Abstract
In a number of mammals muscle dilator nasi (naris) is known as a muscle that reduces nasal airflow resistance by dilating the nostrils. Here we show that in rats the tendon of this muscle inserts into the aponeurosis above the nasal cartilage. Electrical stimulation of this muscle lifts the nose and deflects it sideway towards the side of stimulation, but does not change the size of the nares. In the head-fixed alert rat, electromyographic activity of muscle dilator nasi is tightly coupled to nose motion, not to opening of the nares. Yet, contraction of muscle dilator nasi occurs during the pre-inspiratory phase of the respiratory cycle, suggesting a role in sniffing and sampling odorants. We also show that opening of the nares results from contraction of pars maxillaris profunda of the muscle nasolabialis profundus. This muscle attaches to the outer wall of the nasal cartilage and to the plate of the mystacial pad. Contraction of this muscle exerts a dual action: it pulls the lateral nasal cartilage outwardly, thus dilating the naris, and it drags the plate of the mystacial pad rostralward, provoking a slight retraction of the whiskers. On the basis of these results, we propose that muscle dilator nasi of the rat be renamed muscle deflector nasi, and that pars maxillaris profunda of the muscle nasolabialis profundus be named muscle dilator nasi.
Keywords: facial muscles, dilator nasi, respiration, upper airway, nose movement
Introduction
The transport of odorants to the olfactory epithelium critically depends on nasal airflow, which is driven by the negative pressure generated in the lungs by the respiratory pump muscles (diaphragm, intercostal and abdominal muscles). Nasal airflow is also controlled by the size of the nares, which accounts for over 50% of upper airway flow resistance (Haight and Cole, 1983). In most anatomical studies, muscle (M.) dilator nasi is described as a muscle that dilates the nostrils (Klingener, 1964; Meinertz, 1942; Priddy and Broie, 1948; Rinker, 1954; Ryan, 1989; Whidden, 2002). This muscle has also been referred to as M. dilatator naris (Greene, 1935; Diogo, 2009).
M. dilator nasi takes origin at the orbital edge of the maxilla, and the insertion site of its long tendon varies in different species (see Table 1). In hamsters, it is the nostril (Priddy and Brodie, 1948); in cricetinae and suidea, the rhinarium (Herring, 1972; Rinker, 1954); in sciurus vulgaris, the skin at the tip of the nose (Meinertz, 1942); in rodents, the cartilages of the nose (Klingener, 1964; Ryan, 1989); and in insectivora, the aponeurosis over the nasal cartilages (Whidden, 2002). On the basis of these anatomical data, it is unclear whether M. dilator nasi actually controls nose motion or the shape and size of the nostrils, or both. In the present study we re-examined the anatomical features of M. dilator nasi in rats, and assessed its function by electrical stimulation, electromyographic (EMG) and videographic recordings.
Table 1.
Origin and insertion sites of M. dilator nasi (naris)
| Author | Species | Origin | Insertion |
|---|---|---|---|
| Meinertz, 1942 | Sciuris vul. | Os maxillare, rostral edge of the Foramen infraorbitale | The skin at the tip of the nose |
| Priddy & Brodie, 1948 | Hamster | Dorsal to infraorbital foramen | Dorsal to the nostril |
| Rinker, 1954 | Cricetinae | Medial half of the dorsal margin of the zygomatic Notch | Lateral part at the dorsal border of rhinarium |
| Klingener, 1964 | Rodents | Aponeurosis from the outer surface of outer rim of the infraorbital foramen | Dorsal part of the nasal cartilage |
| Herring, 1972 | Suoidea | Facial crest of the maxilla | Subcutaneous tissue on the side of the rhinarium |
| Ryan, 1989 | Rodentia | Dorsal surface of the zygomatic plate anterior to the eye | The dorsolateral portion of nasal cartilage |
| Whidden, 2002 | Insectivora | Anterior surface of the antorbital ridge and fossa that lies anterior to the orbit | Aponeurosis over the cartilaginous rings of the snout tip |
MATERIALS AND METHODS
Anatomy
Twelve two-week-old, and six adult male albino Wistar rats were used for anatomical studies. The Institute’s Animal Care and Use Committee (The Weizmann Institute of Science) approved all procedures for animal maintenance and experimentation. Rats were anesthetized intraperitonally with urethane (25%; 0.65 ml/100 g body weight), and perfused transcardially (4% paraformaldehyde, and 5% sucrose in 0.1 M phosphate buffer, pH 7.4). The musculature of the snout was visualized from serial sections stained for cytochrome oxidase (CO) activity.
