Abstract
Endurance exercise can lead to systemic improvements in insulin sensitivity and metabolic homeostasis, and is an effective approach to combat metabolic diseases. Pharmacological compounds that recapitulate the beneficial effects of exercise, also known as “exercise mimetics,” have the potential to improve disease symptoms of metabolic syndrome. These drugs, which can increase energy expenditure, suppress hepatic gluconeogenesis, and induce insulin sensitization, have accordingly been highly scrutinized for their utility in treating metabolic diseases including diabetes. Nevertheless, the identity of an efficacious exercise mimetic still remains elusive. In this article, we will highlight several nuclear receptors and cofactors that are putative molecular targets for exercise mimetics, and review recent studies that provide advancements in our mechanistic understanding of how exercise mimetics exert their beneficial effects. We will also discuss evidence from clinical trials utilizing these compounds in human subjects to evaluate their efficacy in treating diabetes.
Keywords: Nuclear Receptors, PPARs, AMPK, PGC1 alpha, Sirtuins, Exercise mimetics, Diabetes
Introduction
Lifestyle interventions such as improving diet and exercise habits can effectively combat the many disease symptoms associated with metabolic syndrome, including obesity, hyperglycemia, insulin resistance, inflammation, hypercholesterolemia, and diabetes (Knowler, et al. 2002; Shin, et al. 2013). Even a small increase in daily physical activity can improve muscle fitness, promote resistance to diet-induced obesity, and reduce systemic inflammation (Warburton, et al. 2006). Although the mechanisms through which exercise improves the symptoms of metabolic diseases are incompletely understood, adaptive changes in skeletal muscle oxidative metabolism and mitochondrial function are believed to play a significant role (Fan, et al. 2013). Endurance training in skeletal muscle can be mechanistically linked to activation of an AMP-sensitive gene expression program coordinated by 5’ adenosine monophosphate-activated protein kinase (AMPK) and the nuclear receptor peroxisome proliferator-activated receptor (PPAR) delta (Narkar, et al. 2008). Two drugs that target these pathways, the AMP-analogue 5-amino-1-β-D-ribofuranosyl-imidazole-4-carboxamide (AICAR) and the PPAR delta ligand GW501516, can greatly improve exercise performance in mice without training (Narkar et al. 2008). Subsequently, other drugs with exercise-mimetic effects have been identified as potential therapeutic avenues for metabolic diseases.
In this review, we will discuss recent findings that address the efficacy of targeting AMPK and/or nuclear receptor-controlled pathways to mimic some of the benefits of exercise, and whether compounds that modulate AMPK and nuclear receptor activity might be useful for treating metabolic diseases such as type II diabetes (T2D). How might one specific exercise mimetic be used as a therapy for a complex disease such as T2D? One apparent mechanism is through the induction of glucose uptake in tissues like skeletal muscle and fat, which removes excess glucose from circulation. Since skeletal muscle is one of the major sites responsible for glucose clearance and utilization in the body, exercise-induced glucose uptake in skeletal muscle is a convenient and straightforward method to lower blood glucose level and improve insulin sensitivity (Baron, et al. 1988). Hepatic gluconeogenesis is another major contributor for hyperglycemia, and interventions that inhibit gluconeogenesis could accordingly be anti-diabetic (Magnusson, et al. 1992). In addition, circulating fatty acids are negatively associated with insulin-dependent glucose uptake, providing one more target to develop anti-diabetic therapies (Boden and Shulman 2002). Exercise is able to reduce hyperglycemia, suppress de novo glucose synthesis, and increase the uptake and metabolism of fatty acids, removing them from circulation. Accordingly, when evaluating exercise mimetics as potential therapies for T2D, we will discuss three separate therapeutic strategies: reducing plasma glucose levels, suppressing gluconeogenesis, and sequestering/oxidizing fatty acids that promote insulin resistance. The safety, specificity, and strategies for therapeutic delivery for each exercise mimetic will also be considered.
PPAR delta: using GW501516 to promote oxidative metabolism in muscle
The PPAR family of nuclear receptors has long been appreciated for its role in regulating lipid metabolism and energetic homeostasis by their direct control of metabolic gene expression (Schoonjans, et al. 1996). Mechanistically, PPARs operate as ligand-inducible transcription factors constitutively bound to consensus response elements on chromatin in a heterodimer with the retinoid X receptors (RXRs) (Barish, et al. 2006). PPAR delta was one of the first nuclear receptors to garner attention as a potential mediator of exercise given its importance in mitochondrial energy metabolism in skeletal muscle (Barish et al. 2006). Transgenic mice overexpressing a constitutively active form of PPAR delta were shown to have a pro-endurance oxidative fiber type shift with induced mitochondrial biogenesis in skeletal muscle. Therefore, PPAR delta ligands naturally surfaced as putative exercise mimetics (Wang, et al. 2003). Originally developed as a drug to treat hyperlipidemia (Oliver, et al. 2001), the PPAR delta-specific ligand GW501516 has been studied and reviewed extensively as an exercise mimetic since it was first demonstrated to synergistically promote endurance with exercise training in mice (Dressel, et al. 2003; Narkar et al. 2008). At present, GW501516 has demonstrated some promise in a handful of phase 1 and phase 2 clinical trials for metabolic disorders such as hypercholesterolemia and dyslipidemia with no published side effects in humans, but has yet to move past this stage of study (Olson, et al. 2012; Ooi, et al. 2011). However, GW501516 was almost immediately and widely used as a doping agent by athletes after its discovery as a potential exercise mimetic, and has subsequently been banned by the world anti-doping agency (Thevis, et al. 2009). This fact is somewhat disconcerting considering that GW501516, in a similar manner to compounds that activate PPAR alpha, has been reported to promote carcinogenesis in some animal models (Wang, et al. 2006). GW501516 has also been linked to a decrease in bone density in ovariectomized rats, similar to side effects seen with PPAR gamma agonists (Mosti, et al. 2014). In addition, GW501516 can potentiate liver fibrosis in response to CCl4-induced injury through a pro-proliferative mechanism (Kostadinova, et al. 2012). Despite the known side effects, GW501516 still presents as an attractive anti-diabetic compound given its profound effects on promoting oxidative metabolism. While oral administration of GW501516 might not be desirable due to its aforementioned side effects, targeted administration of GW501516 to muscle or elsewhere could provide an interesting alternative. A recent example of this strategy utilized topical application of polymer-encapsulated GW501516 to successfully promote healing of diabetic wounds by reducing oxidative stress in the wound microenvironment (Wang, et al. 2015). Future studies, including additional animal studies carefully focusing on the tissue-specific effects of GW501516 will be necessary to establish the utility of this compound as a potential efficacious treatment for diabetes. Alternatively, the development of other PPAR delta ligands with more exercise-specific effects could circumvent the undesirable side effects of GW501516.
