Abstract
In type 2 diabetic patients, the relationships between 25-hydroxyvitamin D and parathyroid hormone levels, and glycemic control, remain unclear. We evaluated associations between 25-hydroxyvitamin D, parathyroid hormone, and hemoglobin A1c levels after adjusting for other covariates, including log transformed 25-hydroxyvitamin D levels and log transformed parathyroid hormone levels, in Korean patients with type 2 diabetes. In total, 1,175 patients with type 2 diabetes were selected from 8,857 individuals who completed the baseline survey of the Dong-gu study, conducted in Korea from 2007 to 2010. After adjusting for other covariates, we found that the mean hemoglobin A1c level was inversely associated with the 25-hydroxyvitamin D level (Q1: 7.47% [7.30–7.63], Q2: 7.25% [7.09–7.40], Q3: 7.17% [7.02–7.32], Q4: 7.19% [7.02–7.35]; p for trend = 0.021, p for between groups = 0.050) and the parathyroid hormone level (Q1: 7.35% [7.19–7.51], Q2: 7.34% [7.19–7.50], Q3: 7.28% [7.13–7.43], Q4: 7.09% [6.94–7.24]; p for trend = 0.022, p for between groups = 0.048). However, the mean fasting glucose level was not associated with either the 25-hydroxyvitamin D or parathyroid hormone level. In conclusion, inverse associations were evident between hemoglobin A1c, 25-hydroxyvitamin D and parathyroid hormone levels in Korean patients with type 2 diabetes. The associations remained significant after adjusting for other covariates, including the log transformed 25-hydroxyvitamin D levels and log transformed parathyroid hormone levels.
Introduction
Diabetes and associated complications are becoming major public health problems. Diabetes affected 422 million subjects in 2014 and total deaths caused by high blood glucose levels in 2012 numbered 3.7 million [1]. Thus, improvement of glycemic control is an increasingly important clinical issue.
Some 80–90% of vitamin D is produced from skin 7-dehydrocholesterol upon adequate exposure to ultraviolet light; only 10–20% is derived from dietary sources such as oily fish, milk, butter, eggs, and various supplements [2]. Vitamin D is first hydroxylated in the liver to 25-hydroxyvitamin D (25[OH]D), which is the major circulating form; it is then further hydroxylated in the kidney, to 1,25-dihydroxyvitamin D (1,25[OH]2D), which is the biologically active form [3]. 1,25(OH)2D is known to regulate glucose metabolism by interacting with the 1-alpha-hydroxylase enzyme and the vitamin D receptor (VDR) in pancreatic β-cells [4,5]. Vitamin D plays an important role in bone and mineral metabolism, and a deficiency thereof is associated with the development of metabolic bone diseases including rickets and osteomalacia [6]. Recently, several health problems have been associated with absence of the non-skeletal actions of vitamin D [7,8].
However, no consensus on any association between vitamin D level and glycemic control has been attained in either observational studies or clinical trials featuring vitamin D supplementation [9]. Several authors have reported inverse associations between the 25(OH)D level and the effectiveness of glycemic control [10,11]; others found no such associations [12]. Moreover, the effect of parathyroid hormone (PTH), a known mediator of vitamin D action, was not considered.
Most previous studies sought to explain the relationship between vitamin D and PTH in the context of hypovitaminosis D and secondary hyperparathyroidism; thus, data on the independent effects of vitamin D and PTH on the HbA1c levels of general populations that do not exhibit hypovitaminosis D are lacking. Koreans are of particular interest because the overall prevalence of vitamin D deficiency in Korea is 1.3-to-1.5-fold greater than in the USA, and the burden of diabetes is increasing in Korea [13]. Therefore, we sought independent associations between 25(OH)D, PTH, and HbA1c levels in Korean patients with type 2 diabetes.
Materials and Methods
Subjects
The Dong-gu study is an ongoing prospective population-based study investigating the prevalence and incidence of, and risk factors for, chronic disease in an elderly urban population. The Dong-gu study complies with all tenets of the Declaration of Helsinki and was approved by the Institutional Review Board of Chonnam National University Hospital. All participants gave written informed consent. Detailed information on the study participants and the measurements performed in relation to this population have been previously published [14]. National resident registration records were used to identify potential study participants. From 2007 to 2010, 34,040 eligible subjects aged ≥50 years who resided in the Dong-gu district of Gwangju Metropolitan City in South Korea (35°N) were invited by telephone to participate in the study, and 9,260 subjects were subsequently enrolled (response rate: 27.2%; 3,713 males and 5,547 females). We selected 1,246 participants who were taking medication to treat type 2 diabetes. After excluding 71 participants because their data were incomplete; no vitamin D data (n = 58) or no HbA1c data (n = 13), we included 1,175 subjects (563 males and 612 females) in the final analyses.
Study measurements
Trained examiners interviewed all participants using a standardized questionnaire to assess smoking status, the use of anti-hypertensive medications, and the duration of diabetes. Height was measured to the nearest 0.1 cm and weight to the nearest 0.1 kg. The body mass index (BMI) was calculated by dividing a subject’s weight (in kg) by the height squared (in m2). Blood samples were drawn from antecubital veins following 12-h overnight fasts; the sera were separated within 30 min, and samples were stored at –70°C prior to analysis. The levels of fasting blood glucose, total cholesterol, triglycerides, and high-density lipoprotein (HDL) cholesterol were measured using enzymatic methods on an automatic analyzer (Hitachi-7600; Hitachi, Ltd., Tokyo, Japan). Low-density lipoprotein (LDL) cholesterol was measured as described by Friedewald et al. [15], except when the triglyceride level exceeded 400 mg/dL. HbA1c levels were analyzed by high-performance liquid chromatography using the VARIANT II system (Bio-Rad, Hercules, CA, USA).
