Variable life histories, species interactions, and historical contingency underlie why some predators recover and others do not.
Keywords: apex predator, Restoration, recovery, intraguild predation, hysteresis, food chain, competition
Abstract
Habitat loss, overexploitation, and numerous other stressors have caused global declines in apex predators. This “trophic downgrading” has generated widespread concern because of the fundamental role that apex predators can play in ecosystem functioning, disease regulation, and biodiversity maintenance. In attempts to combat declines, managers have conducted reintroductions, imposed stricter harvest regulations, and implemented protected areas. We suggest that full recovery of viable apex predator populations is currently the exception rather than the rule. We argue that, in addition to well-known considerations, such as continued exploitation and slow life histories, there are several underappreciated factors that complicate predator recoveries. These factors include three challenges. First, a priori identification of the suite of trophic interactions, such as resource limitation and competition that will influence recovery can be difficult. Second, defining and accomplishing predator recovery in the context of a dynamic ecosystem requires an appreciation of the timing of recovery, which can determine the relative density of apex predators and other predators and therefore affect competitive outcomes. Third, successful recovery programs require designing adaptive sequences of management strategies that embrace key environmental and species interactions as they emerge. Consideration of recent research on food web modules, alternative stable states, and community assembly offer important insights for predator recovery efforts and restoration ecology more generally. Foremost among these is the importance of a social-ecological perspective in facilitating a long-lasting predator restoration while avoiding unintended consequences.
INTRODUCTION: WHY APEX PREDATOR RECOVERY?
Many of the most iconic and charismatic species in the natural world are apex predators, yet they are also often embedded in controversy. Apex predators (for example, whales, cougars, bears, wolves, sharks, and eagles) are consumers that kill their prey during or shortly following an attack, consume many prey over a lifetime, and are not eaten after reaching adult size (1). Apex predators also evoke strong emotional responses in people, varying from wonder and amazement to fear and spite (2). Because of the fundamental roles that apex predators can play in ecosystem functioning, disease regulation, and biodiversity maintenance, ecologists and conservation organizations have repeatedly sounded the alarm about local, regional, and global declines in apex predator (3) and large herbivore populations (4). Consequently, efforts to recover predator populations that have experienced declines are increasingly widespread.
Efforts to recover apex predators are implemented for a variety of reasons that span from the viability of particular populations, to ecosystem functioning, to the resilience of social-ecological systems. In the simplest situation, apex predator recovery is an end in itself. Borrowing from language in the U.S. Endangered Species Act (ESA), a successful recovery consists of a sequence of four events in which the predator population (i) ceases to decline, (ii) stabilizes, (iii) increases, and finally, (iv) occurs at a level that is self-persistent [U.S. Fish and Wildlife Service (USFWS), 2011]. Moreover, considering the key role that apex predators can play in biodiversity maintenance and ecosystem function, full recovery may require not only persistence but also recovery to some level consistent with historical baseline abundances (5). Thus, recovery is a broad term that can be achieved via a variety of mechanisms, including reintroduction and restocking (see Glossary of Terms in Box 1).
Box 1.
Glossary of Terms.
Reintroduction: Intentional movement of an organism into a part of its native range from which it has disappeared or become extirpated in historic times (Armstrong and Seddon, 2007).
Recovery: The first milestone in recovery is halting the decline of the species. Next is stabilizing the species, followed by increasing its numbers and distribution with the ultimate goal of making the species secure in the wild (USFWS, 2011).
Restoration: The process of assisting the recovery of an ecosystem that has been degraded, damaged, or destroyed. It is an intentional activity that initiates or accelerates ecosystem recovery with respect to its health (functional processes), integrity (species composition and community structure), and sustainability (resistance to disturbance and resilience) [Society for Ecological Restoration (SER), 2004].
Restocking: Movement of individuals to build up an existing population (Armstrong and Seddon, 2007).
Reestablishment: Institution of historical ecosystem structure or processes (70).
Carnivore: An animal that feeds on another animal’s body tissue. Synonymous with “predator” in this Review. Community module: A minimum realistic model describing groups of three or four interacting species.
Hysteresis: When the pathway of recovery of an ecosystem differs from its pathway of degradation.
Some efforts to recover apex predators have shown promising signs of sustained success. For example, gray wolves in the Greater Yellowstone Ecosystem [but see the study by Berger et al. (6)], harbor seals in coastal regions of the Northeast Pacific Ocean (7), and cheetahs in Tanzania (8) reached desired increases in abundance and distribution following reductions in densities caused by hunting and culling (Table 1). However, many apex predator recovery efforts have not yet met their potential or have encountered unanticipated problems along the way (Table 2) (9). Apex predators that have not yet successfully recovered despite concerted conservation efforts are widespread across ecosystems (land, freshwater, or marine), taxonomic groups (for example, mammals and birds), and exploitation status. In addition, there are several cases in which a focal predator has recovered successfully in one location (for example, sea otters in central California) but not in another (for example, sea otters in western Alaska). Although Table 2 by no means represents a comprehensive review of apex predator recovery efforts, it suggests that success is not universal [for a recent comprehensive review of marine systems recoveries, see the work by Lotze et al. (10) and Lotze and Worm (11)]. Indeed, the widespread occurrence of unsuccessful or stalled recovery efforts provokes the question: what factors encourage versus impede progress in the recovery of apex predators?
Table 1. Empirical studies of successful predator recoveries.
T, terrestrial; M, marine; F, freshwater; IUCN, International Union for Conservation of Nature; MMPA, Marine Mammal Protection Act.
| Study | Region | System | Predator | Summary of research results |
| (84) | Africa | T | Cheetah | Cheetahs survive with larger predators by seeking areas with low predator densities (spatial segregation from predators and competitors). |
| (84) | Africa | T | Cheetah | Successful reintroduction in Namibia, where larger carnivores were nearly extirpated by hunting [see also the work by Polis and Holt (84)]. |
| (85) | North America | T | Wolf | Wolf-driven declines in coyotes led to a fourfold increase in survival of juvenile pronghorn antelope (Antilocapra americana) in wolf restoration areas in the Greater Yellowstone Ecosystem. |
| (86) | Europe | T | Lynx | In Europe, restored lynx and wolf populations suppress red foxes. |
| (86) | Europe | T | Wolf | In Europe, restored lynx and wolf populations suppress red foxes. |
| (87) | New Zealand | T | Cook’s petrel | Reductions in predatory feral cats and rats and altitude-dependent resource availability promote petrel recovery. |
| (88) | North America (Colorado) |
T | Mountain lion |
Reduced exploitative and interference competition between mountain lions and other historically abundant predators (grizzly bears and wolves), combined with increased ungulate prey abundance, has facilitated mountain lion recovery. |
| (89) | T/M | Polar bear | Appreciation of social-ecological system allowed for subsistence harvest and reduced illegal hunting in shared population between the United States and Russia. |
|
| (90) | North America | F | Bass | Following natural extirpation of bass (Micropterus salmoides) in 1978, reintroduced bass in 1986 led to the return of bass populations despite exploitative competition with dominant mesopredators. |
| (91) | Australia | T | Dingo | Restoration of dingoes in parts of Australia is now being advocated as a necessary condition for the large-scale rees tablishment of declined mammal species (91). |
| (92) | North America | T | Peregrine falcon |
Populations declined globally because of exposure to contaminants and are listed in the United States in 1970 after being extirpated east of the Rockies. Declines of DDT and captive breeding led to rebound, and delisting in 1999. |
| (93) | Europe | T | Brown bear | Populations hunted to near extinction in the 1800s in much of Europe, including Norway and Sweden. Economic in centives and conservation plans have led to a rebound in recent years. |
| (94) | Global | T/M | Sea eagle | Bald and white-tailed eagles were either directly removed or negatively affected by pesticides until the latter half of the 20th century. Since then, populations have recovered worldwide, to the point that these apex predators are having some worrying effects further down the food chain. |
| (95) | Asia | T | Asiatic lion | Following collapse, incentivized pastoral communities to move, which allowed forest/prey populations to recover and lion populations to rebound |
| (96) | North America | F | Alligator | Alligators were depleted as a result of habitat loss and hunting; following protection in 1967, alligators increased nearly exponentially and were delisted in 1987. |
| (97) | Belize | F | Morelet’s crocodile |
Like alligators in North America, these crocodiles were affected by habitat destruction and hunting. Populations have been increasing since IUCN recognition and legal protection in 1981. |
| (98) | Australia | M | Saltwater crocodile |
Intense commercial hunting in the mid-20th century led to a population collapse, from near 100,000 to 500. Legal protection in 1971 and conservation actions have helped the population largely to recover, increasing interactions (mortality) with humans. |
| (99) | Africa | M | Fur seal | Like many pinniped populations (30), fur seals were commercially hunted through part of the 20th century, and portions of this population continue to be harvested; following protection in part of the range, the species has shown increasing trends and range expansion. |
| (100) | Northeast Pacific Ocean |
M | White shark | White sharks were either removed as pest species or taken incidentally in fisheries through most of the 20th century and, in a portion of their range, were negatively affected by contaminants. Recognition of declines and their importance led to reductions in mortality; over the last 20 years, indices of abundance and juvenile growth are increasing. |
| (101) | Northeast Pacific | M | Blue whale | Blue whales were targeted during industrial whaling, leaving them at a fraction of carrying capacity. Following the cessation of whaling, and additional protection, this population is thought to nearly be at historic levels. |
| (102) | Northeast Pacific | M | Sea otter | Sea otters were hunted to local extinction through much of their range but, following protection under the MMPA, has largely rebounded in California. |
| (103) | New Zealand | M | Spiny lobster | Marine reserves were used as a tool to protect spiny lobster habitat. Older reserves were found to yield higher lobster density, as well as larger lobsters. |
| (104) | North America | M | Gray whale | Following the end of whaling and protection under the MMPA, gray whales largely rebounded and were the first marine mammal species delisted from the ESA. |
| (105) | Africa | T | Ethiopian wolf |
Wolves have largely declined as a result of habitat loss. These populations experienced a catastrophic disease-induced collapse 20 years ago but have fully recovered and are no longer affected by Allee dynamics. |
Table 2. Empirical studies of failed and stalled predator recoveries.
