Abstract
Introduction
There is a heavy emphasis in rehabilitation on restoration of function post-stroke at the expense of addressing how to manage the impact of stroke and the environment long term. Management of chronic health conditions is often and effectively addressed using self-management education; however, self-management is mostly focused on managing symptoms and health behaviors, not additional participation and community reintegration issues experienced following stroke. This study evaluated the Improving Participation after Stroke Self-Management Program (IPASS) to improve self-efficacy and participation in everyday life activities for individuals living with the long-term consequences of stroke.
Methods
A multisite, single-blind, exploratory randomized clinical study was conducted with participants with mild-to-moderate chronic stroke (n = 185). Participants were randomized either to receive the IPASS intervention immediately or to a wait list control group. The assessment was completed pre- and post-intervention and at 6–9 months post-intervention follow-up. The primary outcome assessments included measures of self-efficacy to manage chronic health conditions and to participate in everyday life activities.
Results
The results show that there was significant short-term increase in health-related self-efficacy both within-group and between-groups in managing chronic conditions which were retained at follow-up; the average effect size was 0.46, indicating moderate effect overall. Further, a significant short-term increase was found in participation self-efficacy, with an overall moderate effect size of 0.55.
Conclusions
These results provide early support for the use of IPASS to help improve self-efficacy to manage health behaviors and to improve participation post-stroke. Further investigation is warranted to confirm these findings with an active control group and a more sensitive outcome measure to capture participation changes.
Keywords: Stroke, Occupational therapy, Self-management, Participation
Introduction
Approximately 795,000 Americans have a stroke each year in the United States.1 Although the death rate of stroke is declining, there continues to be over seven million people living with stroke in the US, making this disease the leading cause of long-term disability.2 Unfortunately, almost half of the people living in the community following stroke have problems that challenge the activities that support their daily lives particularly those activities that support home, community, and work participation compared to their aged-matched peers.3–7 This is in part due to the rehabilitation community’s focus on short-term stroke recovery and not on supporting their need to actively manage their long-term disability and the environment around them, so they can return to full participation in communities of choice post-rehabilitation.
The chronic disease management literature is mostly focused on self-management to help people improve their ability to develop strategies to manage symptoms/impairments associated with living with a chronic condition, such as diabetes and arthritis.8,9 The chronic disease self-management program (CDSMP) is arguably the most established and evaluated program as it has consistently demonstrated improved emotional and physical health, self-efficacy, quality of life, and a decrease in healthcare utilization in chronic conditions.8–12 The CDSMP is built on the Social Learning Theory and the psychosocial mechanism of self-efficacy.8 Both the theory and the mechanism explain that learning takes place in a social context with peers who are going through the same life experience and through modeling and resource sharing with facilitators, including lay leaders who have chronic conditions themselves. This learning, in turn, can impact self-efficacy or confidence to engage in a behavior. Improvements in self-efficacy ultimately results in behavior change. While self-management has been evaluated and shown to result in positive health behavior change, the application of self-management to those living with a long-term disability, such as mobility impairments, has only recently started to be explored, particularly on extending this focus on returning to and managing participation in the home, community, and workplace.13,14
Systematic reviews of stroke self-management studies provide evidence of short-term increases in self-efficacy14,15; however, to date, self-management focuses on symptom management and related health behaviors (e.g., exercise, communication with healthcare providers and healthy eating). Stroke is a complex neurological condition that impacts a wide variety of body functions, activity engagement, and participation in everyday life activities which should also be included in self-management education. Such an approach supports the individual as he/she learns to manage the interaction between his/her new capacity, skills, and abilities, the activities he/she wants to do, and the environment in which he/she will do the activities. Reintegration in everyday life activities in the home, community, and work following stroke is an essential component of living with stroke, and this participation in meaningful activities can also have a positive impact on health.16,17 There is a great need to develop programs that will support the goals of helping individuals lead healthy, active lives and fully participate in society after stroke. The purpose of this study was to evaluate an intervention approach built on the theoretical tenets of self-management, the Improving Participation after Stroke Self-Management Program (IPASS), active participation, and on symptom, health and participation self-efficacy.
Methods
This study was a single-blind, exploratory randomized clinical study with participants managing daily life issues following stroke. This study was conducted at two University settings both with relationships to free-standing rehabilitation hospitals. All participants were randomized to receive the IPASS immediately or to a wait list control group. This study was reviewed and approved by Washington University in St. Louis, School of Medicine, Human Research Protection Office (HRPO) and University of Illinois at Chicago, Office for the Protection of Research Subjects (OPRS).
