Abstract
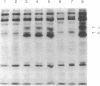
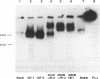
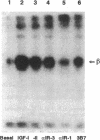
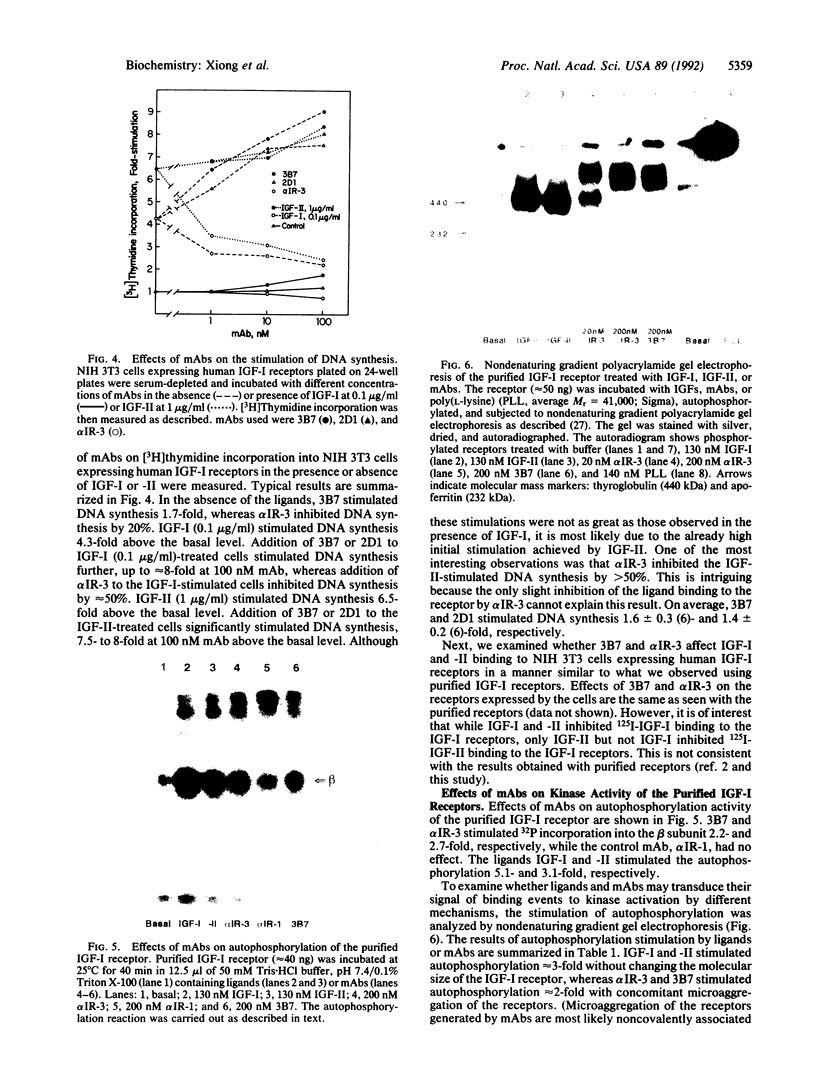
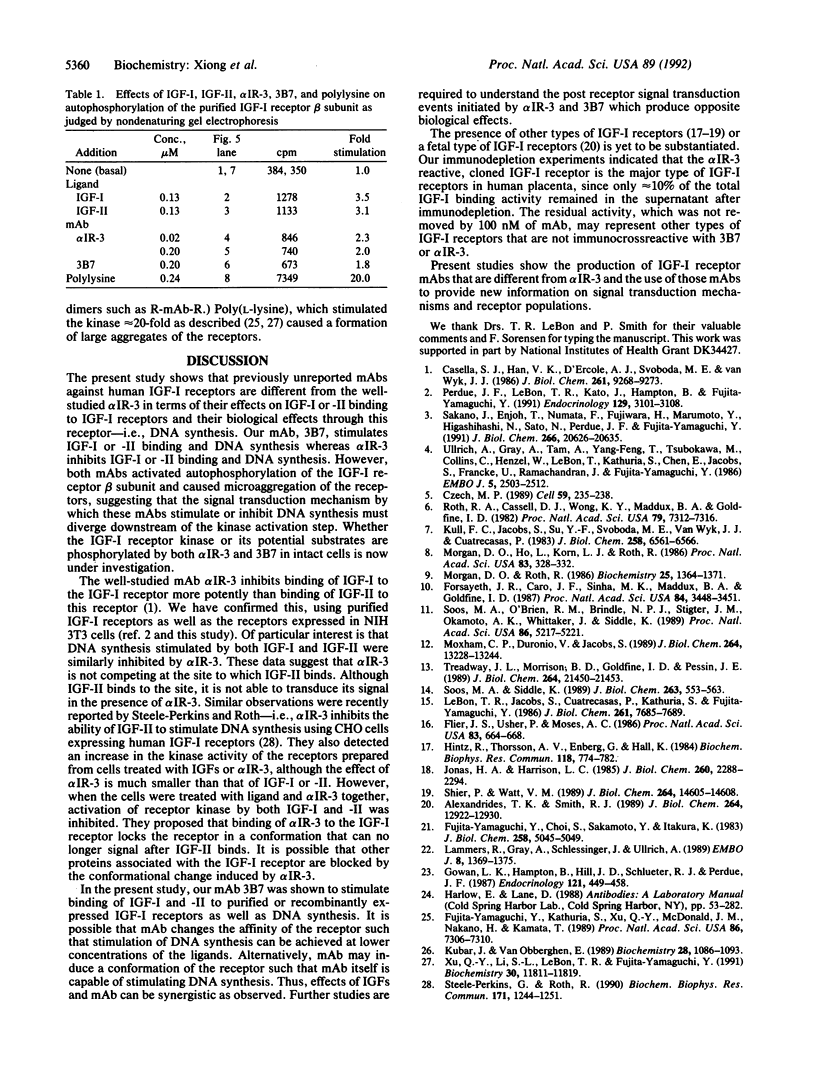
Monoclonal antibodies (mAbs) against purified human placental insulin-like growth factor I (IGF-I) receptors were prepared and characterized. Three IgG mAbs were specific for the human IGF-I receptor and displayed negligible crossreactivity with the human insulin receptor. They stimulated 125I-labeled IGF-I (125I-IGF-I) or 125I-IGF-II binding to purified human placental IGF-I receptors and to IGF-I receptors expressed in NIH 3T3 cells in contrast to the well-studied mAb alpha IR-3, which inhibits 125I-IGF-I or 125I-IGF-II binding to both forms of IGF-I receptors. The mAbs introduced in this study stimulated DNA synthesis in NIH 3T3 cells expressing human IGF-I receptors approximately 1.5-fold above the basal level and the IGF-I- or IGF-II-stimulated level. In contrast, alpha IR-3 inhibited both basal and IGF-I or IGF-II-stimulated DNA synthesis by approximately 30%. Inhibition of IGF-II-stimulated DNA synthesis by alpha IR-3 was as potent as its inhibition of IGF-I-stimulated DNA synthesis, although IGF-II binding to the IGF-I receptors was not inhibited by IGF-II as potently as was IGF-I. With the purified IGF-I receptors, both inhibitory and stimulatory mAbs were shown to activate autophosphorylation of the IGF-I receptor beta subunit and to induce microaggregation of the receptors. These results suggest that conformational changes resulting from receptor dimerization in the presence of either type of mAb may affect the signal-transducing function of the IGF-I receptor differently. These additional mAbs and alpha IR-3 immunoprecipitated nearly 90% of IGF-I binding activity from Triton X-100-solubilized human placental membranes, indicating that IGF-I receptor reactive with these mAbs is the major form of the IGF-I receptor in human placenta.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexandrides T. K., Smith R. J. A novel fetal insulin-like growth factor (IGF) I receptor. Mechanism for increased IGF I- and insulin-stimulated tyrosine kinase activity in fetal muscle. J Biol Chem. 1989 Aug 5;264(22):12922–12930. [PubMed] [Google Scholar]
- Casella S. J., Han V. K., D'Ercole A. J., Svoboda M. E., Van Wyk J. J. Insulin-like growth factor II binding to the type I somatomedin receptor. Evidence for two high affinity binding sites. J Biol Chem. 1986 Jul 15;261(20):9268–9273. [PubMed] [Google Scholar]