| Study | Region | System | Predator | Summary research results |
| (84) | Africa | T | Cheetah | In reserves of Kenya and South Africa, cheetahs have failed to recover because lions and hyenas kill the cheetah cubs. |
| (106) | Antarctica | M | Fur seal | Leopard seal predation on fur seal pups limited recovery. |
| (107) | Southwest Alaska | M | Sea otter | Predation by killer whales on sea otters depressed population recovery. |
| (108) | North America | T | Bobcat | Suggests that bobcats compete with coyotes for resources and are also killed by them. Need to verify that bobcat recovery is desired and that population size trajectory is not as positive as desired. |
| (109) | Eastern Scotian Shelf (Northwest Atlantic) |
M | Cod | Gray seal predation, not competition and predation related to forage fish abundance, may contribute to high mortality rates of older cod, preventing or slowing recovery. |
| (110) | North America (California) | T | Mountain lion | Especially in southwestern California, urban development has fragmented mountain lion habitat, reducing genetic exchange and prey base available to facilitate population growth. |
| (111) | North America (Arizona and New Mexico) |
T | Gray wolf | Mexican wolf reintroductions have been minimally successful compared to other locations, in large part due to human hunting, purportedly in response to wolf predation on livestock. |
| (112) | North America | M | Cook Inlet beluga | Population is small in number and geographically isolated and inhabits a core habitat that is shrinking |
| (113) | North America | M | Southern Resident killer whale |
Juveniles from this population were targeted for aquaria removals until the early 1970s. Because of its small population size, skewed age distribution, and various human threats (contaminants and lack of prey), it is not growing fast enough to meet recovery goals. |
| (114) | North America (Mexico) |
M | Vaquita | IUCN recognized this as the most endangered cetacean species in the world; like beluga, the population size is small (<250) and geographically isolated. Further, vaquita are also incidentally captured in gillnet fisheries and further affected by humans |
| (115) | North America | T | Northern spotted owl |
Stable in portions of the range, but continual declines in others, as a result of habitat loss and interspecific competition with barred owls |
| (116) | North America | T/F | Wood stork | Federally listed wood storks consumed by nonnative introduced Burmese pythons |
| (117) | North America | F | Lake trout | Invasive predator has had severe impacts on threatened Yellowstone cutthroat trout. |
Here, we explore four key aspects of apex predator recovery. In the first section, “Apex Predators Live Slow, Range Widely, and Die Fast,” we focus on how apex predator life history characteristics offer insight into predator recoveries. In the second section, “Ecosystem Context and Apex Predator Recovery,” we focus on the importance of food and habitat limitation, behavior, and species interactions as drivers of predator recovery. In the third section, “When Is as Important as What: Historical Contingency and Predator Recovery,” we discuss the integration of predators into assembly theory and emphasize the role of timing as a key driver in predator recovery. Last, in the fourth section, “Prescriptions for Success? Research Gaps and Ecosystem Restoration,” we emphasize how recovery pathways are not necessarily identical as pathways to decline (that is, hysteresis), discuss theoretical and empirical research gaps, and emphasize the need to consider social as well as ecological systems when seeking targets for apex predator recovery.
APEX PREDATORS LIVE SLOW, RANGE WIDELY, AND DIE FAST
There are a variety of reasons why apex predator recovery programs often are marginally successful or even fail. One of the clearest explanations is that species at the apex of the food chain are known for having slow life history characteristics. Relative to prey, apex predators tend to have slower somatic growth rates, larger size at maturity, and longer generation lengths, all life history traits correlated with fewer offspring (12, 13). A low maximal reproduction rate means that it is difficult to mount compensatory responses via recruitment to enhanced mortality. Although not exclusive to the top of the food chain, apex predators also generally have smaller population sizes and slower population growth rates than their prey (14), making them especially sensitive to extinction from demographic stochasticity (15) and causing slow recovery rates even in cases of positive population trajectories. For example, a review of 198 reintroduction studies found that herbivore reintroductions exhibited a 29% higher success compared to carnivore reintroductions (16). Additionally, a recent synthesis of recovery times for overexploited fisheries suggests that higher-trophic level species are likely to exhibit the slowest recovery rates (17). Indeed, this finding almost certainly generalizes that apex predator recoveries often will be slower than those of lower-trophic level species simply because of a disparity in life history strategies. Such slower recovery times require setting appropriate expectations for the time scale of successful recovery. As we will note below, these life history effects on recovery can be amplified by community and ecosystem interactions.
Directly hostile human activities can exacerbate the negative effects of slow life history strategies on predator recovery time. Continued (over-) exploitation is perhaps the most substantive of these activities [reviewed by Duffy (18)], but other direct and indirect human influences can also play a role. Strong exploitation and poaching often continue during recovery efforts because the predators are themselves valuable (17, 19). For example, tigers are at risk of extinction due to intentional human impacts because of their lucrative commercial value in some Asian medicines (20). In other cases, apex predators are considered to be a threat to valuable species. For example, unexploited shark and wolf populations have been considered threats to wildlife, fisheries, and livestock, often being killed by poachers [for example, Holdo et al. (21)] and, in some cases, even by the agencies mandated to protect them (22). Given low potential recruitment rates, such imposed extra mortality can greatly hamper apex predator recovery.
Even when exploitation rates are reduced to near zero, other human influences can interact with the slow life histories of predators to inhibit their recoveries. Pollution affects many long-lived predators exposed to toxic contaminants via bioaccumulation or accidental poisoning [for example, bald eagles, Arctic wolves, polar bears, ringed seals, and bottlenose dolphins; reviewed by Harrad (23)] and via the chronic stress of anthropogenic noise (24). For instance, Southern Resident killer whales have not been harvested commercially since the 1970s in the North Pacific but remain listed as endangered under the ESA and are exposed to a number of threats, including reductions in prey availability and enormous body burdens of persistent organic pollutants (Ross et al., 2000). The difficulty with pollution is that, even when it is reduced or eliminated (for example, bans on persistent organic pollutants), it can take many years to dissipate from the environment, and long-lived predators with toxins in their tissues can take decades or longer to exhibit signs of recovery (23). Apex predators often have very large home ranges, which implies that they can be exposed to a diverse range of scattered, spatially localized environmental challenges, ranging from exposure to toxins, to vehicular collisions, to hostile ranchers or poachers outside protected areas.
There are also genetic and evolutionary factors that can contribute to poor recovery. If historically outbred apex predators have been pushed to low numbers persisting often in scattered local populations, the remaining individuals may exhibit maladaptation due to inbreeding of related individuals, as well as the depletion of adaptive genetic variation needed to cope with an ever-changing environment. For instance, the severely affected Florida panther has shown many developmental abnormalities associated with an abrupt reduction in effective population size and kin breeding (25), which surely hampers its recovery. However, our focus here will be on ecological dimensions of impaired recovery.
Nevertheless, constraints on successful predator recoveries extend beyond slow life history characteristics, genetic factors, exploitation, and other direct human influences. For instance, the splendid recovery of the bald eagle depended not only on the prohibition of hunting but also on the banning of DDT, improved water quality at many sites, forceful protection of nest sites, and deliberate careful reintroductions from captive-bred populations. Below, we argue that the importance of considering the full community and ecosystem context is underappreciated yet critical to apex predator recovery programs. In particular, we (i) argue that apex predator recovery programs are unique from the better-studied restoration programs for species that are lower on the food chain; (ii) use a simple model to illustrate how successful apex predator recovery requires an appreciation of the types of species interactions that characterize the community, the degree to which mesopredators, if present, are culled, and the extent to which basal resources are supplemented; and (iii) highlight how the rate and degree of apex predator recovery can depend on an ecosystem’s history of trophic downgrading.
ECOSYSTEM CONTEXT AND APEX PREDATOR RECOVERY
Insights into how to enable successful apex predator recovery may be grounded in the lessons learned from ecological restoration practices focused on lower trophic levels, especially plants [reviewed by Young et al. (26)]. The approaches used in ecological restoration fall broadly into three categories, including (i) the noninterventionist, leave-it-to-nature strategy [that is, succession (27)]; (ii) the structure-begets-function or “if you build it they will come” (also known as field-of-dreams) strategy (28); and (iii) the ecosystem function or process-based strategy (29). Modern-day restoration tends to blend elements of strategies (ii) and (iii), often seeking reestablishment of both ecosystem structure and function to achieve restoration of particular species or communities.
Efforts to recover predators seem to be unusual in that they frequently adapt a noninterventionist strategy by simply stopping or reducing hunting and assuming that the system will reequilibrate. Exceptions often involve direct reintroductions, such as red kite and osprey in the UK, or peregrine in the United States; here, breeding programs and reintroductions, in a sense, circumvent the low maximal recruitment possibilities for many apex predators. But often, recovery simply involves dampening in imposed mortality regimes. For example, recovery of many marine mammals (30) has largely been attributed to reduced hunting, with little attention given to reestablishing the structure and function of systems within which marine mammals live. There are several lessons learned in plant-based ecological restoration that go beyond leave-it-to-nature, and may help to speed apex predator recovery and augment recovered predator population sizes.
In restoration aimed primarily at primary producers, ecologists have advocated for an appreciation of species traits, and the ecological processes that affect ecosystem structure and function in the development of restoration strategies (26). A number of ecological processes (for example, dispersal, resource limitation, competition, predation, and mutualism) and traits (for example, life histories, drought tolerance, and herbivore resistance) have been acknowledged and manipulated to facilitate recovery success [reviewed by Young et al. (26)]. For example, plant restoration often involves seed additions and the use of cages over seeds and seedlings to combat recruitment limitation and seed predation. Predator recoveries similarly rely on a suite of ecological processes influenced by a variety of species interactions, but this has not been formally considered in many restoration attempts. Apex predator restoration differs from restoration of lower trophic levels because predators as individuals tend to have greater food resource needs, larger habitat requirements, and different behavioral responses to environmental change than their prey. In addition, and also in contrast to plant restoration ecology, the community context for a predator tends to be usefully conceptualized around trophic interactions first and foremost.
Food resource limitation can constrain apex predator recovery
The high metabolic demand of most apex predators necessitates an abundant, stable, and nutritionally diverse prey base (31). Empirically, populations of large carnivores are most abundant in areas with high prey biomass (32, 33). However, resource availability does not always boost predator abundance and persistence. For example, a recent global synthesis of resource limitation in seabirds describes a threshold under which food availability (of low-trophic level fish) led to reduced and more variable seabird productivity (34), but high abundances above a certain level did not. Changes in food abundance above this threshold would be expected to have negligible effects on seabird recovery programs. Restoration efforts need to carefully consider the nonlinear relationship between consumption and food availability; ensuring some food supply is critical, but the marginal value of higher food supplies may not warrant the extra costs, because consumers can be satiated or limited by factors other than food [for example, resting or nest site provision; cf. Samhouri et al. (35)]. Below, we expand on this idea and provide an explicit analysis of how basal resource carrying capacity modulates predator recovery (see Box 2).
Box 2.
A Model for Understanding How Module Shape Drives Apex Predator Recovery.
A three-species Lotka-Volterra model of an intraguild predation (IGP) module aids in illustrating a number of the concepts regarding the likelihood of success for apex predator recovery programs. This model highlights how the module and strengths of species interactions in which an apex predator is embedded can be important for anticipating the effects of (i) restoring resources (Fig. 2), (ii) culling mesopredators (Fig. 3), and (iii) attempting to reestablish apex predator populations with an insufficient initial number of individuals (Fig. 4). The core of this model, which describes the interplay of predation and competition between an (omnivorous) apex predator and a mesopredator (also known as intraguild prey) for a shared resource, has been extensively studied by many authors and is well understood (47, 56, 84, 118), permitting useful insights in the context of apex predator recovery efforts.