Measurement of 25(OH)D and PTH levels
Serum 25(OH)D and PTH levels were measured with the aid of a microparticle immunoassay system detecting chemiluminescence (Architext i2000; Abbott Diagnostics, Abbott Park, IL, USA). In a comparative study of automated immunoassays and liquid chromatography-tandem mass spectrometry methods (LC-MS/MS) [16], the Abbott 25(OH)D assay was comparable to LC-MS/MS; the concordance correlation coefficient was 0.85, and the mean bias was 4.56 ng/mL. The coefficient of variation for the total analytic precision of this assay was < 10% for 25(OH)D. According to the manufacturer's specifications, the assay has 105% cross-reactivity with 25(OH)D3, 82% cross-reactivity with 25(OH)D2, 12.6% cross-reactivity with 1,25(OH)2D3, 112% cross-reactivity with 24,25(OH)2D3, and minimal cross-reactivity with 3-epimer of 25(OH)D3 (2.7%).
Statistical analysis
Data are presented as means ± standard deviations (SDs) or as percentages by the 25(OH)D and PTH quartiles because no consensus about cut-off value for 25(OH)D and PTH has been achieved [17,18,19]. The 25(OH)D and PTH data were not normally distributed; thus, to approximate normal distributions, the 25(OH)D and PTH data were log-transformed. In addition, the 25(OH)D and PTH quartiles are based on the distribution of the log-25(OH)D and log-PTH values (25[OH]D quartiles: Quartile 1 [Q1]; <12.5 ng/mL, Quartile 2 [Q2]; 12.5–15.2 ng/mL, Quartile 3 [Q3]; 15.3–19.0 ng/mL, and Quartile 4 [Q4]; >19.0 ng/mL, PTH quartiles: Q1; <26.2 pg/mL, Q2; 26.2–34.8 pg/mL, Q3; 34.9–46.1 pg/mL, and Q4; >46.1 pg/mL). Analysis of covariance (ANCOVA) (three models) was used to compare the mean HbA1c and fasting glucose levels by the 25(OH)D and PTH quartiles. Model 1 was adjusted for the month of blood collection, sex, and age. Model 2 included the variables of Model 1 and smoking status, the use of anti-hypertensive medication, the duration of diabetes, and total cholesterol, triglyceride, and HDL cholesterol levels. Model 3 included the variables of Model 2 and the log-PTH and log-25(OH)D data. All statistical analyses were performed using SPSS software version 15.0 (SPSS, Inc., Chicago, IL, USA). A p-value <0.05 was considered to reflect statistical significance.
Results
Characteristics of subjects according to 25(OH)D quartiles
The characteristics of the 1,175 subjects (563 males and 612 females) included in the present study are listed in Table 1. A higher 25(OH)D level tended to be associated with male gender, a greater height, a heavier weight, smoking status, a greater level of physical activity, and lower levels of total cholesterol, triglycerides, LDL cholesterol, HDL cholesterol, HbA1c, and PTH. 25(OH)D levels showed a seasonal pattern being higher in June and July than in April and May.
Table 1. General characteristics of the subjects according to 25(OH)D quartiles.
| Variable | 25(OH)D (ng/mL) | p value | ||||
|---|---|---|---|---|---|---|
| Q1(≤12.40) | Q2(12.50 to15.10) | Q3(15.20 to19.00) | Q4(≥19.10) | Total | ||
| N (%) | 295(25.1) | 288(24.5) | 296(25.2) | 296(25.2) | 1175(100.0) | - |
| Male (%) | 72(24.4) | 102(35.4) | 166(56.1) | 223(75.3) | 563(47.9) | <0.001 |
| Age (years) | 68.0±8.0 | 67.8±6.9 | 67.2±7.4 | 67.1±7.5 | 67.5±7.5 | 0.350 |
| Diabetic duration (years) | 9.8±8.5 | 8.9±7.8 | 9.7±8.7 | 10.2±9.2 | 9.6±8.6 | 0.328 |
| Month of blood collection | <0.001 | |||||
| April | 91(30.8) | 69(24.0) | 54(18.2) | 36(12.2) | 250(21.3) | |
| May | 117(39.7) | 102(35.4) | 93(31.4) | 80(27.0) | 392(33.4) | |
| June | 68(23.1) | 79(27.4) | 97(32.8) | 114(38.5) | 358(30.5) | |
| July | 19(6.4) | 38(13.2) | 52(17.6) | 66(22.3) | 175(14.9) | |
| Height (cm) | 155.9±7.7 | 157.0±8.4 | 160.3±8.6 | 163.1±8.0 | 159.1±8.7 | <0.001 |
| Weight (kg) | 60.3±9.0 | 62.0±9.9 | 64.1±10.1 | 65.6±8.6 | 63.0±9.6 | <0.001 |
