Abstract
Purpose
To determine the impact of obesity on the rate of successful sentinel lymph node (SLN) mapping in patients with uterine cancer undergoing robotic surgery, and compare SLN detection rates using indocyanine green (ICG) versus blue dye.
Methods
We reviewed robotic cases undergoing SLN mapping with a cervical injection from 1/2011–12/2013 using either blue dye or ICG with near-infrared (NIR) fluorescence imaging. Data were stratified by body mass index (BMI) and dye used. Appropriate statistical tests were applied.
Results
Four hundred seventy-two cases were identified. Bilateral mapping was successful in 352 cases (75%), unilateral mapping in 73 cases (15%). Bilateral mapping was achieved in 266 (85%) of 312 ICG cases compared with 86 (54%) of 160 blue dye cases (p<0.001). Cases with successful bilateral mapping had a median BMI of 29.8 kg/m2 (range, 16.3–65.3 kg/m2); cases with no mapping had a median BMI of 34.7 kg/m2 (range, 21.4–60.4 kg/m2) (p=0.001). With increasing BMI, there was a significant decrease in successful bilateral mapping rates for both the ICG (p<0.001) and blue dye groups (p=0.041). However, the use of ICG resulted in better bilateral (p=0.002) and overall (p=0.011) mapping rates compared with the use of blue dye in all BMI groups.
Conclusions
ICG results in a higher overall and bilateral SLN detection than blue dye in women with uterine cancer. Successful mapping decreases with increasing BMI irrespective of dye used; however, it is significantly improved with the use of ICG and NIR fluorescence imaging compared to blue dye.
Introduction
Sentinel lymph node (SLN) mapping in combination with pathologic ultrastaging has gained increasing acceptance in the surgical staging of uterine cancer. According to the most recent National Comprehensive Cancer Network (NCCN) guidelines, a published SLN algorithm incorporating ultrastaging may be considered for the surgical staging of select patients with apparent uterine-confined endometrial cancer (1). The techniques involved in the SLN approach in regards to injection site and preferred dye, however, are still debatable and evolving. There are several reports describing the feasibility and detection rates of SLN mapping using various colorimetric imaging with blue dyes and/or radiocolloid mapping with Technetium (2). Bilateral mapping rates of 40% have been reported with the use of blue dye alone (3), and 69% with blue dye and radioisotope (4). Radiocolloid mapping has disadvantages compared with mapping with blue dye or indocyanine green (ICG) dye as far as the need to coordinate with the nuclear imaging department, potential pain on injection, and exposure to radiation. ICG has recently been used with near-infrared (NIR) fluorescence imaging for lymphatic mapping, and has been shown to be safe, with a low risk of allergic reactions (5).
Recent publications have shown increased success rates for bilateral and overall mapping using ICG dye versus blue dye in patients with uterine cancer (6, 7). However, these same publications have reported decreasing SLN mapping rates with increasing body mass index (BMI). Of note, the study by Sinno and colleagues (6) showed this negative association only with the use of blue dye, and not with ICG. In the current study, we sought to retrospectively investigate the impact of obesity on the rate of successful SLN mapping in patients with uterine cancer and to compare blue dye and ICG SLN detection rates stratified by BMI.