To reveal the sites of muscle attachment to the bones, we used 2-week-old rats because bones are soft enough to be cut with a regular microtome. Postfixation was carried out for the entire rostral part of the snout. In adult rats, CO activity was visualized in sections that were prepared from hemi-snouts because bone decalcification affects CO reactivity. After perfusion, hemi-snouts of adult rats were excised, and placed between two pieces of stainless steel mesh within RCH-44 perforated plastic histology cassettes (Proscitech.com) to prevent tissue curling. The cassettes were placed into 4% paraformaldehyde solution with 30 % (w/v) sucrose for postfixation. After 48 hours of postfixation, tissue samples were sectioned at 30 um in the tangential, horizontal or coronal planes on a freezing microtome (SM 2000R, Leica Instruments, Germany).
Cytochrome oxidase activity was revealed according to our modification (Haidarliu and Ahissar, 2001) of the procedure described by Wong-Riley (1979). Briefly, free-floating slices were incubated in an oxygenated solution of 0.02 % (w/v) cytochrome c (Sigma-Aldrich, St. Louis, MO, USA), catalase (200 μg/mL), and 0.05 % (w/v) diaminobenzidine in 100 mM phosphate buffer at room temperature under constant agitation. When a clear differentiation between highly-reactive and non-reactive tissue structures was observed, the incubation was arrested by adding 0.5 mL of 100 mM phosphate buffer into the incubation wells. Stained slices were washed, mounted on slides, cover-slipped with Entellan (Merck KGaA 64271 Darmstadt, Germany) or Kristalon (Harleco; Lawrence, KS), and examined by light microscopy (Nikon Eclipse 50i microscope). Images were imported in Adobe Photoshop software. When required, minimal adjustments of contrast and brightness were made for clarity.
Physiological experiments
Physiological experiments were carried out in adult Long Evans rats (250–350 g) according to the National Institutes of Health Guidelines, and were approved by the Institutional Animal Care and Use Committee at Laval University and University of California, San Diego. Rats were anesthetized with ketamine (75 mg/kg) - xylazine (5 mg/kg), and placed in a stereotaxic apparatus. Body temperature was maintained at 37.5°C with a thermostatically controlled heating pad. A head post was fixed to the surface of the skull by means of screws and acrylic cement.
Microstimulation of M. dilator nasi was carried out in 4 rats. An incision was made along the midline of the nose, and a cotton tipped applicator was used to separate the medial slip of the nasolabialis muscle from the maxillary bone. Pushing sideway the medial slip of muscle nasolabialis reveals the belly of M. dilator nasi at the level of first arc of vibrissae. A platinum-iridium microelectrode (0.4–0.8 mOhm) was inserted into the muscle and a wick electrode was placed on the dorsal part of the maxillary bone to close the circuit. Respiration was monitored with a piezoelectric film (cantilever type; LDT1 028K; Measurement Specialities) pressing on the rat’s abdomen just caudal to the torso. The effect of stimulation was assessed by videographic recording with a high-speed video camera (100 frames per second; HiSpec 2G Mono camera; Fastec Imaging, CA) equipped with a zoom lens to record nose or vibrissa motion, or with a 2.5× tube lens to capture the opening of the nares. Respiratory signals were sampled at 1 kHz, and synchronized with video frames through a NI USB-6221 acquisition board (National Instruments). Run time commands to the camera were logged with digitized data on the controlling computer. Nose motion and naris dilation were extracted from video recordings using custom scripts written in Matlab.