Model description. Following McCann et al. (119), we illustrate the effects of module type by introducing an apex predator prey preference parameter ω to the basic IGP model. This parameter controls the degree to which the system reflects two further well-studied community modules: the linear food chain (ω = 1, where no competition occurs among predators) (120, 121) and the exploitative competition module (ω = 0, where only competition occurs among predators) (122), in addition to the IGP module (0 < ω < 1, wherein both competition and predation occur) (47, 56, 84, 118, 121). Note that the prey preference parameter may differ substantially between terrestrial and marine apex predators, given that terrestrial apex predators are often highly specialized. For simplicity, we assume that the apex predator’s preference for a given type of resource is fixed rather than responding adaptively to changes in prey density. The population growth rates of the shared basal resource (R), the mesopredator predator (N), and the apex predator (P) are represented by the following three equations.
| (1) |
| (2) |
| (3) |
In this model, r and K are the basal resource’s intrinsic growth rate and carrying capacity, respectively, and e is the trophic efficiency at which consumed prey are converted to predators. The functional responses of both predators are assumed to be linear, with α being the mesopredator attack rate on the resource and a being the apex predator’s total attack rate (distributed between the resource and the mesopredator by the apex predator’s prey preference ω). Parameters δ and d are the respective intrinsic per capita death rates of the mesopredator and apex predator and are assumed to be equal. Increases to δ may be interpreted as the culling of mesopredators by the removal of a constant fraction of individuals. The simplifying assumptions here offer clarity but could be readily modified in a given case study. Just as for the well-studied IGP models without an apex predator prey preference term (118), this model exhibits six possible steady-state equilibria (see fig. S1). Our focus is on two of these: the case in which all three species coexist and the case in which only the apex predator and the resource coexist (with the mesopredator being unable to persist). For illustrative purposes, we restrict our focus to parameter values for which these equilibria exhibit stable dynamics (that is, Re(λ1) < 0). The mesopredator’s attack rate was set to be three times the apex predator’s total attack rate because three-species coexistence necessitates the mesopredator being the superior competitor for the shared resource (65). Note that in the apex predator resource-only state, the parameter ω reflects the apex predator’s efficacy at capturing resources rather than its prey preference per se. Baseline parameters were chosen to be ω = 0.5, K = 0.15, δ = d = 0.01, r = 1, e = 0.1, a = 1, and α = 3, with the effects of varying ω, K, and δ being illustrated in Figs. 2 to 4.
Model Interpretation: Module Shape Determines the Efficacy of Resource Subsidies and Mesopredator Culling. The model demonstrates how expectations and intuition about the most promising approaches for population recovery can depend heavily on an apex predator’s preferences for basal versus mesopredator species (Figs. 2 and 3). As intuition might suggest, an apex predator embedded within a module dominated by exploitative competition should achieve a higher recovered population density than a predator embedded within an IGP or food chain module because less of the system’s energy is lost to the inefficiency of the mesopredator’s trophic conversion rate (Fig. 2). The model also demonstrates how the consequences of mesopredator culling are affected by module shape and how culling may even be counterproductive for the goal of maximizing apex predator densities (Fig. 3). For example, apex predator recovery may require not just partial culling but rather the complete removal of the mesopredator if competition between the mesopredator and apex predator is strong and the apex predator exhibits little preference for consuming the mesopredator (ω < 0.295). That is, without complete mesopredator removal, the mesopredator’s superior ability to exploit basal resources can permit it to preclude the apex predator’s reestablishment outright. At the other extreme, even a weak increase in the mesopredator’s death rate by the implementation of a culling program will decrease a recovered apex predator’s population size when exploitative competition is weak and the module is more like a food chain. Indeed, for nearly all parameter values that allow the coexistence of all three species, a doubling of the mesopredator’s death rate (that is, culling) reduces the apex predator population’s size by almost half. Only within a narrow window of the apex predator’s prey preference, wherein exploitative competition is strong but not strong enough to affect competitive exclusion (0.295 > ω < 0.31), is the culling of the mesopredator expected to lead to an increase in the population size of a recovering apex predator (Fig. 3, inset). The utility of intermediate complexity models, particularly those considering IGP, which is a common structural feature of real food webs (123), is their capacity to capture much of the dynamic properties of food webs as a whole (124, 125). The model presented here offers highlights on how the tractable consideration of strongly interacting species is useful for developing management strategies that take into account ecosystem context [see also discussion of minimum realistic models by Plagányi et al. (126)].
How habitat can constrain predator recovery: “One hill can’t shelter two tigers.”—C. Elton, 1927
Habitat loss and fragmentation are among the primary drivers limiting recovery of predator populations [for example, Crooks and Soulé (36)], and a number of restoration programs continue to struggle from the constraints of insufficient habitat availability (37). Degraded habitats often have limited reproductive sites, which not only contribute to initial predator declines [for example, (38)] but also constrain successful colonization and persistence of recovering or reintroduced predator populations [for example, (39)]. The success of the international Yellowstone to Yukon corridor in facilitating connectivity for large mammalian carnivores (grizzly bear, lynx, wolverine, and wolf) provides evidence for the benefits of large connected expanses of habitat for struggling predator populations that have high minimum area requirements. In contrast, fragmented habitats without wildlife corridors can strongly inhibit recoveries. For example, an eight-lane highway fragments cougar habitat in southern California, leading to a high incidence of vehicle-cougar collisions (40). The importance of habitat availability and fragmentation in driving apex predator recovery is particularly pronounced in terrestrial systems; however, in marine systems, the loss of apex predators due to habitat shifts or connectivity is less well established. The recovery trajectory of predators is therefore dependent on the size and heterogeneity of the landscape within which they are embedded.
Predator behavior can constrain predator recovery
Behavior can play a significant role in determining the potential for predator recovery. Prey preference, breeding habitat preference, aggression, mobility, and social organization are five of the most important behavioral considerations. Populations of predators that are dietary specialists are likely to fluctuate more than generalist predators, which can switch to another prey species if a focal prey becomes rare (41). Therefore, it may be more difficult to recover specialist predators to self-persistent levels. In contrast, the diet breadth that often increases with trophic level [for example, Cohen et al. (42)] offers opportunity for restoration that may not exist at lower trophic levels (though exceptions such as Southern Resident killer whales and sea otters exist). Similarly, predator recovery rates may be constrained despite readily available food if they are limited by other key resources such as access to breeding sites or from intraspecific interference. For example, many predators migrate to breeding sites with smaller recently mature individuals following older, larger individuals who know their way. Overharvesting of older and larger predators may lead to the loss of cultural memory of spawning aggregation location [for example, (43)], a mechanism that likely affects species throughout the food web. Intraspecific aggression among Florida panthers may similarly limit their recovery; the aggression that male mountain lions exhibit towards females with cubs and smaller males is thought to constrain recovery (44). Finally, movement behavior can limit predator recovery rates, such as in cases where dispersal is inhibited by modifications to natural landscapes [for example, (40)], leading to localized food resource depletion. Migrations may also be shorter or avoided at low population densities, creating a type of Allee effect. For instance, endangered African wild dogs generate smaller dispersing cohorts when population densities are low (45). Whereas some of these predator behaviors are relatively inflexible and predictable, others are less so, creating substantial uncertainty about the potential for predator recovery even when food resources are replete.
It takes a community to recover a predator
Like any species subjected to a recovery effort, predators experience constraints driven by a diverse configuration of prey, competitors, and natural enemies (including diseases and parasites). Understanding apex predator dynamics in this light is made difficult by the fact that apex predators are embedded in a complex network of species interactions, with many apex predators being generalist consumers. Whereas theory suggests that the dynamics of generalists may be described by simple single-species models when prey resources are readily abundant (41), prey limitation is one of many hypothesized mechanisms constraining apex predator recovery. Because food webs are often characterized by few strong and many weak interactions (46), full communities can be conceptualized in the form of simpler, digestible, and analytically tractable modules (that is, groups of three to five interacting species) (Fig. 1) (47). Rather than trying to capture the full suite of ecological interactions within a predator’s ecosystem, these modules provide a practical way to narrow the universe of potential influences on a predator’s recovery while going beyond the restricted perspective of resource or habitat limitation alone. For example, modules can focus solely on strongly interacting species within a food web or alternatively can offer a way to simplify a food web by modeling multiple species of similar life history traits as a single node. In Box 2, we focus on the former approach and explore a three-species food web model to consider how different module configurations—ranging from an exploitative competition model, to an IGP module, to a linear food chain—can alter the anticipated recovery of apex predators.
Fig. 1. Three-species community modules: Food chain, exploitative competition, and IGP.

These modules are generic descriptions of common configurations of predator-prey interactions in the natural world (left), each of which corresponds to a predator recovery example (center) that has followed a restoration trajectory corresponding to the module (right).
The model indicates that, although the supplementation of a community’s resource base will increase an apex predator’s recovered population size regardless of the strength of the interactions in any module configuration, the magnitude of this effect will be least pronounced the more the community resembles a linear food chain. Thus, efforts to increase the production rate of basal resources, a strategy practiced in plant restoration (26), will have the largest impact the more the community in which the apex predator is embedded reflects a module of exploitative competition. Moreover, partial mesopredator culling increases recovered predator densities for only a narrow region of parameter space between exploitative competition and linear food chain extremes (Fig. 3). Therefore, culling may be an ineffective tool in all but the most well-understood IGP systems [for example, Persson et al. (48)]. This inference contradicts the historic and current prevalence of predator culling in management [reviewed by Bergstrom et al. (22)] but corroborates studies emphasizing how the culling of apex predators to maximize fisheries yield will not always be effective (2, 49, 50) as well as those highlighting the potential positive population-level effects of increased mortality [reviewed by Schröder et al. (51)]. Other factors, such as the presence of size-structured interactions between mesopredators and apex predators (52, 53) and the existence of additional predator or prey species in the model, can alter model predictions (54). We emphasize that our model of a three-species representation of reality is not a tactical model meant for supporting specific management decisions. Instead, our model is conceptual and strategic, focusing on developing a broad understanding of the ecosystem process and directional patterns of change.
Fig. 3. Module shape alters how apex predator density will respond to the culling of mesopredators, as indicated by the contrast of low (solid lines) and high (dashed lines) mesopredator mortality rates.
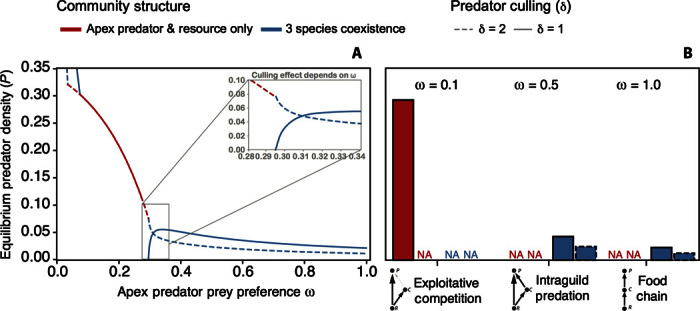
Culling will increase apex predator recovery success when competition is strong. In most cases, culling rates must be sufficiently high such that only the apex predator and the resource persist (red). In contrast, culling will negatively affect the apex predator’s density across most of the range of apex predator prey preference values (ω), when three-species coexistence is desired (blue). Culling of mesopredators only benefits the apex predator when competition is strong but sufficiently weak so as not to cause competitive exclusion (inset). (A) A gradient of predator’s prey preference. (B) Discrete measures of apex predator equilibrium density for discrete models: exploitative competition, IGP, and food chain. NA, not applicable.
