Abstract
Background
Serum caffeine concentrations >20µg/mL (100 µM) in infants treated for apnea of prematurity increases TNF-α and decreases IL-10, change that perhaps is linked to co-morbidities. We hypothesize that this pro-inflammatory cytokine profile may be linked to differential binding of caffeine to adenosine receptor subtypes (AR), inhibition of phosphodiesterases (PDEs), and modulation of toll-like receptors (TLR).
Methods
LPS-activated cord blood monocytes (CBM) from 19 infants were exposed to caffeine (0 to 200 µM) with or without previous exposure to A1R, A3R, or PDE IV antagonists to determine changes in dose-response curves. Cytokines levels (ELISA), intracellular cAMP accumulation (EIA) and TLR gene expression (real time qRT PCR) were measured.
Results
Caffeine at ≤100µM decreased TNF-α levels (~25%, p=0.01) and cAMP. All caffeine concentrations decreased IL-10 levels (17 to 35%, p<0.01). A1R, A3R and PDE blockades decreased TNF-α (31%, 21%, and 88%, p≤0.01), but not IL-10. Caffeine further decreased TNF-α following A3R and PDE blockades. Caffeine concentrations directly correlated to TLR4 gene expression (r=0.84; p<0.001).
Conclusion
Neither A3R, nor PDE blockades are involved in caffeine’s modulation of cytokine release by CBM at any concentration. Besides A1R blockade, caffeine’s up-regulation of TLR4 may promote inflammation at high concentrations.
INTRODUCTION
Adenosine binding to any of the four 7-transmembrane spanning G-protein-coupled receptors, A1R, A2aR, A2bR and A3R, modulates inflammation (1,2). Caffeine, a non-specific adenosine receptor (AR) antagonist, is used to treat apnea in premature infants and at concentration of 50 µM in culture (equivalent to 10 µg/ mL in serum) increases intracellular cAMP accumulation and attenuates TNF-α secretion by blocking A1R on LPS-activated human cord blood monocytes (CBM) (3). Although this mechanism may be operative in the decreased incidence of BPD and neurodevelopmental disabilities observed in infants treated with caffeine (4,5) and in animal models (6), decrease in the anti-inflammatory cytokine, IL-10, along with increase in TNF-α in tracheal aspirates and peripheral blood in preterm infants who have serum caffeine levels ≥ 20 µg/mL (equivalent to 100 µM in culture) raise concerns (7). The mechanisms explaining caffeine’s polar opposite effects in the inflammatory cascade are still unclear, but highly relevant in the design of new strategies to prevent morbidities related to chronic inflammation, like BPD, in premature infants (8).
ARs are either negatively (A1R and likely A3R) or positively (A2Rs) coupled to adenylyl cyclase, decreasing or increasing intracellular cAMP levels, respectively (1,9). Changes in cAMP inversely modulate the expression of transcription factors and their final products, cytokines and chemokines, via protein kinase A (PKA)-mediated pathways (10–13). Caffeine demonstrates the highest affinity for A1R and the lowest affinity for A2bR, which increases or decreases cAMP levels, respectively. At concentrations at least 40 times higher than those needed to antagonize A1Rs, caffeine also inhibits phosphodiesterase (PDE) activity (14), which further increases cAMP accumulation. Additionally, activation of ARs antagonize inflammatory cascades activated by toll-like receptors (TLRs) on mononuclear cells (15,16) as shown by the failure of TLR4 agonists to induce TNF-α release following pre-treatment with A2aR agonists (17). Although caffeine may inhibit TLR-mediated inflammatory cascades in macrophages by suppressing calcium mobilization (18), it may also trigger inflammation by preventing the AR-mediated antagonism of TLRs and perhaps by changing their expression (19). Hence, we hypothesize that the pro-inflammatory cytokine profile observed with high serum caffeine levels (>20 µg/mL, equivalent to > 100 µM in culture) in premature infant at risk for BPD (7) may be linked to differential binding of caffeine to AR subtypes, inhibition of PDEs, and modulation of other components of the inflammatory cascade, such as TLRs.
RESULTS
Cord blood from 19 neonates (mean gestational age ± SD = 39.6 ± 1.2 weeks; birth weight = 3286 ± 505 g; Table 1) was used for experiments in culture. Cord blood serum caffeine levels (mean ± SD = 0.67 ± 1.12 µg/ml) were below the therapeutic range.
TABLE 1.
DEMOGRAPHICS AND SERUM CAFFEINE LEVELS
| Number of subjects | 19 |
| Gestational age (mean ± SD) | 39 4/7 ± 1.2 weeks |
| Birth weight (mean ± SD) | 3286 ± 505 grams |
