results were viewed in Tracer and then combined using the LogCombiner package in BEAST. TreeAnnotator v2.0.3 was used to generate a maximum credibility tree based on this analysis (66).
Constructing a supertree incorporating fossil and extant species
Combining the species relationship from pollen morphology analysis and molecular phylogenetic reconstruction, we created a “supertree” recognizing both extant Beauprea species and fossil species with Beauprea-type pollen. Because there was a small discrepancy between fossil age and molecular estimates of the age of the extinct species that resembles the fossil species (see Results), we assigned the fossil species to a clade containing the closest extant species, and pulled the age of that clade to that of the fossil species but within the 95% HPD of the age estimate for that node. Fossil species were assigned to a region where they were first recorded.
Supplementary Material
Acknowledgments
Special thanks to C. Zongo (deceased) for field assistance during our collecting trip in New Caledonia. We thank S. Isnard, S. McCoy, H. Vandrot, G. Gateble, and D. Letocart for logistic and field assistance for material collection, and L. Milne for insightful discussions about Beauprea fossils. Funding: This work was supported by the Australian Research Council (DP120103389). Author contributions: T.H. and B.B.L. conceived the idea and designed the study. T.H., B.B.L., and B.F. collected experimental material and performed the experiment. T.H. and B.B.L. performed the analysis and wrote the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: DNA sequences generated in this study are accessible from the NCBI database. All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/2/4/e1501648/DC1
table S1. Numerical states for 11 pollen characters assessed on eight Beauprea species, five fossil pollen types, and four other extant genera in the same subfamily (Proteoideae) as Beauprea.
table S2. Species, voucher information, and GenBank accession for all sequences included in this study.
REFERENCES AND NOTES
- 1.Renner S. S., Relaxed molecular clocks for dating historical plant dispersal events. Trends Plant Sci. 10, 550–558 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Crisp M. D., Trewick S. A., Cook L. G., Hypothesis testing in biogeography. Trends Ecol. Evol. 26, 66–72 (2011). [DOI] [PubMed] [Google Scholar]
- 3.Ladiges P. Y., Nelson G., Grimes J.. Subtree analysis, Nothofagus, and Pacific biogeography. Cladistics 13, 125–129 (1997). [DOI] [PubMed] [Google Scholar]
- 4.J. A. Crame, Geological Society Special Publications. No 47: Origins and Evolution of the Antarctic Biota (The Geological Society, London, 1989). [Google Scholar]
- 5.J. E. Francis, A. C. Ashworth, D. J. Cantrill, J. A. Crame, J. Howe, R. Stephens, A. M. Tosolini, V. Thorn, in Proceedings of the 10th International Symposium on Antarctic Earth Sciences, A. K. Cooper, P. J. Barrett, H. Stagg, B. Storey, E. Stump, W. Wise, 10th ISAES Editorial Team, Eds. (The National Academies Press, Washington, DC, 2008), pp. 19–27. [Google Scholar]
- 6.Falcon-Lang H. J., Cantrill D. J., Nichols G. J., Biodiversity and terrestrial ecology of a mid-Cretaceous, high latitude floodplain, Alexander Island, Antarctica. J. Geol. Soc. 158, 709–724 (2001). [Google Scholar]
- 7.Eklund H., Cantrill D. J., Francis J. E., Late Cretaceous plant mesofossils from Table Nunatak, Antarctica. Cretaceous Res. 25, 211–228 (2004). [Google Scholar]
- 8.Winkworth R. C., Hennion F., Prinzing A., Wagstaff S. J., Explaining the disjunct distributions of austral plants: The roles of Antarctic and direct dispersal routes. J. Biogeogr. 42, 1197–1209 (2015). [Google Scholar]
- 9.Schellart W. P., Lister G. S., Toy V. G., A Late Cretaceous and Cenozoic reconstruction of the Southwest Pacific region: Tectonics controlled by subduction and slab rollback processes. Earth Sci. Rev. 76, 191–233 (2006). [Google Scholar]