Methods
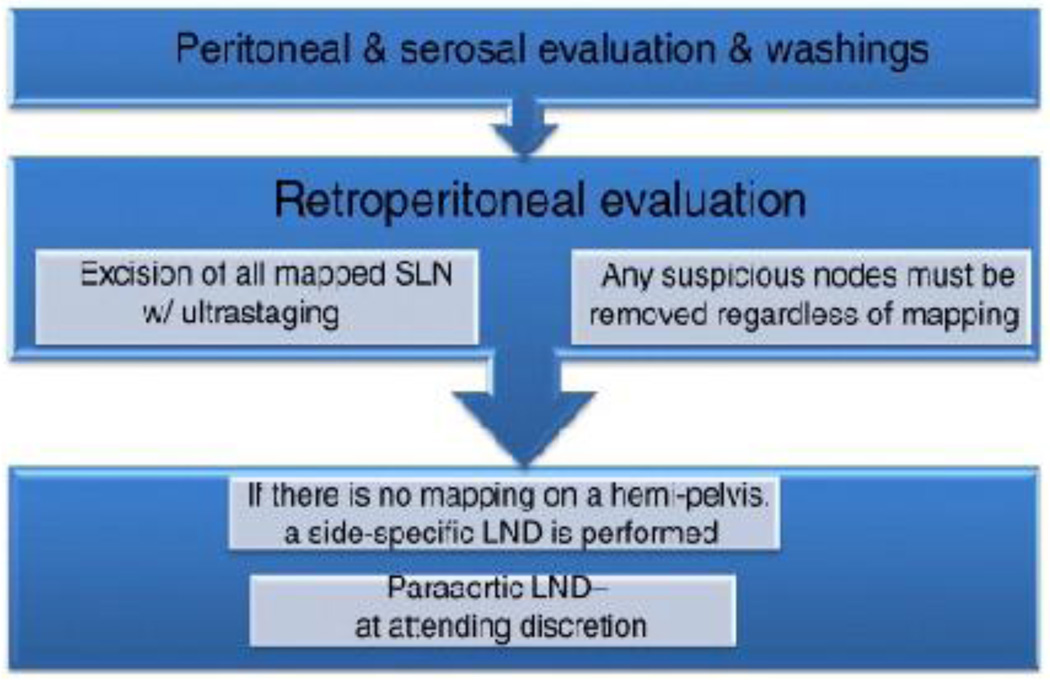
Institutional review board approval was obtained for this retrospective study. We reviewed all robotic cases undergoing SLN mapping with a cervical injection for uterine cancer and complex atypical hyperplasia from 1/2011–12/2013 using either blue dye or ICG with NIR fluorescence imaging. During the study period, our institution changed from primarily using blue dye to using ICG with NIR fluorescence imaging. Patients in whom both dyes were used have been excluded from this analysis. To avoid the possible influence of an SLN learning curve on our data, we elected to only include patients from a time period when our surgeons were experienced with the SLN technique. Our institution has performed SLN mapping since 2005. All surgeons had performed well over the previously published 30-case learning curve with SLN mapping (8) prior to this study-period. Of the 472 cases in this study, our institution has previously reported on 170 (7). All cases were mapped via an intracervical injection. For cases using ICG as a fluorophobe, the concentration used was 1.25 mg/mL. For each patient, a 25-mg vial with ICG powder was diluted in 20 cc of aqueous sterile water. A total of 4 cc of this ICG solution was injected into the cervix at the 3- and 9-o’clock positions, with 1 cc submucosally and 1 cc deep into the stroma after the patient had been sterilely prepped and draped but prior to the insertion of any uterine manipulator or docking of the robot. Blue dye was injected using the same method as described for ICG. Isosulfan blue was our preferred blue dye during the study period. Our previously published SLN algorithm was followed by all surgeons (Figure 1). SLNs were evaluated by pathologic ultrastaging.
Figure 1.
Sentinel lymph node mapping algorithm. LND, lymph node dissection. With permission from Barlin JN, et al. Gynecol Oncol 2012; 125:531-5. (Ref 3)
Demographic and surgical data were collected. Data were stratified by BMI as normal and overweight (BMI <30), obese (BMI ≥30–40), and morbidly obese (BMI ≥40), as well as by dye used. A subset analysis within each dye group to test mapping and BMI relationship was performed. The Fisher exact test or Wilcoxon Rank Sum test was used to test the two-way associations depending on whether a continuous variable was included or not. The mapping, BMI, and dye used (three-way association) was tested using the Cochran-Mantel-Haenszel test. SAS9.2 or R2.3.1 statistical software was used to perform all statistical tests.
Results
Four hundred seventy-two patients with uterine cancer were identified. ICG was used in 312 (66%) of 472 cases, and blue dye was used in 160 (34%) of 472 cases. The median age at time of surgery was 62 years (range, 30–90 years). The final histology and grade did not vary significantly between the two groups. The median operative time was 242 minutes (range, 141–498 minutes) and did not differ dramatically between the ICG and blue dye groups (p=0.045). Of 472 cases, bilateral mapping was successful in 352 (75%), unilateral mapping was successful in 73 (15%), and there was no mapping in 47 (10%). Bilateral mapping was achieved in 266 (85%) of the 312 ICG cases compared with 86 (54%) of the 160 blue dye cases (p<0.001). Demographic and surgical characteristics according to dye used are shown in Table 1.