In additional experiments 2 rats were implanted with a head post fixed to the surface of the skull by means of screws and acrylic cement. A small hole was drilled through the left nasal bone at the level of the frontal-nasal fissure (1 mm lateral from midline) for the placement of a stainless steel cannula (16G) that was lowered over the hole and fixed in place with dental cement. The cannula was sealed with silicone elastomer (Kwik-Cast Sealant; WPI). During the recording sessions the elastomer was removed, and respiration was monitored with a thermocouple (5TC-TT-K-36-36, Omega Engineering) that was lowered into the cannula. Thermocouple measures the cooling and warming of air as it passes through the nasal cavity. Two teflon-coated tungsten wires (diameter: 50 um) were inserted in M. dilator nasi for differential recording of EMG activity (Hill et al., 2008). Rats were allowed to recover for 3 days before the onset of behavioral experiments.
During the recording sessions, rats were placed inside a body-restraining cloth sack and rigid tube, and the animals were head-restrained. A tiny piece of aluminum foil was glue to the tip of the nose with cyanoacrylate to enhance contrast and facilitate tracking of nose motion. A Basler A602f camera equipped with a macro video zoom lens (Edmund Optics) was used to record lateral deflection of the nose from above the head (spatial resolution: 120 um per pixel; 100 frames per second). A mirror was placed at 45 degree in front of the snout, within the field of view of the camera, to capture vertical deflection of the nose. Respiratory signals were synchronized with video frames through a BNC-2090 acquisition board (National Instruments). Nose motion was extracted from video recordings using custom scripts written in Matlab.
Finally, a head post was fixed to the surface of the skull in 3 rats for concurrent recording of naris dilation, whisker motion and breathing. Recordings were carried out as described above while rats recovered from anesthesia.
RESULTS
Anatomical features of M. dilator nasi
Horizontal plane
Horizontal slices of the snout of 2-week-old rats reveal the entire anatomy of M. dilator nasi: origin, belly, tendon, and insertion sites, which lie underneath the most medial row of vibrissae (row A; Fig. 1a). Collagenous fibers of the endomysium, perimysium, and epimysium of the muscle are attached to the collagenous fibers of the periosteum of the maxilla (Fig. 1b and c). The long tendon of the M. dilator nasi inserts in the aponeurosis of the dorsum nasi that separates the skin from the dorsal nasal cartilage. Similar features are observed in horizontal sections of hemi-snouts in adult rats.
Fig. 1.
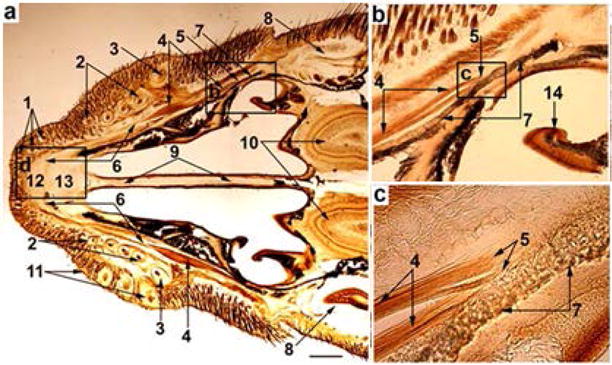
Light microscopy (CO staining) of a horizontal slice of the head of a two-week-old rat at the level of vibrissa row A (a). (b) enlarged boxed area in (a); (c) enlarged boxed area in (b); (d) enlarged area (a); (e) autofluorescence of the region shown in d. (1) nasal vibrissae; (2) follicles of the vibrissal row A; (3) follicle of the vibrissa α; (4) belly of M. dilator nasi, (5) origin, and (6) tendon of M. dilator nasi; (7) maxilla; (8) eyes; (9) septum; (10) olfactory bulbs; (11) follicles of vibrissa row B; (12) aponeurosis above the nasal cartilaginous skeleton (13); (14) ethmoturbinate. Scale bar in a = 1 mm.
Tangential plane
In tangential slices of the snout of two-week-old rats, the belly of M. dilator nasi is about 1.5 mm in diameter. The caudalmost segment of the muscle divides into two parts. The dorsal part originates from the anterior orbital bridge of the maxillary bone, and the ventral part originates from the rostral edge of the zygomatic plate (Fig. 2a). At high magnification, the dual origin of the muscle is clearly seen (Fig. 2b). Figure 2c shows that the endomysial and perimysial collagenous envelopes of the tapered ends of the muscle fibers of the ventral part of the M. dilator nasi are associated with the collagenous structures of the periosteum of the zygomatic process. Both muscle parts run rostralwards separately, but fuse near the rostral end of the muscle, forming a single belly with a multipennate appearance. The tendon forms a single strand. In tangential slices of hemi-snouts of adult rats, the belly and tendon of the M. dilator nasi presents similar features, but are relatively larger, with the belly reaching more than 2 mm in thickness.