PPAR alpha: targeting the liver to promote metabolic fitness through FGF21
In a way similar to PPAR delta, PPAR alpha also regulates the expression of genes that control oxidative metabolism and lipid homeostasis. PPAR alpha has several known endogenous ligands, most of which are fatty acids that can originate from dietary sources, lipolysis of lipid storage in adipose tissues, or de novo lipogenesis (Dreyer, et al. 1993). Whereas PPAR delta is responsible for regulating its target genes most critically in skeletal muscle, PPAR alpha functions in the liver to adapt to fluctuations in metabolic homeostasis (Contreras, et al. 2013). For example, caloric restriction can result in white adipose tissue lipolysis, liberating fatty acids that activate PPAR alpha in liver. Consequently, ligand-activated PPAR alpha can induce the expression of its endocrine hormone target genes that regulate adaptive changes in other tissues (Kersten, et al. 1999). As this fasting response closely resembles metabolic improvements seen with exercise training, could compounds that target PPAR alpha be used as exercise mimetics to treat diabetes?
Given the strong link between PPAR alpha activity and metabolic fitness, recent research efforts have also focused on PPAR alpha target genes that exert its beneficial effects. One such candidate is the endocrine hormone fibroblast growth factor 21 (FGF21), which is produced and secreted by the liver in response to metabolic stresses such as fasting (Inagaki, et al. 2007; Lundasen, et al. 2007). Acute bouts of exercise are able to induce hepatic FGF21 expression and increase circulating FGF21 levels as well (Cuevas-Ramos, et al. 2012). Peripherally, FGF21 promotes a starvation-like state, and can stimulate glucose uptake and fatty acid oxidation in metabolic tissues such as muscle and fat (Mashili, et al. 2011; Potthoff, et al. 2009). Transgenic mice overexpressing FGF21 in the liver as well as mice administered with supra-physiologic doses of FGF21 have significantly improved insulin sensitivity and resistance to metabolic syndrome (Kharitonenkov and Larsen 2011; Zhang, et al. 2012b). Accordingly, FGF21 has been pursued as a potential therapy for metabolic diseases, but off-target effects such as those resulting in bone loss (Wei, et al. 2012) have presented an obstacle. Current research efforts are instead directed towards the development of modified FGF21 variants that lack deleterious side effects. Recent studies have used these variants to improve metabolic homeostasis in diabetic monkeys as well as in a clinical trial involving obese human patients (Adams, et al. 2013; Gaich, et al. 2013). Strategies aimed at inducing FGF21 natively through a physiological metabolic stress while simultaneously circumventing the undesirable side effects of PPAR alpha activation could also be potentially effective anti-diabetes therapies (Bookout, et al. 2013; Wu, et al. 2011).
PPAR gamma: new mechanisms for generating insulin-sensitizing adipokines
PPAR gamma has long been a target for developing therapies for metabolic diseases, and the PPAR gamma-activating thiazolidinedione (TZD) class of drugs have been prolific anti-diabetics since the early 1990s (Spiegelman 1998). PPAR gamma is essential for the development of adipose tissue, and regulates the expression of lipid metabolism genes as well as adipokines with critical metabolic functions such as adiponectin (Berger, et al. 2005). Indeed, energetic stressors such as exercise and fasting can modulate PPAR gamma activity and the expression of its target genes in adipose tissue, highlighting PPAR gamma as a potential target for developing novel exercise mimetics (Butcher, et al. 2008; Vidal-Puig, et al. 1996). Even though the efficacy of PPAR gamma ligands in treating T2D has been firmly established, the precise mechanisms through which they function are still under investigation. Activation of PPAR gamma by TZDs is generally believed to enhance adipocyte development and lipid handling which results in increased absorption of circulating fatty acids into adipose tissue and promotes insulin resistance (Lehrke and Lazar 2005). Recently, TZDs were found to inhibit phosphorylation on the S273 residue of PPAR gamma by cyclin-dependent kinase 5 (CDK5), causing a dysregulated expression pattern of PPAR gamma target genes in white adipose tissue indicative of obesity (Choi, et al. 2010). The phosphorylated S273 can be bound by thyroid hormone receptor associated-protein 3 (Thrap3), which is essential for mediating PPAR gamma activity in obesogenic states (Choi, et al. 2014a). This paradigm has already been explored as a potential therapeutic target for T2D using the non-agonist PPAR gamma ligand UHC1, which exclusively prevents the phosphorylation of S273 on PPAR gamma. As a result, UHC1 treatment not only improved insulin sensitivity but also reduced diet-induced obesity, which is quite different from the TZDs (Choi, et al. 2014b). In addition to providing mechanistic insight into how TZDs and PPAR gamma are involved in the progression of diabetes, these studies also implicate Thrap3 and CDK5 as prospective anti-diabetic targets as well.
In addition to its role in sequestering lipids that promote insulin resistance, PPAR gamma also controls the expression of key adipokines that regulate metabolic homeostasis. For example, adiponectin, a PPAR gamma target gene produced and released from white adipose tissue, is able to suppress hepatic gluconeogenesis, activate glucose uptake in peripheral tissues, increase lipid catabolism, and promote weight loss (Yamauchi, et al. 2001). Interestingly, adipose-derived adiponectin was recently shown to relay the FGF21-mediated systemic improvements in metabolic fitness, proposing a coordinated mechanism by which activation of PPAR alpha in liver and PPAR gamma in fat produce an exercise-like metabolic improvement (Holland, et al. 2013; Lin, et al. 2013). Another adipokine, fibroblast growth factor 1 (FGF1), has also been identified as an adipose-specific PPAR gamma target gene important for adipocyte remodeling and differentiation (Jonker, et al. 2012). Accordingly, FGF1 knockout mice are unable to maintain the plasticity of adipose tissues when challenged with a high-fat dietary stress (Jonker et al. 2012). Although physiological FGF1 functions predominantly in an autocrine manner due to its high affinity for cell surface heparan sulfate proteoglycans, ectopic injection of recombinant FGF1 rapidly lowered glucose levels by 50%, suppressed hepatic glucose production, and improved insulin sensitivity in obese diabetic mice, with the effects sustained for approximately 2 weeks (Suh, et al. 2014). Such metabolic effects of FGF1 are independent of its mitogenic growth hormone-like effects since a non-mitogenic form of FGF1 can still induce similar effects (Suh et al. 2014). Comprehensively, these studies propose the therapeutic use of PPAR gamma-regulated adipokines to improve systemic metabolic homeostasis.