Sampling and Randomization Procedures
Participants were recruited from two existing stroke registries/databases maintained at the Rehabilitation Institute of Chicago in Chicago, Illinois and Barnes-Jewish Hospital in St. Louis, Missouri. Recruiters contacted potential participants from the stroke registries. In addition, flyers were distributed by rehabilitation professionals at rehabilitation hospitals including Rehabilitation Institute of Chicago, University of Illinois hospital, Barnes-Jewish Hospital, and Rehabilitation Institute in St. Louis. Individuals who met the following criteria were included in the study: (1) over 18 year old; (2) have a mild or moderate stroke (i.e., NIH stroke score ≤ 16); (3) at least 3 months post-stroke; (4) reside in a community-based setting; and (5) have completed initial acute/rehabilitation care. Individuals were excluded if they were not medically stable; were moderately or severely cognitively impaired (i.e., short blessed cognitive test score >8); or had severe aphasia (i.e., Boston diagnostic aphasia exam score <9; 15 item Boston naming test <10). The block randomization method was used to randomize participants into an intervention or wait list group.18 A statistician randomized a block of people (7–15 people) to avoid having a large group of people being assigned to the same condition to reduce bias.
Assessment and Intervention Procedures
Interested participants were contacted over the phone to schedule a face-to-face meeting with a study team member to be screened for eligibility based on the inclusion/exclusion criteria. Eligible participants immediately completed a baseline assessment with a blinded research assistant (Time 1). Following the completion of the baseline assessment, the participant was assigned to a study group by the research coordinator at each site. Individuals who were assigned to the treatment group were scheduled to complete the IPASS as soon as the next class was available. Individuals who were assigned to the control group started a 12-week waiting period and did not receive any active research intervention at this time. The control group participants completed another baseline assessment following this 12-week wait, prior to being scheduled to complete the IPASS (Time 2). Following the completion of the intervention, all participants in both groups completed a post-intervention assessment (Time 3). Finally, all participants completed a long-term follow-up assessment 6–9 months after completing the IPASS (Time 4). All assessments were completed by blind raters.
Intervention: Improving Participation after Stroke Self-Management Program (IPASS)
The IPASS Intervention involves a participatory, small group, problem-solving process that is based upon the self-management and environmental management intervention of Lorig and Holman8. All concepts and contents of the chronic disease self-management program (CDSMP) were retained, and an additional seven sessions with an emphasis on home, community, and work management after stroke were supplemented to the original CDSMP.19 The intervention was delivered to small groups of 6–7 participants at a time across 12 sessions, facilitated by an occupational therapist(s) and/or a peer facilitator with stroke (depending on availability) who has completed the CDSMP facilitator training. Table 1 provides a brief overview of the content in each session (Table 1). The program used a structured efficacy building process that focused on medical, emotional, role, and participation management to guide participants to develop skills related to problem-solving, decision-making, resource utilization, client/provider/service partnerships, action planning, and self-tailoring over time.8 For the seven stroke-specific sessions, we utilized an IPASS model to guide the efficacy building process. The IPASS model was adapted from the Person-Environment-Occupational Performance Model.20 This model provided a problem-solving structure for the participants to use to improve their participation by understanding the interaction between their health and participation (i.e. person-centered factors), environmental supports and barriers outside of them (i.e. environmental factors), and what they want to do (i.e. occupational engagement factors). They learned three different strategies (i.e. change the person, change the activity, and/or change the environment) to utilize to manage and support their participation in daily life.
Table 1.
Description of the IPASS intervention content
| Session | Contents | |
|---|---|---|
| 1–5 | Chronic disease self-management program | Original chronic disease self-management programa supplemented with stroke symptom management |
| 6 | Introduction to IPASS | Activity 1: Introduction |
| Activity 2: Importance of being active | ||
| Activity 3: Things that limit what you want to do: inside you | ||
| Activity 4: Things that limit what you want to do: outside you | ||
| Activity 5: Planning what you want to do | ||
| Activity 6: Closing | ||
| 7 | Stroke and home management | Activity 1: Identifying a home activity to work on |
| Activity 2: Taking apart the activity | ||
| Activity 3: Problem-solving your home activity | ||
| Activity 4: Organizing your space | ||
| Activity 5: What keeps you from being organized? | ||
| Activity 6: Organizing an area in your home | ||
| Activity 7: Closing | ||
| 8 | Stroke and meaningful work participation I | Activity 1: Debrief |
| Activity 2: Identifying meaningful work | ||
| Activity 3: Taking apart the job task | ||
| Activity 4: Problem-solving meaningful work | ||
| Activity 5: Closing | ||
| 9 | Stroke and meaningful work management II | Activity 1: Individual work simulation |
| Activity 2: Debrief on reasonable accommodations | ||
| 10 | Stroke and community participation management I | Activity 1: Debrief |
| Activity 2: Requesting reasonable accommodations | ||
| Activity 3: Defining community participation | ||
| Activity 4: Identifying important community activities | ||
| Activity 5: Identifying problems in community activities | ||
| Activity 6: Problem-solving a community activity | ||
| Activity 7: Closing | ||
| 11 | Stroke and community participation management I | Activity 1: Community trip as a group |
| 12 | Communication and long-term action planning to stay engaged | Activity 1: Debrief |
| Activity 2: Communicating with your family and friends | ||
| Activity 3: Setting a communication action plan | ||
| Activity 4: Requesting accommodations in the community | ||
| Activity 5: Looking back and planning for future | ||
| Activity 6: Closing |
Stanford Patient Education Research Center. Chronic disease self-management leader’s manual. Palo Alto, CA: Stanford Patient Education Research Center, 2006.