| Gender | 73% male |
| Race | 78 % AA ; 11% C |
| Apgar 1 minute (median, range) | 8 (6–9) |
| Apgar 5 minute (median, range) | 9 (8–9) |
| Delivery mode | 21% C/S |
| Caffeine level (mean ± SD) | 0.67 ± 1.12 ug/ ml |
AA, African American; Apgars, Apgar score; C, Caucasian; C/S, cesarean section
Baseline changes in cAMP and cytokine mRNA and protein levels
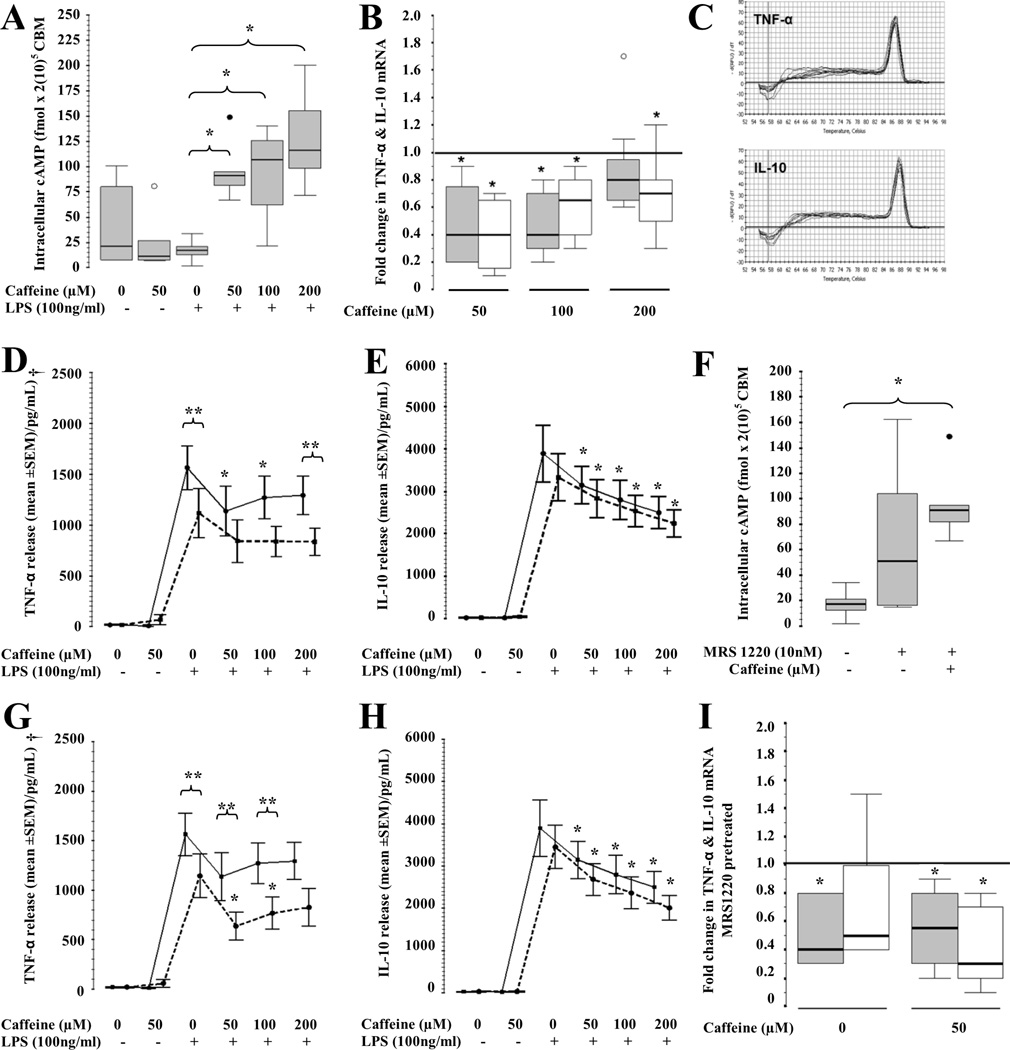
Neither caffeine alone nor LPS alone increased intracellular cAMP accumulation in CBM by 24h. In contrast, caffeine treatment followed by LPS-activation increase cAMP accumulation by 4 to 6-fold (p<0.01 vs. LPS-exposed, Fig. 1A). Consistent with these findings, TNF-α gene expression decreased by 60% following caffeine exposure at concentrations of 50 or 100µM, but at higher concentrations (200 µM) did not decrease TNF-α transcription any longer. Conversely, all tested caffeine concentrations decreased IL-10 gene expression by 25 to 60% (p<0.05 in all cases, Fig.1B and 1C). LPS exposure increased TNF-α and IL-10 release by CBM (p<0.001 vs. no LPS) and caffeine modulated those responses (p=0.01 and p<0.001, respectively). Caffeine at 50 and 100 µM decreased TNF-α levels by 25% (IQR −67 to −5%, p=0.01) and 24% (IQR −30 to −2%, p=0.02); respectively, while at 200 µM did not produce changes (Fig. 1D). Conversely, caffeine at 50, 100 and 200 µM decreased IL-10 by 17% (IQR 34 to 8 %, p=0.006), 27% (IQR 34 to 21%, p=0.003), and 35% (IQR 46 to 25%, p=0.003), respectively (Fig. 1E).
Figure 1.
Effect of caffeine at 50, 100 and 200 µM in (A) intracellular cAMP accumulation (fmol /2(10)5 CBM; n=7). Box and whiskers plot representing median and IQR. *, p<0.05 (Wilcoxon rank test); ○, outliers; ●, extremes. (B) Fold-change in TNF-α (gray) and IL-10 (white) gene expression from reference line (at 1, caffeine at 0 µM; n=9). Box and whiskers plot representing median and IQR. *, p<0.05 (Wilcoxon rank test); ○, outliers; ●, extremes. (C) Melting curves for TNF-α and IL-10 PCR products. (D) TNF-α and (E) IL-10 release from CBM following caffeine exposure (0, 50, 100, 200 µM) alone (continuous line) or combined (dashed line) with DPCPX (A1R antagonist) pre-treatment. Mean ± SEM (n=11). *,p<0.01 vs. LPS-activated CBM exposed to 0µM of caffeine (Bonferroni). **, p<0.01 vs. pre-treatment counterpart. †, overall p=0.02 (Two-way repeat measures ANOVA). (F) Intracellular cAMP concentration (fmol /2(10)5 CBM; n=6) following caffeine (50uM) and MRS1220 alone and combined. Box and whiskers plot representing median and IQR. *, p<0.05 (Wilcoxon rank test); ○, outliers; ●, extremes. (G) TNF-α and (H) IL-10 release from CBM curve in response to caffeine (0, 50, 100, 200 µM) alone (continuous) or combined (dashed) with MRS1220 (A3R antagonist) pre-treatment. Mean ± SEM (n=11). *,p<0.01 vs. LPS-activated CBM exposed to 0µM of caffeine (Bonferroni). **, p<0.01 vs. pre-treatment counterpart. †, overall p=0.002 (Two-way repeat measures ANOVA). (I) Fold change of TNF-α (gray) and IL-10 (white) gene expression after MRS1220 pre-treatment with and without caffeine (50 µM; n=6). *,p<0.05 (Wilcoxon rank test) vs. reference line (at 1, no pre-treatment).
The differential role of A1R and A3R in the modulation of cytokines by caffeine
DPCPX, a specific A1R antagonist (10 nM), decreased TNF-α secretion by 31% from baseline (IQR − 60 to −6 %, p=0.01). The addition of caffeine at 50 and 100 µM did not induce further TNF-α decrease. However, caffeine at 200 µM still significantly decreased TNF-α in CBM when pre-treated with DPCPX, unlike in those not pre-treated (p=0.001, discontinuous line) (Fig 1D). A1R blockade did not modify the effect of caffeine on IL-10 at any concentration (Fig.1E)
In LPS-activated CBM, exposure to caffeine alone (Fig. 1A) or combined with MRS1220 (A3R antagonist, 10 nM) significantly increased intracellular cAMP levels (Fig 1F). MRS1220 alone decreased TNF-α secretion by 21% (−45 to −13%; p=0.008), while the addition of caffeine at 50 and 100 µM produced an additional decrease of 40% (IQR −60 to −16) and 39% (IQR −62 to −15), respectively (p=0.001 in all cases vs. MRS1220 alone) with no additional changes induced by caffeine at 200 µM (Fig. 1G). In contrast, IL-10 was not significantly modulated by A3R blockade (Fig. 1H). While MRS1220 inhibited TNF-α gene expression by 65% (IQR −72 to −10; p=0.02), it did not change IL-10 gene expression. In contrast, caffeine at 50 µM added to MRS1220 inhibited gene expression of both, TNF-α and IL-10 (Fig. 1I).