PGC1 alpha: promoting thermogenic metabolism in brown and beige fat
PPAR gamma is also essential for the development and function of brown adipose tissue (BAT), a mitochondrially dense and highly metabolic fat tissue responsible for adaptive thermogenesis (Cannon and Nedergaard 2004). When stimulated by cold challenge and adrenergic signaling, BAT can rapidly uptake and burn glucose to generate heat through uncoupled mitochondrial oxidative phosphorylation. Induction of thermogenesis in BAT is directly mediated by PPAR gamma coactivator 1 (PGC1) alpha (Wu, et al. 1999). PGC1 alpha is also highly induced in skeletal muscle by exercise, where it activates nuclear receptors such as the PPARs and estrogen related receptors (ERRs) to induce genes that are involved in mitochondrial energy expenditure, fatty acid oxidation, and glucose uptake (Baar, et al. 2002; Finck and Kelly 2006). Therefore, PGC1 alpha has been an attractive target for anti-diabetic therapies aiming to improve metabolic fitness by inducing energy expenditure, and has been extensively studied and reviewed as a putative target for exercise mimetics in fat and muscle (Lin, et al. 2005).
In the last few years, several studies have highlighted the potential to utilize PGC1 alpha-dependent pathways to “brown” subcutaneous white fat pads to create “beige” fat that expresses genes specific for brown fat function and has increased mitochondrial energy expenditure and increased uptake of glucose and lipids (Harms and Seale 2013). Mechanistically, how can browning of white fat be achieved? TZD treatment has been linked to browning, and TZD binding to PPAR gamma facilitates the direct interaction between PPAR gamma and the deacetylase sirtuin 1 (SIRT1). When deacetylated by SIRT1, PPAR gamma induces the expression of genes that mediate thermogenesis in white fat (Qiang, et al. 2012). Accordingly, a SIRT1 gain of function model resulted in a similar browning effect as seen in TZD treatment, which significantly improved insulin sensitivity (Qiang et al. 2012). Browning has also been recently associated with inflammation, with macrophages recruited to white fat during cold challenge facilitating its transition to the beige state (Qiu, et al. 2014). IL-4 treatment alone was also sufficient to increase the amount of beige fat and subsequently reduce the symptoms of obesity in mice (Qiu et al. 2014). Metabolic endocrine hormones, including FGF21, have also been closely associated with browning in mice and humans, but conflicting evidence exists as to their actual specific contribution to the development of beige fat (Lee, et al. 2014). One such hormone that has been hotly contested recently in the browning field is irisin, a myokine that is secreted from skeletal muscle by the cleavage of fibronectin type III domain-containing protein 5 (FNDC5). The expression of FNDC5 and secretion of irisin are induced by exercise in skeletal muscle in a PGC1 alpha-dependent manner. In mouse, irisin acts on white adipose tissues and turns on its browning program, which induces energy expenditure and protects against diet-induced obesity and diabetes (Bostrom, et al. 2012). Given these findings, recombinant irisin certainly seems like an attractive candidate exercise mimetic useful for the treatment of T2D. However, mixed findings have been recently published regarding the relevance of irisin to browning in humans. For example, irisin levels in circulation as well as in muscle and fat have been inversely correlated with obesity in human subjects, and human white adipose cell lines have yet to show sensitivity to irisin-mediated browning (Elsen, et al. 2014). In addition, emerging evidence suggests irisin may originate from adipose tissue in addition to muscle (Crujeiras, et al. 2014). Further investigation into the molecular mechanisms that govern browning in human and animal models will be required to determine if this process can be efficaciously targeted by exercise mimetics.
AMPK: targeting the central regulator of energy homeostasis
Long considered to be at the nexus of metabolic signaling pathways, AMP-activated protein kinase (AMPK) relays fluctuations in intracellular energy supply to functional cellular energetics by simultaneously activating catabolic and repressing anabolic processes (Hardie 2011). Mechanistically, AMP interacts with AMPK’s gamma subunit, facilitating an activating phosphorylation of the alpha subunit by upstream regulatory kinases such as LKB1. This allows AMPK to phosphorylate its downstream targets, many of which are key participants or regulators of energy metabolism pathways including nuclear receptor cofactors such as PGC1 alpha (Gwinn, et al. 2008; Jager, et al. 2007; Shaw, et al. 2005). Accumulating evidence has established a “global” role for AMPK in relaying energetic stress to physiological changes from a wide array of environmental stimuli such as caloric restriction, exercise, and disease conditions such as T2D (Canto and Auwerx 2009; Narkar et al. 2008). As such, pharmacological modulation of AMPK activity has developed into an attractive and widely studied therapeutic avenue for metabolic disorders.
To date, several AMPK-targeted exercise mimetic compounds have been designed and exhaustively tested with this aim in mind. At the forefront is AICAR, an adenosine analogue whose intracellular metabolite directly interacts with and activates AMPK (Sullivan, et al. 1994). AICAR has been shown to induce glucose uptake in skeletal muscle via AMPK-dependent stimulation of the translocation of the cellular glucose transporter Glut4 onto cell membranes (Russell, et al. 1999). When administered to sedentary mice, AICAR alone can significantly enhance exercise performance without training through facilitating an oxidative fiber-type switch and mitochondrial biogenesis in skeletal muscle (Narkar et al. 2008). More recently, AICAR has also been used to recapitulate exercise’s mood-improving effects while simultaneously improving insulin sensitivity in a mouse model of depression and diet-induced obesity (Liu, et al. 2014). Despite the known benefits, AICAR has yet to demonstrate its anti-diabetic efficacy in humans due to it being rapidly metabolized once administered, and its marginal oral activity (Musi and Goodyear 2002). In addition, AICAR administration in humans has been linked to lactic acidosis, an undesirable side effect which has shelved other anti-diabetic compounds in the past (Musi and Goodyear 2002). Even though these issues have not been resolved to date, AICAR still has potential and continues to be pursued as a possible anti-diabetic agent.