- Czech M. P. Signal transmission by the insulin-like growth factors. Cell. 1989 Oct 20;59(2):235–238. doi: 10.1016/0092-8674(89)90281-x. [DOI] [PubMed] [Google Scholar]
- Flier J. S., Usher P., Moses A. C. Monoclonal antibody to the type I insulin-like growth factor (IGF-I) receptor blocks IGF-I receptor-mediated DNA synthesis: clarification of the mitogenic mechanisms of IGF-I and insulin in human skin fibroblasts. Proc Natl Acad Sci U S A. 1986 Feb;83(3):664–668. doi: 10.1073/pnas.83.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsayeth J. R., Caro J. F., Sinha M. K., Maddux B. A., Goldfine I. D. Monoclonal antibodies to the human insulin receptor that activate glucose transport but not insulin receptor kinase activity. Proc Natl Acad Sci U S A. 1987 May;84(10):3448–3451. doi: 10.1073/pnas.84.10.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita-Yamaguchi Y., Choi S., Sakamoto Y., Itakura K. Purification of insulin receptor with full binding activity. J Biol Chem. 1983 Apr 25;258(8):5045–5049. [PubMed] [Google Scholar]
- Fujita-Yamaguchi Y., Kathuria S., Xu Q. Y., McDonald J. M., Nakano H., Kamata T. In vitro tyrosine phosphorylation studies on RAS proteins and calmodulin suggest that polylysine-like basic peptides or domains may be involved in interactions between insulin receptor kinase and its substrate. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7306–7310. doi: 10.1073/pnas.86.19.7306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowan L. K., Hampton B., Hill D. J., Schlueter R. J., Perdue J. F. Purification and characterization of a unique high molecular weight form of insulin-like growth factor II. Endocrinology. 1987 Aug;121(2):449–458. doi: 10.1210/endo-121-2-449. [DOI] [PubMed] [Google Scholar]
- Hintz R. L., Thorsson A. V., Enberg G., Hall K. IGF-II binding on human lymphoid cells: demonstration of a common high affinity receptor for insulin like peptides. Biochem Biophys Res Commun. 1984 Feb 14;118(3):774–782. doi: 10.1016/0006-291x(84)91462-1. [DOI] [PubMed] [Google Scholar]
- Jonas H. A., Harrison L. C. The human placenta contains two distinct binding and immunoreactive species of insulin-like growth factor-I receptors. J Biol Chem. 1985 Feb 25;260(4):2288–2294. [PubMed] [Google Scholar]