The effect of caffeine in cytokines is independent of PDE IV blockade
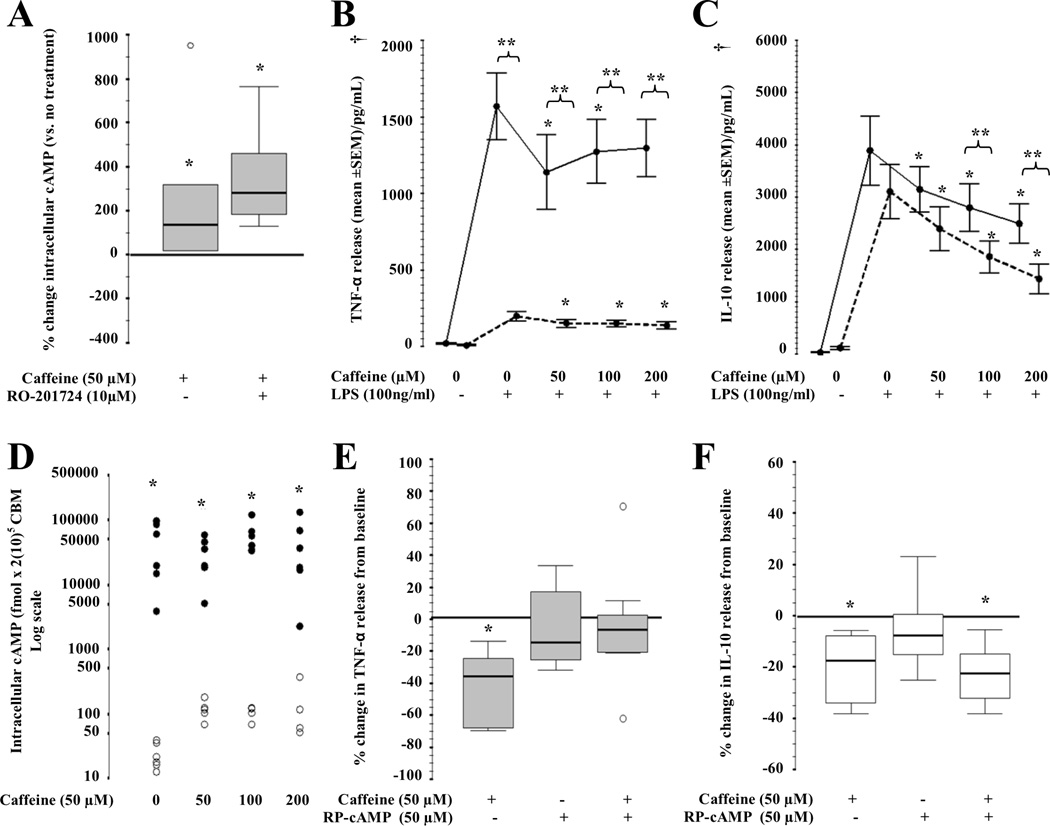
RO-201724 (PDE IV inhibitor, 10µM) increased intracellular cAMP levels by 134% (20 to 476; p=0.02), while the combined treatment with caffeine (50 µM) increased cAMP levels by 283% (167 to 535; p=0.02; Fig. 2A). In CBM, pre-treatment with RO-201724 produced an 88% (IQR −90 to −84) decrease in the release of TNF-α (p<0.001 vs. no pre-treatment). In the presence of RO-201724, the addition of caffeine at 50, 100 and 200 uM further decreased TNF-α release by CBM by 24% (IQR −49 to − 8); 19% (IQR −39 to −5) and 21% (IQR −44 to −20) (p<0.001, Fig. 2B). On the other hand, PDE IV inhibition alone did not modify IL-10 release by CBM; however, in the presence of caffeine at 100 or 200 µM, IL-10 levels were 39% and 46% lower than with caffeine exposure alone, (p<0.01). Caffeine, at all concentrations, decreased IL-10 secretion regardless of PDE inhibition (p<0.01, Fig 2C).
Figure 2.
(A) Intracellular cAMP accumulation (fmol /2(10)5 CBM; n=6) following RO-201724 (PDE IV inhibitor) alone and combined with caffeine (50µM). Box and whiskers plot representing median and IQR. *,p<0.05 (Wilcoxon rank test). ○, outliers. (B) TNF-α and (C) IL-10 release from CBM curve in response to caffeine (0, 50, 100, 200 µM) alone (continuous line) or combined (dashed line) with RO-201724 pre-treatment. Mean ± SEM (n=11). *,p<0.01 vs. LPS-activated CBM exposed to 0µM of caffeine (Bonferroni). **, p<0.05 vs. pre-treatment counterpart. †, overall p<0.05 (Two-way repeat measures ANOVA). (D) Intracellular cAMP accumulation (fmol /2(10)5 CBM; log scale; n=6) following caffeine exposure (0, 50, 100, 200 uM) alone (○) and combined (●) with RP-cAMPs (PKA inhibitor; 50 uM) pre-treatment. *,p<0.05 (Wilcoxon rank test). (E) TNF-α (gray) and (F) IL-10 (white) release from CBM curve in response to RP-cAMP and/or caffeine (n=7). *,p<0.05 (Wilcoxon rank test) vs. no treatments. ○, outliers.
Unlike IL-10, TNF-α modulation by caffeine depends on PKA activity
To determine the role of cAMP-dependent PKA in the pathway inhibiting the transcription of TNF-α and IL-10 in response to caffeine, CBM were pretreated with RP-cAMPs (50 µM), a PKA inhibitor, prior to caffeine and LPS exposure. Following pre-treatment with RP-cAMPs, intracellular cAMP accumulation increased by 200-fold (Fig. 2D) in CBM. Despite inducing high intracellular cAMP levels, RP-cAMP blocked the decrease in TNF-α induced by caffeine (Fig. 2E). In contrast, RP-cAMPs did not modify the effect produced by caffeine on IL-10 secretion (Fig. 2F).