As illustrated through these examples, community modules can be used to calibrate qualitative expectations for the recovery of a focal predator population. Just as in plant-based ecological restoration, predator recovery efforts that focus on restoring key ecological processes—including species interactions—are likely to be those that meet with the greatest success. Of course, identification of the community module that best characterizes a predator and its ecosystem may be difficult at the outset of a recovery effort. Additionally, the results of short-term recovery efforts likely represent transient dynamics and may differ from equilibrium expectations (55). Furthermore, the structure of community modules and the strength of the interactions embedded within them can change with environmental context and the loss or introduction of new species (56) and can depend crucially on the details of population structure (for example, size-dependent predation) (53). Thus, conservation and management structures are likely to meet with greater success if they are shaped by the idea that the conceptualization of a community module will require modification over time (57).
WHEN IS AS IMPORTANT AS WHAT: HISTORICAL CONTINGENCY AND PREDATOR RECOVERY
The community module in which a predator is embedded at the beginning of a recovery effort provides critical information about the potential of different recovery strategies. Recent advances in community assembly theory suggest that successful ecological restoration may also require careful consideration of the order and timing in which species come together to form a community. This idea, that historical contingencies can influence community assembly, has been well described in the basic ecology and restoration literatures for primary producers (26). However, for larger carnivorous species, the role of historical contingency in driving community assembly remains poorly studied.
Borrowing from these other literatures, we suggest that the importance of correctly timing the occurrence of ecological processes can be summarized with two principles. First, biophysical processes that modify the environment to promote establishment of one or more target species in a restoration effort are critical. For instance, early colonizing plants can engineer hospitable soil conditions that facilitate the colonization success of other, less drought-tolerant, plant species [for example, (58)]. Thus, ensuring drought-tolerant plants established early is necessary, but not sufficient, for restoring drought-intolerant plants. Predator recoveries are similarly sensitive to ordered nonconsumptive interactions. For example, recovery of endangered salmon populations in the U.S. Pacific Northwest likely requires an increase in the quantity and/or quality of available habitat (which is small relative to historical levels) (59). Promising avenues include geomorphological modifications to in-stream conditions (for example, sediment grain size and flow rates) for salmon during the freshwater phase of the life cycle and improved access to spawning habitat (for example, via removal of hydropower dams) (59).
The second timing principle holds that, at the outset of a recovery effort, the density of predators relative to their competitors and/or prey can have a strong effect on the outcome. This concept, known as priority effects in the ecological literature (60), has been well documented in the context of competitive interactions, especially among plants and in plant restoration (26). If one species builds up a sufficiently large relative abundance before another species attempting to join the community, the species in high abundance can prevent the competitor from establishing. The competitor’s abundance that is sufficient to preclude a focal species’ establishment need not be greater than the abundance at which the focal species is reintroduced.
Both competitive priority effects and predation-mediated priority effects can have long-lasting effects on communities. Indeed, priority effects can provoke alternative community states that persist indefinitely (61, 62). In the context of recovering apex predators, the appropriate initial densities to facilitate predator recovery depend on which community module is most representative of the system (63). Here, too, the IGP model (see Box 2; Eqs. 1 to 3) provides informative insight. In the case of a simple food chain module, the appropriate initial densities within the community are clear: predators are most likely to succeed when resources are most readily available (Fig. 2). Indeed, ensuring sufficient prey availability first is critical to avoiding a predator pit (63, 64). In contrast, to recover a predator population embedded within an IGP module, determining appropriate reintroduction densities, or culling competitors before predator reintroduction of the apex predator commences, can be key to establishing persistent predator populations (Fig. 4) (61, 65).
Fig. 2. Module shape alters how an apex predator’s abundance will respond to the restoration of basal resources, as indicated by the contrast of low (solid lines) and high (dashed lines) resource carrying capacities (red lines, apex predator resource-only state; blue lines, three-species coexistence state).
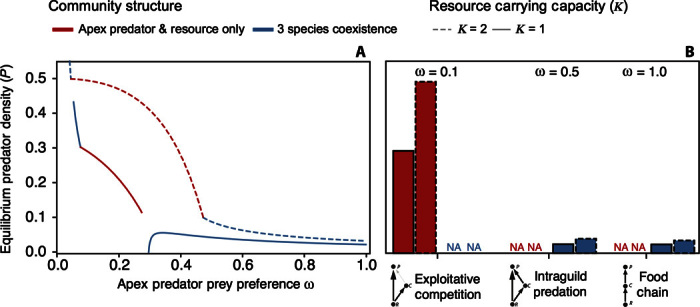
When ω has intermediate values, increases in resource productivity benefit the apex predator’s abundance to the detriment of the mesopredator because the mesopredator’s competetive advantage becomes superseded by the predation pressure that it experiences from the apex predator. (A and B) A continuous gradient of predator’s prey preference (ω) (A) and discrete measures of apex predator equilibrium density for characteristic models, including exploitative competition, IGP, and a food chain (B). For additional model details, see fig. S1 (baseline parameters here are as follows: ω = 0.5, r = 1, e = 0.1, a = 1, and α = 3).
Fig. 4. Priority effects occur when final equilibrium population sizes are dependent on initial population sizes, even though all other parameter values (that is, environmental conditions) remain unchanged.
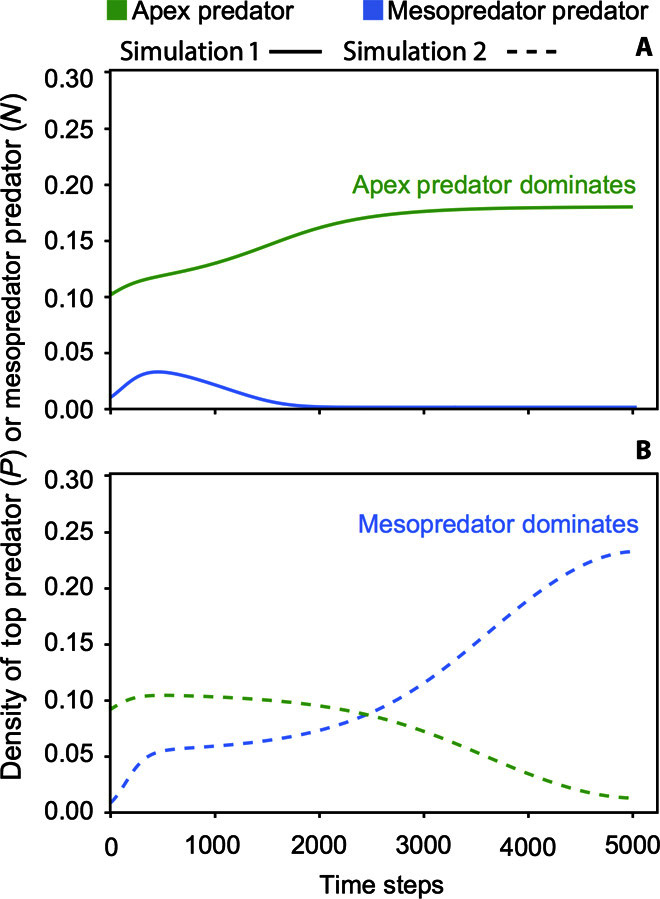
Such priority effects occur in the IGP module when competition between the apex predators and the mesopredators is strongest, illustrated here with two simulations that differ only in the initial abundance of the apex predator. (A) The dynamics illustrate the scenario where the apex predator’s initial population size (green, P0 > 0.1) is sufficient to affect the extinction of the mesopredator (blue, N0 = 0.01). (B) In contrast, the dynamics illustrate a scenario where the apex predator’s initial population size (P0 < 0.1) is insufficient to avoid extinction due to exclusion by the mesopredator (N0 = 0.01) (that is, a failed restoration). Parameters are as in Figs. 2 and 3 but with ω = 0.225 reflecting an IGP module in which exploitative competition is strong.
More broadly, the growth and stability of reintroduced predator populations can vary depending on the timing of predator recruitment to a community. For example, predator recovery will likely have maximum success if prey are readily available early in their reintroduction. However, it has only recently become clear that colonization history also drives dynamics and stability of multiple trophic levels (66). For example, in a microcosm study, Olito and Fukami (66) showed that predators reached higher population abundance if they were introduced early during community assembly rather than late, as would at first seem more intuitive. In the context of conservation and management actions, these theoretical and empirical findings together underscore the importance of controlling putative competitor populations before predator recovery implementation, introducing high densities of predators relative to their competitors, and doing so earlier rather than later.
PRESCRIPTIONS FOR SUCCESS? RESEARCH GAPS AND ECOSYSTEM RESTORATION
Above, we demonstrated three major lessons in promoting successful apex predator recoveries. First, we argued that, relative to species lower on the food chain, some apex predators are likely to respond slowly to recovery efforts. Apex predators exhibit slow life history characteristics, are particularly prone to human pressures, and can require large amounts of available resources and habitat before they can succeed (67). In the face of these numerous challenges, it is necessary (but not always sufficient: see third lesson below) to identify and counteract the initial driver(s) of the predator decline.
Second, we emphasized the importance of ecological context in driving variation in apex predator recovery dynamics. Like primary producers, apex predators are embedded within a complex network of interacting species, where resources, competitors, and pathogens can affect population dynamics. The configuration of these community modules (Fig. 1) can play a key role in defining the appropriate strategy for recovery success. Because the prescription for recovery can differ markedly among these modules, practitioners will benefit from defining the appropriate module for their system at the outset. Existing uncertainty in the number, strength, and shape of food webs (68) may make it difficult to forecast on the time scale of predator recovery; therefore, it may be more beneficial to consider a suite of potential modules and evaluate recovery strategies by integrating over different modules (for example, adding an invasive species and culling a mesopredator). From a practical perspective, defining community modules involves a variety of tactics, including gut content studies and scat samples, to discern predator diet preference, diet flexibility, and foraging behavior. Although certainly challenging, new tools that combine laboratory studies and field observations point to the possibility of quantifying the various aspects of predator foraging behavior in the wild [for example, Wootton and Emmerson (46) and Novak (56)].
The third major lesson relates to a role for ecosystem history in evaluating effectiveness of alternative recovery strategies. The trajectory of an apex predator population recovery may depend closely on the way that predator was harvested, the density of predators, competitors, and resources when a recovery program begins, and the likelihood with which a degraded community/ecosystem becomes entrained by an alternative stable state (see below).