Assessments
The table below provides an overview and description of the assessments used in this study (Table 2). The initial three screening measures were administered immediately following informed consent. The remaining outcome measures were measured at all other time points (Time 1–4). To be eligible to be included in the final analysis, participants had to participate in at least eight of the 12 total sessions for IPASS. Participants were allowed to make up a session with the facilitator prior to the next session.
Table 2.
Description of assessments
| Assessment | Description | Time Completed |
|---|---|---|
| Screening | ||
| Short blessed test (SBT) | The SBT assesses one’s cognitive ability using 6 items. Each item has a weighted score based on the number of errors made. The total score can range from 0 to 28. The cutoff score for screening was 9 and higher which indicates that the person has cognitive impairment26 | Screening before randomization |
| Boston diagnostic aphasia exam (BDAE) | The BDAE was used to screen aphasia. Examinees are asked to follow three commands and get one point for each correct action. The total score ranges from 0 to 10. The cutoff score was 8 and lower27 | Screening before randomization |
| Boston naming test (BNT) | The 15-item short form BNT was used to screen aphasia. Testers are asked to name 15 pictures on cards and get one point for each correctly named object. The total score ranges from 0 to 15. The cutoff score was 9 and lower28 | Screening before randomization |
| Primary outcome measures | ||
| Chronic disease self-efficacy scale (CDSES) | This scale measures an individual’s confidence in performing specific tasks or behaviors to manage medical conditions. The scale consists of 20 questions and is rated on a Likert scale of 1–10 with higher scores indicating greater self-efficacy. The scale has shown high internal consistency coefficients (ranging from 0.77 to 0.92) and test–retest coefficients (from 0.72 to 0. 89)25 | Baseline, post-intervention, long-term follow-up interviews |
| Participation strategies self-efficacy scale (PS-SES) | The PS-SES measures self-efficacy in using strategies that help participation in home, community, work, and social activities. The scale consists of 35 questions and is rated on a Likert scale of 1–10 with higher scores indicating greater self-efficacy. The measure has strong internal consistency24 | Baseline, post-intervention, long-term follow-up interviews |
| Secondary outcome measures | ||
| Community participation indicators (CPI) | The CPI includes objective ratings of participation across major life areas (i.e., frequency of engagement) with subjective ratings of participation values (i.e., importance and satisfaction) and enfranchisement (e.g., inclusion, choice, opportunity, social capital membership). Findings from the factor analyses and Item Response Theory (IRT) analysis support validity of the instrument29 | Baseline, post-intervention, long-term follow-up interviews |
| Reintegration to normal living (RNL) | The RNL covers participation in areas such as recreational and social participation, community mobility, family roles, and other relationships.30 It consists of 11 items with a Likert scale of 0–10. Higher total score indicates stronger integration into the community. RNL has a strong inter-rater reliability (r>0.91). Construct validity was examined comparing RNL with Barthel Index (r = 0.42), Short Form 36 (r = 0.74), and Frenchay Activities Index (r=0.69). | Baseline, post-intervention, long-term follow-up interviews |
| Activity card sort (ACS) | The ACS assesses a person’s engagement in activities. This measure can show how many activities a person is involved in. Using 89 activity cards with pictures, participants indicate their current involvement using following response options: “didn’t do before stroke,” “do now,” “do less,” “given up,” and “started after stroke” Percent of retained activities are calculated. The measure has excellent test–retest reliability and excellent concurrent validity with community dwelling adults31 | Baseline, post-intervention, long-term follow-up interviews |
| WHO quality of life scale (WHOQOL-BREF) | The WHOQOL-BREF comprises 26 items measuring physical health, psychological health, social relationships, and environment. Cronbach’s alpha ranged from 0.66 to 0.84, demonstrating good internal consistency32 | Baseline, post-intervention, long-term follow-up interviews |
| Stroke impact scale (SIS) | The SIS has 60 items that assess the impact of stroke using a 5-point difficulty Likert scale. The SIS has seven subcategories: strength, function, mobility, emotion, communication, cognition, and participation. The measure has an adequate to excellent internal consistency with chronic stroke survivors33 | Baseline, post-intervention, long-term follow-up interviews |
Analysis
Data were entered and managed using REDCap electronic data capture tools hosted in the Biostatistics Division of Washington University School of Medicine.21 Analyses were conducted using SPSS 17.0 and Excel. Descriptive analyses, including frequency, means, and standard deviations, were conducted to summarize the sample’s demographic characteristics.22 Repeated measures ANOVA was used to investigate the impact of the intervention on two different groups. Between-groups and within-group analyses were conducted using the general linear model procedure. Between-groups analysis compared the waiting period of the control group (T1–T2) with the intervention period of the immediate intervention group (T1–T3). The within-group analysis compared the waiting period (T1–T2) to the intervention period (T2–T3) of the control group. Long-term impact of the intervention was calculated using a paired t-test. Means at post-intervention (T3) and at 6–9 months follow-up (T4) were compared in order to investigate whether the short-term impact of the IPASS program lasted. Statistical significance was determined based on the a priori α-level of p = 0.05. In addition, effect sizes (i.e., Cohen’s d) were calculated to further show the level of change between groups by dividing the mean difference of the waiting period of the control group and intervention period of the immediate intervention group by the standard deviation of the mean difference.22 Confidence intervals of 95% were calculated on the effect sizes.