Fig. 2.
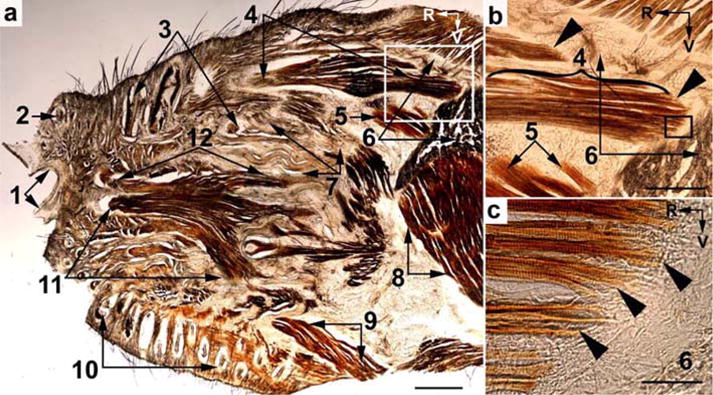
Light microscopy of a tangential slice of the snout of a 2-week-old rat (a). (b) and (c), igher magnification of the boxed areas in (a) and (b), respectively. The slice was stained for CO activity. Arrow heads point at the tapered muscle fibers at the origin of the M. dilator nasi. (1) Nostril; (2) follicle of a nasal vibrissa; (3) follicles of vibrissae in row A; (4) belly of M. dilator nasi; (5) anterior unit of the medial layer of the masseter muscle; (6) zygomatic notch and transversal profile at the base of the processus zygomaticus of the maxilla; (7) subcapsular fibrous mat; (8) anterior part of the masseter muscle; (9) Pars orbicularis oris of the M. buccinatorius; (10) furry buccal pad; (11) and (12) Partes maxillares profunda and superficialis, respectively, of the M. nasolabialis profundus. R, rostral; V, ventral. Scale bars = 1 mm in (a), 0.5 mm in (b), and 0.1 mm (c).
Coronal plane
In coronal slices, the belly of the M. dilator nasi is easily distinguishable by its strong CO reactivity. The tendon does not stain for CO activity, but appears as an oval compact formation among other collagenous structures. At the level of the straddlers vibrissae, M. dilator nasi appears as an elongated structure in dorso-ventral direction. The muscle consists of short fragments of muscle fibers that are cut obliquely. Most of the muscle fibers have a concentric orientation. In more rostral sections, the muscle contains an increasing number of tendinous fibers that eventually fuse in a single tendon.
Function of muscle dilator nasi
The function of M. dilator nasi was assessed by monitoring the motion of the nose, vibrissae and nares following electrical stimulation in lightly anesthetized rats. Low-intensity (100–200 uA) stimulation of M. dilator nasi exclusively produces upward and lateral deflection of the tip of the nose towards the side of stimulation (Fig. 3A). As seen from above, the upward deflection appears as a retraction. Vibrissa motion was only elicited at stimulus intensity 2–3 times above threshold intensity for nose motion (about 800 uA), which suggests the recruitment of additional muscles or nerves by current spread.
Fig. 3.