Caffeine alters TLR transcription
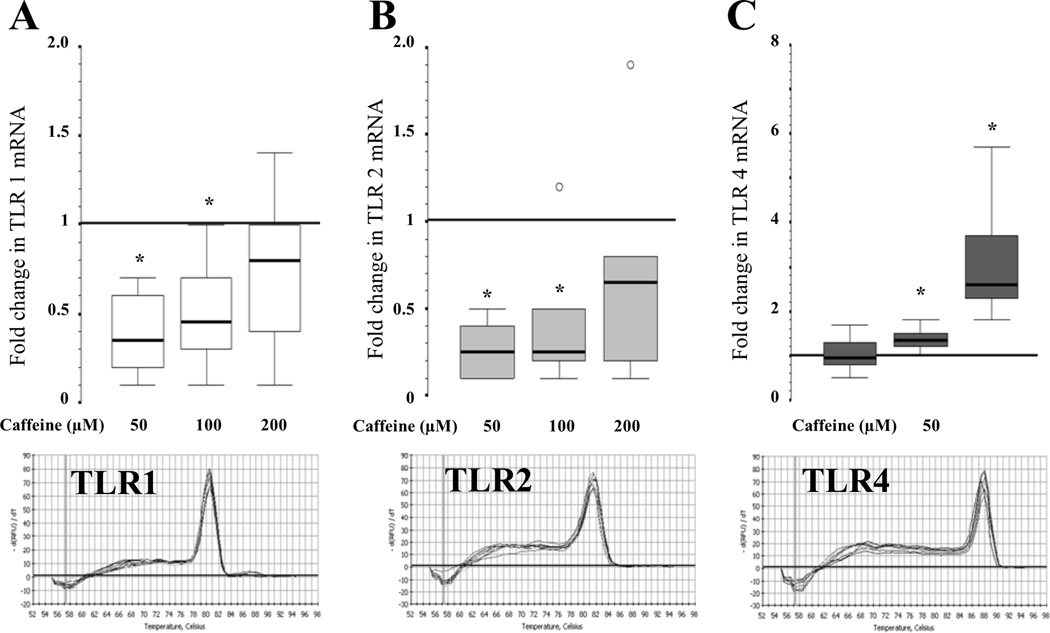
While TLR3 mRNA was not detected in CBM by real time qRT-PCR, TLR1, TLR2 and TLR4 gene expression was detected and modified by caffeine in a concentration-dependent manner. Caffeine at 50 and 100 µM down-regulated the expression of TLR1 by 65% (83 to 37%; p=0.02) and 55% (75 to 23%; p=0.04), and TLR2 by 75% (90 to 57%; p=0.02) and 74% (83% to 33%; p=0.04); respectively (Fig.3A and 3B). Caffeine at 200 µM did not alter TLR1 or TLR2 mRNA levels. TLR4 gene expression was directly correlated with the concentration of caffeine (r=0.84; p<0.001; Spearman’s correlation). While TLR4 gene expression was unchanged in CBM exposed to 50 µM of caffeine, it was up-regulated by 2.6-fold (2.2 to 4.2; p=0.02) when exposed to 200 µM (Fig. 3C).
Figure 3.
Fold change in (A) TLR1; (B) TLR2 and (C) TLR4 gene expression induced by caffeine at 50, 100 and 200 µM (n=7). *,p<0.05 (Wilcoxon rank test) vs. reference line (at 1, caffeine at 0 µM). ○, outliers. Melting curves for PCR products are shown.
DISCUSSION
Caffeine at 50 µM (equivalent to 10 µg/mL in serum) decreases TNF-α secretion by CBM via A1R blockade (3), but in-vivo data suggest that higher serum caffeine concentrations are associated higher TNF-α, IL-8, IL-6 and lower IL-10, suggesting a pro-inflammatory profile potentially linked to BPD and other comorbidities (7). The mechanisms may involve not only non-specific ARs blockade, but also inhibition of PDE IV and alteration in TLR expression. Using a culture treatment paradigm modeled to replicate the impaired hepatic metabolism of caffeine in neonates and the relevant time and concentrations of caffeine in infants receiving daily doses to achieve serum concentrations between 10 to 20 µg/ mL or higher, we show that caffeine at concentrations of 50 and perhaps 100 µM (equivalent to 10 and 20 µg/mL, respectively) decrease TNF-α and IL-10 secretions by CBM, while at concentrations greater than 100 µM only decrease IL-10 secretion and gene expression. The blockade of A1R, but not A3R, is operative in the effect of caffeine on TNF-α secretion via a PKA-mediated mechanism, while neither of these receptors is involved in the effects on IL-10. PDE IV inhibition does not play a significant role in caffeine’s immunomodulatory effects. Additionally, caffeine may promote the activation of TLR-mediated inflammatory pathways relevant to sick neonates, by up-regulating TLR 4 in a concentration dependent manner.
A1R expression is up-regulated on CBM following LPS exposure (a TLR4 agonist), suggesting the important role of this receptor in the control of TLR-mediated inflammation in neonates (3). A1R blockade or A2aR activation produces an equally significant increase in cAMP accumulation and decrease in TNF-α production by adult monocytes (20). In accordance with these findings, our results using CBM suggest that low caffeine concentrations (50 µM – equivalent to 10 µg/mL in serum) preferentially block A1R, probably allowing adenosine to bind to A2aR (Gαs-coupled), thereby increasing cAMP production (1). At increasing concentrations, this preferential A1R blockade by caffeine may be lost.
A3R is highly expressed in inflammatory tissues and mononuclear cells (3,21), but the interaction with adenylyl cyclase (protein Gi or Gq) varies by cellular type (1,20). In either case, the net effect of A3R blockade is cAMP accumulation and decrease cytokine expression. Similar to the effect described in adult peripheral blood monocytes (20), our results show that A3R blockade (MRS1220) increases cAMP accumulation and decreases TNF-α production. Caffeine significantly intensifies the effect produced by MRS1220 alone, suggesting caffeine effects are independent of A3R blockade in CBM.
Xanthines also decrease TNF-α and IL-10 release from mononuclear cells via PDE inhibition and cAMP accumulation (14,22–24). However, because caffeine has a considerably higher IC50 for PDE inhibition than for cytokine modulation (23), serum concentrations 25 times higher than those achieved during standard treatment for apnea of prematurity would be required to elicit PDE inhibition in vivo (4,14). Here, we show that although RO-201724 decreases TNF-α secretion by CBM, caffeine further decreases this cytokine suggesting that PDE inhibition does not explain the effect of caffeine on TNF-α. In contrast, the inhibition of PDE IV and the subsequent increase in cAMP accumulation does not significantly modify IL-10 levels, suggesting that the pathway mediating the effect of caffeine in IL-10 is not primarily cAMP-dependent.