Hysteresis
Appreciating the context dependency of apex predator dynamics is particularly key where environmental conditions or resource extraction counteracts recovery efforts. There are an increasing number of examples of ecosystems in which critical thresholds (that is, tipping points) to less-preferred states have been crossed (69). In these systems, hysteresis effects (that is, when recovery pathways differ from degradation pathways) may exacerbate the challenge of achieving full ecosystem recovery (26, 70). As noted above, different initial species abundances can promote dynamics, leading to different states of the system even when environmental conditions themselves remain unchanged. The hysteresis associated with the presence of these alternative stable states involving predator-prey interactions (71, 72) increases the difficulty of getting back to the original preferred high predator density state (Fig. 4). Knowing that thresholds between alternative stable states exist can also be beneficial for staying in preferred high predator density states (26, 70). Therefore, following the initial loss of an apex predator, it is important to know what appropriate initial starting densities of predators, as well as their competitors and prey, can be critical to the long-term success of a predator recovery program. For example, the existence of alternative states has been shown to be exacerbated if one or both of the apex predator and mesopredator consume one another’s juveniles (52). Such size-structured interactions in which predators and prey exhibit role reversals are relatively common to fisheries (73) where lower-trophic level forage fish are often competitors of the young life stages of larger predatory fishes. More generally, the exploitation history of a system connected through trophic links can affect assembly and recovery, requiring harvest rules that are more stringent than those that originally caused a population collapse to overcome the hysteresis of the system [for example, Collie et al. (74)].
Research gaps and opportunities
Our capacity to take these important lessons and use them to meet recovery goals for depleted apex predator populations requires understanding basic predator-prey assembly ecology. Historically, consumer-resource interactions have been understudied in the context of restoration ecology (26), and in some cases, researchers who study trophic ecology choose to address basic biology questions and ignore more conservation-oriented research (75). Therefore, increased communication between conservation practitioners and research biologists is necessary.
Furthermore, it is inevitable that we will develop insights into how to reconstruct intact communities by gaining a deeper understanding of the role of predators in community assembly and succession theory (62, 66, 76, 77). In the past, assembly models have either ignored predators or explored the effects of predation as an exogenous force on prey communities. Just like prey, predators experience variable population and community dynamics due to stochastic colonization, and this variability in colonization is essentially mimicked, intentionally or not, in a restoration program. Ecologists are only at the early stages of understanding the role of predator-prey interactions in driving assembly theory. Predator reintroduction programs offer ideal opportunities to learn more about basic predator-prey assembly ecology by conducting mensurative studies that would otherwise be too difficult to conduct at large spatial scales.
Social-ecological context of predator recoveries
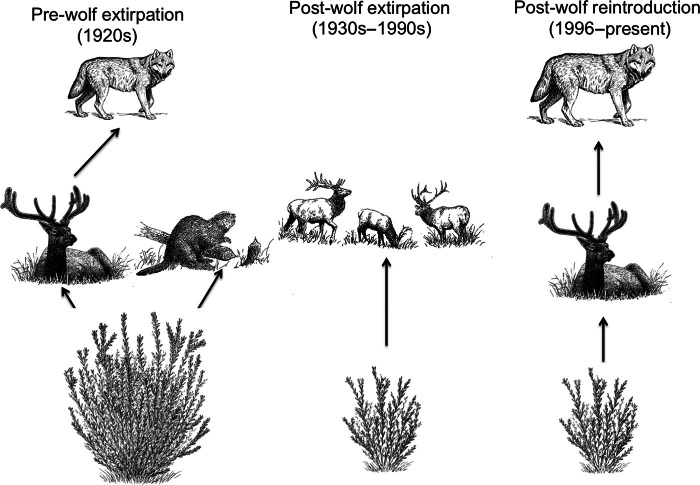
Restoration goals often extend beyond a single apex predator species into the broader ecosystem and social-ecological system within which predators are embedded. From an ecosystem perspective, some predators may be considered keystone species that can maintain biodiversity, control disease, modify their ecosystem’s biogeochemistry, and provide resistance to invasion (3, 78). If restoration practitioners broaden their goal to define restoration success beyond just apex predator recovery and include the ecosystem structure and function within which that predator is embedded as well, a complex suite of interactions between species and environmental conditions will determine recovery “success” (79). Box 3 provides an example of such an ecosystem context for apex predator recoveries in the Greater Yellowstone Ecosystem, where successfully reintroduced wolf populations have been insufficient to recover riparian willow communities along small streams to date (Fig. 5). This example serves to highlight the promise and complexity of using predator recoveries to achieve ecosystem-level and social-ecological goals. More than anything, it demonstrates that predator recoveries demand an evaluation of trade-offs, especially in light of the potential for human-wildlife conflict (80).
Box 3.
Yellowstone tipping points and wolf recoveries: An ecosystem perspective.
The reintroduction of the gray wolf to the Greater Yellowstone Ecosystem is exemplary of success among predator restoration programs (Table 1) (127). The USFWS along with the National Park Service reintroduced 31 wolves in the northern section of Yellowstone in 1995–1996. The wolf population initially expanded to more than 170 individuals in 2002 and subsequently stabilized around 100 individuals in recent years (127). Since reintroducing this apex predator, the northern range elk herd, which peaked at about 12,000 animals in 1995, has declined to around 4000 individuals (128, 129). The elk population decline has been attributed to multiple causes that include wolves, severe winters, and changes to hunting practices (130) Early research found support for cascading trophic effects from wolves to elk to vegetation, documenting increased growth in plants after 1996 [for example, (131, 132)].
Despite the success of this predator restoration and early findings on positive ecosystem consequences, recent work suggests that a classic food chain module is insufficient to describe the dominant interactions, particularly for riparian willows (9, 133, 134). Willows are the dominant woody vegetation along small streams on the northern range and across the semiarid Rocky Mountains. Willows are also an important food source for elk in winter, when snow covers the grassy range. Before wolves were extirpated from Yellowstone, willows also supported a large beaver population on the northern range. Beavers used willow and aspen stems as building material for dams in small streams, flooding areas to create pools in which they submerged large winter food caches, also made of willow and aspen stems (135). Beaver dams raise water tables and lead to flooding upstream and downstream, increasing water availability for willows and creating opportunities for seedling establishment (133, 136). During the 70 years wolves were absent from the ecosystem, elk populations increased markedly, which had negative effects on herbaceous and woody vegetation on the northern range (137, 138). Beavers abandoned much of the northern range by the 1950s, likely because they were outcompeted by the abundant elk population (133, 139). Recent experimental work has shown that positive feedbacks from beaver dams are at least as important as top-down effects from browsing ungulates in regulating willow growth (Marshall et al., 2013).
The wolf reintroduction was predicted to improve conditions for vegetation on the northern range, assuming that a simple food chain captured the most important dynamics in the ecosystem. At least for willows along small streams, the effects of wolf reintroduction have been limited because restoring the apex predator has not restored important feedbacks between willows and beavers required for tall willow communities. These plant communities demonstrate asymmetric effects of predator removal and reintroduction, which translates to nonlinear state transitions, and perhaps alternate stable states (Marshall et al., 2013). Restoration of the predator has not yet equated to restoration of this part of the ecosystem.
Recovery of the gray wolf was a necessary but not sufficient condition for recovery of the pre-wolf extirpation state. However, recovery must be defined in a broader social-ecological context. Changes in land-use patterns and a warmer, drier climate may preclude ecosystem recovery to the 1920s state. Further, wolf predation on livestock creates conflict between conservation objectives and rancher objectives (140). Trade-offs may also occur between conservation interests and recreational hunting when hunters and predators rely on the same prey populations (141). More conflicts may arise between multiple endangered species in this ecosystem, modulated through food web interactions (142). Explicit ecosystem recovery goals will need to be defined by stakeholders to evaluate trade-offs and find optimal solutions that efficiently meet the needs of multiple invested parties.
Fig. 5. Time-varying modules of riparian corridors along small streams within the northern range of the Greater Yellowstone Ecosystem from the 1920s to present.
Northern-range riparian areas have exhibited (at least) three different major types of communities since 1920. Before wolf extinctions (1920s), riparian areas included wolves, elk, beavers, and willows. Following wolf extinctions (1930s to 1990s), these areas were reduced to just elk and willow. Most recently (1990 to present), wolf reintroductions have produced a system with wolves, elk, and willow but few beavers. Qualitatively, these different modules exhibit fundamentally different dynamics, exemplify temporal variability in a single system’s characteristic module, and meet different ecological and social services. [Illustration by Shannon Hennessey, Oregon State University].
Indeed, apex predator population recovery and the associated ecosystem responses are not always well received. The concept of rewilding is controversial (81), and increases in predator abundance or protection of existing populations can produce human-environment and human-human conflicts due to the strong cultural and economic linkages between predators, their impacts, and humans. For example, some fishermen consider their livelihoods to be threatened by increasing populations of whales and seals that eat key fisheries species like cod, salmon, or herring [for example, Yodzis (2)]. Similarly, ranchers have expressed concern over increasing populations of wolves and cougars that eat livestock (22). Therefore, the persistence of predators, and the target biomass at which successful recovery is defined, requires an explicit acknowledgment of the social-ecological trade-offs at play (80). In the case of a protected area, we might define success on the basis of estimates of historic apex predator densities or ecosystem characteristics. In contrast, successful apex predator restoration in the presence of heavy human-wildlife conflict may look substantially different, with lower target predator densities that reduce conflict but maintain a reasonable facsimile of the biodiversity and ecosystem function stewardship that predators can afford.
The future of apex predator restoration will therefore require compromise among multiple stakeholders who have different ideas for what successful restoration will mean (43). Although this may generate some conflicts among stakeholders, there are also glimmers of hope for creative solutions that allow the persistence of higher predator densities while avoiding human-wildlife conflict. For example, Can et al. (82) developed a toolbox for minimizing conflict between humans and bears, and Bruskotter and Wilson (83) recently called on psychological theory to generate tolerance for predator recoveries (for example, through increased signage to avoid dangers and increased education about the benefits of large carnivores). The dynamic nature of food webs and the dynamic social preferences for the definition of ecosystem health highlight the need to identify common and conflicting goals of restoration, define ecosystem and social goals, and develop policies that adapt to constantly changing social-ecological systems.
Supplementary Material
Acknowledgments
We thank M. Mangel, O. Shelton, D. McCauley, and two anonymous reviewers for constructive feedback. Funding: J.F.S. and A.C.S. were funded in part by the Ocean Tipping Points Grant 2897.01 from the Gordon and Betty Moore Foundation and R.D.H. by the University of Florida Foundation. Author contributions: A.C.S. and J.F.S. conceived and designed the study, M.N. and A.C.S. adapted and analyzed the theoretical model, E.J.W. conducted the literature search and synthesis, and K.N.M. contributed the case studies. A.C.S., J.F.S., and M.N. wrote the manuscript, and all authors contributed substantive revisions and conceptual advances. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/2/5/e1501769/DC1
fig. S1. Table of equilibrium solutions for three-species Lotka-Volterra model of IGP for the basal resource (R), the mesopredator (N), and the apex predator (P).
fig. S2. Response of the equilibrium densities of the apex predator (P) and mesopredator (N) to increases in the resource’s carrying capacity (K) for stable equilibria in which all three species coexist (RNP), only the resource and mesopredator coexist (RN), and only the resource and the apex predator coexist (RP).
fig. S3. Stable (solid line) and unstable (dashed line) equilibrium densities for the apex predator (P) and mesopredator (N) across a range of the apex predator’s prey preferences.