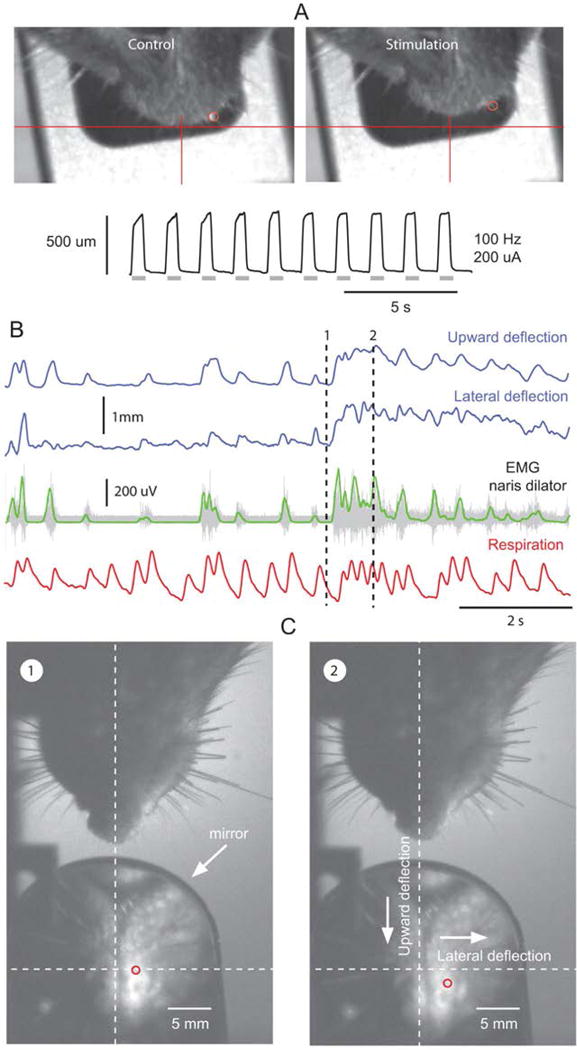
Muscle dilator nasi in rats deflects the nose. A, electrical stimulation of the left dilator nasi induces retraction of the nose and lateral deflection towards the side of stimulation. Gray bars indicate stimulus trains. B, recording of nose movements, muscle dilator nasi EMG (raw recording in grey and smoothed rectified EMG in green), and respiration (inspiration up) in a head-fixed alert rat. Video frames in C correspond to time points labeled 1 and 2 in B. Nose movement was tracked from images of the rhinarium reflected in a mirror placed at an angle of 45° in front of the rat. Red circles show the displacement of the reference point used to track nose motion.
To further assess the exclusive role of M. dilator nasi in nose motion, we carried out EMG and videographic recordings in head-fixed alert rats. We observe that, irrespective of the respiratory frequency, M. dilator nasi EMG activity is tightly coupled to nose motion (Fig. 3B,C). Yet, bursts of EMG activity do not occur in a one-to-one manner with each breath, and steady deflection of the nose is associated with sustained EMG activity. Together these observations indicate that contraction of M. dilator nasi does not actually open the nares, but that it deflects the nose.
Muscle involved in opening the nares
A prior anatomical study of facial musculature in rats identified a muscle termed pars maxillaris of muscle nasolabialis profundus (Haidarliu et al., 2010; Fig. 4) that attaches rostrally to the outer wall of the nasal cartilage, and caudally to the plate of the mystacial pad. It was suggested that this muscle exerts a dual action: (1) dilation of the nares by pulling the lateral nasal cartilage outwardly, and (2) retraction of the whiskers by pulling the proximal part of vibrissa follicles that inserts in the mystacial plate. Accordingly, one would expect naris dilation to be associated in a one-to-one manner with whisker retraction. We thus tracked the opening of the nares and whisker motion when rats recover from anesthesia. Naris dilation was not observed under deep anesthesia, which was assessed by the absence of a withdrawal reflex to pinch of the hindlimbs. As anesthesia vanishes, the lateral wall of the atrium turbinate starts moving outwardly in association with retraction of the large caudal vibrissae (up to arc 3; Fig. 5A). Retractions are of small amplitude (2–3°), synchronous on both sides of the face, and tightly coupled with naris dilation (see cross correlogram, Fig. 5B). This coordinate activity occurs at the onset of inhalation, and remains clearly observable until the animal starts moving its whiskers and nose actively. In addition, both naris dilation and whisker retraction were abolished during apnea induced by applying a puff of ammonia to the snout (Fig. 5C). Critically, both naris dilation and whisker retraction recovered synchronously when the rat begins to breathe again after apnea. In sum, these physiological results fully agree with the proposal that pars maxillaris profunda of muscle nasolabialis profundus is the main muscle involved in opening the nares in rats.
