The detection of glycine and phosphorus in the coma of 67P shows that comets contain all ingredients to help spark life on Earth.
Keywords: Origins of life, chemistry, astronomy, comets, prebiotic molecules, amino acid, 67P/Churyumov-Gerasimenko
Abstract
The importance of comets for the origin of life on Earth has been advocated for many decades. Amino acids are key ingredients in chemistry, leading to life as we know it. Many primitive meteorites contain amino acids, and it is generally believed that these are formed by aqueous alterations. In the collector aerogel and foil samples of the Stardust mission after the flyby at comet Wild 2, the simplest form of amino acids, glycine, has been found together with precursor molecules methylamine and ethylamine. Because of contamination issues of the samples, a cometary origin was deduced from the 13C isotopic signature. We report the presence of volatile glycine accompanied by methylamine and ethylamine in the coma of 67P/Churyumov-Gerasimenko measured by the ROSINA (Rosetta Orbiter Spectrometer for Ion and Neutral Analysis) mass spectrometer, confirming the Stardust results. Together with the detection of phosphorus and a multitude of organic molecules, this result demonstrates that comets could have played a crucial role in the emergence of life on Earth.
INTRODUCTION
The possibilities that organic molecules were brought to the early Earth through impacts of small bodies and that these molecules contributed to spark the emergence of life have been the subject of significant debates (1). Many primitive meteorites contain amino acids (2), and it is generally believed that these are formed by aqueous alterations either in the parent body or during the analytical process (3). The organic inventory in comets is of particular interest because comets represent a reservoir of primitive materials in the solar system. Comets most likely consist of interstellar materials that have been moderately to heavily processed in the protosolar nebulae (4). Although more than 140 molecules have been detected in molecular clouds and more than 25 parent molecules in cometary comae (5), glycine is not among them. In contrast, methylamine has been observed in the interstellar medium (ISM) (6). There was a rigorous attempt to verify the presence of glycine in the ISM by Snyder et al. (7) after a tentative detection by Kuan et al. (8). This attempt concluded that the observed lines do not prove the existence of glycine in the ISM. The sublimation temperature of glycine is below 150°C (9), making it a very rare species in the gas phase and therefore hard to detect. Glycine has been searched for without success in the comae of comets Hale-Bopp and Hyakutake, with calculated upper limits of [glycine]/[H2O] of 0.15 (10). In dust samples from comet Wild 2 brought back by the Stardust mission (11), the simplest amino acid, glycine, has been found together with precursor molecules methylamine and ethylamine. However, the detection is based on the isotopic signature 13C, because there were problems associated with terrestrial contamination. The same authors also found other amino acids that they declared to be, most probably, a terrestrial contaminant, with the exception of β-alanine above background levels, suggesting a possible cometary source. There was not enough material to make carbon isotope measurements, but the authors do note that β-alanine could be cometary. The β-alanine/glycine ratio on the Stardust foils ranged from 0.05 to 0.5 (11). The authors claim that even for glycine, only approximately 40% is in free form, with the remaining 60% produced during the hydrolysis extraction from the foil from acid labile precursors (for example, HCN).
Here, we report the presence of volatile glycine accompanied bymethylamine and ethylamine in the coma of 67P/Churyumov-Gerasimenko measured by the ROSINA (Rosetta Orbiter Spectrometer for Ion and Neutral Analysis) mass spectrometer (12) on numerous occasions while the comet was approaching perihelion (for details on the data sample, see Materials and Methods). ROSINA’s double focusing mass spectrometer (DFMS) ionizes the incoming volatiles by electron impact ionization and detects the corresponding positively charged fragments. Unlike for meteorites or Stardust grains, there is no chemical sample preparation involved. Furthermore, the absence of a terrestrial source of glycine from the spacecraft is verified from observations before arrival at the comet. Therefore, glycine detected by DFMS has to be in this form already in the coma of the comet and is clearly not the result of contamination.