Results
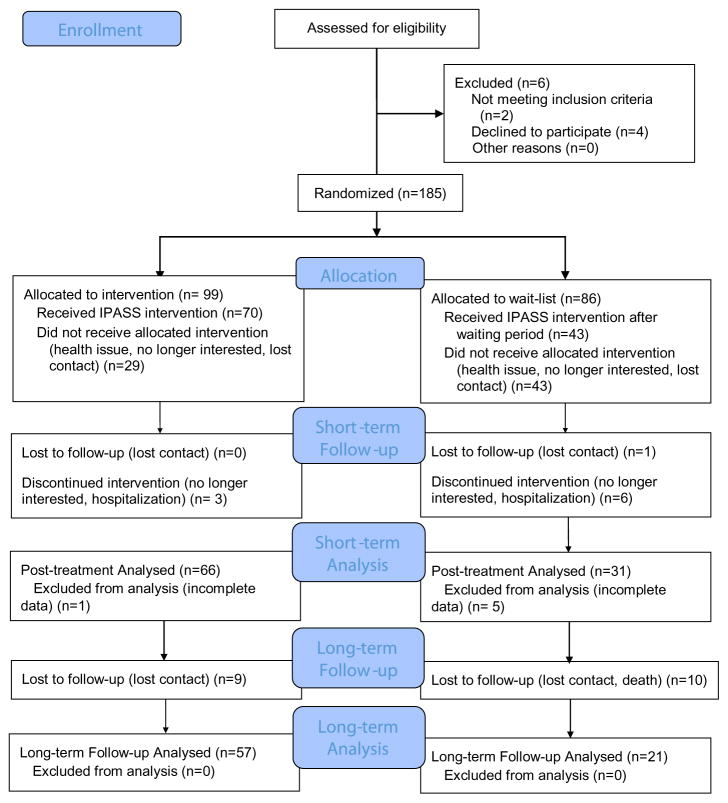
Recruitment occurred between November 2010 and June 2013. Of 191 individuals who were eligible to participate, 185 met inclusion criteria, were randomized, and completed baseline assessments; 65 completed the IPASS intervention immediately and were assessed at one week post-intervention (Figure 1). Of 86 participants who were assigned to a wait list group, 36 completed an assessment after the waiting period and completed the IPASS intervention and the post-intervention assessment. Although the overall attrition rates were relatively high at 35% in the intervention group and 58% in the wait list group, once individuals started the IPASS intervention, the completion rate was high. Ninety-five percent of immediate intervention group and 86% of the wait list group completed the intervention. There were no important adverse events. Six to nine months after the intervention was completed, a total of 78 participants across the two groups completed the long-term assessment. Seven from the immediate intervention group and 12 from the wait list group were lost during the 6- to 9-month period.
Figure 1.
CONSORT diagram showing participant flow through the study
Data from 31 participants from the wait list and 66 participants from the intervention participants were analyzed. For both groups, there was almost an even split between male and female participants. The majority of the participants in both groups were African-Americans and educated with at least a GED. The mean score of the National Institute of Health Stroke Scale (NIHSS) was 4.8 (SD = 2.75) and 4.9 (SD = 3.04), respectively, indicating mild impairment. Both groups represent a variety of experiences with stroke, with the majority having had the stroke less than one year followed by 1–5, 5–10 years, and more than 10 years. Baseline scores on participant descriptive variables were not significantly different between the intervention and wait list groups (see Table 3).
Table 3.