- Kubar J., Van Obberghen E. Oligomeric states of the insulin receptor: binding and autophosphorylation properties. Biochemistry. 1989 Feb 7;28(3):1086–1093. doi: 10.1021/bi00429a024. [DOI] [PubMed] [Google Scholar]
- Kull F. C., Jr, Jacobs S., Su Y. F., Svoboda M. E., Van Wyk J. J., Cuatrecasas P. Monoclonal antibodies to receptors for insulin and somatomedin-C. J Biol Chem. 1983 May 25;258(10):6561–6566. [PubMed] [Google Scholar]
- Lammers R., Gray A., Schlessinger J., Ullrich A. Differential signalling potential of insulin- and IGF-1-receptor cytoplasmic domains. EMBO J. 1989 May;8(5):1369–1375. doi: 10.1002/j.1460-2075.1989.tb03517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBon T. R., Jacobs S., Cuatrecasas P., Kathuria S., Fujita-Yamaguchi Y. Purification of insulin-like growth factor I receptor from human placental membranes. J Biol Chem. 1986 Jun 15;261(17):7685–7689. [PubMed] [Google Scholar]
- Morgan D. O., Ho L., Korn L. J., Roth R. A. Insulin action is blocked by a monoclonal antibody that inhibits the insulin receptor kinase. Proc Natl Acad Sci U S A. 1986 Jan;83(2):328–332. doi: 10.1073/pnas.83.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. O., Roth R. A. Mapping surface structures of the human insulin receptor with monoclonal antibodies: localization of main immunogenic regions to the receptor kinase domain. Biochemistry. 1986 Mar 25;25(6):1364–1371. doi: 10.1021/bi00354a026. [DOI] [PubMed] [Google Scholar]
- Moxham C. P., Duronio V., Jacobs S. Insulin-like growth factor I receptor beta-subunit heterogeneity. Evidence for hybrid tetramers composed of insulin-like growth factor I and insulin receptor heterodimers. J Biol Chem. 1989 Aug 5;264(22):13238–13244. [PubMed] [Google Scholar]
- Perdue J. F., LeBon T. R., Kato J., Hampton B., Fujita-Yamaguchi Y. Binding specificities and transducing function of the different molecular weight forms of insulin-like growth factor-II (IGF-II) on IGF-I receptors. Endocrinology. 1991 Dec;129(6):3101–3108. doi: 10.1210/endo-129-6-3101. [DOI] [PubMed] [Google Scholar]
- Roth R. A., Cassell D. J., Wong K. Y., Maddux B. A., Goldfine I. D. Monoclonal antibodies to the human insulin receptor block insulin binding and inhibit insulin action. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7312–7316. doi: 10.1073/pnas.79.23.7312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakano K., Enjoh T., Numata F., Fujiwara H., Marumoto Y., Higashihashi N., Sato Y., Perdue J. F., Fujita-Yamaguchi Y. The design, expression, and characterization of human insulin-like growth factor II (IGF-II) mutants specific for either the IGF-II/cation-independent mannose 6-phosphate receptor or IGF-I receptor. J Biol Chem. 1991 Nov 5;266(31):20626–20635. [PubMed] [Google Scholar]