| BMI (kg/m2) | 24.8±3.2 | 25.1±3.1 | 24.9±3.1 | 24.6±2.4 | 24.9±3.0 | 0.241 |
| Current smoker (%) | 22(7.5) | 32(11.1) | 48(16.2) | 48(16.3) | 150(12.8) | <0.001 |
| aPhysically active (%) | 37(13.5) | 42(15.0) | 65(22.3) | 82(28.2) | 226(19.9) | <0.001 |
| Hypertensive medication (%) | 174(59.0) | 167(58.0) | 178(60.1) | 163(55.1) | 682(58.0) | 0.633 |
| Total cholesterol (mg/dL) | 204.6±49.3 | 194.8±40.0 | 181.5±41.2 | 175.9±37.5 | 189.1±43.6 | <0.001 |
| Triglycerides (mg/dL) | 185.6±219.1 | 166.4±121.3 | 146.1±88.4 | 147.7±92.6 | 161.4±141.4 | 0.002 |
| LDL cholesterol (mg/dL) | 120.6±40.9 | 115.1±35.6 | 104.8±37.2 | 100.4±33.9 | 110.1±37.8 | <0.001 |
| HDL cholesterol (mg/dL) | 50.6±11.3 | 48.9±12.6 | 48.7±11.5 | 46.4±10.2 | 48.6±11.5 | <0.001 |
| Fasting glucose (mg/dL) | 145.7±43.5 | 144.4±44.4 | 142.7±39.0 | 142.2±37.8 | 143.7±41.2 | 0.725 |
| Hemoglobin A1c (%) | 7.5±1.5 | 7.2±1.3 | 7.1±1.3 | 7.2±1.3 | 7.3±1.4 | 0.004 |
| PTH (pg/mL) | 46.2±36.9 | 41.2±19.3 | 37.2±21.3 | 33.5±13.4 | 39.5±24.7 | <0.001 |
All values are given as n (%) or mean ± standard deviation.
BMI, body mass index; 25(OH)D, 25-hydroxyvitamin D; LDL, low-density lipoprotein; HDL, high-density lipoprotein; PTH, parathyroid hormone
aSubjects who performed 30 min or more of moderate activity at least 5 days a week or 20 min of vigorous physical activity at least 3 days a week were regarded as doing physical activity
Characteristics of subjects according to PTH quartiles
Subject characteristics by PTH quartile are listed in Table 2. A higher PTH level tended to be associated with female gender, older age, a lower height, a higher BMI, non-smoking status, greater use of anti-hypertensive medications, lower levels of fasting glucose, HbA1c, and 25(OH)D.
Table 2. General characteristics of the subjects according to PTH quartiles.
| Variable | PTH (pg/mL) | p value | ||||
|---|---|---|---|---|---|---|
| Q1(≤26.1) | Q2(26.2 to 34.8) | Q3(34.9 to 46.1) | Q4(≥46.2) | Total | ||
| N (%) | 294(25.0) | 293(25.0) | 295(25.1) | 292(24.9) | 1174(100.0) | |
| Male (%) | 163(55.4) | 139(47.4) | 140(47.5) | 121(41.4) | 563(48.0) | 0.009 |
| Age (years) | 67.1±7.3 | 66.2±7.6 | 67.7±7.2 | 69.1±7.5 | 67.5±7.5 | <0.001 |
| Diabetic duration (years) | 10.2±9.0 | 9.8±8.1 | 9.0±8.0 | 9.5±9.1 | 9.6±8.6 | 0.328 |
| Month of blood collection | 0.494 | |||||
| April | 60(20.4) | 64(21.8) | 58(19.7) | 67(22.9) | 249(21.2) | |
| May | 91(31.0) | 88(30.0) | 105(35.6) | 108(37.0) | 392(33.4) | |
| June | 96(32.7) | 90(30.7) | 89(30.2) | 83(28.4) | 358(30.5) | |
| July | 47(16.0) | 51(17.4) | 43(14.6) | 34(11.6) | 175(14.9) | |
| Height (cm) | 160.2±8.6 | 159.3±8.8 | 158.9±8.7 | 158.0±8.5 | 159.1±8.7 | 0.018 |
| Weight (kg) | 62.7±9.3 | 63.2±9.2 | 63.5±10.3 | 62.6±9.7 | 63.0±9.6 | 0.600 |
| BMI (kg/m2) | 24.4±2.7 | 24.9±2.9 | 25.1±3.0 | 25.1±3.2 | 24.9±3.0 | 0.010 |
| Current smoker (%) | 56(19.0) | 32(11.0) | 32(10.8) | 30(10.3) | 150(12.8) | 0.003 |
| aPhysically active (%) | 69(24.3) | 60(21.4) | 46(16.1) | 51(17.8) | 226(19.9) | 0.068 |
| Hypertensive medication (%) | 141(48.0) | 158(53.9) | 188(63.7) | 194(66.4) | 681(58.0) | <0.001 |
| Total cholesterol (mg/dL) | 190.3±46.7 | 188.7±43.6 | 189.0±42.3 | 188.4±42.1 | 189.1±43.7 | 0.955 |
| Triglycerides (mg/dL) | 164.6±154.8 | 166.0±178.4 | 157.9±114.5 | 157.4±106.4 | 161.5±141.5 | 0.831 |
| LDL cholesterol (mg/dL) | 110.8±38.3 | 110.1±36.5 | 110.2±37.9 | 109.3±38.7 | 110.1±37.8 | 0.974 |
| HDL cholesterol (mg/dL) | 48.4±11.2 | 47.9±10.7 | 48.7±11.5 | 49.4±12.7 | 48.6±11.5 | 0.456 |
| Fasting glucose (mg/dL) | 146.8±44.3 | 144.7±40.7 | 145.4±39.2 | 137.9±39.8 | 143.7±41.2 | 0.042 |
| Hemoglobin A1c (%) | 7.4±1.3 | 7.3±1.5 | 7.2±1.3 | 7.1±1.3 | 7.3±1.4 | 0.033 |
| 25(OH)D (ng/mL) | 17.7±5.8 | 16.3±5.5 | 16.3±5.3 | 14.4±4.5 | 16.2±5.4 | <0.001 |
All values are given as n (%) or mean ± standard deviation.