- 10.Neall V. E., Trewick S. A., The age and origin of the Pacific islands: A geological overview. Philos. Trans. R. Soc. London Ser. B 363, 3293–3308 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaffré T., Morat P. H., Veillon J. M., Rigault F., Dagostini G., Composition and characterization of the native flora of New Caledonia. Centre IRD. Doc. Sci. Tech. II4, 1–121 (2001). [Google Scholar]
- 12.Boyer S. L., Clouse R. M., Benavides L. R., Sharma P., Schwendinger P. J., Karunarathna I., Giribet G., Biogeography of the world: A case study from cyphophthalmid Opiliones, a globally distributed group of arachnids. J. Biogeogr. 34, 2070–2085 (2007). [Google Scholar]
- 13.Lee D. E., Lee W. G., Mortimer N., Where and why have all the flowers gone? Depletion and turnover in the New Zealand Cenozoic angiosperm flora in relation to palaeogeography and climate. Aust. J. Bot. 49, 341–356 (2001). [Google Scholar]
- 14.Grandcolas P., Murienne J., Robillard T., Desutter-Grandcolas L., Jourdan H., Guilbert E., Deharveng L., New Caledonia: A very old Darwinian island? Philos. Trans. R. Soc. London Ser. B 363, 3309–3317 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murienne J., Grandcolas P., Puilachs M. D., Bellés X., D’Haese C., Legendre F., Pellens R., Guilbert E.. Evolution on a shaky piece of Gondwana: Is local endemism recent in New Caledonia? Cladistics 21, 2–7 (2005). [DOI] [PubMed] [Google Scholar]
- 16.Espeland M., Murienne J., Diversity dynamics in New Caledonia: Towards the end of the museum model? BMC Evol. Biol. 11, 254 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buckley T. R., Attanayake D., Park D., Ravindran S., Jewell T. R., Normark B. B., Investigating hybridization in the parthenogenetic New Zealand stick insect Acanthoxyla (Phasmatodea) using single-copy nuclear loci. Mol. Phylogenet. Evol. 47, 335–349 (2008). [DOI] [PubMed] [Google Scholar]
- 18.Murienne J., Pellens R., Grandcolas P., Short-range endemism in New Caledonia. Distribution and new species in the genus Lauraesilpha. Zool. Neocaledonica 197, 261–271 (2008). [Google Scholar]
- 19.Swenson U., Nylinder S., Munzinger J., Sapotaceae biogeography supports New Caledonia being an old Darwinian island. J. Biogeogr. 41, 797–809 (2014). [Google Scholar]
- 20.Sharma P., Giribet G., A relict in New Caledonia: Phylogenetic relationships of the family Troglosironidae (Opiliones: Cyphophthalmi). Cladistics 25, 279–294 (2009). [DOI] [PubMed] [Google Scholar]
- 21.Burckhardt D., Taxonomy and phylogeny of the Gondwanan moss bugs or Peloridiidae (Hemiptera, Coleorrhyncha). Dtsch. Entomol. Z. 56, 173–235 (2009). [Google Scholar]
- 22.van Doesburg P. H., Cassis G., Monteith G. B., Discovery of a living fossil: A new xylastodorine species from New Caledonia (Heteroptera: Thaumastocoridae) and first record of the subfamily from the eastern Hemisphere. Zool. Med. Leiden 84, 93–115 (2010). [Google Scholar]
- 23.G. W. Gibbs, D. C. Lees, in Zoologia Neocaledonica 8. Biodiversity Studies in New Caledonia, E. Guilberté, T. Robillard, H. Jourdan, P. Grandcolas, Eds. (Muséum National d’Histoire Naturelle, Paris, 2014), pp. 239–266. [Google Scholar]
- 24.Cluzel D., Adams C. J., Maurizot P., Meffre S.. Detrital zircon records of Late Cretaceous syn-rift sedimentary sequences of New Caledonia: An Australian provenance questioned. Tectonophysics 501, 17–27 (2011). [Google Scholar]
- 25.McGlone M. S., Duncan R. P., Heenan P. B., Endemism, species selection and the origin and distribution of the vascular plant flora of New Zealand. J. Biogeogr. 28, 199–216 (2001). [Google Scholar]
- 26.Winkworth R. C., Robertson A. W., Ehrendorfer F., Lockhart P. J., The importance of dispersal and recent speciation in the flora of New Zealand. J. Biogeogr. 26, 1323–1325 (1999). [Google Scholar]
- 27.Pole M., The New Zealand flora—Entirely long-distance dispersal? J. Biogeogr. 21, 625–635 (1994). [Google Scholar]
- 28.Landis C. A., Campbell H. J., Begg J. G., Mildenhall D. C., Paterson A. M., Trewick S. A., The Waipounamu Erosion Surface: Questioning the antiquity of the New Zealand land surface and terrestrial fauna and flora. Geol. Mag. 145, 173–197 (2008). [Google Scholar]