Demographics (n = 97)
| Characteristics | Control (n=31) | Intervention (n=66) | ||
|---|---|---|---|---|
| Count | Percent | Count | Percent | |
| Sex | ||||
| Male | 15 | 48.4 | 31 | 47.0 |
| Female | 16 | 51.6 | 35 | 53.0 |
| Race | ||||
| Caucasian/White | 10 | 32.3 | 23 | 35.4 |
| African-American | 19 | 61.3 | 35 | 53.8 |
| Hispanic/Latino | 1 | 3.2 | 5 | 7.7 |
| Asian-American | 1 | 3.2 | 2 | 3.2 |
| Marital status | ||||
| Single, widowed, separated, or divorced | 15 | 48.4 | 45 | 68.2 |
| Married or partner | 16 | 51.6 | 21 | 31.8 |
| Education | ||||
| Less than high school | 4 | 12.9 | 6 | 9.1 |
| High school completed/GED | 13 | 41.9 | 31 | 47.0 |
| College (some, Associate, Bachelor’s degree) | 9 | 29.0 | 23 | 34.8 |
| Graduate degree and above | 5 | 16.1 | 6 | 9.1 |
| Time since stroke | ||||
| less than 1 year | 13 | 41.9 | 24 | 36.9 |
| 1–5 years | 8 | 25.8 | 19 | 29.2 |
| 5–10 years | 6 | 19.4 | 15 | 23.1 |
| more than 10 years | 4 | 12.9 | 7 | 10.8 |
| Insurance status | ||||
| No insurance | 2 | 6.7 | 4 | 6.1 |
| Subsidized insurance | 14 | 46.7 | 34 | 51.5 |
| Private insurance | 14 | 46.7 | 28 | 42.4 |
| Mean (Range) | SD | Mean (Range) | SD | |
| Age (years) | 59 (45–80) | 7.7 | 57 (32–93) | 10.0 |
| Months since stroke | 50 (2–211) | 58.0 | 54 (3–295) | 66.4 |
| NIH Stroke Scale | 4.8 (1–12) | 2.75 | 4.7 (1–12) | 3.04 |
| Total number of medical conditions | 5.6 (1–14) | 2.70 | 6.6 (1–18) | 3.90 |
Note: None of the characteristics showed significant difference between the intervention and wait list groups.
The result in Table 4 shows that there was a statistically significant effect of the IPASS intervention on self-efficacy in managing chronic conditions, as measured by the chronic disease self-efficacy scale (CDSES)23 In six subcategories of the CDSES (i.e., exercise regularly, obtain help from others, communicate with physicians, manage disease in general, control/manage depression, and do chores), the between-groups analysis showed that there was a significantly greater increase in CDSES outcomes after the intervention in the intervention group than during the waiting period of the control group (p < 0.05). During the same time periods, the intervention group showed positive change in the CDSS scores, and the wait list control group showed negative changes while waiting for the intervention. Eight of 10 subcategories showed moderate to large effect sizes (0.37–0.75), indicating the positive impact of the intervention on chronic disease self-management self-efficacy when control and intervention groups are compared. The average of all effect sizes was 0.53, indicating moderate effect overall. The within-group analysis of the control group also showed statistically significant different changes in six categories of CDSES after the intervention when compared to the waiting period (p < 0.05). On the long-term follow-up, none of the changes were significant (p > 0.05) indicating that the mean of all subcategories remained improved without significant decreases over the time. Effect sizes of the long-term impact were in negative directions but showed small effect sizes (<0.2), suggesting that the short-term increase after the intervention did not change considerably (Table 4).
Table 4.
Chronic Disease Self-Efficacy Scale (CDSES)
| Subcategories | Short-term effect (n=97) | Long-term Effect (n=78) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
|
F (p-value)
|
Effect size a | 95% CI | t (p-value) | Effect size a | ||
| Between-groups | Within-group | |||||
| Exercise regularly | 7.280* (0.008) | 5.542* (0.026) | 0.57 | 013,1.00 | −.684 (0.496) | −0.07 |
| Get information about disease | 1.406 (0.239) | 1.148 (0.293) | 0.34 | −0.10,0.77 | −1.489 (0.143) | −0.20 |
| Obtain help from others | 4.139* (0.045) | 8.544* (0.007) | 0.44 | 0.00,0.88 | −.053 (0.958) | −0.01 |
| Communicate with physician | 6.968* (0.010) | 1.090 (0.305) | 0.61 | 0.16,1.05 | −.324 (0.747) | −0.03 |
| Manage disease in general | 13.851* (0.000) | 13.605* (0.001) | 0.74 | 0.29,1.18 | −.918 (0.362) | −0.09 |
| Manage symptoms | 3.695 (0.058) | 3.322 (0.079) | 0.45 | 0.01,0.89 | −.953 (0.343) | −0.10 |
| Manage shortness of breath | 2.010 (0.160) | 2.949 (0.097) | 0.34 | −0.10,0.78 | −.738 (0.463) | −0.12 |
| Control/manage depression | 7.454* (0.008) | 6.038* (0.020) | 0.66 | 0.21,1.10 | −1.107 (0.272) | −0.10 |
| Do chores | 11.634* (0.001) | 7.837* (0.009) | 0.75 | 0.30,1.19 | −.412 (0.681) | −0.05 |
| Social/recreational activities | 2.431 (0.122) | 14.639* (0.001) | 0.37 | −0.07,0.80 | −.836 (0.406) | −0.10 |
p < 0.05.
Moderate and big effect sizes are bolded. The outcome has a moderate effect if 0.35 ≤ D < 0.65; big effect if D ≥ 0.65.