RESULTS AND DISCUSSION
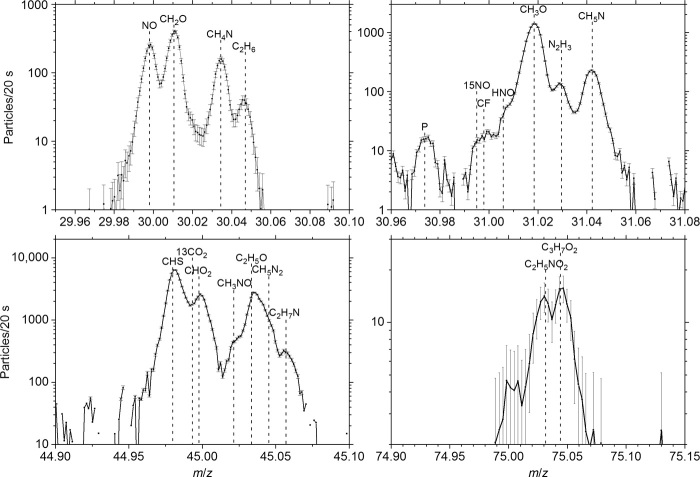
A sample mass spectrum at 75, 45, 31, and 30 dalton is shown in Fig. 1. The number of ionized particles registered on the detector is given as a function of the position on the detector, which corresponds to mass/charge ratio (m/z). Glycine (C2H5NO2), methylamine (CH5N), and ethylamine (C2H7N) can be found on mass 75, 31, and 45 dalton, respectively. CH4N, the most abundant fragment and a product of the electron impact ionization of glycine, can be found on mass 30 dalton. This is also a fragment of both amines. All species are separated from neighboring mass peaks belonging to other parent molecules. For example, we find C3H7O2 on mass 75 dalton per electron charge (Da/e), which might be a fragment of propylene glycol (C3H8O2) or any of its isomers and/or of even heavier species like butanediol (C4H10O2). Details on the data analysis for ROSINA DFMS can be found in a study by Le Roy et al. (13) and in Materials and Methods.
Fig. 1. ROSINA DFMS mass spectra (9 July 2015) for masses 30, 31, 45, and 75 dalton.
Integration time is 20 s per spectrum. Error bars represent 1-σ counting statistics.
In mass spectrometry, isomers can only be distinguished by their fragmentation pattern due to electron impact ionization. The fragmentation pattern reflects the structure of a molecule because it splits preferentially along the weakest bonds. There are several isomers with the same formula and therefore the exact same mass as glycine. Careful bookkeeping of the fragments allows identification of the molecules, including their structure. The measured fragmentation pattern (fig. S1) is clearly compatible with glycine and ethylamine. Methylamine has no isomer. Even taking into account the fragmentation pattern, it is not possible from our measurements to state whether the detected glycine was present in its zwitterionic form or in its noncharged form. For details, refer to the Supplementary Materials.
Glycine was detected around the nucleus for the first time during the 10-km bound orbits of Rosetta in October 2014, just before lander delivery. At that time, the comet was at 3 astronomical units (AU) from the Sun. The next time glycine was observed was during a close flyby of the comet at 15 km on 28 March 2015 at a heliocentric distance of 2 AU. The flyby speed was 1.1 m/s, flying over the summer hemisphere from morning to afternoon local time. Glycine could be detected in the mass spectra between 30 km and the closest approach both incoming and outgoing. Although the local time and therefore the illumination conditions were changing, profiles of density versus distance indicate a distributed source of glycine associated with dust. Figure 2 shows the total neutral gas signal, as measured by ROSINA Comet Pressure Sensor (COPS), as well as the glycine signal (C2H5NO2+; mass, 75.0315 dalton), both multiplied by the distance squared.
Fig. 2. Total neutral gas density and glycine abundance (arbitrary units) multiplied by distance squared inbound and outbound during a close flyby on 28 March 2015 as a function of distance from the comet.