Metformin, a drug of the biguanide class known to function in an AMPK-dependent manner, is one of the most widely used anti-diabetes drug on the market today (Knowler et al. 2002; Zhou, et al. 2001). Despite the broad usage of metformin to this end due to its efficacious, if transient, ability to improve insulin sensitivity, the precise mechanism by which metformin exerts its effects is imperfectly understood (Luengo, et al. 2014). One proposed mechanism for metformin is as an inhibitor of the mitochondrial electron transport chain (ETC) – by interacting with the ETC directly, metformin disrupts the normal process of cellular ATP generation, creating cellular energy deficit (Andrzejewski, et al. 2014), which in turn induces mitochondrial energy metabolism by activating AMPK and its subsequent downstream targets (Zhou et al. 2001). Recently metformin has also been linked to bile acid homeostasis in liver through its activation of AMPK. Upon activation, AMPK directly phosphorylates and activates the nuclear receptor farnesoid X receptor (FXR) in the liver, promoting metabolic homeostasis (Lien, et al. 2014). Interestingly, structural studies have also suggested that metformin might interact directly with AMPK’s gamma subunit in a similar manner to AMP (Zhang, et al. 2012a). Recent evidence, however has proposed alternative mechanisms for metformin’s action. For example, metformin was found to directly inhibit the mitochondrial enzyme glycerophosphate dehydrogenase, which is involved in the gluconeogenic pathway in liver (Madiraju, et al. 2014). This inhibition disrupted the redox-sensitive ratio of lactate to pyruvate, resulting in suppressed hepatic gluconeogenesis (Madiraju et al. 2014). Similar phenotypes were also seen in mice deficient for glycerophosphate dehydrogenase (Madiraju et al. 2014). These findings are supported by independent studies demonstrating metformin’s ability to inhibit hepatic gluconeogenesis in an AMPK-independent manner (Foretz, et al. 2010). While metformin’s precise mechanism of action remains muddled, the compound is still highly relevant to treating metabolic disorders such as T2D, which is likely linked to some interaction with an AMPK-dependent pathway.
SIRT1: relaying mitochondrial redox states to metabolic improvements
Cellular energetic state is highly associated with its oxidation-reduction (redox) state through the coupling of multiple redox nodes with mitochondrial energy metabolism. One of the key redox nodes is that of NAD+/NADH, which are critical cofactors that transport electrons during mitochondrial oxidative phosphorylation and function as coenzymes in key metabolic reactions NAD+ has also been proposed to function as a cofactor for the deacetylase sirtuin 1 (SIRT1), which deacetylates and activates PGC1a, promoting mitochondrial biogenesis and energy metabolism in a way similar to exercise (Rodgers, et al. 2005). Moreover, AMPK activation elevates the intracellular NAD+/NADH ratio and induces the activity of SIRT1 to activate PGC1a, which mediates the energetic functions of AMPK on top of its direct phosphorylation of PGC1a (Canto et al. 2009). Accordingly, drugs that impact the NAD+/NADH ratio or target SIRT1 directly have the potential to create exercise-like effects. Recent studies have explored these strategies, using compounds in this manner to increase oxidative metabolism and combat diet-induced obesity in mice. For example, supplementing mice with the NAD+ precursor nicotinamide riboside increased intracellular NAD+ levels and enhanced mitochondrial function in skeletal muscle and BAT, comprehensively resulting in improved exercise performance (Canto, et al. 2012). Similarly, inhibition of the NAD+ consuming DNA repair enzyme poly (adp-ribose) polymerase 1 (PARP-1) conferred resistance to diet-induced metabolic defects through improvements in mitochondrial function and oxidative metabolism in skeletal muscle (Pirinen, et al. 2014). Interestingly, genetic deletion of PARP-1 or treatment with PARP inhibitors was able to correct the phenotype of mice with dysfunctional synthesis of cytochrome c, a model of mitochondrial disease (Cerutti, et al. 2014). Analogously, administration of nicotinamide riboside to the Twinkle mitochondrial DNA deletor mouse model of mitochondrial disease increased mitochondrial biogenesis in skeletal muscle and blunted the progression of mitochondrial myopathy (Khan, et al. 2014). Collectively, compounds that can be used to modulate the cellular redox state by increasing the NAD+/NADH ratio can create an exercise-like, PGC1a-dependent induction of mitochondrial and metabolic fitness, which might be useful for combating obesity and T2D.
How else might the SIRT1-PGC1a pathway be exploited to create exercise-like effects? Resveratrol, a naturally occurring polyphenol produced by grapes, is a well-established activator of SIRT1 that can promote mitochondrial function in muscle, increase exercise endurance, and is also linked to longevity (Lagouge, et al. 2006). Subsequently, resveratrol has been proposed to combat diseases ranging from cardiac dysfunction to cancer through activation of SIRT1, although its exact mechanism of action is not known (Baur and Sinclair 2006). Does resveratrol have potential to be used as an exercise mimetic to efficaciously treat diabetes? Resveratrol has undergone a few recent clinical trials to address this question. One trial revealed that administering resveratrol to 10 obese type-2 diabetic males induced the expression of both AMPK and SIRT1 in skeletal muscle, coupled with an increase in resting metabolic rate (Goh, et al. 2014). Another trial demonstrated that after 6 months of resveratrol treatment, patients receiving the compound had approximately 10% higher levels of circulating adiponectin, coupled with reduced expression of inflammatory markers (Tome-Carneiro, et al. 2013). These results suggest that resveratrol treatment might exert beneficial effects on white adipose tissue, potentially mediated through PPAR gamma. Unfortunately, another recent clinical trial administering high doses of resveratrol to 24 obese male subjects for 4 weeks did not show any improvements in insulin sensitivity, glucose homeostasis, or oxidative metabolism (Poulsen, et al. 2013). Resveratrol treatment also appeared to suppress exercise-dependent improvements in aerobic respiration in a trial of 27 inactive aged men (Gliemann, et al. 2013). Although conflicting clinical evidence exists as to the efficacy of resveratrol to treat diabetes, the compound unquestionably highlights the promise of SIRT1 as a putative anti-diabetic target.