Table 1.
Clinicopathologic demographics according to dye used
| All N=472 |
ICG n=312 |
Blue Dye n=160 |
p* | |
|---|---|---|---|---|
| Age at Surgery | ||||
| Median, years (Mean) | 62 (61.4) | 62 (61.5) | 62 (61.2) | 0.984* |
| Range | 30–90 | 30–90 | 34–87 | |
| BMI | ||||
| Median, kg/m2 (Mean) | 30.4 (31.9) | 30.6 (32.2) | 30 (31.3) | 0.506* |
| Range | 16.3–65.3 | 17.7–65.3 | 16.3–52.3 | |
| BMI <30 | 223 (47%) | 144 (46%) | 79 (49%) | |
| BMI 30–39 | 178 (38%) | 113 (36%) | 65 (41%) | |
| BMI≥40 | 71 (15%) | 55 (18%) | 16 (10%) | |
| Final Histology | ||||
| CAH/Endometrioid | 387 (82%) | 257 (82%) | 130 (81%) | 0.801 |
| Other | 85 (18%) | 55 (18%) | 30 (19%) | |
| Operative Time | ||||
| Median, minutes (Mean) | 242 (254.1) | 240 (247.7) | 248 (266.6) | 0.045* |
| Range | 141–498 | 145–444 | 141–498 | |
| SLN Operative Time | ||||
| Median, minutes (Mean) | 26 (29.4) | 26 (29) | 26 (30.4) | 0.984* |
| Range | 3–98 | 3–98 | 6–90 | |
| Intraoperative Mapping | ||||
| No mapping | 47 (10%) | 17 (5%) | 30 (19%) | <0.001 |
| Unilateral mapping | 73 (15%) | 29 (9%) | 44 (28%) | |
| Bilateral mapping | 352 (75%) | 266 (85%) | 86 (54%) | |
| Additional Lymphadenectomy | ||||
| No | 252 (53%) | 190 (61%) | 62 (39%) | <0.001 |
| Yes | 220 (47%) | 122 (39%) | 98 (61%) |
ICG, indocyanine green; BMI, body mass index; CAH, complex atypical hyperplasia
obtained using the Wilcoxon-rank sum test; other p-values were obtained using the Fisher exact test.
Of the 413 patients who had at least 1 SLN detected on final pathology, the median SLN count was 3 (range, 1–15). In the 59 patients who did not have a SLN detected, the median number of lymph nodes removed was 3 (range, 0–46). The majority of the patients who required additional lymph node dissection were in the blue dye group (61% vs. 39% in the ICG group; p<0.001).
Of the 425 patients who had either bilateral or unilateral intraoperative mapping, operative time spent specifically on SLN mapping was available for 279 patients (66%). The median SLN time was 26 minutes for the ICG group (range, 3–98 minutes) and 26 minutes (range, 6–90 minutes) for the blue dye group (p=0.984). When stratified by BMI, SLN time did significantly correlate with BMI for the entire cohort. When stratified by dye used and BMI, SLN time did not significantly correlate to BMI in the blue dye group, and it did not reach significance in the ICG group either (Table 2).
Table 2.