Fig. 4.
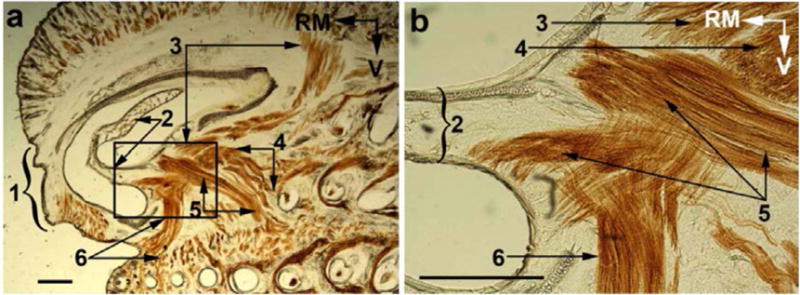
Muscle attachments to the cartilages of the nasal sidewall. The boxed area in a is shown at a higher magnification in b. (1) rhinarium; (2) atrioturbinate; (3–5) superficial, pseudointrinsic and posterior slips of the Pars interna respectively; and (6) Pars anterior of the M. nasolabialis profundus. RM, rostromedial; V, ventral. Scale bar: 1 mm. (Sebastian, can you provide another picture of maxillaris profunda of the muscle nasolabialis profundus.)The one above was taken from one of your prior papers)
Fig. 5.
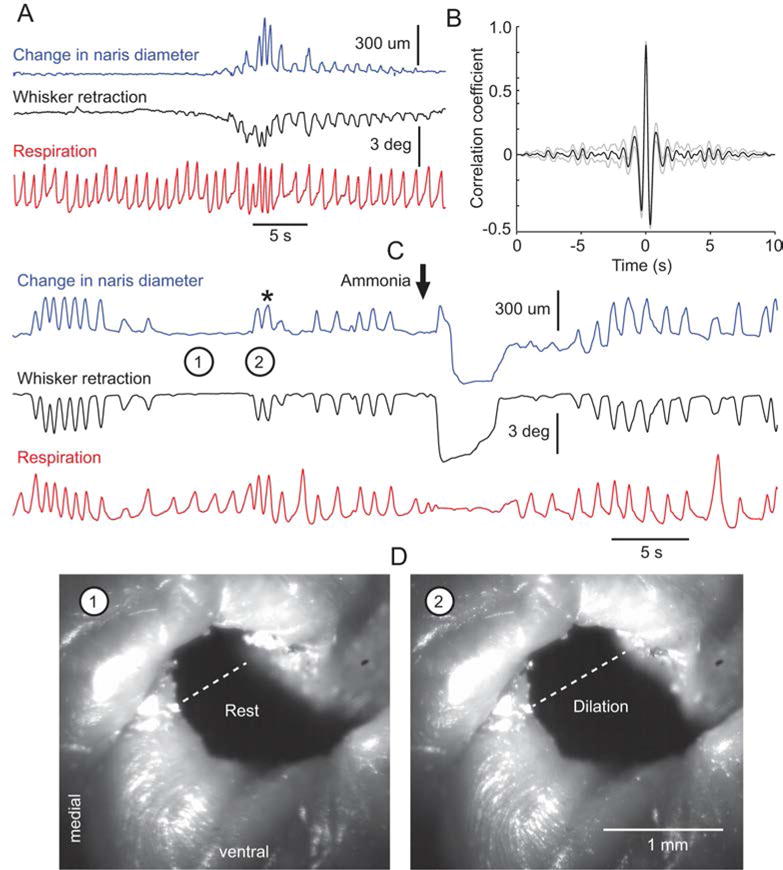
Opening of the naris is coupled with whisker retraction in the lightly anesthetized rat. A, as rats recover from anesthesia, the onset of naris dilation occurs in synchrony with the onset of whisker retraction. Both actions are time locked to the inspiratory phase of the respiratory cycle. B, cross correlation between change in naris diameter and whisker retraction. Averaged cross correlograms were computed from 30 sequences of 10 s each in 2 rats. Gray lines indicate 95 % confidence intervals. The one-to one relationship between naris dilation and whisker retraction is further revealed after an apneic reaction induced by applying a puff of ammonia to the snout. Note the synchronous recovery of both naris dilation and whisker retraction when respiration resumes. Video frames in D show naris size corresponding to time points 1 and 2 (asterisk) in C. The dotted line indicates how naris diameter was measured.