Conclusions and Perspectives
The push to develop novel drugs to treat diabetes has never been stronger in the metabolism field, and AMPK/nuclear receptor-regulated pathways continue to remain in the limelight with regard to artificially recapitulating the benefits of exercise. The specific molecular mechanisms dictating how these exercise mimetics work are just beginning to emerge, despite many of these compounds being used to combat diabetes for more than a decade. Future studies aimed at fleshing out a concrete link between exercise, AMPK, nuclear receptors, and improvements in metabolic homeostasis will facilitate the development of new exercise mimetics with improved tissue specificity, increased stability, and minimal side effects. Given the amount of recent findings that critically advance our understanding of these drugs, we believe that an exercise mimetic capable of efficaciously treating T2D should be in our near future.
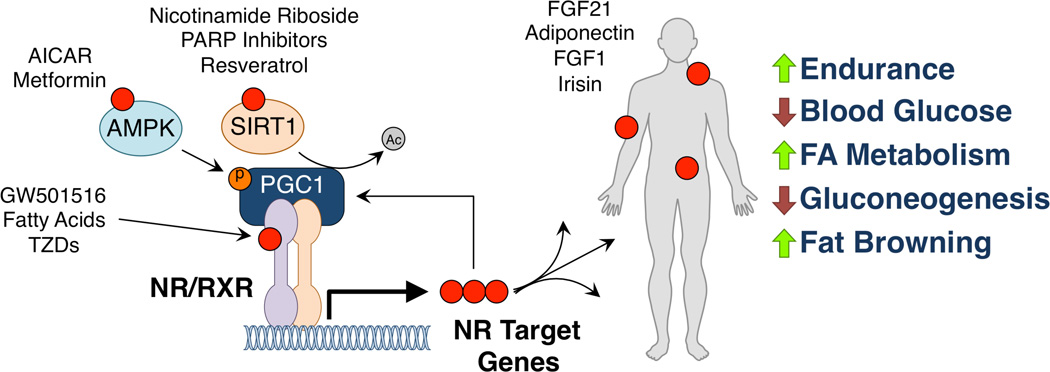
Figure 1. Unified pathways for exercise mimetics.
Compounds, either endogenous or synthetic, that can recapitulate the benefits of exercise (red dots) interact with several different parts of a unified nuclear receptor/cofactor transcriptional complex that promotes the expression of genes that maintain metabolic homeostasis. Direct ligands for nuclear receptors (GW501516, fatty acids, and TZDs), activators of the nuclear receptor cofactor PGC1 alpha AMPK and SIRT1 (AICAR and metformin, and nicotinamide riboside, PARP inhibitors, and resveratrol respectively) promote the expression of nuclear receptor target genes (FGF21, adiponectin, FGF1, and irisin) which can be secreted to function as endocrine hormones with peripheral effects. Comprehensively, these exercise mimetics can promote endurance in skeletal muscle, lower blood glucose levels, increase fatty acid metabolism, suppress hepatic gluconeogenesis, and cause browning of white fat into beige fat.
Acknowledgments
We thank L. Ong and C. Brondos for administrative assistance. R.M.E. is an Investigator of the Howard Hughes Medical Institute at the Salk Institute and March of Dimes Chair in Molecular and Developmental Biology. This work was supported by US National Institutes of Health grants (DK057978, DK090962, HL088093, HL105278, CA014195 and ES010337), the Glenn Foundation for Medical Research, the Leona M. and Harry B. Helmsley Charitable Trust (#2012-PG-MED002), Ipsen/Biomeasure, and the Ellison Medical Foundation.
Footnotes
The authors declare no conflicts of interest.
References
- Adams AC, Halstead CA, Hansen BC, Irizarry AR, Martin JA, Myers SR, Reynolds VL, Smith HW, Wroblewski VJ, Kharitonenkov A. LY2405319, an Engineered FGF21 Variant, Improves the Metabolic Status of Diabetic Monkeys. PLoS One. 2013;8:e65763. doi: 10.1371/journal.pone.0065763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrzejewski S, Gravel SP, Pollak M, St-Pierre J. Metformin directly acts on mitochondria to alter cellular bioenergetics. Cancer Metab. 2014;2:12. doi: 10.1186/2049-3002-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, Kelly DP, Holloszy JO. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J. 2002;16:1879–1886. doi: 10.1096/fj.02-0367com. [DOI] [PubMed] [Google Scholar]
- Barish GD, Narkar VA, Evans RM. PPAR delta: a dagger in the heart of the metabolic syndrome. J Clin Invest. 2006;116:590–597. doi: 10.1172/JCI27955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron AD, Brechtel G, Wallace P, Edelman SV. Rates and tissue sites of non-insulin- and insulin-mediated glucose uptake in humans. Am J Physiol. 1988;255:E769–E774. doi: 10.1152/ajpendo.1988.255.6.E769. [DOI] [PubMed] [Google Scholar]
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Berger JP, Akiyama TE, Meinke PT. PPARs: therapeutic targets for metabolic disease. Trends Pharmacol Sci. 2005;26:244–251. doi: 10.1016/j.tips.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest. 2002;32(Suppl 3):14–23. doi: 10.1046/j.1365-2362.32.s3.3.x. [DOI] [PubMed] [Google Scholar]
- Bookout AL, de Groot MH, Owen BM, Lee S, Gautron L, Lawrence HL, Ding X, Elmquist JK, Takahashi JS, Mangelsdorf DJ, et al. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat Med. 2013;19:1147–1152. doi: 10.1038/nm.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher LR, Thomas A, Backx K, Roberts A, Webb R, Morris K. Low-intensity exercise exerts beneficial effects on plasma lipids via PPARgamma. Med Sci Sports Exerc. 2008;40:1263–1270. doi: 10.1249/MSS.0b013e31816c091d. [DOI] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Canto C, Auwerx J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol. 2009;20:98–105. doi: 10.1097/MOL.0b013e328328d0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, Fernandez-Marcos PJ, Yamamoto H, Andreux PA, Cettour-Rose P, et al. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012;15:838–847. doi: 10.1016/j.cmet.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti R, Pirinen E, Lamperti C, Marchet S, Sauve AA, Li W, Leoni V, Schon EA, Dantzer F, Auwerx J, et al. NAD(+)-dependent activation of Sirt1 corrects the phenotype in a mouse model of mitochondrial disease. Cell Metab. 2014;19:1042–1049. doi: 10.1016/j.cmet.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH, Banks AS, Estall JL, Kajimura S, Bostrom P, Laznik D, Ruas JL, Chalmers MJ, Kamenecka TM, Bluher M, et al. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARgamma by Cdk5. Nature. 2010;466:451–456. doi: 10.1038/nature09291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH, Choi SS, Kim ES, Jedrychowski MP, Yang YR, Jang HJ, Suh PG, Banks AS, Gygi SP, Spiegelman BM. Thrap3 docks on phosphoserine 273 of PPARgamma and controls diabetic gene programming. Genes Dev. 2014a;28:2361–2369. doi: 10.1101/gad.249367.