Three-way table of SLN time by dye and BMI (N=279)
| All N=279 |
SLN time (minutes) Median (Range) N=279 |
SLN time (minutes) Median (Range) ICG N=195 |
SLN time (minutes) Median (Range) Blue dye N=84 |
|
|---|---|---|---|---|
| BMI<30 | 136 | 25 (3–73) | 24(3–73) | 25.5(6–67) |
| BMI: 30–40 | 106 | 29 (7–98) | 29(8–98) | 28(7–90) |
| BMI>=40 | 37 | 26 (10–73) | 26(10–73) | 26.5(11–36) |
| P* | 0.04 | 0.07 | 0.4 |
BMI, body mass index; SLN, sentinel lymph node; ICG, indocyanine green dye
p-value is obtained using Kruskal-Wallis test
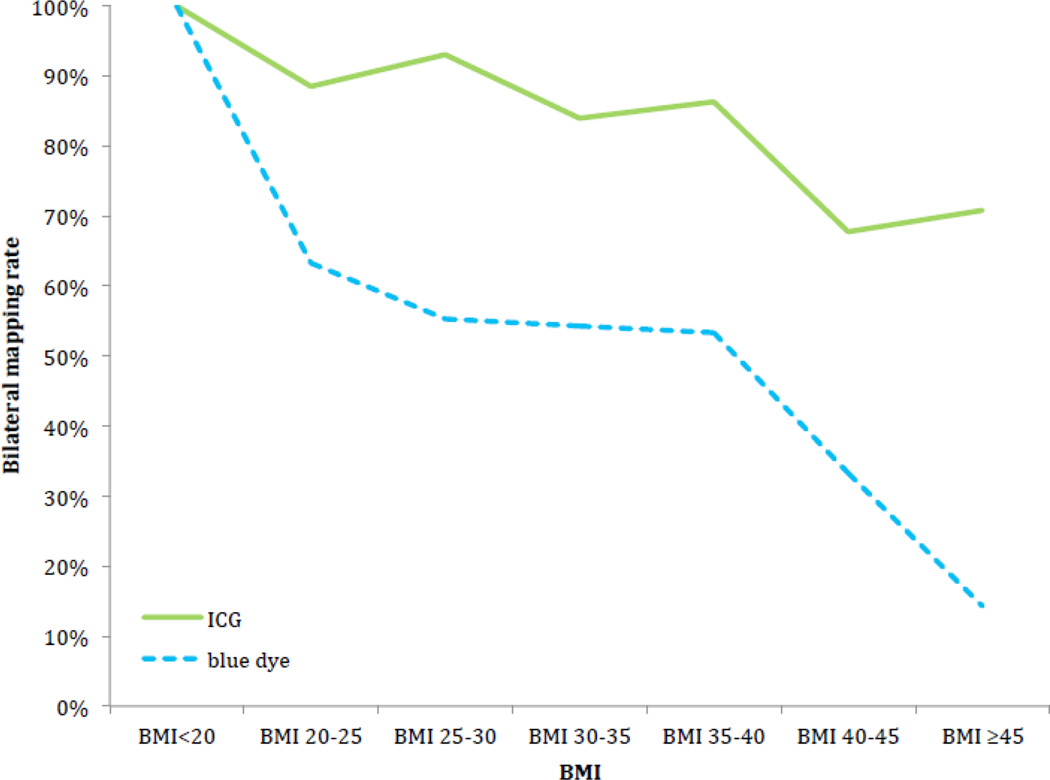
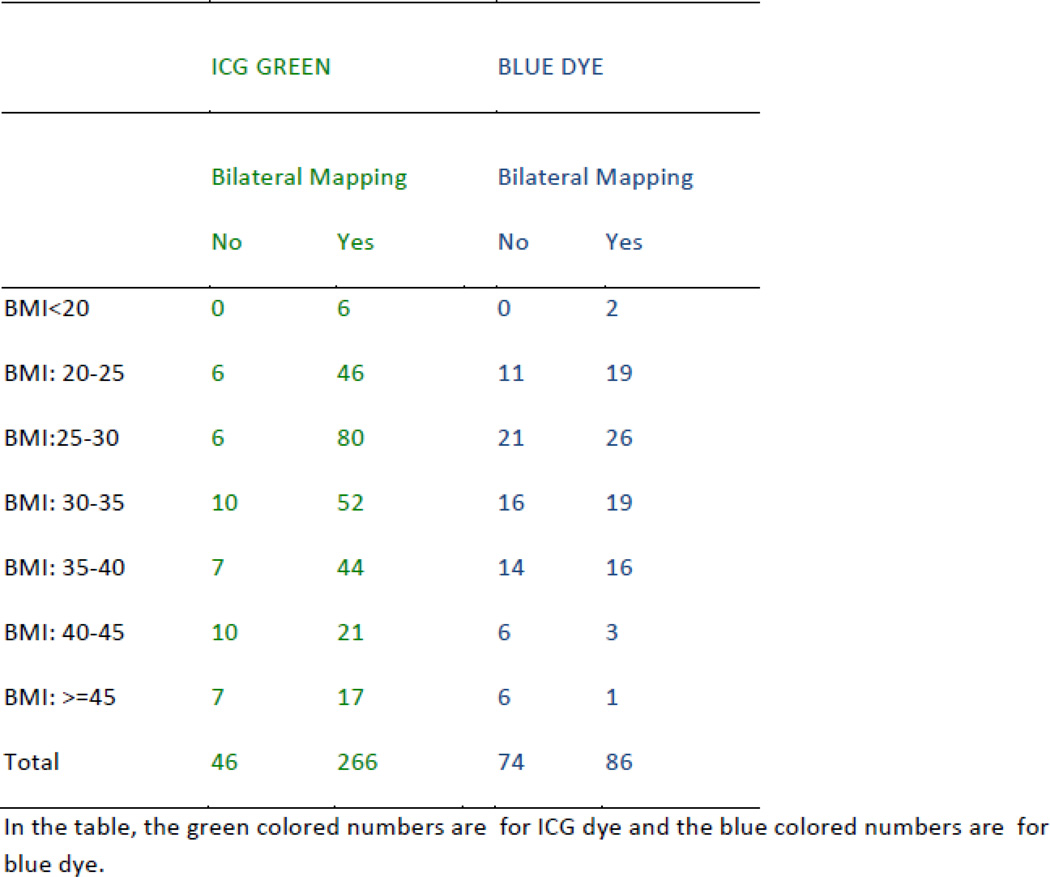
The median BMI for the entire cohort was 30.4 kg/m2 (range, 16.3–65.3 kg/m2) and was similar between the ICG and blue dye cohorts (p=0.51). Seventy-one (15%) of all patients were morbidly obese (BMI ≥40); the majority (55/71, 77%) of these patients were in the ICG group (p=0.03). Patients with successful bilateral mapping had a median BMI of 29.8 kg/m2 (range, 16.3–65.3 kg/m2) compared with patients with unsuccessful mapping, in whom the median BMI was 34.7 kg/m2 (range, 21.4–60.4 kg/m2) (p=0.001). There was a significant decrease in the rate of successful bilateral mapping for both the ICG (p<0.001) and blue dye groups (p=0.041) with increasing BMI. Overall mapping also decreased with increasing BMI in the ICG (p=0.008) and blue dye groups (p=0.036). However, use of ICG resulted in significantly better bilateral (p=0.002) and overall (p=0.011) mapping rates compared with the use of blue dye in all BMI groups (Table 3). The bilateral mapping rates for ICG and blue dye are illustrated in Figure 2.