DISCUSSION
Origin and insertion site of M. dilator nasi
In several species M. dilator nasi was reported to originate at the orbit or the zygomatic plate of the maxilla (Table 1). None of these studies, however, identified the precise sites of contact of this muscle with the skull. In some species, M. dilator nasi may have two separate bellies and multiple tendons, as described in Suidea by Herring (1972). In rats, we found that the caudal end of M. dilator nasi divides into two parts: (i) a dorsal part that attaches to the anterior orbital ridge of the maxilla, and (ii) a ventral part that attaches to the rostral edge of the zygomatic process. Near the rostral end of the muscle, these two parts apparently fuse, and the muscle looks like a multipennate muscle that possesses a single-strand long tendon. It is likely that the ventral and dorsal slips of muscle dilator nasi exert different actions: the dorsal slip may raise the nose, while the ventral slip may deflect the nose laterally. Yet, the small size of these muscles renders this hypothesis difficult to test by selective stimulation or EMG recording.
The insertion site of the M. dilator nasi seems to vary in different species (Table 1). In Suidea, the insertion site is located within the rhinarium, laterally to the nostrils, which suggests that contraction of this muscle can indeed dilate the nares (Figs 4 and 5 in Herring, 1972). In the other studies mentioned in Table 1, it is not clear whether contraction of M. dilator nasi can produce naris dilation, because the insertion sites are located mostly in the dorsal part of the tip of the nose (i.e., the aponeurosis, or the dorsal part of the nasal cartilage). These structures are not directly connected to the nostrils or to the cartilaginous processes that maintain the shape of the nasal openings.
Effect of M. dilator nasi contraction
It has been proposed that M. dilator nasi is a multifunctional muscle that takes part in three different motor plants: vibrissa motion, nose movement, and olfactomotor activity (i.e., sniffing; Haidarliu et al., 2012). On the basis of electrical stimulation with transcutaneous electrodes, it was shown that this muscle deflects the nose, and protracts the rostral arcs of vibrissae. The role of this muscle as a component of olfactomotor plant (i.e., naris dilation) was not directly assessed, but rather inferred from previous anatomical studies. However, it appears likely that motion of the rostral vibrissae as reported in that study, is attributable to current spread to neighboring muscles or nerves. In the present study, we dissected facial tissue to ensure that M. dilator nasi was selectively stimulated, and in accord with the anatomical data (origin and insertion sites), M. dilator nasi stimulation solely produced nose motion. This result was confirmed by EMG recordings, which reveal M. dilator nasi activity associated with nose motion, but not with nares dilation. Yet, it is worth noting that nose motion is an integral part of the sniffing behavior. Therefore, nose motion, whisking and breathing are expected to be coordinated as initially reported by Welker (1964).
Our results show that naris dilation is tightly coupled to small amplitude whisker retractions, which are only discernable when the rat is quiet, almost drowsy, or when it recovers from anesthesia. Such a coupling was already anticipated on the basis of the attachment points of pars maxillaris profunda of the muscle nasolabialis profundus. This muscle slip attaches to the outer wall of the nasal cartilage and to the plate of the mystacial pad. Therefore, contraction of this muscle pulls the lateral nasal cartilage outwardly, thus dilating the nostrils, and also pulls the plate rostrally, provoking a slight retraction of the vibrissae. It is unlikely that the latter action plays any significant role in whisking; rather these slight retractions appear as a side effect of the action of this muscle on opening the naris.the anchoring point in the mystacial plate is compliant elastic compliance of collagenous tissue. It should be noted that other small muscles in the rhinarium may take part in naris dilation or nose movement. Interested readers should refer to the papers by Haidarliu et al. (2010, 2012) for a detailed account of the layout of these muscles.