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SS, Kim ES, Koh M, Lee SJ, Lim D, Yang YR, Jang HJ, Seo KA, Min SH, Lee IH, et al. A novel non-agonist peroxisome proliferator-activated receptor gamma (PPARgamma) ligand UHC1 blocks PPARgamma phosphorylation by cyclin-dependent kinase 5 (CDK5) and improves insulin sensitivity. J Biol Chem. 2014b;289:26618–26629. doi: 10.1074/jbc.M114.566794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras AV, Torres N, Tovar AR. PPAR-alpha as a key nutritional and environmental sensor for metabolic adaptation. Adv Nutr. 2013;4:439–452. doi: 10.3945/an.113.003798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crujeiras AB, Pardo M, Casanueva FF. Irisin: ‘fat’ or artefact. Clin Endocrinol (Oxf) 2014 doi: 10.1111/cen.12627. [DOI] [PubMed] [Google Scholar]
- Cuevas-Ramos D, Almeda-Valdes P, Meza-Arana CE, Brito-Cordova G, Gomez-Perez FJ, Mehta R, Oseguera-Moguel J, Aguilar-Salinas CA. Exercise increases serum fibroblast growth factor 21 (FGF21) levels. PLoS One. 2012;7:e38022. doi: 10.1371/journal.pone.0038022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressel U, Allen TL, Pippal JB, Rohde PR, Lau P, Muscat GE. The peroxisome proliferator-activated receptor beta/delta agonist, GW501516, regulates the expression of genes involved in lipid catabolism and energy uncoupling in skeletal muscle cells. Mol Endocrinol. 2003;17:2477–2493. doi: 10.1210/me.2003-0151. [DOI] [PubMed] [Google Scholar]
- Dreyer C, Keller H, Mahfoudi A, Laudet V, Krey G, Wahli W. Positive regulation of the peroxisomal beta-oxidation pathway by fatty acids through activation of peroxisome proliferator-activated receptors (PPAR) Biol Cell. 1993;77:67–76. doi: 10.1016/s0248-4900(05)80176-5. [DOI] [PubMed] [Google Scholar]
- Elsen M, Raschke S, Eckel J. Browning of white fat: does irisin play a role in humans? J Endocrinol. 2014;222:R25–R38. doi: 10.1530/JOE-14-0189. [DOI] [PubMed] [Google Scholar]
- Fan W, Atkins AR, Yu RT, Downes M, Evans RM. Road to exercise mimetics: targeting nuclear receptors in skeletal muscle. J Mol Endocrinol. 2013;51:T87–T100. doi: 10.1530/JME-13-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finck BN, Kelly DP. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest. 2006;116:615–622. doi: 10.1172/JCI27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foretz M, Hebrard S, Leclerc J, Zarrinpashneh E, Soty M, Mithieux G, Sakamoto K, Andreelli F, Viollet B. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest. 2010;120:2355–2369. doi: 10.1172/JCI40671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaich G, Chien JY, Fu H, Glass LC, Deeg MA, Holland WL, Kharitonenkov A, Bumol T, Schilske HK, Moller DE. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab. 2013;18:333–340. doi: 10.1016/j.cmet.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Gliemann L, Schmidt JF, Olesen J, Bienso RS, Peronard SL, Grandjean SU, Mortensen SP, Nyberg M, Bangsbo J, Pilegaard H, et al. Resveratrol blunts the positive effects of exercise training on cardiovascular health in aged men. J Physiol. 2013;591:5047–5059. doi: 10.1113/jphysiol.2013.258061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh KP, Lee HY, Lau DP, Supaat W, Chan YH, Koh AF. Effects of resveratrol in patients with type 2 diabetes mellitus on skeletal muscle SIRT1 expression and energy expenditure. Int J Sport Nutr Exerc Metab. 2014;24:2–13. doi: 10.1123/ijsnem.2013-0045. [DOI] [PubMed] [Google Scholar]
- Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG. Sensing of energy and nutrients by AMP-activated protein kinase. Am J Clin Nutr. 2011;93:891S–896S. doi: 10.3945/ajcn.110.001925. [DOI] [PubMed] [Google Scholar]
- Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19:1252–1263. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- Holland WL, Adams AC, Brozinick JT, Bui HH, Miyauchi Y, Kusminski CM, Bauer SM, Wade M, Singhal E, Cheng CC, et al. An FGF21-adiponectin-ceramide axis controls energy expenditure and insulin action in mice. Cell Metab. 2013;17:790–797. doi: 10.1016/j.cmet.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab. 2007;5:415–425. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonker JW, Suh JM, Atkins AR, Ahmadian M, Li P, Whyte J, He M, Juguilon H, Yin YQ, Phillips CT, et al. A PPARgamma-FGF1 axis is required for adaptive adipose remodelling and metabolic homeostasis. Nature. 2012;485:391–394. doi: 10.1038/nature10998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten S, Seydoux J, Peters JM, Gonzalez FJ, Desvergne B, Wahli W. Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. J Clin Invest. 1999;103:1489–1498. doi: 10.1172/JCI6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan NA, Auranen M, Paetau I, Pirinen E, Euro L, Forsstrom S, Pasila L, Velagapudi V, Carroll CJ, Auwerx J, et al. Effective treatment of mitochondrial myopathy by nicotinamide riboside, a vitamin B3. EMBO Mol Med. 2014;6:721–731. doi: 10.1002/emmm.201403943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharitonenkov A, Larsen P. FGF21 reloaded: challenges of a rapidly growing field. Trends Endocrinol Metab. 2011;22:81–86. doi: 10.1016/j.tem.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM Diabetes Prevention Program Research G. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostadinova R, Montagner A, Gouranton E, Fleury S, Guillou H, Dombrowicz D, Desreumaux P, Wahli W. GW501516-activated PPARbeta/delta promotes liver fibrosis via p38-JNK MAPK-induced hepatic stellate cell proliferation. Cell Biosci. 