Adult monocytes express TLR1, TLR2 and TLR4, among many others; however, the expression profile on CBM has not been well defined. Contrary to TLR2 expression on CBM, TLR4 directly correlates with gestational age (25,26), and further expression is induced by LPS (27). Because LPS is a TLR4 agonist and caffeine mediates the inhibition of LPS – induced TNF-α production by CBM, we hypothesized that changes in TLR expression may account for some of caffeine immunomodulatory effects. Here, we report the direct correlation between caffeine concentrations and TLR4 expression, as well as the inhibitory effect of caffeine at 50 and 100 µM on TLR1 and TLR2 expression. In LPS-activated CBM, the increase TLR4 expression induced by caffeine at 200 µM, may up-regulate TNF-α expression via MyD88-NFkB pathway (10,13). The implications of TLR1 and TLR2 down-regulation induced by caffeine remain unexplored but this novel mechanism may explain some of the anti-inflammatory properties derived from clinical studies demonstrating decrease incidence of BPD (4).
The pathways mediated by TLR-NFκB (21,28) and cAMP-PKA (10,20) are the main biochemical cascades regulating TNF-α production by adult monocytes. NFκB, a transcription factor for TNF-α, also up-regulates A2R expression following LPS exposure as a delayed feedback attenuating persistent inflammation (29). The decrease in TNF-α production following AR activation can be abolished by the blockade of PKA (20). Similarly, we show that caffeine inhibitory effect on TNF-α transcription in CBM is suppressed by RP-cAMPs, suggesting that this process is PKA mediated.
Adenosine decreases TNF-α and increases IL-10 production by monocytes, although the specific signaling cascade remains unknown (30,31). AR activation induces accumulation of the IL-10 transcription factor C/EBPβ (enhancing-binding protein) (32). Transient MAPK-mediated phosphorylation of histone H3 in the IL-10 promoter may facilitate C/EBPβ binding via a cAMP-PKA independent pathway (33,34). Here, we show that caffeine produces a transcriptional down-regulation of IL-10, which is not mediated by A1R or A3R blockade and unlike TNF-α is PKA independent. We speculate that the modulation of A2R expression or function is involved in the effect of caffeine on IL-10 and the mechanisms may include: i) the prevention of LPS-induced up-regulation of A2R expression by caffeine (3), which by decreasing A2R availability, decreases transcription factors (C/EBPβ) and IL-10 production; and ii) the non-specific blockade of A2aR, as well as A1R by caffeine, which at increasing concentrations further decrease IL-10 and increase TNF-α production, as observed in our experiments. The effects of caffeine in other steps of the IL-10 signaling pathway are still unexplored.
In conclusion, caffeine decreases the release of TNF-α and IL-10 via distinct mechanisms. A1R, but not A3R, is operative in the effect of caffeine on TNF-α, while neither of these receptors modulates IL-10 production by CBM. Similarly, caffeine’s immunomodulatory effects are not primarily produced by PDE inhibition. The mechanism responsible for the decrease of IL-10 produced by caffeine is still unclear, but is potentially related to the modulation of A2R expression and/or function. Here, we also describe for the first time the inhibition of TLR1 and TLR2, and the induction of TLR4 expression on LPS-activated CBM exposed to caffeine, which extends our understanding about the immunomodulatory effects of this drug in the newborn population. The understanding of the distinct effects of caffeine on cytokines is relevant in the context of the potential side effects of its use in the clinical setting in a population at high risk for BPD and other morbidities linked to inflammation.
METHODS
Subjects
This study complied with the Guidelines for Human Experimentation from the U.S. Department of Health and Human Services and received approval from the Johns Hopkins Medicine Institutional Review Board (NA_00002034). The work has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) (35). Only cord blood of subjects whose parents provided informed consent for the study was considered for experiments. CBM were isolated from full-term infants (≥ 37 weeks gestation). Cord blood was collected after repeat cesarean section without labor or vaginal delivery without evidence of chorioamnionitis. We excluded births with known genetic disorder, intrauterine growth restriction or small for gestational age (birth weight < 10th percentile for gestational age), and suspected viral infection (based on serological or clinical findings). Infants who subsequently received antibiotics or had illness sufficient to be admitted to the neonatal intensive care unit (NICU) were removed from final experiments. Serum caffeine levels were obtained on all blood samples and those with levels > 4 µg/ mL were removed from final analysis.
CBM isolation
Cord blood was collected in EDTA and in no-additive tubes for monocyte isolation and caffeine level determination, respectively (BD Biosciences, Franklin Lakes,NJ). Blood samples were centrifuged at 2400 × g for 10 m at 25°C, cellular portion was reconstituted in Dubelcco’s phosphate-buffered saline (DPBS, pH 7.4; Mediatech Inc, Herndon,VA), and CBM were isolated using Ficoll-Hypaque gradient (GE Healthcare Biosciences AB, Uppsala, Sweden). CBM were then washed and reconstituted in 4× RPMI 1640 media containing 8% (v/v) human AB serum, penicillin/streptomycin (400 IU/ml /400 ug/ml), and 8 mM L-glutamine (Sigma-Aldrich, St. Louis, MO). Cell count and viability was determined using ammonium-chloride-potassium lysing buffer (Quality Biological, Inc., Gaithersburg,MD) and the trypan blue exclusion method (Gibco, Grand Island,NY), respectively (36). Viable CBM were cultured and used in experiments, as outlined below.
Treatment protocol in culture
Neonates treated with caffeine citrate for apnea of prematurity receive daily maintenance doses to achieve serum levels of 10 to 20 µg/ mL. Due to immaturity of the cytochrome P-450 system in neonates, the hepatic metabolism of caffeine is limited for several weeks after birth (37,38). To model the array of caffeine’s effects in cytokine levels in the blood of neonates with limited hepatic clearance, we used a single dose of caffeine in citrated buffer to achieve comparable concentrations in culture (50 to 100 µM) and since some neonates achieve levels > 20 µg/ mL, which may switch the cytokine expression response7, we also tested a concentration of 200 µM.