BMI, body mass index; PTH, parathyroid hormone; LDL, low-density lipoprotein; HDL, high-density lipoprotein; 25(OH)D, 25-hydroxyvitamin D
aSubjects who performed 30 min or more of moderate activity at least 5 days a week or 20 min of vigorous physical activity at least 3 days a week were regarded as doing physical activity
Comparison of mean HbA1c and fasting glucose values according to 25(OH)D quartiles
The mean HbA1c values (95% confidence intervals [CIs]) by 25(OH)D quartiles are listed in Table 3. After adjusting for the month of blood collection, sex, age, BMI, smoking status, the extent of physical activity, the use of anti-hypertensive medications, the duration of diabetes, and the levels of total cholesterol, triglycerides, HDL cholesterol, and the log-PTH level (Model 3), the association between HbA1c and 25(OH)D levels remained significant (Q1: 7.47% [7.30–7.63], Q2: 7.25% [7.09–7.40], Q3: 7.17% [7.02–7.32], Q4: 7.19% [7.02–7.35]; p for trend = 0.021, p for between groups = 0.050). The mean fasting glucose levels did not vary by the 25(OH)D quartile after adjusting for all covariates and the log-PTH level (p for trend = 0.608, p for between groups = 0.945).
Table 3. Comparison of mean HbA1c and fasting glucose according to 25(OH)D quartiles.
| 25(OH)D level | Model 1 | Model 2 | Model 3 | |
|---|---|---|---|---|
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | ||
| Fasting glucose (mg/dL) | Quartile 1 | 145.1(140.2–150.0) | 144.4 (139.5–149.3) | 144.7 (139.7–149.7) |
| Quartile 2 | 144.4(139.7–149.2) | 144.8 (140.1–149.5) | 145.1 (140.4–149.8) | |
| Quartile 3 | 142.7(138.1–147.4) | 144.1 (139.5–148.7) | 143.9 (139.3–148.4) | |
| Quartile 4 | 142.7(137.8–147.7) | 143.6 (138.7–148.4) | 143.0 (138.1–147.9) | |
| p for trend | 0.468 | 0.787 | 0.608 | |
| p for between groups | 0.893 | 0.988 | 0.945 | |
| Hemoglobin A1c (%) | Quartile 1 | 7.48 (7.32–7.64)* | 7.45 (7.29–7.61) | 7.47 (7.30–7.63)* |
| Quartile 2 | 7.20 (7.04–7.36) | 7.24 (7.08–7.39) | 7.25 (7.09–7.40) | |
| Quartile 3 | 7.13 (6.98–7.29) | 7.18 (7.03–7.33) | 7.17 (7.02–7.32) | |
| Quartile 4 | 7.19 (7.03–7.36) | 7.20 (7.05–7.36) | 7.19 (7.02–7.35) | |
| p for trend | 0.018 | 0.043 | 0.021 | |
| p for between groups | 0.018 | 0.083 | 0.050 |
Model 1, Adjusted by month of blood collection, sex and age
Model 2, Adjusted by Model 1 plus BMI, smoking, physical activity, hypertension medications, diabetic duration, total cholesterol, triglyceride and HDL cholesterol
Model 3, Adjusted by Model 2 plus log-PTH
*p<0.005 compared with Quartile 3 with the Bonferroni adjustment for multiple comparison
Comparison of mean HbA1c and fasting glucose values according to PTH quartiles
The mean HbA1c values (95% CIs) by PTH quartiles are listed in Table 4. After adjusting for the covariates described above and the log-25(OH)D value (Model 3), the association between HbA1c and PTH levels became significant (Q1: 7.35% [7.19–7.51], Q2: 7.34% [7.19–7.50], Q3: 7.28% [7.13–7.43], Q4: 7.09% [6.94–7.24]; p for trend = 0.022, p for between groups = 0.048). The mean fasting glucose value did not differ by PTH quartile after adjusting for all covariates and the log-25(OH)D value (p for trend = 0.104, p for between groups = 0.088).