REFERENCES AND NOTES
- 1.Sergio F., Schmitz O. J., Krebs C. J., Holt R. D., Heithaus M. R., Wirsing A. J., Ripple W. J., Ritchie E., Ainley D., Oro D., Jhala Y., Hiraldo F., Korpimäki E., Towards a cohesive, holistic view of top predation: A definition, synthesis and perspective. Oikos 123, 1234–1243 (2014). [Google Scholar]
- 2.Yodzis P., Must top predators be culled for the sake of fisheries? Trends Ecol. Evol. 16, 78–84 (2001). [DOI] [PubMed] [Google Scholar]
- 3.Estes J. A., Terborgh J., Brashares J. S., Power M. E., Berger J., Bond W. J., Carpenter S. R., Essington T. E., Holt R. D., Jackson J. B. C., Marquis R. J., Oksanen L., Oksanen T., Paine R. T., Pikitch E. K., Ripple W. J., Sandin S. A., Scheffer M., Schoener T. W., Shurin J. B., Sinclair A. R. E., Soulé M. E., Virtanen R., Wardle D. A., Trophic downgrading of planet Earth. Science 333, 301–306 (2011). [DOI] [PubMed] [Google Scholar]
- 4.Ripple W. J., Newsome T. M., Wolf C., Dirzo R., Everatt K. T., Galetti M., Hayward M. W., Kerley G. I. H., Levi T., Lindsey P. A., Macdonald D. W., Malhi Y., Painter L. E., Sandom C. J., Terborgh J., Van Valkenburgh B., Collapse of the world’s largest herbivores. Sci. Adv. 1, e1400103 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soulé M. E., Estes J. A., Berger J., del Rio C. M., Ecological effetiveness: Conservation goals for interactive species. Conserv. Biol. 17, 1238–1250 (2003). [Google Scholar]
- 6.Berger K. M., Gese E. M., Berger J., Indirect effects and traditional trophic cascades: A test involving wolves, coyotes, and pronghorn. Ecology 89, 818–828 (2008). [DOI] [PubMed] [Google Scholar]
- 7.P. F. Olesiuk, An Assessment of Population Trends and Abundance of Harbour Seals (Phoca vitulina) in British Columbia (Fisheries and Oceans Canada, Ottawa, 2010). [Google Scholar]
- 8.Kelly M. J., Laurenson M. K., FitzGibbon C. D., Collins D. A., Durant S. M., Frame G. W., Bertram B. C., Caro T. M., Demography of the Serengeti cheetah (Acinonyx jubatus) population: The first 25 years. J. Zool. 244, 473–488 (1998). [Google Scholar]
- 9.Marshall K. N., Hobbs N. T., Cooper D. J., Stream hydrology limits recovery of riparian ecosystems after wolf reintroduction. Proc. Biol. Sci. 280, 20122977 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lotze H. K., Coll M., Magera A. M., Ward-Paige C., Airoldi L., Recovery of marine animal populations and ecosystems. Trends Ecol. Evol. 26, 595–605 (2011). [DOI] [PubMed] [Google Scholar]
- 11.Lotze H. K., Worm B., Historical baselines for large marine animals. Trends Ecol. Evol. 24, 254–262 (2009). [DOI] [PubMed] [Google Scholar]
- 12.Purvis A., Gittleman J. L., Cowlishaw G., Mace G. M., Predicting extinction risk in declining species. Proc. Biol. Sci. 267, 1947–1952 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cardillo M., Biological determinants of extinction risk: Why are smaller species less vulnerable? Anim. Conserv. 6, 63–69 (2003). [Google Scholar]
- 14.Wilbur H. M., Tinkle D. W., Collins J. P., Environmental certainty, trophic level, and resource availability in life history evolution. Am. Nat. 108, 805–817 (1974). [Google Scholar]
- 15.Pimm S. L., Jones H. L., Diamond J., On the risk of extinction. Am. Nat. 132, 757–785 (1988). [Google Scholar]
- 16.Griffith B., Scott J. M., Carpenter J. W., Reed C., Translocation as a species conservation tool: Status and strategy. Science 245, 477–480 (1989). [DOI] [PubMed] [Google Scholar]
- 17.Neubauer P., Jensen O. P., Hutchings J. A., Baum J. K., Resilience and recovery of overexploited marine populations. Science 340, 347–349 (2013). [DOI] [PubMed] [Google Scholar]
- 18.Duffy J. E., Biodiversity loss, trophic skew, and ecosystem functioning. Ecol. Lett. 6, 680–687 (2003). [Google Scholar]
- 19.Hall R. J., Milner-Gulland E. J., Courchamp F., Endangering the endangered: The effects of perceived rarity on species exploitation. Conserv. Lett. 1, 75–81 (2008). [Google Scholar]
- 20.Graham-Rowe D., Biodiversity: Endangered and in demand. Nature 480, S101–S103 (2011). [DOI] [PubMed] [Google Scholar]
- 21.Holdo R. M., Galvin K. A., Knapp E., Polasky S., Hilborn R., Holt R. D., Responses to alternative rainfall regimes and antipoaching in a migratory system. Ecol. Appl. 20, 381–397 (2010). [DOI] [PubMed] [Google Scholar]
- 22.Bergstrom B. J., Arias L. C., Davidson A. D., Ferguson A. W., Randa L. A., Sheffield S. R., License to kill: Reforming federal wildlife control to restore biodiversity and ecosystem function. Conserv. Lett. 7, 131–142 (2014). [Google Scholar]
- 23.S. Harrad, Persistent Organic Pollutants (Wiley Online Library, Chichester, 2010). [Google Scholar]
- 24.W. J. Richardson, C. R. Greene Jr., C. I. Malme, D. H. Thomson, Marine Mammals and Noise (Academic Press, San Diego, CA, 1995). [Google Scholar]
- 25.Johnson W. E., Onorato D. P., Roelke M. E., Land E. D., Cunningham M., Belden R. C., McBride R., Jansen D., Lotz M., Shindle D., Howard J., Wildt D. E., Penfold L. M., Hostetler J. A., Oli M. K., O’Brien S. J., Genetic restoration of the Florida panther. Science 329, 1641–1645 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Young T. P., Petersen D. A., Clary J. J., The ecology of restoration: Historical links, emerging issues and unexplored realms. Ecol. Lett. 8, 662–673 (2005). [Google Scholar]
- 27.L. R. Walker, J. Walker, R. J. Hobbs, Linking Restoration and Ecological Succession (Springer, New York, 2007). [Google Scholar]
- 28.Stier A. C., Osenberg C. W., Propagule redirection: Habitat availability reduces colonization and increases recruitment in reef fishes. Ecology 91, 2826–2832 (2010). [DOI] [PubMed] [Google Scholar]
- 29.B. Fry, Stable Isotope Ecology (Springer, New York, 2006), vol. 521. [Google Scholar]
- 30.Magera A. M., Mills Flemming J. E., Kaschner K., Christensen L. B., Lotze H. K., Recovery trends in marine mammal populations. PLOS One 8, e77908 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holt R. D., Lawton J. H., Polis G. A., Martinez N. D., Trophic rank and the species-area relationship. Ecology 80, 1495–1504 (1999). [Google Scholar]
- 32.Carbone C., Gittleman J. L., A common rule for the scaling of carnivore density. Science 295, 2273–2276 (2002). [DOI] [PubMed] [Google Scholar]
- 33.Hatton I. A., McCann K. S., Fryxell J. M., Davies T. J., Smerlak M., Sinclair A. R. E., Loreau M., The predator-prey power law: Biomass scaling across terrestrial and aquatic biomes. Science 349, aac6284 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Cury P. M., Boyd I. L., Bonhommeau S., Anker-Nilssen T., Crawford R. J. M., Furness R. W., Mills J. A., Murphy E. J., Österblom H., Paleczny M., Piatt J. F., Roux J.-P., Shannon L., Sydeman W. J., Global seabird response to forage fish depletion—One-third for the birds. Science 334, 1703–1706 (2011). [DOI] [PubMed] [Google Scholar]
- 35.Samhouri J. F., Levin P. S., Ainsworth C. H., Identifying thresholds for ecosystem-based management. PLOS One 5, e8907 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crooks K. R., Soulé M. E., Mesopredator release and avifaunal extinctions in a fragmented system. Nature 400, 563–566 (1999). [Google Scholar]
- 37.M. W. Hayward, M. Somers, Reintroduction of Top-Order Predators (John Wiley and Sons, New York, 2009). [Google Scholar]
- 38.Terborgh J., Lopez L., Nuñez P., Rao M., Shahabuddin G., Orihuela G., Riveros M., Ascanio R., Adler G. H., Lambert T. D., Balbas L., Ecological meltdown in predator-free forest fragments. Science 294, 1923–1926 (2001). [DOI] [PubMed] [Google Scholar]
- 39.Hein A. M., Gillooly J. F., Predators, prey, and transient states in the assembly of spatially structured communities. Ecology 92, 549–555 (2011). [DOI] [PubMed] [Google Scholar]
- 40.Riley S. P. D., Serieys L. E. K., Pollinger J. P., Sikich J. A., Dalbeck L., Wayne R. K., Ernest H. B., Individual behaviors dominate the dynamics of an urban mountain lion population isolated by roads. Curr. Biol. 24, 1989–1994 (2014). [DOI] [PubMed] [Google Scholar]
- 41.Murdoch W. W., Kendall B. E., Nisbet R. M., Briggs C. J., McCauley E., Bosler R., Single-species models for many-species food webs. Nature 417, 541–543 (2002). [DOI] [PubMed] [Google Scholar]
- 42.Cohen J. E., Pimm S. L., Yodzis P., Saldaña J., Body sizes of animal predators and animal prey in food webs. J. Anim. Ecol. 62, 67–78 (1993). [Google Scholar]
- 43.Petitgas P., Secor D. H., McQuinn I., Huse G., Lo N., Stock collapses and their recovery: Mechanisms that establish and maintain life-cycle closure in space and time. ICES J. Mar. Sci. 67, 1841–1848 (2010). [Google Scholar]
- 44.Taylor S. K., Buergelt C. D., Roelke-Parker M. E., Homer B. L., Rotstein D. S., Causes of mortality of free-ranging Florida panthers. J. Wildlife Dis. 38, 107–114 (2002). [DOI] [PubMed] [Google Scholar]
- 45.Creel S., Creel N. M., Communal hunting and pack size in African wild dogs, Lycaon pictus. Anim. Behav. 50, 1325–1339 (1995). [Google Scholar]
- 46.Wootton J. T., Emmerson M., Measurement of interaction strength in nature. Annu. Rev. Ecol. Evol. Syst., 419–444 (2005). [Google Scholar]
- 47.R. D. Holt, in Multitrophic Interactions in Terrestrial Systems, A. C. Gange, V. K. Brown, Eds. (Blackwell, Oxford, 1997), pp. 333–350. [Google Scholar]
- 48.Persson L., Amundsen P.-A., De Roos A. M., Klemetsen A., Knudsen R., Primicerio R., Culling prey promotes predator recovery—Alternative states in a whole-lake experiment. Science 316, 1743–1746 (2007). [DOI] [PubMed] [Google Scholar]
- 49.Gerber L. R., Morissette L., Kaschner K., Pauly D., Should whales be culled to increase fishery yield. Science 323, 880–881 (2009). [DOI] [PubMed] [Google Scholar]