Caffeine, A1R antagonist (DPCPX), A3R antagonist (MRS1220), PDE IV inhibitor (RO-201724), and cAMP-dependent PKA antagonist (RP-cAMPs) (Table 2) were used to investigate the changes in the dose-response curves of increasing caffeine concentrations in TNF-α and IL-10 release by CBM. Caffeine was reconstituted in citric acid monohydrate based water (pH 7.3), which in solution forms caffeine citrate salts. All other antagonists were reconstituted in DMSO (cell-culture-concentration: 2.7 × 10−6 g/ml). RO-201724 was purchased from Calbiochem (San Diego, CA), and all other drugs were purchased from Sigma Chemical Co (St. Louis, MO). Although caffeine is a non-specific phosphodiesterase inhibitor at high concentrations, PDE IV is the most abundant in monocytes and has the greatest cAMP degradation activity. Thus, we selected RO-201724 to target this subfamily of PDEs.
TABLE 2.
LIST OF ANTAGONISTS AND INHIBITORS
| Antagonist | Synonym | Dose (s) | Target |
|---|---|---|---|
| Caffeine | 1,3,7-Trimethylxanthine | 50, 100, 200 µM | A1 > A2 > A3 |
| DPCPX | 1,3-Dipropyl-8cyclopentylxanthine | 10 nM | A1 |
| MRS1220 | 9-Chloro-2-(2-furanyl)-5-(phenylacetyl amino)-[1,2,4]triazolo[1,5-c] quinazoline | 10 nM | A3 |
| RO-201724 | 4- (3- Butoxy- 4- methoxybenzyl)- 2- imidazolidinone | 10 µM | PDE IV |
| RP-cAMPs | Rp-Adenosine 3′,5′-cyclic monophosphorothioate triethylammonium salt hydrate | 50 µM | cAMP-PKA |
A1, A2a, A2b, A3, adenosine receptor subtypes; cAMP, cyclic adenosine monophosphate; PDE, phosphodiesterase; PKA, protein kinase A
CBM were treated with DPCPX, MRS1220, RO-201724 or RP-cAMPs 1 h before exposure to caffeine at 0, 50, 100 and 200 µM. Following an additional hour in culture (37°C /5%CO2), CBM were activated using Escherichia coli K235 LPS (100 ng/ml; Sigma-Aldrich). Twenty-four hours after LPS-activation, media were collected, centrifugated at 2000 × g for 5 min, and supernatants were used to measure cytokines levels by ELISA, while CBM attached to the well were recovered using trypsin-EDTA 0.05%, phenol free (Life Technology Corp. Carlsbad, CA), evaluated for viability using Trypan-blue exclusion method as described above, and then lysed for RNA isolation and real-time RT PCR or to measure intracellular cAMP accumulation by EIA.
CBM viability was assessed by their property to adhere to the plastic at 24h after LPS-activation. LPS activated CBM were observed under the inverted microscope to evaluate the morphological appearance and the degree of debris and to ascertain good quality of the culture. If any concerns, a 1 µl aliquot of recovered CBM from each well was evaluated using the trypan blue exclusion method. No difference in cell viability was observed between treatments. However, those culture plates that show increased cell death were discarded and not used for analysis.
Enzyme-Linked Immunosorbent Assay (ELISA) / Enzyme Immunoassay (EIA)
TNF-α and IL-10 concentrations were measured by ELISA using multiplex kits according to manufacturer’s protocol and concentrations were calculated using the Luminex detection platform (Millipore, Billerica, MA).
Intracellular cAMP levels were measured to confirm function of ARs during exposure to caffeine and other treatments. After exposure, CBM were lysed using 2.5% dodecyltrimethylammonium bromide in assay buffer (pH 5.8; 0.05 M sodium acetate buffer and 0.02% bovine serum albumin). Intracellular cAMP levels were measured using the Amersham cAMP EIA System (GE Healthcare, Little Chalfont, Buckinghamshire, UK) following manufacturer’s protocol as described previously (3), and results were reported corrected for 2 × 105 viable CBM.
Real Time Quantitative Reverse-Transcription Polymerase-Chain-Reaction (Real time qRT- PCR)
Total RNA was extracted from LPS-activated CBM to determine changes in cytokines (TNF-α, IL-10) and TLRs (TLR1, TLR2, TLR3, TLR4) gene expression following caffeine (50, 100 and 200 µM vs 0 µM) and MRS1220 (10nM, specific A3R antagonist) treatment alone and combined. RNA mini kit (Invitrogen, Carlsbad, CA) was used according to specifications. Approximately 1µg of total RNA was used for generation of cDNA using iScript cDNA synthesis kit (BioRad, Hercules, CA). Reverse transcription protocol included 5 minutes at 25°C; 30 minutes at 42°C and 5 minutes at 85°C. cDNA was then used to amplify target genes by real time qRT-PCR using primers at 300 nM [Table 3, (39)]. SYBR Green Supermix (BioRad) was used for signal detection by PCR Thermocycler (BioRad). The amplification protocols included 40 cycles of: 1 minute at 95.0 °C, 1 minute at 61.0 °C (cytokines) or 59.0 °C (TLRs), and 1 minute and 15 seconds at 72.0 °C. GAPDH (glyceraldehyde phosphate dehydrogenase), β-actin and G6PDH (glucose-6 phosphate dehydrogenase) were preliminarily tested to establish gene expression stability under experimental conditions using the BestKeeper approach (40). Based on these results GAPDH was used as the reference gene to calculate fold difference in gene expression using the Pfaffl method (41) as reported previously (3). Melting curves were used to ascertain purity of PCR products.
TABLE 3.
PRIMERS FOR REAL TIME qRT-PCR.
| Gene | Direction | Sequence (5’-3’) | Product | UniSTS |
|---|---|---|---|---|
| TNF-α | S | CACTAAGAATTCAAACTGGGGC | 166 bp | 28864 |
| AS | GAGGAAGGCCTAAGGTCCAC | |||
| IL-10 | S | ACCTGGGTTGCCAAGCCTTGTC | 158 bp | Ref 39a |
| AS | AAATCGATGACAGCGCCGTAGC | |||
| TLR1 | S | CACATCAAGTGAAAAATATTCCTCC | 151 bp | 24274 |
| AS | TAAATGGTGAACTGCGACCC | |||
| TLR2 | S | CTACTGGGTGGAGAACCTTATGGT | 76 bp | 1092 |
| AS | CCGCTTATGAAGACACAACTTGA | |||
| TLR3 | S | TCCTAGAAGAGATGTAATTG | 169 bp | 66831 |
| AS | CCCAAAAACTCTGTACATTA | |||
| TLR4 | S | GTTTCTGAGCAGTCGTGCAG | 172 bp | 480158 |
| AS | CAGGGCTTTTCTGAGTCGTC | |||
| GAPDH | S | AACAGCGACACCCACTCCTC | 258 bp | 270428 |
| AS | GGAGGGGAGATTCAGTGTGGT |
, not listed at UniSTS
AS, antisense; bp, base pair; GADPH, glyceraldehyde phosphate dehydrogenase; IL-10, interleukin-10; qRT-PCR, quantitative reverse-transcription polymerase-chain reaction; S, sense; TLR, Toll-like receptor; TNF, tumor necrosis factor
Statistical analysis
Nonparametric statistics including Bonferroni adjusted- Wilcoxon signed rank test and Friedman two-way ANOVA for ranks were applied. Results are reported as median with IQR (25th to 75th percentile) and represented as box-and-whisker plots with outliers (boxes symbolize IQR) for cAMP and RT-PCR data. To better represent dose-response curves for measured cytokines, these data are represented as mean ± SEM. Comparison between curves was made by two-way repeat measures ANOVA with Bonferroni correction for pair analysis. Significance was assigned by p ≤ 0.01. Analysis was performed using IBM SPSS 18.0 software (IBM corp., Armonk NY).