Table 4. Comparison of mean HbA1c and fasting glucose according to PTH quartiles.
| PTH level | Model 1 | Model 2 | Model 3 | |
|---|---|---|---|---|
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | ||
| Fasting glucose (mg/dL) | Quartile 1 | 146.7(142.0–151.4) | 146.2 (141.6–150.9) | 146.2 (141.6–150.9) |
| Quartile 2 | 144.0(139.3–148.6) | 144.0 (139.4–148.7) | 144.0 (139.4–148.7) | |
| Quartile 3 | 145.5(140.9–150.1) | 147.1 (142.5–151.7) | 147.1 (142.5–151.7) | |
| Quartile 4 | 138.5(133.8–143.2) | 139.2 (134.6–143.9) | 139.2 (134.6–143.9) | |
| p for trend | 0.033 | 0.092 | 0.104 | |
| p for between groups | 0.080 | 0.080 | 0.088 | |
| Hemoglobin A1c (%) | Quartile 1 | 7.39(7.24–7.55)* | 7.32 (7.16–7.47) | 7.35 (7.19–7.51)* |
| Quartile 2 | 7.32(7.17–7.48) | 7.35 (7.19–7.50) | 7.34 (7.19–7.50) | |
| Quartile 3 | 7.21(7.05–7.36) | 7.28 (7.13–7.43) | 7.28 (7.13–7.43) | |
| Quartile 4 | 7.08(6.92–7.23) | 7.12 (6.97–7.27) | 7.09 (6.94–7.24) | |
| p for trend | 0.003 | 0.067 | 0.022 | |
| p for between groups | 0.021 | 0.124 | 0.048 |
Model 1, Adjusted by month of blood collection, sex and age
Model 2, Adjusted by Model 1 plus BMI, smoking, physical activity, hypertension medications, diabetic duration, total cholesterol, triglyceride and HDL cholesterol
Model 3, Adjusted by Model 2 plus log-25(OH)D
*p<0.005 compared with Quartile 4 with the Bonferroni adjustment for multiple comparison
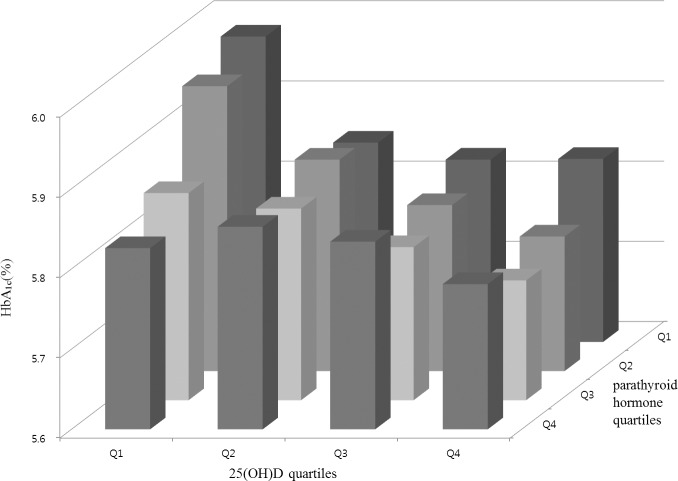
Mean HbA1c values according to the 25(OH)D and PTH quartiles
Fig 1 shows how the mean HbA1c value varied among the 25(OH)D and PTH quartiles. The mean HbA1c value tended to decrease in the higher 25(OH)D and PTH quartiles and to increase in the lower quartiles.
Fig 1. Mean HbA1c levels by the 25(OH)D and PTH quartiles.
Discussion
Inverse associations were evident between the HbA1c, 25(OH)D and PTH levels in Korean patients with type 2 diabetes. These associations remained significant after adjustment for other covariates, including the log-25(OH)D and log-PTH values. However, no association was evident between the fasting glucose level and either the 25(OH)D or PTH level.
Consistent with earlier reports, we found that the 25(OH)D level was influenced by month of blood collection [20], physical activity [21], lipid profiles [22,23] and PTH [24,25]. Unlike the findings of previous studies [20,26], BMI was not associated with the 25(OH)D level. The Longitudinal Aging Study conducted in Amsterdam [27] sought associations between 25(OH)D levels and bodily composition variables, including BMI, waist-to-hip ratio, waist circumference, total body fat, and total body fat percentage. The association of the 25(OH)D level with the total body fat percentage was stronger than were the associations with other bodily composition variables. This indicates that adiposity, and not simply body weight or BMI per se, is associated with the 25(OH)D concentration [27]. When we analyzed association between the 25(OH)D levels with body fat percentage in our data (data are not shown), body fat percentage is inversely associated with 25(OH)D quartiles (Q1: 34.6±7.2%, Q2: 33.6±7.3%, Q3: 31.0±7.7%, Q4: 29.2±6.3%, p<0.001) like previous study [27].
Many studies have explored associations between 25(OH)D levels and the prevalence of diabetes; lower 25(OH)D levels were associated with a higher prevalence of type 2 diabetes [10,28,29]. However, any relationship between the 25(OH)D level and the HbA1c level remains unclear. Some authors reported positive relationships between the 25(OH)D level and the HbA1c level [30]; others presented no association [12]; the others reported inverse associations [10,11,31].
We found that the association between the HbA1c and 25(OH)D levels was significant, but no association was apparent between the fasting glucose and 25(OH)D levels. In a previous study with 668 older subjects [10], an association was evident between 25(OH)D and HbA1c levels, but not between 25(OH)D and fasting glucose levels (as also found by us). The 25(OH)D level is related to the HbA1c level but not to the fasting glucose level, because the HbA1c level is a long-term marker of glycemic control, reflecting changes that take place over about 2–3 months [32]. Such changes are difficult to capture using single measurements of fasting glucose levels.