The between-groups analysis of the Participation Strategies Self-Efficacy Scale (PSES)24 shows that there was statistically significant difference between the control group waiting period and intervention group’s post-intervention results in five of six subcategories (p < 0.05): (1) managing home, (2) staying organized, (3) managing community, (4) managing work and productivity, and (5) advocating for resources. All of these categories also showed moderate to large effect sizes ranging from 0.37 to 0.97. The average effect size across all subcategories was 0.58, indicating moderate overall impact of the intervention on participation management self-efficacy when control and intervention groups were compared. On the long-term follow-up, the scores of “managing home” and “staying organized” remained improved without statistically significant decreases. However, “managing community” and “advocating for resources” dropped significantly (p < 0.05), although the effect size of the decrease was small. The “managing work and productivity” score showed significant decrease in the score with a moderate effect size (Table 5).
Table 5.
Participation Strategies Self-Efficacy Scale (PS-SES)
| Subcategories | Short-term effect (n=97) | Long-term effect (n=78) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
|
F (p-value)
|
Effect size a | 95% CI | t (p-value) | Effect size a | ||
| Between-groups | Within-group | |||||
| Managing home | 8.856* (0.004) | 2.054 (0.162) | 0.76 | 0.31,1.19 | −0.49 (0.961) | −0.00 |
| Staying organized | 5.327* (0.023) | 2.134 (0.155) | 0.56 | 0.12,0.99 | −1.521 (0.132) | −0.15 |
| Managing community | 16.143*(0.000) | 2.603 (0.118) | 0.97 | 0.51,1.41 | −2.036** (0.045) | −0.17 |
| Managing work and productivity | 4.219*(0.043) | 5.548* (0.025) | 0.37 | –0.06,0.81 | −2.934** (0.004) | −0.35 |
| Managing communication | 1.026 (0.314) | 2.742 (0.109) | 0.19 | –0.24,0.62 | −0.414 (0.680) | −0.03 |
| Advocating for resources | 10.073* (0.002) | 5.460* (0.027) | 0.65 | 0.20,1.09 | −2.411** (0.018) | −0.25 |
p < 0.05.
Moderate and big effect sizes are bolded. The outcome has a moderate effect if 0.35 ≤ D < 0.65; big effect if D ≥ 0.65.
Discussion
While self-management education has been prominent in health care for several years, individuals with neurological injuries with executive cognitive impairments (e.g., stroke) have largely been overlooked and there is limited data to support whether or not these individuals can participate in these programs or whether they can benefit from a self-efficacy, problem-solving intervention. 14 The data from this study support that individuals with chronic stroke can participate in a self-management education program that addresses the everyday life issues that they must learn to manage, in addition to managing their health and symptoms. The participants in this program showed improvement in self-efficacy in managing their health and also their participation. Demonstrating the feasibility of this intervention approach with the long-term consequences of stroke can open up new opportunities for the continued development of stroke-specific, community-based, participation-focused, self-management programs like IPASS.
The improvement in participants’ self-efficacy to manage their chronic health conditions is consistent with other published outcomes of studies using the CDSMP25 and stroke-specific self-management programs. These gains in self-efficacy were maintained at follow-up, which demonstrates that IPASS had a long-term impact. In addition to these findings, the exploratory aim of this study was to evaluate the impact of IPASS on participation in everyday life activities. To accomplish this, we first evaluated the impact of IPASS on the individual’s self-efficacy to participate in everyday life activities. The data demonstrate that the IPASS was able to improve the participants’ self-efficacy to manage and participate in home, community, and work activities, and these gains were maintained at follow-up. Communication-related self-efficacy, however, did not show short-term improvement, and there was a negative moderate long-term effect in the work subcategory. Second, we also evaluated actual changes in participation. The data suggest that actual changes in participation may be present but the data are inconclusive. These findings have some implications for future research.
It is impossible at this time to determine whether the lack of an effect on the secondary participation outcomes was due to insensitivity of the outcome measures or changes that are necessary to the IPASS intervention; however, based on this study, there are some further considerations that should be addressed. Future studies should first investigate the use of other participation outcome measures to determine which measure best fits with participation-focused interventions such as IPASS. Given the exploratory nature of this study, measures were selected to try to capture broad changes in participation vs. more targeted changes in specific areas, which may be more prominent. Second, future studies with IPASS may need to increase content on improving participation in social and work contexts, specifically offering more opportunities to problem solve and negotiate social and system level barriers people with stroke are facing as they transition out of rehabilitation back into society and the competitive workforce. Ideally, this content would help individuals further build confidence and skills related to advocating, communicating needs, navigating health and social systems, and requesting reasonable accommodations that will support them in returning to meaningful work of their choice. Finally, while some of the gains in self-efficacy were maintained at follow-up, some of the effect diminished over time. With this in mind, it may be necessary to implement strategies, such as periodic booster sessions, in the future evaluation of the IPASS intervention to help mitigate this loss in effect.