2012;2:34. doi: 10.1186/2045-3701-2-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Lee P, Linderman JD, Smith S, Brychta RJ, Wang J, Idelson C, Perron RM, Werner CD, Phan GQ, Kammula US, et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 2014;19:302–309. doi: 10.1016/j.cmet.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrke M, Lazar MA. The many faces of PPARgamma. Cell. 2005;123:993–999. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- Lien F, Berthier A, Bouchaert E, Gheeraert C, Alexandre J, Porez G, Prawitt J, Dehondt H, Ploton M, Colin S, et al. Metformin interferes with bile acid homeostasis through AMPK-FXR crosstalk. J Clin Invest. 2014;124:1037–1051. doi: 10.1172/JCI68815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005;1:361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Lin Z, Tian H, Lam KS, Lin S, Hoo RC, Konishi M, Itoh N, Wang Y, Bornstein SR, Xu A, et al. Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metab. 2013;17:779–789. doi: 10.1016/j.cmet.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Liu W, Zhai X, Li H, Ji L. Depression-like behaviors in mice subjected to co-treatment of high-fat diet and corticosterone are ameliorated by AICAR and exercise. J Affect Disord. 2014;156:171–177. doi: 10.1016/j.jad.2013.11.025. [DOI] [PubMed] [Google Scholar]
- Luengo A, Sullivan LB, Heiden MG. Understanding the complex-I-ty of metformin action: limiting mitochondrial respiration to improve cancer therapy. BMC Biol. 2014;12:82. doi: 10.1186/s12915-014-0082-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundasen T, Hunt MC, Nilsson LM, Sanyal S, Angelin B, Alexson SE, Rudling M. PPARalpha is a key regulator of hepatic FGF21. Biochem Biophys Res Commun. 2007;360:437–440. doi: 10.1016/j.bbrc.2007.06.068. [DOI] [PubMed] [Google Scholar]
- Madiraju AK, Erion DM, Rahimi Y, Zhang XM, Braddock DT, Albright RA, Prigaro BJ, Wood JL, Bhanot S, MacDonald MJ, et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature. 2014;510:542–546. doi: 10.1038/nature13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson I, Rothman DL, Katz LD, Shulman RG, Shulman GI. Increased rate of gluconeogenesis in type II diabetes mellitus. A 13C nuclear magnetic resonance study. J Clin Invest. 1992;90:1323–1327. doi: 10.1172/JCI115997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashili FL, Austin RL, Deshmukh AS, Fritz T, Caidahl K, Bergdahl K, Zierath JR, Chibalin AV, Moller DE, Kharitonenkov A, et al. Direct effects of FGF21 on glucose uptake in human skeletal muscle: implications for type 2 diabetes and obesity. Diabetes Metab Res Rev. 2011;27:286–297. doi: 10.1002/dmrr.1177. [DOI] [PubMed] [Google Scholar]
- Mosti MP, Stunes AK, Ericsson M, Pullisaar H, Reseland JE, Shabestari M, Eriksen EF, Syversen U. Effects of the peroxisome proliferator-activated receptor (PPAR)-delta agonist GW501516 on bone and muscle in ovariectomized rats. Endocrinology. 2014;155:2178–2189. doi: 10.1210/en.2013-1166. [DOI] [PubMed] [Google Scholar]
- Musi N, Goodyear LJ. Targeting the AMP-activated protein kinase for the treatment of type 2 diabetes. Curr Drug Targets Immune Endocr Metabol Disord. 2002;2:119–127. [PubMed] [Google Scholar]
- Nakamaru K, Matsumoto K, Taguchi T, Suefuji M, Murata Y, Igata M, Kawashima J, Kondo T, Motoshima H, Tsuruzoe K, et al. AICAR, an activator of AMP-activated protein kinase, down-regulates the insulin receptor expression in HepG2 cells. Biochem Biophys Res Commun. 2005;328:449–454. doi: 10.1016/j.bbrc.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Narkar VA, Downes M, Yu RT, Embler E, Wang YX, Banayo E, Mihaylova MM, Nelson MC, Zou Y, Juguilon H, et al. AMPK and PPARdelta agonists are exercise mimetics. Cell. 2008;134:405–415. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver WR, Jr, Shenk JL, Snaith MR, Russell CS, Plunket KD, Bodkin NL, Lewis MC, Winegar DA, Sznaidman ML, Lambert MH, et al. A selective peroxisome proliferator-activated receptor delta agonist promotes reverse cholesterol transport. Proc Natl Acad Sci U S A. 2001;98:5306–5311. doi: 10.1073/pnas.091021198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson EJ, Pearce GL, Jones NP, Sprecher DL. Lipid effects of peroxisome proliferator-activated receptor-delta agonist GW501516 in subjects with low high-density lipoprotein cholesterol: characteristics of metabolic syndrome. Arterioscler Thromb Vasc Biol. 2012;32:2289–2294. doi: 10.1161/ATVBAHA.112.247890. [DOI] [PubMed] [Google Scholar]
- Ooi EM, Watts GF, Sprecher DL, Chan DC, Barrett PH. Mechanism of action of a peroxisome proliferator-activated receptor (PPAR)-delta agonist on lipoprotein metabolism in dyslipidemic subjects with central obesity. J Clin Endocrinol Metab. 2011;96:E1568–E1576. doi: 10.1210/jc.2011-1131. [DOI] [PubMed] [Google Scholar]