We found that, after adjustment for the log-PTH level, the association between the HbA1c and 25(OH)D levels remained significant. To the best of our knowledge, only a few previous studies have explored the relationship between the 25(OH)D level and the extent of glycemic control after adjusting for the PTH level. In the NHANES study (2001–2006) on 3,958 subjects [33], associations of the 25(OH)D level with fasting glucose and insulin levels were independent of the serum PTH concentration. In another study using NHANES data collected from 2003–2006 [11], an analysis of 9,773 subjects showed that the 25(OH)D level was inversely associated with the HbA1c level after adjusting for other covariates including the PTH level, which is similar to what we found. 1,25 dihydroxyvitamin D3 (1,25[OH]2D3) regulates insulin synthesis/secretion by controlling calcium homeostasis via a mechanism involving PTH [34,35], and it may also (in a PTH-independent manner) influence glucose metabolism via activation of VDRs on pancreatic β-cells [36] and protects β-cells from immune attack [37]. Recently, it has been shown that vitamin D3 can be transformed by the steroidogenic enzyme cytochrome P450scc (CYP11A) into not only 25(OH)D3 and 1,25(OH)2D3, but also the novel metabolites 20(OH)D3, 22(OH)D3, 20,22(OH)2D3, and 20,23(OH)2D3 [38,39]. The major product of CYP11A acting on vitamin D3, 20(OH)D3 is noncalcemic [40], and it may regulate 1,25(OH)2D3 production in a PTH-independent manner [41].
We found that the HbA1c level was inversely associated with the PTH level, independently of 25(OH)D status. This is surprising; previous studies associated elevated PTH levels with glucose intolerance and insulin insensitivity [42,43]. This discrepancy may not be attributable to differences in PTH distribution. The mean (with standard deviation [SD]) level of PTH in one study was 42.1±15.7 pg/mL [43], and the mean (with standard error [SE]) level in another study was 40.11(1.74) pg/mL [42], whereas the mean ± SD and mean (SE) levels in our study were 39.5±24.7 pg/mL and 39.5 (0.72) pg/mL, respectively. The precise mechanisms how increased PTH is associated with decreased HbA1c level are not clear. Under normal physiological conditions, low levels of vitamin D should stimulate PTH secretion, and low levels of PTH should increase calcium uptake from both the gut and skeleton [44]. Therefore, high-level PTH reflects a low calcium level, which may improve glucose-tolerance [45], and ameliorate both insulin sensitivity and insulin secretion [46].
The primary strengths of this study lie in its population based design and use of a relatively large sample size, which minimized selection bias and provided sufficient statistical power. However, a number of limitations should also be considered. First, the present study employed a cross-sectional design. Second, it was comparatively limited in its ability to explain seasonal changes in 25(OH)D, partly due to a lack of information regarding sun exposure during seasonal cycles. Finally, only a single serum 25(OH) D measurement was taken, and the data therefore reflect only a single point in time rather than long-term exposure.
Conclusion
Inverse associations were evident between HbA1c, 25(OH)D and PTH levels in Korean patients with type 2 diabetes. Such associations remained significant after adjustment for covariates, including the log-25(OH)D and log-PTH values.
Data Availability
All relevant data are within the paper.
Funding Statement
The authors received no specific funding for this work.
References
- 1.World Health Organization. Global report on diabetes Geneva: WHO Press; 2016. [Google Scholar]
- 2.Holick MF. Evolution, biologic functions, and recommended dietary allowances for vitamin D In: Procedings of Md MFH (ed) Vitamin D. Humana Press; 1999. pp. 1–16 [Google Scholar]
- 3.Inouye K, Sakaki T. Enzymatic studies on the key enzymes of vitamin D metabolism; 1 alpha-hydroxylase (CYP27B1) and 24-hydroxylase (CYP24). Biotechnol Annu Rev. 2001;7: 179–94. [DOI] [PubMed] [Google Scholar]
- 4.Bland R, Markovic D, Hills CE, Hughes SV, Chan SL, Squires PE, et al. Expression of 25-hydroxyvitamin D3-1alpha-hydroxylase in pancreatic islets. J Steroid Biochem Mol Biol. 2004;89-90(1–5):121–5. [DOI] [PubMed] [Google Scholar]
- 5.Johnson JA, Grande JP, Roche PC, Kumar R. Immunohistochemical localization of the 1,25(OH)2D3 receptor and calbindin D28k in human and rat pancreas. Am J Physiol. 1994; 267(3 Pt 1):E356–60. [DOI] [PubMed] [Google Scholar]
- 6.Christodoulou S, Goula T, Ververidis A, Drosos G. Vitamin D and bone disease. Biomed Res Int. 2013;396541 10.1155/2013/396541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87(4):1080S–6S. [DOI] [PubMed] [Google Scholar]