- 50.Yodzis P., Local trophodynamics and the interaction of marine mammals and fisheries in the Benguela ecosystem. J. Anim. Ecol. 67, 635–658 (1998). [Google Scholar]
- 51.Schröder A., van Leeuwen A., Cameron T. C., When less is more: Positive population-level effects of mortality. Trends Ecol. Evol. 29, 614–624 (2014). [DOI] [PubMed] [Google Scholar]
- 52.Chase J. M., Food web effects of prey size refugia: Variable interactions and alternative stable equilibria. Am. Nat. 154, 559–570 (1999). [DOI] [PubMed] [Google Scholar]
- 53.De Roos A. M., Persson L., Size-dependent life-history traits promote catastrophic collapses of top predators. Proc. Natl. Acad. Sci. U.S.A. 99, 12907–12912 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nishijima S., Takimoto G., Miyashita T., Roles of alternative prey for mesopredators on trophic cascades in intraguild predation systems: A theoretical perspective. Am. Nat. 183, 625–637 (2014). [DOI] [PubMed] [Google Scholar]
- 55.Yodzis P., The indeterminacy of ecological interactions as perceived through perturbation experiments. Ecology 69, 508–515 (1988). [Google Scholar]
- 56.Novak M., Trophic omnivory across a productivity gradient: Intraguild predation theory and the structure and strength of species interactions. Proc. Biol. Sci. 280, 20131415 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walters C. J., Hilborn R., Ecological optimization and adaptive management. Annu. Rev. Ecol. Syst. 9, 157–188 (1978). [Google Scholar]
- 58.Yelenik S. G., C. M. D’Antonio, Self-reinforcing impacts of plant invasions change over time. Nature 503, 517–520 (2013). [DOI] [PubMed] [Google Scholar]
- 59.Ruckelshaus M. H., Levin P., Johnson J. B., Kareiva P. M., The Pacific salmon wars: What science brings to the challenge of recovering species. Annu. Rev. Ecol. Syst. 33, 665–706 (2002). [Google Scholar]
- 60.Shulman M. J., Ogden J. C., Ebersole J. P., McFarland W. N., Miller S. L., Wolf N. G., Priority effects in the recruitment of juvenile coral-reef fishes. Ecology 64, 1508–1513 (1983). [Google Scholar]
- 61.Chase J. M., Community assembly: When should history matter? Oecologia 136, 489–498 (2003). [DOI] [PubMed] [Google Scholar]
- 62.Chase J. M., Biro E. G., Ryberg W. A., Smith K. G., Predators temper the relative importance of stochastic processes in the assembly of prey metacommunities. Ecol. Lett. 12, 1210–1218 (2009). [DOI] [PubMed] [Google Scholar]
- 63.McCann K., Hastings A., Re–evaluating the omnivory–stability relationship in food webs. Proc. Biol. Sci. 264, 1249–1254 (1997). [Google Scholar]
- 64.Sinclair A. R. E., Pech R. P., Dickman C. R., Hik D., Mahon P., Newsome A. E., Predicting effects of predation on conservation of endangered prey. Conserv. Biol. 12, 564–575 (1998). [Google Scholar]
- 65.Holt R. D., Polis G. A., A theoretical framework for intraguild predation. Am. Nat., 745–764 (1997). [Google Scholar]
- 66.Olito C., Fukami T., Long-term effects of predator arrival timing on prey community succession. Am. Nat. 173, 354–362 (2009). [DOI] [PubMed] [Google Scholar]
- 67.J. A. Musick, Life in the Slow Lane: Ecology and Conservation of Long-Lived Marine Animals (American Fisheries Society, Bethesda, MD, 1999), vol. 23. [Google Scholar]
- 68.Stier A. C., Samhouri J. F., Gray S., Martone R. G., Mach M. E., Halpern B. S., Kappel C. V., Scarboroughand C., Levin P. S., Integrating expert perceptions into food web conservation and management. Conserv. Lett., in press. [Google Scholar]
- 69.Scheffer M., Carpenter S. R., Lenton T. M., Bascompte J., Brock W., Dakos V., van de Koppel J., van de Leemput I. A., Levin S. A., van Nes E. H., Pascual M., Vandermeer J., Anticipating critical transitions. Science 338, 344–348 (2012). [DOI] [PubMed] [Google Scholar]
- 70.Suding K. N., Gross K. L., Houseman G. R., Alternative states and positive feedbacks in restoration ecology. Trends Ecol. Evol. 19, 46–53 (2004). [DOI] [PubMed] [Google Scholar]
- 71.Holling C. S., Resilience and stability of ecological systems. Annu. Rev. Ecol. Syst. 1–23 (1973). [Google Scholar]
- 72.May R. M., Simple mathematical models with very complicated dynamics. Nature 261, 459–467 (1976). [DOI] [PubMed] [Google Scholar]
- 73.Walters C., Kitchell J. F., Cultivation/depensation effects on juvenile survival and recruitment: Implications for the theory of fishing. Can. J. Fish. Aquat. Sci. 58, 39–50 (2001). [Google Scholar]
- 74.Collie J., Rochet M.-J., Bell R., Rebuilding fish communities: The ghost of fisheries past and the virtue of patience. Ecol. Appl. 23, 374–391 (2013). [DOI] [PubMed] [Google Scholar]
- 75.Balme G. A., Lindsey P. A., Swanepoel L. H., Hunter L. T. B., Failure of research to address the rangewide conservation needs of large carnivores: Leopards in South Africa as a case study. Conserv. Lett. 7, 3–11 (2014). [Google Scholar]
- 76.Stier A. C., Geange S. W., Hanson K. M., Bolker B. M., Predator density and timing of arrival affect reef fish community assembly. Ecology 94, 1057–1068 (2013). [DOI] [PubMed] [Google Scholar]
- 77.Price J. E., Morin P. J., Colonization history determines alternate community states in a food web of intraguild predators. Ecology 85, 1017–1028 (2004). [Google Scholar]
- 78.Ripple W. J., Estes J. A., Beschta R. L., Wilmers C. C., Ritchie E. G., Hebblewhite M., Berger J., Elmhagen B., Letnic M., Nelson M. P., Schmitz O. J., Smith D. W., Wallach A. D., Wirsing A. J., Status and ecological effects of the world’s largest carnivores. Science 343, (2014). [DOI] [PubMed] [Google Scholar]
- 79.Ritchie E. G., Elmhagen B., Glen A. S., Letnic M., Ludwig G., McDonald R. A., Ecosystem restoration with teeth: What role for predators? Trends Ecol. Evol. 27, 265–271 (2012). [DOI] [PubMed] [Google Scholar]
- 80.Hirsch P. D., Adams W. M., Brosius J. P., Zia A., Bariola N., Dammert J. L., Acknowledging conservation trade-offs and embracing complexity. Conserv. Biol. 25, 259–264 (2011). [DOI] [PubMed] [Google Scholar]
- 81.Nogués-Bravo D., Simberloff D., Rahbek C., Sanders N. J., Rewilding is the new Pandora’s box in conservation. Curr. Biol. 26, R87–R91 (2016). [DOI] [PubMed] [Google Scholar]
- 82.Can Ö. E., N. D’Cruze, Garshelis D. L., Beecham J., Macdonald D. W., Resolving human-bear conflict: A global survey of countries, experts, and key factors. Conserv. Lett. 7, 501–513 (2014). [Google Scholar]
- 83.Bruskotter J. T., Wilson R. S., Determining where the wild things will be: Using psychological theory to find tolerance for large carnivores. Conserv. Lett. 7, 158–165 (2014). [Google Scholar]
- 84.Polis G. A., Holt R. D., Intraguild predation: The dynamics of complex trophic interactions. Trends Ecol. Evol. 7, 151–154 (1992). [DOI] [PubMed] [Google Scholar]
- 85.Berger J., Undetected species losses, food webs, and ecological baselines: A cautionary tale from the Greater Yellowstone Ecosystem, USA. Oryx 42, 139–142 (2008). [Google Scholar]
- 86.Elmhagen B., Rushton S. P., Trophic control of mesopredators in terrestrial ecosystems: Top-down or bottom-up? Ecol. Lett. 10, 197–206 (2007). [DOI] [PubMed] [Google Scholar]
- 87.Rayner M. J., Hauber M. E., Clout M. N., Breeding habitat of the Cook’s Petrel (Pterodoma cookii) on Little Barrier Island (Hauturu): Implications for the conservation of a New Zealand endemic. Emu 107, 59–68 (2007). [Google Scholar]
- 88.J. C. Devos, T. McKinney, Recent trends in North American mountain lion populations: A hypothesis, in Colorado Plateau II: Biophysical, Socioeconomic, and Cultural Research, C. van Riper III, D. J. Mattson, Eds. (University of Arizona Press, Tucson, AZ, 2005), pp. 297–307. [Google Scholar]
- 89.Peacock E., Derocher A. E., Thiemann G. W., Stirling I., Conservation and management of Canada’s polar bears (Ursus maritimus) in a changing Arctic. Can. J. Zool. 89, 371–385 (2011). [Google Scholar]
- 90.Mittelbach G. G., Turner A. M., Hall D. J., Rettig J. E., Osenberg C. W., Perturbation and resilience: A long-term, whole-lake study of predator extinction and reintroduction. Ecology 76, 2347–2360 (1995). [Google Scholar]
- 91.C. R. Dickman, A. S. Glen, M. Letnic, in Reintroduction of Top-Order Predators, M. W. Hayward, M. J. Somers, Eds. (Wiley-Blackwell, Oxford, 2009). [Google Scholar]
- 92.Wootton J. T., Bell D. A., A metapopulation model of the Peregrine Falcon in California: Viability and management strategies. Ecol. Appl. 2, 307–321 (1992). [DOI] [PubMed] [Google Scholar]
- 93.Swenson J. E., Wabakken P., Sandegren F., Söderberg A., The near extinction and recovery of brown bears in Scandinavia in relation to the bear management policies of Norway and Sweden. Wildlife Biol. 1, 11–25 (1995). [Google Scholar]
- 94.Hipfner J. M., Blight L. K., Lowe R. W., Wilhelm S. I., Robertson G. J., Barrett R. T., Unintended consequences: How the recovery of sea eagle Haliaeetus spp. populations in the northern hemisphere is affecting seabirds. Marine Ornithol. 40, 39–52 (2012). [Google Scholar]
- 95.Singh H. S., Gibson L., A conservation success story in the otherwise dire megafauna extinction crisis: The Asiatic lion (Panthera leo persica) of Gir forest. Biol. Conserv. 144, 1753–1757 (2011). [Google Scholar]
- 96.Abbitt R. J. F., Scott J. M., Examining differences between recovered and declining endangered species. Conserv. Biol. 15, 1274–1284 (2001). [Google Scholar]
- 97.Platt S. G., Thorbjarnarson J. B., Population status and conservation of Morelet’s crocodile, Crocodylus moreletii, in northern Belize. Biol. Conserv. 96, 21–29 (2000). [Google Scholar]
- 98.Fukuda Y., Webb G., Manolis C., Delaney R., Letnic M., Lindner G., Whitehead P., Recovery of saltwater crocodiles following unregulated hunting in tidal rivers of the northern territory, Australia. J. Wildlife Manage. 75, 1253–1266 (2011). [Google Scholar]
