Abstract
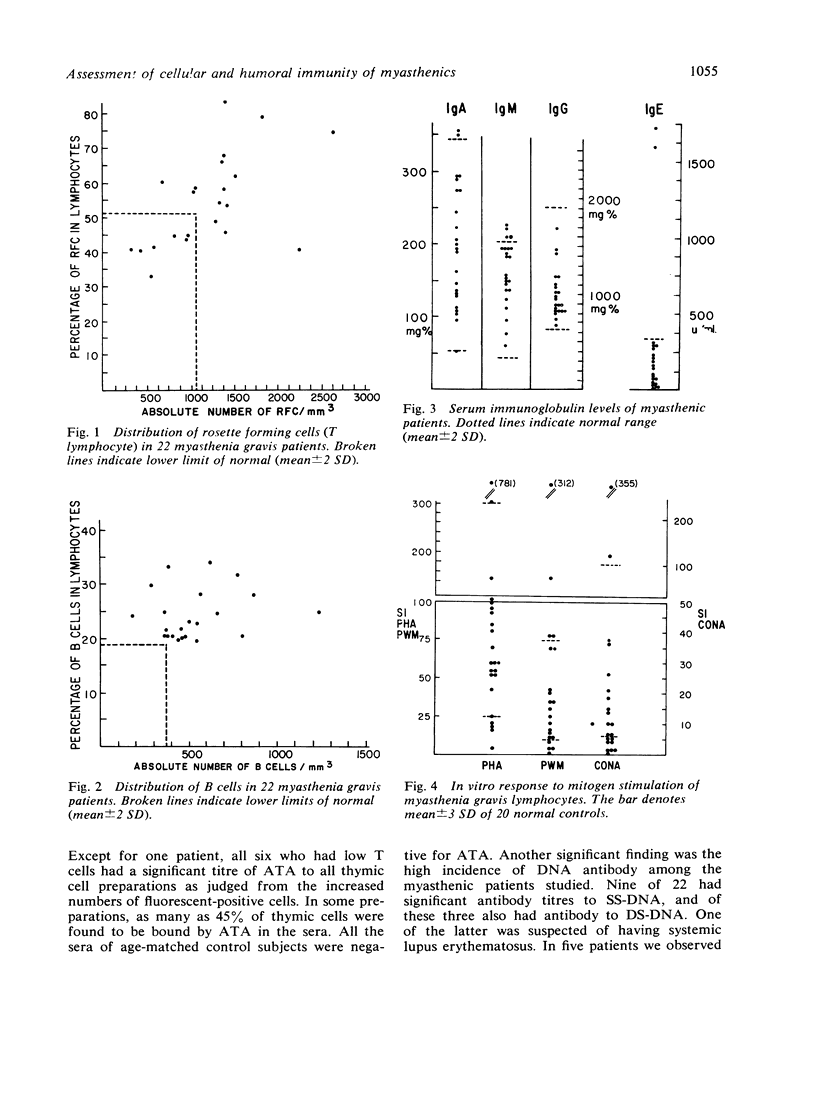
A close association of autoimmune diseases or autoimmune phenomena in myasthenia gravis is well known. A comprehensive immunological study of 22 patients with myasthenia gravis showed that changes in the immune system mainly involve the thymus-derived lymphocytes (T cells). Anti-thymus antibody was present in 90% of the patients, and it paralleled the frequency of thymic abnormality in myasthenia gravis. It is postulated that in myasthenia gravis the altered T cell functions caused by anti-thymus antibody result in the formation of an array of autoantibodies including the factor which blocks the neuromuscular transmission.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdou N. I., Lisak R. P., Zweiman B., Abrahamsohn I., Penn A. S. The thymus in myasthenia gravis. Evidence for altered cell populations. N Engl J Med. 1974 Dec 12;291(24):1271–1275. doi: 10.1056/NEJM197412122912403. [DOI] [PubMed] [Google Scholar]
- Adner M. M., Isé C., Schwab R., Sherman J. D., Dameshek W. Immunologic studies of thymectomized and nonthymectomized patients with myasthenia gravis. Ann N Y Acad Sci. 1966 Jan 26;135(1):536–554. doi: 10.1111/j.1749-6632.1966.tb45501.x. [DOI] [PubMed] [Google Scholar]
- Aharonov A., Tarrab-Hazdai R., Abramsky O., Fuchs S. Immunological relationship between acetylcholine receptor and thymus: a possible significance in myasthenia gravis. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1456–1459. doi: 10.1073/pnas.72.4.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammann A. J., Pelger R. J. Determination of antibody to pneumococcal polysaccharides with chromic chloride-treated human red blood cells and indirect hemagglutination. Appl Microbiol. 1972 Nov;24(5):679–683. doi: 10.1128/am.24.5.679-683.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel S. H., Almon R. R., Levy N. Acetylcholine receptor antibodies in myasthenia gravis. N Engl J Med. 1975 Oct 9;293(15):760–761. doi: 10.1056/NEJM197510092931508. [DOI] [PubMed] [Google Scholar]
- Armstrong R. M., Nowak R. M., Falk R. E. Thymic lymphocyte function in myasthenia gravis. Neurology. 1973 Oct;23(10):1078–1083. doi: 10.1212/wnl.23.10.1078. [DOI] [PubMed] [Google Scholar]
- BEUTNER E. H., WITEBSKY E., RICKEN D., ADLER R. H. Studies on autoantibodies in myasthenia gravis. JAMA. 1962 Oct 6;182:46–58. [PubMed] [Google Scholar]
- Bender A. N., Ringel S. P., Engel W. K., Daniels M. P., Vogel Z. Myasthenia gravis: a serum factor blocking acetylcholine receptors of the human neuromuscular junction. Lancet. 1975 Mar 15;1(7907):607–609. doi: 10.1016/s0140-6736(75)91886-3. [DOI] [PubMed] [Google Scholar]
- Field E. J., Bates D., Shaw D. A., Griffin S. G., Shenton B. K., Smith K. Lymphocyte sensitisation in myasthenia gravis: function of the adult thymus gland. Lancet. 1973 Sep 22;2(7830):675–676. doi: 10.1016/s0140-6736(73)92510-5. [DOI] [PubMed] [Google Scholar]
- Goust J. M., Castaigne A., Moulias R. Delayed hypersensitivity to muscle and thymus in myasthenia gravis and polymyositis. Clin Exp Immunol. 1974 Sep;18(1):39–47. [PMC free article] [PubMed] [Google Scholar]