- 8.Pludowski P, Holick MF, Pilz S, Wagner CL, Hollis BW, Grant WB, et al. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality-a review of recent evidence. Autoimmun Rev. 2013;12(10):976–89. 10.1016/j.autrev.2013.02.004 [DOI] [PubMed] [Google Scholar]
- 9.Chagas CEA, Borges MC, Martini LA, Rogero MM. Focus on vitamin D, inflammation and type 2 diabetes. Nutrients. 2012;4(1):52–67. 10.3390/nu4010052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dalgård C, Petersen MS, Weihe P, Grandjean P. Vitamin D status in relation to glucose metabolism and type 2 diabetes in Septuagenarians. Diabetes Care. 2011;34(6):1284–8. 10.2337/dc10-2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kositsawat J, Freeman VL, Gerber BS, Geraci S. Association of A1C levels with vitamin D status in U.S. adults: data from the National Health and Nutrition Examination Survey. Diabetes Care. 2010;33(6):1236–8. 10.2337/dc09-2150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joergensen C, Gall MA, Schmedes A, Tarnow L, Parving HH, Rossing P. Vitamin D levels and mortality in type 2 diabetes. Diabetes Care. 2010;33(10):2238–43. 10.2337/dc10-0582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oh YJ, Kim M, Lee H, Lee JP, Kim H, Kim S, et al. A threshold value of estimated glomerular filtration rate that predicts changes in serum 25-hydroxyvitamin D levels: 4th Korean National Health and Nutritional Examination Survey 2008. Nephrol Dial Transplant. 2012;27(6):2396–403. 10.1093/ndt/gfr763 [DOI] [PubMed] [Google Scholar]
- 14.Kweon SS, Shin MH, Jeong SK, Nam HS, Lee YH, Park KS, et al. Cohort Profile: The Namwon Study and the Dong-gu Study. Int J Epidemiol. 2014;43(2):558–67. 10.1093/ije/dys244 [DOI] [PubMed] [Google Scholar]
- 15.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 16.Farrell CJ, Martin S, McWhinney B, Straub I, Williams P, Herrmann M. State-of-the-art vitamin D assays: a comparison of automated immunoassays with liquid chromatography-tandem mass spectrometry methods. Clin Chem. 2012;58(3):531–42. 10.1373/clinchem.2011.172155 [DOI] [PubMed] [Google Scholar]
- 17.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81(3):353–73. [DOI] [PubMed] [Google Scholar]
- 18.Rosen CJ. Clinical practice. Vitamin D insufficiency. N Engl J Med. 2011;364(3):248–54. 10.1056/NEJMcp1009570 [DOI] [PubMed] [Google Scholar]
- 19.Souberbielle JC, Cormier C, Kindermans C, Gao P, Cantor T, Forette F, et al. Vitamin D status and redefining serum parathyroid hormone reference range in the elderly. J Clin Endocrinol Metab. 2001;86(7):3086–90. [DOI] [PubMed] [Google Scholar]
- 20.Liu E, Meigs JB, Pittas AG, Economos CD, McKeown NM, Booth SL, et al. Predicted 25-hydroxyvitamin D score and incident type 2 diabetes in the Framingham Offspring Study. Am J Clin Nutr. 2010;91(6):1627–33. 10.3945/ajcn.2009.28441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Dam RM, Snijder MB, Dekker JM, Stehouwer CD, Bouter LM, Heine RJ, et al. Potentially modifiable determinants of vitamin D status in an older population in the Netherlands: the Hoorn Study. Am J Clin Nutr. 2007;85(3):755–61. [DOI] [PubMed] [Google Scholar]
- 22.Sun X, Cao ZB, Tanisawa K, Ito T, Oshima S, Ishimi Y, et al. Associations between the serum 25(OH)D concentration and lipid profiles in Japanese men. J Atheroscler Thromb. 2015; 22(4):355–62. 10.5551/jat.26070 [DOI] [PubMed] [Google Scholar]
- 23.Zhang MC, Li HX, Liu HM, Lei H, Han L, Gao M, et al. Serum vitamin D is low and inversely associated with LDL cholesterol in the Kazak ethnic population: a cross-sectional study. Med Sci Monit. 2014;20:1274–83. 10.12659/MSM.890930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sahota O, Mundey MK, San P, Godber IM, Lawson N, Hosking DJ. The relationship between vitamin D and parathyroid hormone: calcium homeostasis, bone turnover, and bone mineral density in postmenopausal women with established osteoporosis. Bone. 2004;35(1):312–9. [DOI] [PubMed] [Google Scholar]
- 25.Mezquita-Raya P, Muñoz-Torres M, Luna JD, Luna V, Lopez-Rodriguez F, Torres-Vela E, et al. Relation between vitamin D insufficiency, bone density, and bone metabolism in healthy postmenopausal women. J Bone Miner Res. 2001;16(8):1408–15. [DOI] [PubMed] [Google Scholar]
- 26.Bischof MG, Heinze G, Vierhapper H. Vitamin D status and its relation to age and body mass index. Horm Res. 2006;66(5):211–5. [DOI] [PubMed] [Google Scholar]
- 27.Snijder MB, van Dam RM, Visser M, Deeg DJ, Dekker JM, Bouter LM, et al. Adiposity in relation to vitamin D status and parathyroid hormone levels: a population-based study in older men and women. J Clin Endocrinol Metab. 2005;90(7):4119–23. [DOI] [PubMed] [Google Scholar]