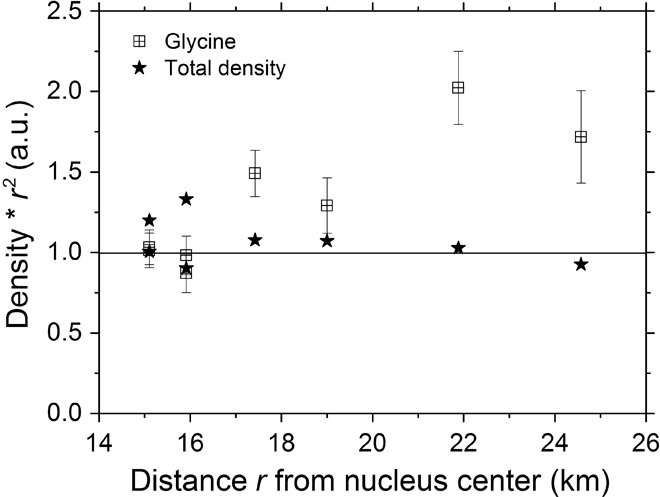
For a point source, we expect these quantities to be constant. This is true for the total neutral density, which is dominated by water, but seems to be questionable for glycine. It means that glycine is most probably released, at least partly, from dust grain mantles, which heat up in the coma of the comet. Other occasions where glycine was found in the coma are mostly correlated to cometary outbursts near perihelion (9 July to 4 August 2015), when Rosetta was more than 200km from the nucleus and surrounded by a dust cloud that subsequently forced the spacecraft to go to large distances for safety reasons. Glycine is not a very volatile species with a sublimation temperature slightly below 150°C (9), and probably, very little of it sublimates from the nucleus (sub) surface due to cold temperatures (14). However, temperatures of the dark small grains released in the coma may be higher (15). It cannot be excluded that glycine is also partly embedded in the cometary ice and then released together with other volatiles.
The abundances found for glycine relative to water range between 0 and 0.0025. Glycine is only observed when overall densities are high, that is, when the spacecraft is close to the nucleus or when there is a lot of dust in the vicinity of the spacecraft after outbursts. The ratios of methylamine to glycine and ethylamine to glycine are 1.0 ± 0.5 and 0.3 ± 0.2, respectively. If we assume that glycine is mostly connected to dust and not to ice, the ratio relative to water is not very significant. ROSINA is not able to measure refractories; therefore, we cannot give a relative abundance to, for example, Mg, which would be more representative of dust.
Methylamine and ethylamine are seen in the mass spectra only when glycine is also detected. The three molecules seem to be closely related, which is not surprising. The presence of amino acids in comets, where aqueous alterations involving liquid water are unlikely to occur, is explained by chemistry in interstellar icy dust mantles (16) or ultraviolet irradiation of ice (17, 18) and subsequent conservation in the comet. Chemical models predict that glycine could form in dust grain ices via three radical addition mechanisms at temperatures ranging from 40 to 120 K (16), which are compatible with hot core temperatures. Methylamine is part of this process. Direct gas phase chemistry of glycine seems to be insignificant (16). The pathway described by Bossa et al. (18) includes photochemistry of methylamine and CO2.
So far, glycine is the only amino acid that has shown a capability to form without liquid water, and because of this, it is therefore likely to be the only amino acid in comets where aqueous alterations are highly implausible. It is therefore probably not surprising that no other amino acid has been found in the ROSINA data to date, despite the fact that the volatility of alanine is in the same range as glycine (9) and more than 80 different amino acids have been isolated in meteorites (19). Our findings are therefore fully compatible with the proposed pathways of glycine formation in the ISM or in the protosolar nebula on dust grain ices (20).