- Hobbs J. R. Immune imbalance in dysgammaglobulinaemia type IV. Lancet. 1968 Jan 20;1(7534):110–114. doi: 10.1016/s0140-6736(68)92721-9. [DOI] [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junge U., Hoekstra J., Wolfe L., Deinhardt F. Microtechnique for quantitative evaluation of in vitro lymphocyte transformation. Clin Exp Immunol. 1970 Sep;7(3):431–437. [PMC free article] [PubMed] [Google Scholar]
- Kao I., Drachman D. B. Thymic muscle cells bear acetylcholine receptors: possible relation to myasthenia gravis. Science. 1977 Jan 7;195(4273):74–75. doi: 10.1126/science.831257. [DOI] [PubMed] [Google Scholar]
- Koffler D., Carr R. I., Agnello V., Fiezi T., Kunkel H. G. Antibodies to polynucleotides: distribution in human serums. Science. 1969 Dec 26;166(3913):1648–1649. doi: 10.1126/science.166.3913.1648. [DOI] [PubMed] [Google Scholar]
- Koziner B., Bloch K. J., Perlo V. P. Distribution of peripheral blood latex-ingesting cells, T cells, and B cells in patients with myasthenia gravis. Ann N Y Acad Sci. 1976;274:411–420. doi: 10.1111/j.1749-6632.1976.tb47702.x. [DOI] [PubMed] [Google Scholar]
- Lisak R. P., Abdou N. I., Zweiman B., Zmijewski C., Penn A. S. Aspects of lymphocyte function in myasthenia gravis. Ann N Y Acad Sci. 1976;274:402–410. doi: 10.1111/j.1749-6632.1976.tb47701.x. [DOI] [PubMed] [Google Scholar]
- Mittag T., Kornfeld P., Tormay A., Woo C. Detection of anti-acetylcholine receptor factors in serum and thymus from patients with myasthenia gravis. N Engl J Med. 1976 Mar 25;294(13):691–694. doi: 10.1056/NEJM197603252941303. [DOI] [PubMed] [Google Scholar]
- Mittal K. K., Rossen R. D., Sharp J. T., Lidsky M. D., Butler W. T. Lymphocyte cytotoxic antibodies in systemic lupus erythematosus. Nature. 1970 Mar 28;225(5239):1255–1256. doi: 10.1038/2251255a0. [DOI] [PubMed] [Google Scholar]
- Miyawaki S., Ritchie R. F. Heterogeneity of antinucleolar antibody and IgE antinuclear antibody in patients with systemic rheumatic diseases. J Immunol. 1974 Oct;113(4):1346–1352. [PubMed] [Google Scholar]
- Mori R., Kawanami S. Destruction of cultured thymus cells by autologous lymphocytes from a patient with myasthenia gravis. Lancet. 1973 Jan 27;1(7796):210–210. doi: 10.1016/s0140-6736(73)90051-2. [DOI] [PubMed] [Google Scholar]
- NASTUK W. L., PLESCIA O. J., OSSERMAN K. E. Changes in serum complement activity in patients with myasthenia gravis. Proc Soc Exp Biol Med. 1960 Oct;105:177–184. doi: 10.3181/00379727-105-26050. [DOI] [PubMed] [Google Scholar]
- NASTUK W. L., PLESCIA O. J., OSSERMAN K. E. Changes in serum complement activity in patients with myasthenia gravis. Proc Soc Exp Biol Med. 1960 Oct;105:177–184. doi: 10.3181/00379727-105-26050. [DOI] [PubMed] [Google Scholar]
- Richman D. P., Patrick J., Arnason B. G. Cellular immunity in myasthenia gravis. Response to purified acetylcholine receptor and autologous thymocytes. N Engl J Med. 1976 Mar 25;294(13):694–698. doi: 10.1056/NEJM197603252941304. [DOI] [PubMed] [Google Scholar]