Acknowledgments
We thank Ariel Mason and Veronica Bostic for their administrative assistance as well as the obstetric team at Johns Hopkins Hospital for their support.
FINANCIAL SUPPORT: The National Institutes of Health (NIH) grant number HL-072748 (E.B.G); The Thomas Wilson Sanitarium for Children of Baltimore City (RCV) and The Sheila S. and Lawrence C. Pakula, M.D. Endowment for Neonatal Research (RCV).
Footnotes
DISCLOSURE: The authors declare no financial ties to products in the study or conflicts of interest.
REFERENCES
- 1.Hasko G, Pacher P, Deitch EA, Vizi ES. Shaping of monocyte and macrophage function by adenosine receptors. Pharmacol Ther. 2007;113:264–275. doi: 10.1016/j.pharmthera.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sipka S, Kovacs I, Szanto S, et al. Adenosine inhibits the release of interleukin-1beta in activated human peripheral mononuclear cells. Cytokine. 2005;31:258–263. doi: 10.1016/j.cyto.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Chavez-Valdez R, Wills-Karp M, Ahlawat R, Cristofalo EA, Nathan A, Gauda EB. Caffeine modulates TNF-alpha production by cord blood monocytes: the role of adenosine receptors. Pediatric research. 2009;65:203–208. doi: 10.1203/PDR.0b013e31818d66b1. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt B, Roberts RS, Davis P, et al. Caffeine therapy for apnea of prematurity. N Engl J Med. 2006;354:2112–2121. doi: 10.1056/NEJMoa054065. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt B, Roberts RS, Davis P, et al. Long-term effects of caffeine therapy for apnea of prematurity. N Engl J Med. 2007;357:1893–1902. doi: 10.1056/NEJMoa073679. [DOI] [PubMed] [Google Scholar]
- 6.Koroglu OA, MacFarlane PM, Balan KV, et al. Anti-inflammatory effect of caffeine is associated with improved lung function after lipopolysaccharide-induced amnionitis. Neonatology. 2014;106:235–240. doi: 10.1159/000363217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chavez Valdez R, Ahlawat R, Wills-Karp M, Nathan A, Ezell T, Gauda EB. Correlation between serum caffeine levels and changes in cytokine profile in a cohort of preterm infants. The Journal of pediatrics. 2011;158:57–64. e1. doi: 10.1016/j.jpeds.2010.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Shea TM, Shah B, Allred EN, et al. Inflammation-initiating illnesses, inflammation-related proteins, and cognitive impairment in extremely preterm infants. Brain Behav Immun. 2013;29:104–112. doi: 10.1016/j.bbi.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krump E, Lemay G, Borgeat P. Adenosine A2 receptor-induced inhibition of leukotriene B4 synthesis in whole blood ex vivo. Br J Pharmacol. 1996;117:1639–1644. doi: 10.1111/j.1476-5381.1996.tb15334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bshesh K, Zhao B, Spight D, et al. The A2A receptor mediates an endogenous regulatory pathway of cytokine expression in THP-1 cells. J Leukoc Biol. 2002;72:1027–1036. [PubMed] [Google Scholar]
- 11.Martin L, Pingle SC, Hallam DM, Rybak LP, Ramkumar V. Activation of the adenosine A3 receptor in RAW 264.7 cells inhibits lipopolysaccharide-stimulated tumor necrosis factor-alpha release by reducing calcium-dependent activation of nuclear factor-kappaB and extracellular signal-regulated kinase 1/2. J Pharmacol Exp Ther. 2006;316:71–78. doi: 10.1124/jpet.105.091868. [DOI] [PubMed] [Google Scholar]
- 12.Sajjadi FG, Takabayashi K, Foster AC, Domingo RC, Firestein GS. Inhibition of TNF-alpha expression by adenosine: role of A3 adenosine receptors. J Immunol. 1996;156:3435–3442. [PubMed] [Google Scholar]
- 13.Zhang JG, Hepburn L, Cruz G, Borman RA, Clark KL. The role of adenosine A2A and A2B receptors in the regulation of TNF-alpha production by human monocytes. Biochem Pharmacol. 2005;69:883–889. doi: 10.1016/j.bcp.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 14.van Furth AM, Seijmonsbergen EM, Langermans JA, van der Meide PH, van Furth R. Effect of xanthine derivates and dexamethasone on Streptococcus pneumoniae-stimulated production of tumor necrosis factor alpha, interleukin-1 beta (IL-1 beta), and IL-10 by human leukocytes. Clin Diagn Lab Immunol. 1995;2:689–692. doi: 10.1128/cdli.2.6.689-692.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olah ME, Caldwell CC. Adenosine receptors and mammalian toll-like receptors: synergism in macrophages. Molecular interventions. 2003;3:370–374. doi: 10.1124/mi.3.7.370. [DOI] [PubMed] [Google Scholar]
- 16.Wald D, Qin J, Zhao Z, et al. SIGIRR, a negative regulator of Toll-like receptor-interleukin 1 receptor signaling. Nature immunology. 2003;4:920–927. doi: 10.1038/ni968. [DOI] [PubMed] [Google Scholar]
- 17.Mukhopadhyay S, Herre J, Brown GD, Gordon S. The potential for Toll-like receptors to collaborate with other innate immune receptors. Immunology. 2004;112:521–530. doi: 10.1111/j.1365-2567.2004.01941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ren H, Teng Y, Tan B, et al. Toll-like receptor-triggered calcium mobilization protects mice against bacterial infection through extracellular ATP release. Infection and immunity. 2014;82:5076–5085. doi: 10.1128/IAI.02546-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tunc T, Aydemir G, Karaoglu A, et al. Toll-like receptor levels and caffeine responsiveness in rat pups during perinatal period. Regul Pept. 2013;182:41–44. doi: 10.1016/j.regpep.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 20.Hamano R, Takahashi HK, Iwagaki H, et al. Stimulation of adenosine A2A receptor inhibits LPS-induced expression of intercellular adhesion molecule 1 and production of TNF-alpha in human peripheral blood mononuclear cells. Shock. 2008;29:154–159. doi: 10.1097/shk.0b013e31812385da. [DOI] [PubMed] [Google Scholar]