Another detection made by ROSINA DFMS is phosphorus (m/z, 31; Fig. 1). Phosphorus complements the detection of glycine. It is a key element in all living organisms, which is found in adenosine 5′-triphosphate, in the backbone of DNA and RNA, and in cell membranes. PN (21), CP (22), HCP (23), and PO (24) have been detected in the ISM. Traces of phosphorus may have been present in the dust signature of comet Halley, but no phosphorus was found in Stardust grains (25). Until the observations in Fig. 1, no phosphorus has been detected in the cometary coma. In the mass spectra of ROSINA (fig. S1), there is a clear peak at mass 30.973 dalton, the exact mass of ionized phosphorus. However, at the moment, we cannot state what the origin of phosphorus is. No clear signatures of PO, PN, CP, or HCP have been found so far in the ROSINA data. It is therefore not clear which parent molecule produces the fragment seen in the DFMS spectra. Assuming that it is mainly PH3, and taking the fragmentation pattern from the National Institute of Standards and Technology (NIST) database, one arrives at an abundance of PH3 (1.8 × 10−3) relative to water for the spectrum shown in fig. S1. This is close to the solar system value of 5 × 10−4 (26), considering the uncertainties of our value, the assumption on the parent of P being PH3, and taking into account that we measure only the volatile content for O and P.
The presence of glycine, phosphorus, and a multitude of organic molecules (13), including hydrogen sulfide (H2S) and hydrogen cyanide (HCN), seen in the coma of 67P/Churyumov-Gerasimenko supports the idea that comets delivered key molecules for prebiotic chemistry throughout the solar system and, in particular, to the early Earth (27), drastically increasing the concentration of life-related chemicals by impact on a closed water body. The simultaneous presence of methylamine and the correlation between dust and glycine also suggest that the pathways for glycine formation on dust grain ices, as described for the ISM or the protosolar nebula, could also account for the cometary glycine.
MATERIALS AND METHODS
Data sample
Mass 75 Da/e was measured regularly over the course of the mission, starting with the arrival at the comet in August 2014. A full mass spectrum of DFMS between 13 and 100 Da/e takes approximately 40 min. The instrument is in standby only during thruster firings, which account for about 10% of the total time. This means that each mass in this mass range is measured 30 times per day and more than a 1000 times since arrival at the comet. However, the spacecraft is not always in a position or altitude to register rare species like glycine, mostly because of large distances to the comet forced by the dense dust coma and by pointing away from the comet to make measurements possible for other instruments. For this study, we analyzed approximately 50 mass spectra containing glycine from periods that were most suited to this analysis, namely, the two close flybys in February and March 2015 and the period in June or July when the spacecraft encountered a significant amount of dust. Traces of glycine were also seen outside of this period, for example, in the prelander phase already at >3 AU heliocentric distance. It does not make sense to produce an average over the entire time spent near the comet because too many parameters change during the mission, for example, sub-spacecraft latitude and longitude, cometocentric and heliocentric distances, overall comet activity, and dust/gas ratio. Therefore, the relative abundances have to be understood not as uncertainties but as a range of abundances due to changing conditions on the comet.
Relative abundances
The density of a species n1 is calculated by
where ie is the electron current, σ is the ionization cross section, and ni/t is the number of ions per second corrected for the fragmentation and instrument-specific sensitivities (energy-dependent), electron charge, and bulk velocity of the gas (v). The relative abundances are therefore given by
For the total ionization cross section, we used the following theoretical values: 10 Å2 for glycine (28), 6.12 Å2 for methylamine (29), 9 Å2 for ethylamine (30), and 1.57 Å2 for water (31). The abundances found for glycine relative to water range between 0 and 0.16%. The ratio of methylamine to glycine is 1.0 ± 0.5, whereas that of ethylamine to glycine is 0.3 ± 0.2. The relative abundances vary significantly. Especially, the relative abundance of glycine compared to water is in the range from 0% (no traces of glycine in the mass spectra) to 0.16%, probably reflecting the fact that glycine is more correlated to dust than to ice. The variations in the methylamine/glycine and ethylamine/glycine ratios are partly due to statistical reasons, with generally a low signal for glycine, because DFMS is much less sensitive for high masses than for low masses. It may also reflect the time difference between the single mass measurements, which can be up to 1 hour depending on the measurement mode.