- 29.Kamp P. J. J., Tripathi A. R. P., Nelson C. S., Paleogeography of Late Eocene to earliest Miocene Te Kuiti Group, central-western North Island, New Zealand. N. Z. J. Geol. Geophys. 57, 128–148 (2014). [Google Scholar]
- 30.Mildenhall D. C., Mortimer N., Bassett K. N.. , Kennedy E. M., Oligocene paleogeography of New Zealand: Maximum marine transgression. N. Z. J. Geol. Geophys. 57, 107–109 (2014). [Google Scholar]
- 31.R. Virot, Flore de la Nouvelle-Calédonie et Dependances. 2. Protéacées (Museum National d’Histoire Naturelle, Paris, 1968). [Google Scholar]
- 32.Pocknall D. T., Crosbie Y. M., Pollen morphology of Beauprea (Proteaceae): Modern and fossil. Rev. Palaeobot. Palynol. 53, 305–327 (1988). [Google Scholar]
- 33.Dettmann M. E., Jarzen D. M., The early history of the Proteaceae in Australia: The pollen record. Aust. Syst. Bot. 11, 401–438 (1998). [Google Scholar]
- 34.A. Sandiford, Palynology and tephrostratigraphy of Quaternary coverbed sequences of the Auckland area, New Zealand, thesis, The University of Auckland Library, Auckland (2001). [Google Scholar]
- 35.Macphail M. K., Stone M. S., Age and palaeoenvironmental constraints on the genesis of the Yandi channel iron deposits, Marillana Formation, Pilbara, northwestern Australia. Aust. J. Earth Sci. 51, 497–520 (2004). [Google Scholar]
- 36.Mack C. L., Milne L. A., Eocene palynology of the Mulga Rocks deposits, southern Gunbarrel Basin, Western Australia. Alcheringa 39, 444–458 (2015). [Google Scholar]
- 37.Askin R. A., Cryptogam spores from the Upper Campanian and Maastrichtian of Seymour Island, Antarctica. Micropaleontology 36, 141–156 (1990). [Google Scholar]
- 38.Keating J. M., Spencer-Jones M., Newham S., The stratigraphical palynology of the Kotick Point and Whisky Bay formations, Gustav Group (Cretaceous), James Ross Island. Antarct. Sci. 4, 279–292 (1992). [Google Scholar]
- 39.Pross J., Contreras L., Bijl P. K., Greenwood D. R., Bohaty S. M., Schouten S., Bendle J. A., Röhl U., Tauxe L., Raine J. I., Huck C. E., van de Flierdt T., Jamieson S. S. R., Stickley C. E., van de Schootbrugge B., Escutia C., Brinkhuis H.; Integrated Ocean Drilling Program Expedition 318 Scientists, Persistent near-tropical warmth on the Antarctic continent during the early Eocene epoch. Nature 488, 73–77 (2012). [DOI] [PubMed] [Google Scholar]
- 40.Askin R. A., Baldoni A. M., The Santonian through Paleogene record of Proteaceae in the southern South America–Antarctic peninsula region. Aust. Syst. Bot. 11, 373–390 (1998). [Google Scholar]
- 41.Sniderman J. M. K., Haberle S. G., Fire and vegetation change during the Early Pleistocene in southeastern Australia. J. Quaternary Sci. 27, 307–317 (2012). [Google Scholar]
- 42.Sauquet H., Weston P. H., Anderson C. L., Barker N. P., Cantrill D. J.. Mast A. R., Savolainen V., Contrasted patterns of hyperdiversification in Mediterranean hotspots. Proc. Natl. Acad. Sci. U.S.A. 106, 221–225 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Truswell E. M., Recycled Cretaceous and Tertiary pollen and spores in Antarctic marine sediments: A catalogue. Palaeontogr. Abt. B. 186, 121–174 (1983). [Google Scholar]
- 44.M. E. Dettmann, in The Origin and Evolution of the Antarctic Biota, J. A. Crame, Ed. (Geological Society Special Publications, Brassmill, Bath, 1989), vol. 47, 8 pp. [Google Scholar]
- 45.B. A. R. Mohr, in Proceedings of the Ocean Drilling Program, Scientific Results, P. F. Barker, J. P. Kennett, Eds. (College Station publishing, TX, 1990), pp. 449–464. [Google Scholar]
- 46.R. A. Askin, in The Antarctic Paleoenvironment: A Perspective on Global Change: Part One. Antarctic Research Series, J. P. Kennett, D. A. Warkne, Eds. (AGU, Washington, DC, 1992), vol. 56, pp. 61–73. [Google Scholar]
- 47.A. M. Baldoni, in Geologia de la Isla James Ross (Instituto Antártico Argentino, Buenos Aires, 1992), pp. 359–374. [Google Scholar]