- 99.Kirkman S. P., Yemane D., Oosthuizen W. H., Meÿer M. A., Kotze P. G. H., Skrypzeck H., Vaz Velho F., Underhill L. G., Spatio-temporal shifts of the dynamic Cape fur seal population in southern Africa, based on aerial censuses (1972–2009). Mar. Mammal Sci. 29, 497–524 (2013). [Google Scholar]
- 100.R. S. Collier, Suggested protocol for the scientific investigation of shark attacks, in Field Guide to the Great White Shark (Reef Quest Centre for Shark Research, Vancouver, 2003). [Google Scholar]
- 101.Monahan C. C., Branch T. A., Punt A. E., Do ship strikes threaten the recovery of endangered eastern North Pacific blue whales? Mar. Mammal Sci. 31, 279–297 (2015). [Google Scholar]
- 102.Lubina J. A., Levin S. A., The spread of a reinvading species: Range expansion in the California sea otter. Am. Nat. 131, 526–543 (1988). [Google Scholar]
- 103.Kelly S., Scott D., MacDiarmid A. B., Babcock R. C., Spiny lobster, Jasus edwardsii, recovery in New Zealand marine reserves. Biol. Conserv. 92, 359–369 (2000). [Google Scholar]
- 104.Keller A. C., Gerber L. R., Monitoring and the Endangered Species Act: Revisiting the Eastern North Pacific Gray Whale. Endangered Species Update 20, 87–92 (2004). [Google Scholar]
- 105.Marino J., Sillero-Zubiri C., Macdonald D. W., Trends, dynamics and resilience of an Ethiopian wolf population. Anim. Conserv. 9, 49–58 (2006). [Google Scholar]
- 106.Boveng P. L., Hiruki L. M., Schwartz M. K., Bengtson J. L., Population growth of Antarctic fur seals: Limitation by a top predator, the leopard seal? Ecology 79, 2863–2877 (1998). [Google Scholar]
- 107.Estes J. A., Tinker M. T., Williams T. M., Doak D. F., Killer whale predation on sea otters linking oceanic and nearshore ecosystems. Science 282, 473–476 (1998). [DOI] [PubMed] [Google Scholar]
- 108.Fedriani J. M., Fuller T. K., Sauvajot R. M., York E. C., Competition and intraguild predation among three sympatric carnivores. Oecologia 125, 258–270 (2000). [DOI] [PubMed] [Google Scholar]
- 109.Swain D. P., Mohn R. K., Forage fish and the factors governing recovery of Atlantic cod (Gadus morhua) on the eastern Scotian Shelf. Can. J. Fish. Aquat. Sci. 69, 997–1001 (2012). [Google Scholar]
- 110.Ernest H. B., Boyce W. M., Bleich V. C., May B., Stiver S. J., Torres S. G., Genetic structure of mountain lion (Puma concolor) populations in California. Conserv. Genet. 4, 353–366 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wayne R., Hedrick P., Genetics and wolf conservation in the American West: Lessons and challenges. Heredity 107, 16–19 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Moore S. E., DeMaster D. P., Cook Inlet Belugas, Delphinapterus leucas: Status and overview. Mar. Fish. Rev. 62, 1–5 (2000). [Google Scholar]
- 113.R. Hilborn, S. P. Cox, F. M. D. Gulland, D. G. Hankin, N. T. Hobbs, D. E. Schindler, A. W. Trites, The Effects of Salmon Fisheries on Southern Resident Killer Whales: Final Report of the Independent Science Panel, prepared with the assistance of D. R. Marmorek and A. W. Hall (ESSA Technologies Ltd., Vancouver, British Columbia, Canada) for National Marine Fisheries Service (Seattle, WA) and Fisheries and Oceans Canada (Vancouver, British Columbia, Canada), xv + 61 pp. + Appendices (2012). [Google Scholar]
- 114.Rojas-Bracho L., Reeves R. R., Jaramillo-Legorreta A., Conservation of the vaquita Phocoena sinus. Mammal Rev. 36, 179–216 (2006). [Google Scholar]
- 115.Revised Recovery Plan for the Northern Spotted Owl (Strix occidentalis caurina) (U.S. Fish and Wildlife Service, Portland, OR, 2011). [Google Scholar]
- 116.Dove C. J., Snow R. W., Rochford M. R., Mazzotti F. J., Birds consumed by the Invasive Burmese Python (Python Molurus Bivittatus) in Everglades National Park, Florida, USA. Wilson J. Ornithol. 123, 126–131 (2011). [Google Scholar]
- 117.Ruzycki J. R., Beauchamp D. A., Yule D. L., Effects of introduced lake trout on native cutthroat trout in yellowstone lake. Ecol. Appl. 13, 23–37 (2003). [Google Scholar]
- 118.Diehl S., Feißel M., Effects of enrichment on three-level food chains with omnivory. Am. Nat. 155, 200–218 (2000). [DOI] [PubMed] [Google Scholar]
- 119.McCann K., Hastings A., Huxel G. R., Weak trophic interactions and the balance of nature. Nature 395, 794–798 (1998). [Google Scholar]
- 120.Chase J. M., Leibold M. A., Downing A. L., Shurin J. B., The effects of productivity, herbivory, and plant species turnover in grassland food webs. Ecology 81, 2485–2497 (2000). [Google Scholar]
- 121.Oksanen L., Fretwell S. D., Arruda J., Niemela P., Exploitation ecosystems in gradients of primary productivity. Am. Nat. 118, 240–261 (1981). [Google Scholar]
- 122.Lotka A., Fluctuations in the abundance of a species considered mathematically (with comment by V. Volterra). Nature 119, 12–13 (1927). [Google Scholar]
- 123.Bascompte J., Melián C. J., Simple trophic modules for complex food webs. Ecology 86, 2868–2873 (2005). [Google Scholar]
- 124.Bascompte J., Melián C. J., Sala E., Interaction strength combinations and the overfishing of a marine food web. Proc. Natl. Acad. Sci. U.S.A. 102, 5443–5447 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kondoh M., Building trophic modules into a persistent food web. Proc. Natl. Acad. Sci. U.S.A. 105, 16631–16635 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Plagányi É. E., Punt A. E., Hillary R., Morello E. B., Thébaud O., Hutton T., Pillans R. D., Thorson J. T., Fulton E. A., Smith A. D. M., Smith F., Bayliss P., Haywood M., Lyne V., Rothlisberg P. C., Multispecies fisheries management and conservation: Tactical applications using models of intermediate complexity. Fish Fish. 15, 1–22 (2014). [Google Scholar]
- 127.Smith D. W., Peterson R. O., Houston D. B., Yellowstone after wolves. Bioscience 53, 330–340 (2003). [Google Scholar]
- 128.D. B. Houston, The Northern Yellowstone Elk: Ecology and Management (Macmillan, New York, 1982). [Google Scholar]
- 129.D. W. Smith, D. R. Stahler, E. Stahler, M. Metz, K. Quimby, R. McIntyre, C. Ruhl, H. Martin, R. Kindermann, N. Bowersock, M. McDevitt, Yellowstone Wolf Project: Annual Report, 2012 (National Park Service, Washington, DC, 2013). [Google Scholar]
- 130.Vucetich J. A., Smith D. W., Stahler D. R., Influence of harvest, climate and wolf predation on Yellowstone elk, 1961–2004. Oikos 111, 259–270 (2005). [Google Scholar]
- 131.Ripple W. J., Beschta R. L., Restoring Yellowstone’s aspen with wolves. Biol. Conserv. 138, 514–519 (2007). [Google Scholar]
- 132.Beschta R. L., Ripple W. J., Increased willow heights along northern Yellowstone’s Blacktail Deer Creek following wolf reintroduction. West. N. Am. Naturalist 67, 613–617 (2007). [Google Scholar]
- 133.Wolf E. C., Cooper D. J., Hobbs N. T., Hydrologic regime and herbivory stabilize an alternative state in yellowstone national park. Ecol. Appl. 17, 1572–1587 (2007). [DOI] [PubMed] [Google Scholar]
- 134.Bilyeu D. M., Cooper D. J., Hobbs N. T., Water tables constrain height recovery of willow on Yellowstone’s northern range. Ecol. Appl. 18, 80–92 (2008). [DOI] [PubMed] [Google Scholar]
- 135.B. W. Baker, E. P. Hill, in Wild Mammals of North America: Biology, Management, and Conservation, G. A. Feldhamer, B. C. Thompson, J. A. Chapman, Eds. (Johns Hopkins University Press, Baltimore, MD, 2003), pp. 288–310. [Google Scholar]
- 136.Westbrook C. J., Cooper D. J., Baker B. W., Beaver dams and overbank floods influence groundwater-surface water interactions of a Rocky Mountain riparian area. Water Resour. Res. 42, W06404 (2006). [Google Scholar]
- 137.Singer F. J., Mark L. C., Cates R. C., Ungulate herbivory of willows on Yellowstone northern winter range. J. Range Manage. 47, 435–443 (1994). [Google Scholar]
- 138.Singer F. J., Effects of grazing by ungulates on upland bunchgrass communities of the northern winter range of Yellowstone National Park. Northwest Sci. 69, 191–203 (1995). [Google Scholar]
- 139.Baker B. W., Ducharme H. C., Mitchell D. C. S., Stanley T. R., Peinetti H. R., Interaction of beaver and elk herbivory reduces standing crop of willow. Ecol. Appl. 15, 110–118 (2005). [Google Scholar]
- 140.E. E. Bangs, J. A. Fontaine, M. D. Jimenez, T. J. Meier, E. H. Bradley, C. C. Niemeyer, D. W. Smith, C. M. Mack, V. Asher, J. K. Oakleaf, Managing wolf-human conflict in the Northwestern United States, in People and Wildlife: Conflict or Coexistence? R. Woodroffe, S. Thirgood, A. Rabinowitz, Eds. (Cambridge Univ. Press, Cambridge, 2005), vol. 9, pp. 340–356. [Google Scholar]
- 141.Graham K., Beckerman A. P., Thirgood S., Human–predator–prey conflicts: Ecological correlates, prey losses and patterns of management. Biol. Conserv. 122, 159–171 (2005). [Google Scholar]
- 142.Marshall K. N., Stier A. C., Samhouri J. F., Kelly R. P., Ward E. J., Conservation challenges of predator recovery. Conserv. Lett. 9, 70–78 (2016). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/2/5/e1501769/DC1
fig. S1. Table of equilibrium solutions for three-species Lotka-Volterra model of IGP for the basal resource (R), the mesopredator (N), and the apex predator (P).
fig. S2. Response of the equilibrium densities of the apex predator (P) and mesopredator (N) to increases in the resource’s carrying capacity (K) for stable equilibria in which all three species coexist (RNP), only the resource and mesopredator coexist (RN), and only the resource and the apex predator coexist (RP).
fig. S3. Stable (solid line) and unstable (dashed line) equilibrium densities for the apex predator (P) and mesopredator (N) across a range of the apex predator’s prey preferences.