Fragmentation pattern
The number of detected ionized particles on the detector of ROSINA DFMS depends on the density of the species considered at the entrance of DFMS, on the fragmentation pattern, on the ionization cross section, on the geometric sensitivity of the instrument for the different masses, and on the detector yield. DFMS is less sensitive to heavy masses than to light masses because of its fixed magnetic field. Therefore, we applied a post-acceleration voltage to the ions for masses >69 dalton to improve the detection of heavier masses. With all of these complications, there is no convenient correlation between the density of the species and the number of counts on the detector. The data analysis method for ROSINA DFMS is discussed in detail by Le Roy et al. (13), and the instrument details are discussed by Balsiger et al. (12). The most common methods in mass spectrometry use electron impact ionization with electron energies of 70 eV to ionize neutral incoming gas, which is also the basis of the NIST Standard Reference Database No. 69 used for most of the molecules in this study. This ionization method yields not only the ionized parent molecule but also the many daughter fragments. The fragments and their abundances depend on the structure of the molecule and on the electron energy. The fragmentation pattern can thus be used to defer the structure of the molecule. The coma of the comet’s several parent molecules present in a gas mixture can contribute to the same fragments. Careful bookkeeping of fragments is therefore necessary to arrive at a (unique) solution for the fragments seen in the mass spectra, which is compatible with the parent molecules present in the gas mixture. For a discussion of the specific fragmentation pattern, see the Supplementary Materials. In principle, this fragmentation pattern depends on the specific instrument used. ROSINA DFMS uses electron energy of only 45 eV, which fragments less and therefore yields a higher amount of the ionized parent molecule compared to the daughter species. As we had to use the NIST Chemistry WebBook database because DFMS was not calibrated for most of the compounds discussed here, this therefore adds an uncertainty in the relative abundances given below. Other uncertainties are due to the detector sensitivity for the different species and due to the fact that DFMS measures single masses sequentially, which means that the sub-spacecraft longitude and the local time change during a measurement of a full mass spectrum, which takes up to 1 hour. The data were normalized to the total density measured by COPS to at least partially compensate for this. The total uncertainty in the relative abundances is estimated to be a factor of 2.
Supplementary Material
Acknowledgments
ROSINA would not give such outstanding results without the work of the many engineers, technicians, and scientists involved in the mission, in the Rosetta spacecraft, and in the ROSINA instrument team over the last 20 years, whose contributions are gratefully acknowledged. Rosetta is a European Space Agency (ESA) mission with contributions from its member states and NASA. We acknowledge herewith the work of the whole ESA Rosetta team. Funding: Work at University of Bern was funded by the State of Bern, the Swiss National Science Foundation, and the ESA PRODEX (PROgramme de Développement d’Expériences scientifiques) program. Work at Max Planck Institute for Solar System Research (MPS) was funded by the Max Planck Society and the Federal Ministry