- Pirinen E, Canto C, Jo YS, Morato L, Zhang H, Menzies KJ, Williams EG, Mouchiroud L, Moullan N, Hagberg C, et al. Pharmacological Inhibition of poly(ADP-ribose) polymerases improves fitness and mitochondrial function in skeletal muscle. Cell Metab. 2014;19:1034–1041. doi: 10.1016/j.cmet.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potthoff MJ, Inagaki T, Satapati S, Ding X, He T, Goetz R, Mohammadi M, Finck BN, Mangelsdorf DJ, Kliewer SA, et al. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc Natl Acad Sci U S A. 2009;106:10853–10858. doi: 10.1073/pnas.0904187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen MM, Vestergaard PF, Clasen BF, Radko Y, Christensen LP, Stodkilde-Jorgensen H, Moller N, Jessen N, Pedersen SB, Jorgensen JO. High-dose resveratrol supplementation in obese men: an investigator-initiated, randomized, placebo-controlled clinical trial of substrate metabolism, insulin sensitivity, and body composition. Diabetes. 2013;62:1186–1195. doi: 10.2337/db12-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang L, Wang L, Kon N, Zhao W, Lee S, Zhang Y, Rosenbaum M, Zhao Y, Gu W, Farmer SR, et al. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Ppargamma. Cell. 2012;150:620–632. doi: 10.1016/j.cell.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, Nguyen KD, Odegaard JI, Cui X, Tian X, Locksley RM, Palmiter RD, Chawla A. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell. 2014;157:1292–1308. doi: 10.1016/j.cell.2014.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- Russell RR, 3rd, Bergeron R, Shulman GI, Young LH. Translocation of myocardial GLUT-4 and increased glucose uptake through activation of AMPK by AICAR. Am J Physiol. 1999;277:H643–H649. doi: 10.1152/ajpheart.1999.277.2.H643. [DOI] [PubMed] [Google Scholar]
- Schoonjans K, Staels B, Auwerx J. Role of the peroxisome proliferator-activated receptor (PPAR) in mediating the effects of fibrates and fatty acids on gene expression. J Lipid Res. 1996;37:907–925. [PubMed] [Google Scholar]
- Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, Cantley LC. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JA, Lee JH, Lim SY, Ha HS, Kwon HS, Park YM, Lee WC, Kang MI, Yim HW, Yoon KH, et al. Metabolic syndrome as a predictor of type 2 diabetes, and its clinical interpretations and usefulness. J Diabetes Investig. 2013;4:334–343. doi: 10.1111/jdi.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman BM. PPAR-gamma: adipogenic regulator and thiazolidinedione receptor. Diabetes. 1998;47:507–514. doi: 10.2337/diabetes.47.4.507. [DOI] [PubMed] [Google Scholar]
- Suh JM, Jonker JW, Ahmadian M, Goetz R, Lackey D, Osborn O, Huang Z, Liu W, Yoshihara E, van Dijk TH, et al. Endocrinization of FGF1 produces a neomorphic and potent insulin sensitizer. Nature. 2014;513:436–439. doi: 10.1038/nature13540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JE, Brocklehurst KJ, Marley AE, Carey F, Carling D, Beri RK. Inhibition of lipolysis and lipogenesis in isolated rat adipocytes with AICAR, a cell-permeable activator of AMP-activated protein kinase. FEBS Lett. 1994;353:33–36. doi: 10.1016/0014-5793(94)01006-4. [DOI] [PubMed] [Google Scholar]
- Thevis M, Beuck S, Thomas A, Kortner B, Kohler M, Rodchenkov G, Schanzer W. Doping control analysis of emerging drugs in human plasma - identification of GW501516, S-107, JTV-519, and S-40503. Rapid Commun Mass Spectrom. 2009;23:1139–1146. doi: 10.1002/rcm.3987. [DOI] [PubMed] [Google Scholar]
- Tome-Carneiro J, Gonzalvez M, Larrosa M, Yanez-Gascon MJ, Garcia-Almagro FJ, Ruiz-Ros JA, Tomas-Barberan FA, Garcia-Conesa MT, Espin JC. Grape resveratrol increases serum adiponectin and downregulates inflammatory genes in peripheral blood mononuclear cells: a triple-blind, placebo-controlled, one-year clinical trial in patients with stable coronary artery disease. Cardiovasc Drugs Ther. 2013;27:37–48. doi: 10.1007/s10557-012-6427-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Puig A, Jimenez-Linan M, Lowell BB, Hamann A, Hu E, Spiegelman B, Flier JS, Moller DE. Regulation of PPAR gamma gene expression by nutrition and obesity in rodents. J Clin Invest. 1996;97:2553–2561. doi: 10.1172/JCI118703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Wang H, Guo Y, Ning W, Katkuri S, Wahli W, Desvergne B, Dey SK, DuBois RN. Crosstalk between peroxisome proliferator-activated receptor delta and VEGF stimulates cancer progression. Proc Natl Acad Sci U S A. 2006;103:19069–19074. doi: 10.1073/pnas.0607948103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Sng MK, Foo S, Chong HC, Lee WL, Tang MB, Ng KW, Luo B, Choong C, Wong MT, et al. Early controlled release of peroxisome proliferator-activated receptor beta/delta agonist GW501516 improves diabetic wound healing through redox modulation of wound microenvironment. J Control Release. 2015;197:138–147. doi: 10.1016/j.jconrel.2014.11.001. [DOI] [PubMed] [Google Scholar]
- Wang YX, Lee CH, Tiep S, Yu RT, Ham J, Kang H, Evans RM. Peroxisome-proliferator-activated receptor delta activates fat metabolism to prevent obesity. Cell. 2003;113:159–170. doi: 10.1016/s0092-8674(03)00269-1. [DOI] [PubMed] [Google Scholar]
- Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. CMAJ. 2006;174:801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Dutchak PA, Wang X, Ding X, Wang X, Bookout AL, Goetz R, Mohammadi M, Gerard RD, Dechow PC, et al. Fibroblast growth factor 21 promotes bone loss by potentiating the effects of peroxisome proliferator-activated receptor gamma. Proc Natl Acad Sci U S A. 2012;109:3143–3148. doi: 10.1073/pnas.1200797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu AL, Kolumam G, Stawicki S, Chen Y, Li J, Zavala-Solorio J, Phamluong K, Feng B, Li L, Marsters S, et al. Amelioration of type 2 diabetes by antibody-mediated activation of fibroblast growth factor receptor 1. Sci Transl Med. 2011;3:113ra126. doi: 10.1126/scitranslmed.3002669. [DOI] [PubMed] [Google Scholar]
- Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang Y, Bao C, Xu Y, Shen H, Chen J, Yan J, Chen Y. Metformin interacts with AMPK through binding to gamma subunit. Mol Cell Biochem. 2012a;368:69–76. doi: 10.1007/s11010-012-1344-5. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xie Y, Berglund ED, Coate KC, He TT, Katafuchi T, Xiao G, Potthoff MJ, Wei W, Wan Y, et al. The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. Elife. 2012b;1:e00065. doi: 10.7554/eLife.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]