Note on terminology
The present study reveals an inconsistency between the name “M. dilator nasi (naris)”, and the actual role of this muscle as assessed by electrical stimulation and EMG recordings. Our results provide direct evidence that this muscle has no effect on the size or shape of the nostrils, but that its function is to lift the nose and deflect it sideway. A similar inconsistency between the name “M. dilatator naris” and the function of this muscle was reported in other animals. In moose, Clifford and Witmer (2004) found that M. dilatator naris medialis, according to its origin and insertion sites, constricts rather than dilates the nostril as its name suggests. We therefore propose that this muscle in rats be termed M. deflector nasi. The name dilator nasi should refer to pars maxillaris profunda of the muscle nasolabialis profundus.
References
- Clifford AB, Witmer LM. Case studies in novel narial anatomy: 2. The enigmatic nose of moose (Artiodactyla: Cervidae: Alces alces) J Zool Lond. 2004;262:339–360. [Google Scholar]
- Deschênes M, Moore JD, Kleinfeld D. Sniffing and whisking in rodents. Curr Opin Neurobiol. 2012;22:243–250. doi: 10.1016/j.conb.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diogo R. The head and neck muscles of the \philippine colugo (Dermoptera: Cynocephalus volans), with a comparison to tree-shrews, primates, and other mammals. J Morph. 2009;270:14–51. doi: 10.1002/jmor.10666. [DOI] [PubMed] [Google Scholar]
- Greene EC. Anatomy of the rat. New York: Hafner Publishing Co; 1935. [Google Scholar]
- Haidarliu S, Ahissar E. Size gradients of barreloids in the rat thalamus. J Comp Neurol. 2001;429:372–387. doi: 10.1002/1096-9861(20010115)429:3<372::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Haidarliu S, Simony E, Golomb D, Ahissar E. Muscle architecture in the mystacial pad of the rat. Anat Rec. 2010;293:1192–1206. doi: 10.1002/ar.21156. [DOI] [PubMed] [Google Scholar]
- Haidarliu S, Golomb D, Kleinfeld D, Ahissar E. Dorsorostral snout muscles in the rat subserve coordinated movement for whisking and sniffing. Anat Rec. 2012;295:1181–1191. doi: 10.1002/ar.22501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haight JS, Cole P. The site and function of the nasal valve. Laryngoscope. 1983;93:49–55. doi: 10.1288/00005537-198301000-00009. [DOI] [PubMed] [Google Scholar]
- Herring SW. The facial musculature of the Suoidea. J Morph. 1972;137:49–62. doi: 10.1002/jmor.1051370104. [DOI] [PubMed] [Google Scholar]
- Hill DN, Bermejo R, Zeigler HP, Kleinfeld D. Biomechanics of the vibrissa motor plant in rat: rhythmic whisking consists of triphasic neuromuscular activity. J Neurosci. 2008;28:3438–3455. doi: 10.1523/JNEUROSCI.5008-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingener D. The comparative myology of four dipodoid rodents (Genera Zapus, Napeozapus, Sicista, and Jaculus) Misc Publ Mus Zool Univ Michigan. 1964;124:1–100. [Google Scholar]
- Priddy RB, Brodie AF. Facial musculature, nerves and blood vessels of the hamster in relation to the cheek pouch. J Morphol. 1948;83:149–180. doi: 10.1002/jmor.1050830202. [DOI] [PubMed] [Google Scholar]
- Rinker GC. The comparative myology of the mammalian genera Sigmodon, Oryzomys, Neotoma, and Peromyscus (Cricetinae), with remarks on their intergeneric relationships. Misc Publ Mus Zool Univ Michigan. 1954;83:1–125. [Google Scholar]
- Ryan JM. Comparative myology and polygenetic systematics of the Heteromyidae (Mammalia, Rodentia) Misc Publ Mus Zool Univ Michigan. 1989;176:1–103. [Google Scholar]
- Wachowiak M. All in a sniff: olfaction as a model for active sensing. Neuron. 2011;71:962–973. doi: 10.1016/j.neuron.2011.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker WI. Analysis of sniffing of the white rat. Behavior. 1964;22:223–244. [Google Scholar]
- Wong-Riley M. Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res. 1979;171:11–28. doi: 10.1016/0006-8993(79)90728-5. [DOI] [PubMed] [Google Scholar]