- 21.Ochaion A, Bar-Yehuda S, Cohn S, et al. Methotrexate enhances the anti-inflammatory effect of CF101 via up-regulation of the A3 adenosine receptor expression. Arthritis Res Ther. 2006;8:R169. doi: 10.1186/ar2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horrigan LA, Kelly JP, Connor TJ. Immunomodulatory effects of caffeine: friend or foe? Pharmacol Ther. 2006;111:877–892. doi: 10.1016/j.pharmthera.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Semmler J, Gebert U, Eisenhut T, et al. Xanthine derivatives: comparison between suppression of tumour necrosis factor-alpha production and inhibition of cAMP phosphodiesterase activity. Immunology. 1993;78:520–525. [PMC free article] [PubMed] [Google Scholar]
- 24.Semmler J, Wachtel H, Endres S. The specific type IV phosphodiesterase inhibitor rolipram suppresses tumor necrosis factor-alpha production by human mononuclear cells. Int J Immunopharmacol. 1993;15:409–413. doi: 10.1016/0192-0561(93)90052-z. [DOI] [PubMed] [Google Scholar]
- 25.Forster-Waldl E, Sadeghi K, Tamandl D, et al. Monocyte toll-like receptor 4 expression and LPS-induced cytokine production increase during gestational aging. Pediatric research. 2005;58:121–124. doi: 10.1203/01.PDR.0000163397.53466.0F. [DOI] [PubMed] [Google Scholar]
- 26.Sadeghi K, Berger A, Langgartner M, et al. Immaturity of infection control in preterm and term newborns is associated with impaired toll-like receptor signaling. The Journal of infectious diseases. 2007;195:296–302. doi: 10.1086/509892. [DOI] [PubMed] [Google Scholar]
- 27.Yerkovich ST, Wikstrom ME, Suriyaarachchi D, Prescott SL, Upham JW, Holt PG. Postnatal development of monocyte cytokine responses to bacterial lipopolysaccharide. Pediatric research. 2007;62:547–552. doi: 10.1203/PDR.0b013e3181568105. [DOI] [PubMed] [Google Scholar]
- 28.Navarro-Peran E, Cabezas-Herrera J, Sanchez-Del-Campo L, Garcia-Canovas F, Rodriguez-Lopez JN. The anti-inflammatory and anti-cancer properties of epigallocatechin-3-gallate are mediated by folate cycle disruption, adenosine release and NF-kappaB suppression. Inflamm Res. 2008 doi: 10.1007/s00011-008-8013-x. [DOI] [PubMed] [Google Scholar]
- 29.Murphree LJ, Marshall MA, Rieger JM, MacDonald TL, Linden J. Human A(2A) adenosine receptors: high-affinity agonist binding to receptor-G protein complexes containing Gbeta(4) Mol Pharmacol. 2002;61:455–462. doi: 10.1124/mol.61.2.455. [DOI] [PubMed] [Google Scholar]
- 30.Link AA, Kino T, Worth JA, et al. Ligand-activation of the adenosine A2a receptors inhibits IL-12 production by human monocytes. J Immunol. 2000;164:436–442. doi: 10.4049/jimmunol.164.1.436. [DOI] [PubMed] [Google Scholar]
- 31.Nemeth ZH, Lutz CS, Csoka B, et al. Adenosine augments IL-10 production by macrophages through an A2B receptor-mediated posttranscriptional mechanism. J Immunol. 2005;175:8260–8270. doi: 10.4049/jimmunol.175.12.8260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Csoka B, Nemeth ZH, Virag L, et al. A2A adenosine receptors and C/EBPbeta are crucially required for IL-10 production by macrophages exposed to Escherichia coli. Blood. 2007;110:2685–2695. doi: 10.1182/blood-2007-01-065870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang BC, Lin HK, Hor WS, et al. Mediation of enhanced transcription of the IL-10 gene in T cells, upon contact with human glioma cells, by Fas signaling through a protein kinase A-independent pathway. J Immunol. 2003;171:3947–3954. doi: 10.4049/jimmunol.171.8.3947. [DOI] [PubMed] [Google Scholar]
- 34.Zhang X, Edwards JP, Mosser DM. Dynamic and transient remodeling of the macrophage IL-10 promoter during transcription. J Immunol. 2006;177:1282–1288. doi: 10.4049/jimmunol.177.2.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lundberg GD. The International Code of Medical Ethics of the World Medical Association. Med Gen Med : Medscape general medicine. 2004;6:37. [PMC free article] [PubMed] [Google Scholar]
- 36.Strober W. Trypan blue exclusion test of cell viability. Curr Protoc Immunol. 2001 doi: 10.1002/0471142735.ima03bs21. Appendix 3:Appendix 3B. [DOI] [PubMed] [Google Scholar]
- 37.Aranda JV, Collinge JM, Zinman R, Watters G. Maturation of caffeine elimination in infancy. Arch Dis Child. 1979;54:946–949. doi: 10.1136/adc.54.12.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pons G, Blais JC, Rey E, et al. Maturation of caffeine N-demethylation in infancy: a study using the 13CO2 breath test. Pediatric research. 1988;23:632–636. doi: 10.1203/00006450-198806000-00021. [DOI] [PubMed] [Google Scholar]
- 39.Kandulski A, Wex T, Kuester D, et al. Naturally occurring regulatory T cells (CD4+, CD25high, FOXP3+) in the antrum and cardia are associated with higher H. pylori colonization and increased gene expression of TGF-beta1. Helicobacter. 2008;13:295–303. doi: 10.1111/j.1523-5378.2008.00612.x. [DOI] [PubMed] [Google Scholar]
- 40.Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper--Excel-based tool using pair-wise correlations. Biotechnology letters. 2004;26:509–515. doi: 10.1023/b:bile.0000019559.84305.47. [DOI] [PubMed] [Google Scholar]
- 41.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]