- Ross G. D., Rabellino E. M., Polley M. J., Grey H. M. Combined studies of complement receptor and surface immunoglobulin-bearing cells and sheep erythrocyte rosette-forming cells in normal and leukemic human lymphocytes. J Clin Invest. 1973 Feb;52(2):377–385. doi: 10.1172/JCI107194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp G. C., Irvin W. S., LaRoque R. L., Velez C., Daly V., Kaiser A. D., Holman H. R. Association of autoantibodies to different nuclear antigens with clinical patterns of rheumatic disease and responsiveness to therapy. J Clin Invest. 1971 Feb;50(2):350–359. doi: 10.1172/JCI106502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai T., Yoshiki T., Mellors R. C. Thymus dependence of cells in peripheral lymphoid tissues and in the circulation sensitive to natural thymocytotoxic autoantibody in NZB mice. J Immunol. 1972 Jul;109(1):32–37. [PubMed] [Google Scholar]
- Simpson J. A., Behan P. O., Dick H. M. Studies on the nature of autoimmunity in myasthenia gravis. Evidence for an immunodeficiency type. Ann N Y Acad Sci. 1976;274:382–389. doi: 10.1111/j.1749-6632.1976.tb47699.x. [DOI] [PubMed] [Google Scholar]
- Simpson J. A. Myasthenia gravis as an autoimmune disease: clinical aspects. Ann N Y Acad Sci. 1966 Jan 26;135(1):506–516. doi: 10.1111/j.1749-6632.1966.tb45499.x. [DOI] [PubMed] [Google Scholar]
- Stobo J. D., Talal N., Paul W. E. Lymphocyte classes in New Zealand mice. II. Decreased frequency of immunoglobulin-bearing lymphocytes and increased frequency of lymphocytes lacking detectable theta or immunoglobulin determinants. J Immunol. 1972 Oct;109(4):701–710. [PubMed] [Google Scholar]
- Strauss A. J., Kemp P. G., Douglas S. D. Myasthenia gravis. Lancet. 1966 Apr 2;1(7440):772–773. doi: 10.1016/s0140-6736(66)90931-7. [DOI] [PubMed] [Google Scholar]
- Stutman O. Lymphocyte subpopulations in NZB mice: deficit of thymus-dependent lymphocytes. J Immunol. 1972 Sep;109(3):602–611. [PubMed] [Google Scholar]
- Terasaki P. I., Mottironi V. D., Barnett E. V. Cytotoxins in disease. Autocytotoxins in lupus. N Engl J Med. 1970 Oct 1;283(14):724–728. doi: 10.1056/NEJM197010012831403. [DOI] [PubMed] [Google Scholar]
- Vyas G. N., Perkins H. A., Fudenberg H. H. Anaphylactoid transfusion reactions associated with anti-IgA. Lancet. 1968 Aug 10;2(7563):312–315. doi: 10.1016/s0140-6736(68)90527-8. [DOI] [PubMed] [Google Scholar]
- Waldmann T. A., Bull J. M., Bruce R. M., Broder S., Jost M. C., Balestra S. T., Suer M. E. Serum immunoglobulin E levels in patients with neoplastic disease. J Immunol. 1974 Jul;113(1):379–386. [PubMed] [Google Scholar]
- Wekerle H., Ketelsen U. P. Intrathymic pathogenesis and dual genetic control of myasthenia gravis. Lancet. 1977 Mar 26;1(8013):678–680. doi: 10.1016/s0140-6736(77)92118-3. [DOI] [PubMed] [Google Scholar]
- Wernet P., Kunkel H. G. Antibodies to a specific surface antigen of T cells in human sera inhibiting mixed leukocyte culture reactions. J Exp Med. 1973 Oct 1;138(4):1021–1026. doi: 10.1084/jem.138.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. C., Jr, DeBoard J. R., Mellbye O. J., Messner R. P., Lindström F. D. Studies of T- and B-lymphocytes in patients with connective tissue diseases. J Clin Invest. 1973 Feb;52(2):283–295. doi: 10.1172/JCI107184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunis E. J., Stutman O., Good R. A. Thymus, immunity and autoimmunity. Ann N Y Acad Sci. 1971 Sep 15;183:205–220. doi: 10.1111/j.1749-6632.1971.tb30752.x. [DOI] [PubMed] [Google Scholar]
- van der Geld H. W., Strauss A. J. Myasthenia gravis. Immunological relationship between striated muscle and thymus. Lancet. 1966 Jan 8;1(7428):57–60. doi: 10.1016/s0140-6736(66)92356-7. [DOI] [PubMed] [Google Scholar]