- 28.Lim S, Kim MJ, Choi SH, Shin CS, Park KS, Jang HC, et al. Association of vitamin D deficiency with incidence of type 2 diabetes in high-risk Asian subjects. Am J Clin Nutr. 2013;97(3):524–30. 10.3945/ajcn.112.048496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pittas AG, Sun Q, Manson JE, Dawson-Hughes B, Hu FB. Plasma 25-hydroxyvitamin D concentration and risk of incident type 2 diabetes in women. Diabetes Care. 2010;33(9):2021–3. 10.2337/dc10-0790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miñambres I, Sánchez-Quesada JL, Vinagre I, Sánchez-Hernández J, Urgell E, de Leiva A, et al. Hypovitaminosis D in type 2 diabetes: relation with features of the metabolic syndrome and glycemic control. Endocr Res. 2015;40(3):160–5. 10.3109/07435800.2014.982326 [DOI] [PubMed] [Google Scholar]
- 31.El Lithy A, Abdella RM, El-Faissal YM, Sayed AM, Samie RM. The relationship between low maternal serum vitamin D levels and glycemic control in gestational diabetes assessed by HbA1c levels: an observational cross-sectional study. BMC Pregnancy Childbirth. 2014;14:362 10.1186/1471-2393-14-362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woerle HJ, Pimenta WP, Meyer C, Gosmanov NR, Szoke E, Szombathy T, et al. Diagnostic and therapeutic implications of relationships between fasting, 2-hour postchallenge plasma glucose and hemoglobin a1c values. Arch Intern Med. 2004;164(15):1627–32. [DOI] [PubMed] [Google Scholar]
- 33.Fraser A, Williams D, Lawlor DA. Associations of serum 25-hydroxyvitamin D, parathyroid hormone and calcium with cardiovascular risk factors: analysis of 3 NHANES cycles (2001–2006). PLoS One. 2010;5(11):e13882 10.1371/journal.pone.0013882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.d'Emden MC, Dunlop M, Larkins RG, Wark JD. The in vitro effect of 1 alpha,25-dihydroxyvitamin D3 on insulin production by neonatal rat islets. Biochem Biophys Res Commun. 1989;164(1):413–8. [DOI] [PubMed] [Google Scholar]
- 35.Boland ARD, Norman AW. Influx of Extracellular Calcium Mediates 1,25-Dihydroxyvitamin D3-Dependent Transcaltachia (the Rapid Stimulation of Duodenal Ca2+ Transport). Endocrinology. 1990;127(5):2475–80. [DOI] [PubMed] [Google Scholar]
- 36.Maestro B, Campión J, Dávila N, Calle C. Stimulation by 1,25-dihydroxyvitamin D3 of insulin receptor expression and insulin responsiveness for glucose transport in U-937 human promonocytic cells. Endocr J. 2000;47(4): 383–91. [DOI] [PubMed] [Google Scholar]
- 37.van Etten E, Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts. J Steroid Biochem Mol Biol. 2005;97(1–2): 93–101. [DOI] [PubMed] [Google Scholar]
- 38.Slominski AT, Kim TK, Shehabi HZ, Semak I, Tang EK, Nguyen MN, et al. In vivo evidence for a novel pathway of vitamin D3 metabolism initiated by P450scc and modified by CYP27B1. FASEB J. 2012;26(9):3901–15. 10.1096/fj.12-208975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slominski AT, Kim TK, Li W, Tuckey RC. Classical and non-classical metabolic transformation of vitamin D in dermal fibroblasts. Exp Dermatol 2016;25(3):231–2. 10.1111/exd.12872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Slominski AT, Kim TK, Li W, Postlethwaite A, Tieu EW, Tang EK, et al. Detection of novel CYP11A1-derived secosteroids in the human epidermis and serum and pig adrenal gland. Sci Rep. 2015;5:14875 10.1038/srep14875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zbytek B, Janjetovic Z, Tuckey RC, Zmijewski MA, Sweatman TW, Jones E, et al. 20-hydroxyvitamin D3, a product of vitamin D3 hydroxylation by cytochrome P450scc, stimulates keratinocyte differentiation. J Invest Dermatol. 2008;128(9):2271–80. 10.1038/jid.2008.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chiu KC, Chuang L- M, Lee NP, Ryu JM, McGullam JL, Tsai GP, et al. Insulin sensitivity is inversely correlated with plasma intact parathyroid hormone level. Metabolism. 2000;49(11):1501–5. [DOI] [PubMed] [Google Scholar]
- 43.Alemzadeh R, Kichler J (2012) Parathyroid hormone is associated with biomarkers of insulin resistance and inflammation, independent of vitamin D status, in obese adolescents. Metab Syndr Relat Disord. 2012;10(6):422–9. 10.1089/met.2012.0056 [DOI] [PubMed] [Google Scholar]
- 44.Brändstedt J, Almquist M, Ulmert D, Manjer J, Malm J. Vitamin D, PTH, and calcium and tumor aggressiveness in prostate cancer: a prospective nested case-control study. Cancer Causes Control. 2016;27(1):69–80. 10.1007/s10552-015-0684-3 [DOI] [PubMed] [Google Scholar]
- 45.Wareham NJ, Byrne CD, Carr C, Day NE, Boucher BJ, Hales CN. Glucose intolerance is associated with altered calcium homeostasis: A possible link between increased serum calcium concentration and cardiovascular disease mortality. Metabolism. 1997;46(10):1171–7. [DOI] [PubMed] [Google Scholar]
- 46.Hagström E, Hellman P, Lundgren E, Lind L, Arnlöv J. Serum calcium is independently associated with insulin sensitivity measured with euglycaemic-hyperinsulinaemic clamp in a community-based cohort. Diabetologia. 2007;50(2):317–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.