- Shier P., Watt V. M. Primary structure of a putative receptor for a ligand of the insulin family. J Biol Chem. 1989 Sep 5;264(25):14605–14608. [PubMed] [Google Scholar]
- Soos M. A., O'Brien R. M., Brindle N. P., Stigter J. M., Okamoto A. K., Whittaker J., Siddle K. Monoclonal antibodies to the insulin receptor mimic metabolic effects of insulin but do not stimulate receptor autophosphorylation in transfected NIH 3T3 fibroblasts. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5217–5221. doi: 10.1073/pnas.86.14.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soos M. A., Siddle K. Immunological relationships between receptors for insulin and insulin-like growth factor I. Evidence for structural heterogeneity of insulin-like growth factor I receptors involving hybrids with insulin receptors. Biochem J. 1989 Oct 15;263(2):553–563. doi: 10.1042/bj2630553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele-Perkins G., Roth R. A. Monoclonal antibody alpha IR-3 inhibits the ability of insulin-like growth factor II to stimulate a signal from the type I receptor without inhibiting its binding. Biochem Biophys Res Commun. 1990 Sep 28;171(3):1244–1251. doi: 10.1016/0006-291x(90)90819-9. [DOI] [PubMed] [Google Scholar]
- Treadway J. L., Morrison B. D., Goldfine I. D., Pessin J. E. Assembly of insulin/insulin-like growth factor-1 hybrid receptors in vitro. J Biol Chem. 1989 Dec 25;264(36):21450–21453. [PubMed] [Google Scholar]
- Ullrich A., Gray A., Tam A. W., Yang-Feng T., Tsubokawa M., Collins C., Henzel W., Le Bon T., Kathuria S., Chen E. Insulin-like growth factor I receptor primary structure: comparison with insulin receptor suggests structural determinants that define functional specificity. EMBO J. 1986 Oct;5(10):2503–2512. doi: 10.1002/j.1460-2075.1986.tb04528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q. Y., Li S. L., LeBon T. R., Fujita-Yamaguchi Y. Aggregation of IGF-I receptors or insulin receptors and activation of their kinase activity are simultaneously caused by the presence of polycations or K-ras basic peptides. Biochemistry. 1991 Dec 24;30(51):11811–11819. doi: 10.1021/bi00115a011. [DOI] [PubMed] [Google Scholar]