for Economic Affairs and Energy (BMWI) under contract 50QP1302. Work at Southwest Research Institute was supported by subcontract #1496541 from the Jet Propulsion Laboratory (JPL). Work at the Royal Belgian Institute for Space Aeronomy (BIRA-IASB) was supported by the Belgian Science Policy Office via PRODEX/ROSINA PRODEX Experiment Arrangement 90020. This work has been carried out thanks to the support of the A*MIDEX project (no. ANR-11-IDEX-0001-02) funded by the “Investissements d’Avenir” French Government program, managed by the French National Research Agency (ANR). This work was supported by CNES (Centre National d’Etudes Spatiales) grants at IRAP (Institut de Recherche en Astrophysique et Planétologie), LATMOS (Laboratoire Atmosphères, Milieux, Observations Spatiales), LPC2E (Laboratoire de Physique et Chimie de l’Environnement et de l’Espace), LAM (Laboratoire d’Astrophysique de Marseille), LISA (Laboratoire Interuniversitaire des Systèmes Atmosphériques), and CRPG (Centre de Recherches Pétrographiques et Géochimiques) and by the European Research Council (grant no. 267255 to B.M.). A.B.-N. thanks the Ministry of Science and the Israel Space Agency. Work at the University of Michigan was funded by NASA under contract JPL-1266313. Work by J.H.W. at Southwest Research Institute was funded by the NASA JPL subcontract NAS703001TONMO710889. Author contributions: K.A. managed the ROSINA project and wrote the manuscript with assistance from H.C., C.B., P.B., B.M., O.M., and T.O. A.B., U.C., S.G., M.H., A.J., E.K., L.L.R., M.R., T.S., and C.-Y.T. contributed to instrument calibration and operations. H.B., J.-J.B., M.R.C., J.D.K., F.D., B.F., S.A.F., T.I.G., K.C.H., A.K., U.M., H.R., J.H.W., and P.W. were responsible for hardware subsystem development and construction. All authors contributed to data interpretation and commented on the paper. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All ROSINA data have been/will be released to the Planetary Science Archive of ESA (http://www.sciops.esa.int/index.php?project=PSA&page=home) and to the Planetary Data System archive of NASA (https://pds.nasa.gov/). All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/2/5/e1600285/DC1
Fragmentation pattern
Search for the amino acid alanine
Phosphorus
fig. S1. Contribution of the main fragments of glycine, methylamine, and ethylamine for a DFMS mass spectrum on Aug. 3, 2015.
fig. S2. Mass spectra of mass 89 Da/e, taken adjacent to the mass spectra in Fig. 1.
fig. S3. Mass spectra of masses 31 and 34 Da/e.
REFERENCES AND NOTES
- 1.Cobb A. K., Pudritz R. E., Pearce B. K. D., Nature’s starships. II. Simulating the synthesis of amino acids in meteorite parent bodies. Astrophys. J. 809, 6 (2015). [Google Scholar]
- 2.Cobb A. K., Pudritz R. E., Nature’s starships. I. Observed abundances and relative frequencies of amino acids in meteorites. Astrophys. J. 783, 140 (2014). [Google Scholar]
- 3.Burton A. S., Stern J. C., Elsila J. E., Glavin D. P., Dworkin J. P., Understanding prebiotic chemistry through the analysis of extraterrestrial amino acids and nucleobases in meteorites. Chem. Soc. Rev. 41, 5459–5472 (2012). [DOI] [PubMed] [Google Scholar]
- 4.P. Ehrenfreund, W. A. Schutte, in Astrochemistry: From Molecular Clouds to Planetary Systems, Y. C. Minh, E. F. van Dishoeck, Eds. (Astronomical Society of the Pacific, San Francisco, CA, 2000), p. 135. [Google Scholar]
- 5.D. Bockelée-Morvan, J. Crovisier, M. J. Mumma, H. A. Weaver, The composition of cometary volatiles, in Comets II, M. C. Festou, H. U. Keller, H. A. Weaver, Eds. (University of Arizona Press, Tucson, 2004), pp. 391–423. [Google Scholar]