There were several limitations with this study. First, the wait list crossover design resulted in several individuals as lost-to-follow-up especially in the wait list group, which was not engaged early on in the study process. Future studies should explore other design options to evaluate the effect of IPASS compared to an active control group. As previously mentioned, the study lacked sensitive participation outcome measures to evaluate targeted improvements in specific areas of participation targeted by IPASS (e.g., work). Additional participation assessments should be evaluated for use in future studies evaluating IPASS. Also, participants’ participation in other rehabilitation services was not tracked in this study. While participants had to state that their recommended post-stroke rehabilitation, services were completed prior to enrolling in this study, if they pursued or received other services after enrolling, this was not tracked and may have impacted the results.
Acknowledgments
Funding
This study was supported by the National Institute on Disability and Rehabilitation Research within the Rehabilitation Research and Training Center on enhancing the functional and employment outcomes of individuals who experience a stroke #H133B080031. Timothy Wolf received salary support from the National Center for Medical Rehabilitation Research (NCMRR) in the National Institute of Child Health and Human Development (NICHD) of the National Institutes of Health (NIH) [award numbers K12HD055931 and K23HD073190].
Footnotes
Conflict of interests
There is no conflict of interest to report.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclaimer Statements
Contributors
TJ.F is an associate professor and chair at University of Missouri in the Department of Occupational Therapy. His research interests include investigating the use of strategy- based intervention approaches to support individuals with neurological injuries and improve their performance in everyday life activities. His recent publications appear in Neurorehabilitation and Neural Repair, the American Journal of Occupational Therapy, and Disability and Rehabilitation.
CM.B is the Elias Michael Executive Director and professor at the Washington University School of Medicine Program in Occupational Therapy. Her research interests include better understanding the relationship between brain function, behavior, and performance in persons with stroke. Her recent publications appear in Archives of Physical Medicine and Rehabilitation and American Journal of Occupational Therapy.
DL is a PhD candidate in the Disability Studies program and a project coordinator in the Department of Occupational Therapy at the University of Illinois at Chicago. Her research interests include community living and participation of adults aging with a disability. Her recent publications appear in Disability and Rehabilitation. JH is a professor and Wade-Meyer Endowed Chair at the University of Illinois at Chicago in the Occupational Therapy Department, and Joint Doctoral Programs in Disability Studies and in Rehabilitation Sciences. Her research interests include community-based participatory research focused on increasing community living, community participation, and work/economic opportunities with people with long-term disabilities. Her recent publications appear in Archives of Physical Medicine and Rehabilitation and Disability and Rehabilitation.
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics – 2014 update a report from the American Heart Association. Circulation. 2014;129(3):e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Stroke Association. Survivors: Are you a stroke survivor. Centennial, CO: 2014. [Google Scholar]
- 3.Alzahrani M, Ada L, Dean C. Duration of physical activity is normal but frequency is reduced after stroke: An observational study. J Physiother. 2011;57(1):47–51. doi: 10.1016/S1836-9553(11)70007-8. [DOI] [PubMed] [Google Scholar]
- 4.Appelros P, Samuelsson M, Karlsson-Tivenius S, Lokander M, Terént A. A national stroke quality register: 12 years experience from a participating hospital. Eur J Neurol. 2007;14(8):890–894. doi: 10.1111/j.1468-1331.2007.01826.x. [DOI] [PubMed] [Google Scholar]
- 5.Cott CA, Wiles R, Devitt R. Continuity, transition and participation: Preparing clients for life in the community post-stroke. Disabil Rehabil. 2007;29(20–21):1566–1574. doi: 10.1080/09638280701618588. [DOI] [PubMed] [Google Scholar]
- 6.Hackett M, Glozier N, Jan S, Lindley R. Returning to paid employment after stroke: The psychosocial outcomes in stroke (poise) cohort study. PLoS ONE. 2012;7(7):e41795. doi: 10.1371/journal.pone.0041795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayo NE, Wood-Dauphinee S, Cote R, Durcan L, Carlton J. Activity, participation, and quality of life 6 months poststroke. Arch Phys Med Rehabil. 2002;83(8):1035–1042. doi: 10.1053/apmr.2002.33984. [DOI] [PubMed] [Google Scholar]
- 8.Lorig KR, Holman H. Self-management education: History, definition, outcomes, and mechanisms. Ann Behav Med. 2003;26(1):1–7. doi: 10.1207/S15324796ABM2601_01. [DOI] [PubMed] [Google Scholar]