- 48.Dettmann M. E., Jarzen D. M., Pollen of proteaceous-type from the latest Cretaceous sediments, southeastern Australia. Alcheringa 20, 103–160 (1996). [Google Scholar]
- 49.Barker N. P., Weston P. H., Rutschmann F., Sauquet H., Molecular dating of the ‘Gondwanan’ plant family Proteaceae is only partially congruent with the timing of the break-up of Gondwana. J. Biogeogr. 34, 2012–2027 (2007). [Google Scholar]
- 50.Kula J., Tulloch A., Spell T. L., Wells M. L., Two-stage rifting of Zealandia-Australia-Antarctica: Evidence from 40Ar/39Ar thermochronometry of the Sisters shear zone, Stewart Island, New Zealand. Geology 35, 411–414 (2007). [Google Scholar]
- 51.Wardle P., Origin of the New Zealand mountain flora, with special reference to trans-Tasman relationships. N. Z. J. Bot. 16, 535–550 (1978). [Google Scholar]
- 52.Barreda V. D., Palazzesi L., Telleria M. C., Olivero E. B., Raine J. I., Forest F., Early evolution of the angiosperm clade Asteraceae in the Cretaceous of Antarctica. Proc. Natl. Acad. Sci. U.S.A. 112, 10989–10994 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lockwood J. L., Cassey P., Blackburn T. M., The more you introduce the more you get: The role of colonization pressure and propagule pressure in invasion ecology. Divers. Distrib. 15, 904–910 (2009). [Google Scholar]
- 54.Stramma L., Peterson R. G., Tomczak M., The South Pacific Current. J. Phys. Oceanogr. 25, 77–91 (1995). [Google Scholar]
- 55.Pillon Y., Time and tempo of diversification in the flora of New Caledonia. Bot. J. Linn. Soc. 170, 288–298 (2012). [Google Scholar]
- 56.Hopper S. D., OCBIL theory: Towards an integrated understanding of the evolution, ecology and conservation of biodiversity on old-climatically buffered, infertile landscapes. Plant Soil 322, 49–86 (2009). [Google Scholar]
- 57.Heads M., Biological disjunction along the West Caledonian fault, New Caledonia: A synthesis of molecular phylogenetics and panbiogeography. Bot. J. Linn. Soc. 158, 470–488 (2008). [Google Scholar]
- 58.J. V. Crisci, L. Katinas, P. Posadas, Historical Biogeography: An Introduction (Harvard University Press, Cambridge, MA, 2003), 262 pp. [Google Scholar]
- 59.M. E. Dettmann, in History of the Australian Vegetation: Cretaceous to Recent, R. S. Hill, Ed. (Cambridge Univ. Press, Cambridge, 1994), pp. 143–170. [Google Scholar]
- 60.Swenson U., Backlund A., McLoughlin S., Hill R. S., Nothofagus biogeography revisited with special emphasis on the enigmatic distribution of subgenus Brassospora in New Caledonia. Cladistics 17, 28–47 (2001). [Google Scholar]
- 61.Hill R. S., Scriven L. J., The angiosperm-dominated woody vegetation of Antarctica: A review. Rev. Palaeobot. Palynol. 86, 175–198 (1995). [Google Scholar]
- 62.Carpenter R. J., Macphail M. K., Jordan G. J., Hill R. S., Fossil evidence for open, Proteaceae-dominated heathlands and fire in the Late Cretaceous of Australia. Am. J. Bot. 102, 2092–2107 (2015). [DOI] [PubMed] [Google Scholar]
- 63.D. L. Swofford, PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods). Version 4 (Sinauer Associates, Sunderland, MA, 2003).
- 64.Drummond A. J., Rambaut A., BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 7, 214 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.R. Morgan, Albian to Sentonian Palynology of Site 364, Angola Basin (Geological and Mining Museum, New South Wales, Australia, 1978), pp. 915–951. [Google Scholar]
- 66.Drummond A. J., Ho S. Y. W., Phillips M. J., Rambaut A., Relaxed phylogenetics and dating with confidence. PLOS Biol. 4, e88 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Couper R. A., Upper Mesozoic and Cainozoic spores and pollen grains from New Zealand. N. Z. Geol. Surv. Paleontol. Bull 22, 1–77 (1953). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/2/4/e1501648/DC1
table S1. Numerical states for 11 pollen characters assessed on eight Beauprea species, five fossil pollen types, and four other extant genera in the same subfamily (Proteoideae) as Beauprea.
table S2. Species, voucher information, and GenBank accession for all sequences included in this study.