- 6.Ehrenfreund P., Charnley S. B., Organic molecules in the interstellar medium, comets, and meteorites: A voyage from dark clouds to the early earth. Ann. Rev. Astron. Astrophys. 38, 427–483 (2000). [Google Scholar]
- 7.Snyder L. E., Lovas F. J., Hollis J. M., Friedel D. N., Jewell P. R., Remijan A., Ilyushin V. V., Alekseev E. A., Dyubko S. F., A rigorous attempt to verify interstellar glycine. Astrophys. J. 619, 914–930 (2005). [Google Scholar]
- 8.Kuan Y.-J., Charnley S. B., Huang H.-C., Tseng W.-L., Kisiel Z., Interstellar glycine. Astrophys. J. 593, 848–867 (2003). [Google Scholar]
- 9.Gross D., Grodsky G., On the sublimation of amino acids and peptides. J. Am. Chem. Soc. 77, 1678–1680 (1955). [Google Scholar]
- 10.Crovisier J., Bockelée-Morvan D., Colom P., Biver N., Despois D., Lis D. C.; Team for target-of-opportunity radio observations of comet, The composition of ices in comet C/1995 O1 (Hale-Bopp) from radio spectroscopy. A&A 418, 1141–1157 (2004). [Google Scholar]
- 11.Elsila J. E., Glavin D. P., Dworkin J. P., Cometary glycine detected in samples returned by Stardust. Meteorit. Planet. Sci. 44, 1323–1330 (2009). [Google Scholar]
- 12.Balsiger H., Altwegg K., Bochsler P., Eberhardt P., Fischer J., Graf S., Jäckel A., Kopp E., Langer U., Mildner M., Müller J., Riesen T., Rubin M., Scherer S., Wurz P., Wüthrich S., Arijs E., Delanoye S., De Keyser J., Neefs E., Nevejans D., Rème H., Aoustin C., Mazelle C., Médale J.-L., Sauvaud J. A., Berthelier J.-J., Bertaux J.-L., Duvet L., Illiano J.-M., Fuselier S. A., Ghielmetti A. G., Magoncelli T., Shelley E. G., Korth A., Heerlein K., Lauche H., Livi S., Loose A., Mall U., Wilken B., Gliem F., Fiethe B., Gombosi T. I., Block B., Carignan G. R., Fisk L. A., Waite J. H., Young D. T., Wollnik H., Rosina—Rosetta Orbiter Spectrometer for Ion and Neutral Analysis. Space Sci. Rev. 128, 745–801 (2007). [Google Scholar]
- 13.Le Roy L., Altwegg K., Balsiger H., Berthelier J.-J., Bieler A., Briois C., Calmonte U., Combi M. R., De Keyser J., Dhooghe F., Fiethe B., Fuselier S. A., Gasc S., Gombosi T. I., Hässig M., Jäckel A., Rubin M., Tzou C.-Y., Inventory of the volatiles on comet 67P/Churyumov-Gerasimenko from Rosetta/ROSINA. A&A 583, A1 (2015). [Google Scholar]
- 14.Choukroun M., Keihm S., Schloerb F. P., Gulkis S., Lellouch E., Leyrat C., von Allmen P., Biver N., Bockelée-Morvan D., Crovisier J., Encrenaz P., Hartogh P., Hofstadter M., Ip W.-H., Jarchow C., Janssen M., Lee S., Rezac L., Beaudin G., Gaskell B., Jorda L., Keller H. U., Sierks H., Dark side of comet 67P/Churyumov-Gerasimenko in Aug.–Oct. 2014. MIRO/Rosetta continuum observations of polar night in the southern regions. A&A 583, A28 (2015). [Google Scholar]
- 15.Lien D. J., Dust in comets. I - Thermal properties of homogeneous and heterogeneous grains. Astrophys. J. 355, 680–692 (1990). [Google Scholar]
- 16.Garrod R. T., A three-phase chemical model of hot cores: The formation of glycine. Astrophys. J. 765, 60 (2013). [Google Scholar]
- 17.Meierhenrich U. J., Muñoz Caro G. M., Schutte W. A., Thiemann W. H.-P., Barbier B., Brack A., Precursors of biological cofactors from ultraviolet irradiation of circumstellar/interstellar ice analogs. Chem. Eur. J. 11, 4895–4900 (2005). [DOI] [PubMed] [Google Scholar]
- 18.Bossa J.-B., Borget F., Duvernay F., Theulé P., Chiavassa T., How a usual carbamate can become an unusual intermediate: A new chemical pathway to form glycinate in the interstellar medium. J. Phys. Org. Chem. 23, 333–339 (2010). [Google Scholar]