- 9.Lorig KR, Sobel DS, Stewart AL, et al. Evidence suggesting that a chronic disease self-management program can improve health status while reducing hospitalization: A randomized trial. Med Care. 1999;37(1):5–14. doi: 10.1097/00005650-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Kennedy A, Reeves D, Bower P, et al. The effectiveness and cost effectiveness of a national lay-led self care support programme for patients with long-term conditions: A pragmatic randomised controlled trial. J Epidemiol Community Health. 2007;61(3):254–261. doi: 10.1136/jech.2006.053538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lorig K, Ritter P, Pifer C, Werner P. Effectiveness of the chronic disease self-management program for persons with a serious mental illness: A translation study. Community Ment Health J. 2014;50(1):96–103. doi: 10.1007/s10597-013-9615-5. [DOI] [PubMed] [Google Scholar]
- 12.Ritter P, Ory M, Laurent D, Lorig K. Effects of chronic disease selfmanagement programs for participants with higher depression scores: Secondary analyses of an on-line and a small-group program. Transl Behav Med. 2014;4(4):398–406. doi: 10.1007/s13142-014-0277-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ipsen C, Ravesloot C, Seekins T, Seninger S. A financial cost – benefit analysis of a health promotion program for individuals with mobility impairments. J Disabi Policy Stud. 2006;16(4):220–228. [Google Scholar]
- 14.Jones F, Riazi A. Self-efficacy and self-management after stroke: A systematic review. Disabi Rehabil. 2011;33(10):797–810. doi: 10.3109/09638288.2010.511415. [DOI] [PubMed] [Google Scholar]
- 15.Lennon S, McKenna S, Jones F. Self-management programmes for people post stroke: A systematic review. Clin Rehabil. 2013 doi: 10.1177/0269215513481045. 0269215513481045. [DOI] [PubMed] [Google Scholar]
- 16.Glass T, de Leon C, Marottoli R, Berkman L. Population based study of social and productive activities as predictors of survival among elderly Americans. BMJ. 1999;319(7208):478–483. doi: 10.1136/bmj.319.7208.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stav W, Hallenen T, Lane J, Arbesman M. Systematic review of occupational engagement and health outcomes among community-dwelling older adults. Am J Occup Ther. 2012;66(3):301–310. doi: 10.5014/ajot.2012.003707. [DOI] [PubMed] [Google Scholar]
- 18.Suresh K. An overview of randomization techniques: An unbiased assessment of outcome in clinical research. J Hum Reprod Sci. 2011;4(1):8. doi: 10.4103/0974-1208.82352. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Lorig K, Ritter P, Laurent D, Plant K. Internet-based chronic disease self-management: a randomized trial. Med Care. 2006;44(11): 964–971. doi: 10.1097/01.mlr.0000233678.80203.c1. [DOI] [PubMed] [Google Scholar]
- 20.Christiansen C, Baum C, Bass-Haugen J. Occupational therapy: Performance, participation and well-being. Thorofare, NJ: Slack; 2005. Person-environment-occupation-performance: An occupation-based framework for practice; pp. 243–259. [Google Scholar]
- 21.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Info. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munro B. Statistical methods for health care research. Vol. 1. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 23.Lorig K, Steward A, Ritter P, Gonzalez V, Laurent D, Lynch J. Outcome measures for health education and other health care interventions. Thousand Oaks, CA: Sage; 1996. [Google Scholar]
- 24.Lee D, Fogg L, Hammel J, Baum C, Wolf T. Validation of the Participation Strategies Self-efficacy Scale (PS-SES) Disabil Rehabil. doi: 10.1080/09638288.2016.1242172. In review. [DOI] [PubMed] [Google Scholar]
- 25.Lorig K, Sobel D, Stewart A, et al. Evidence suggesting that a chronic disease self-management program can improve health status while reducing hospitalization: A randomized trial. Med Care. 1999;37(1):5–14. doi: 10.1097/00005650-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short orientation-memory-concentration test of cognitive impairment. Am J Psych. 1983;140:734–739. doi: 10.1176/ajp.140.6.734. [DOI] [PubMed] [Google Scholar]
- 27.Goodglass H, Kaplan E. The assessment of aphasia and related disorders. Philadelphia, PA: Lea & Febiger; 1983. [Google Scholar]
- 28.Lansing AE, Ivnik RJ, Cullum CM, Randolph C. An empirically derived short form of the Boston naming test. Arch Clin Neuropsychol. 1999;14(6):481–487. [PubMed] [Google Scholar]
- 29.Heinemann A, Lai J, Magasi S, et al. Measuring participation enfranchisement. Arch Phys Med Rehabil. 2011;92(4):564–571. doi: 10.1016/j.apmr.2010.07.220. [DOI] [PubMed] [Google Scholar]
- 30.Wood-Dauphinee SL, Opzoomer MA, Williams JI, Marchand B, Spitzer WO. Assessment of global function: The reintegration to normal living index. Arch Phys Med Rehabil. 1988;69:583–590. [PubMed] [Google Scholar]
- 31.Baum C. The contribution of occupation to function in persons with Alzheimer’s disease. J Occup Sci. 1995;2(2):59–67. [Google Scholar]
- 32.Hawthorne G, Herrman H, Murphy B. Interpreting the WHOQOL-bref: Preliminary population norms and effect sizes. Soc Indic Res. 2006;77(1):37–59. [Google Scholar]
- 33.Duncan PW, Wallace D, Lai SM, Johnson D, Embretson S, Laster LJ. The stroke impact scale version 2.0: Evaluation of reliability, validity, and sensitivity to change. Stroke. 1999;30:2131–2140. doi: 10.1161/01.str.30.10.2131. [DOI] [PubMed] [Google Scholar]