- 19.Glavin D. P., Callahan M. P., Dworkin J. P., Elsila J. E., The effects of parent body processes on amino acids in carbonaceous chondrites. Meteorit. Planet. Sci. 45, 1948–1972 (2010). [Google Scholar]
- 20.Ciesla F. J., Sandford S. A., Organic synthesis via irradiation and warming of ice grains in the solar nebula. Science 336, 452–454 (2012). [DOI] [PubMed] [Google Scholar]
- 21.Ziurys L. M., Detection of interstellar PN—The first phosphorus-bearing species observed in molecular clouds. Astrophys. J. 321, L81–L85 (1987). [DOI] [PubMed] [Google Scholar]
- 22.Guélin M., Cernicharo J., Paubert G., Turner B. E., Free CP in IRC + 10216. A&A 230, L9–L11 (1990). [Google Scholar]
- 23.Agúndez M., Cernicharo J., Guélin M., Discovery of phosphaethyne (HCP) in space: Phosphorus chemistry in circumstellar envelopes. Astrophys. J. 662, L91 (2007). [Google Scholar]
- 24.Tenenbaum E. D., Woolf N. J., Ziurys L. M., Identification of phosphorus monoxide (X2Πr) in VY Canis Majoris: Detection of the first P-O bond in space. Astrophys. J. 666, L29 (2007). [Google Scholar]
- 25.Macia E., The role of phosphorus in chemical evolution. Chem. Soc. Rev. 34, 691–701 (2005). [DOI] [PubMed] [Google Scholar]
- 26.K. Lodders, H. Palme, H.-P. Gail, in Landolt-Börnstein, New Series VI/4B, J. E. Trümper, Ed. (Springer, Berlin, 2009). [Google Scholar]
- 27.Oró J., Comets and the formation of biochemical compounds on the primitive earth. Nature 190, 389–390 (1961). [DOI] [PubMed] [Google Scholar]
- 28.Scheer A. M., Mozejko P., Gallup G. A., Burrow P. D., Total dissociative electron attachment cross sections of selected amino acids. J. Chem. Phys. 126, 174301 (2007). [DOI] [PubMed] [Google Scholar]
- 29.Vinodkumar M., Limbachiya C., Joshipura K. N., Vaishnav B., Gangopadhyay S., Computation of total electron scattering cross sections for molecules of astrophysical relevance. J. Phys. Conf. Ser. 115, 012013 (2008). [Google Scholar]
- 30.Goesmann F., Rosenbauer H., Bredehöft J., Cabane M., Ehrenfreund P., Gautier T., Giri C., Krüger H., Le Roy L., MacDermott A. J., McKenna-Lawlor S., Meierhenrich U. J., Muñoz Caro G. M., Raulin F., Roll R., Steele A., Steininger H., Sternberg R., Szopa C., Thiemann W., Ulamec S., Organic compounds on comet 67P/Churyumov-Gerasimenko revealed by COSAC mass spectrometry. Science 349, aab0689 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Schutten J., de Heer F. J., Moustafa H. R., Boerboom A. J. H., Kistemaker J., Gross- and partial-ionization cross sections for electrons on water vapor in the energy range 0.1–20 keV. J. Chem. Phys. 44, 3924 (1966). [Google Scholar]
- 32.Bossa J.-B., Borget F., Duvernay F., Theulé P. , Chiavassa T., Formation of neutral methylcarbamic acid (CH3NHCOOH) and methylammonium methylcarbamate [CH3NH3+][CH3NHCO2-] at low temperature. J. Phys. Chem. A 112, 5113–5120 (2008). [DOI] [PubMed] [Google Scholar]
- 33.Märk T. D., Egger F., Ionization of phosphine and deuterated phosphine by electron impact from threshold up to 180 eV. J. Chem. Phys. 67, 2629 (1977). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/2/5/e1600285/DC1
Fragmentation pattern
Search for the amino acid alanine
Phosphorus
fig. S1. Contribution of the main fragments of glycine, methylamine, and ethylamine for a DFMS mass spectrum on Aug. 3, 2015.
fig. S2. Mass spectra of mass 89 Da/e, taken adjacent to the mass spectra in Fig. 1.
fig. S3. Mass spectra of masses 31 and 34 Da/e.