Table 3.
Overall and bilateral mapping according to body mass index and dye used
| Number of Patients N=472 |
ICG n=312 |
Blue Dye n=160 |
**p-value | |
|---|---|---|---|---|
| Overall Mapping | 425 (90%) | |||
| BMI <30 ≥30–40 ≥40 |
207 162 56 |
140/144 (97%) 108/113 (96%) 47/55 (86%) |
67/79 (85%) 54/65 (83%) 9/16 (56%) |
0.011 |
| *p-value | 0.008 | 0.036 | ||
| Bilateral Mapping | 352 (75%) | |||
| BMI <30 ≥30–40 ≥40 |
179 131 42 |
132/144 (92%) 96/113 (85%) 38/55 (69%) |
47/79 (59%) 35/65 (54%) 4/16 (25%) |
0.002 |
| *p-value | <0.001 | 0.041 |
ICG, indocyanine green; BMI, body mass index
p-value: subset analysis within each dye group using the Fisher exact test to test mapping and BMI relationship
p-value: Three-way association Cochran Mantel Haenszel test p-values
Figure 2.
Bilateral mapping rate in relation to body mass index (BMI).
Discussion
Greater than 35% of adult women in the United States are obese (BMI ≥30) (9). Obesity is a major risk factor for uterine cancer, and in our study, 53% of the patients had a BMI ≥30. The high prevalence of obesity in this patient population makes it imperative to find reliable techniques for the surgical staging of obese and morbidly obese women in this setting. SLN mapping with pathologic ultrastaging is emerging as an acceptable approach for the surgical management of patients with uterine cancer, as it has showed high nodal metastatic detection rates (4, 10). The identification of nodal disease allows for tailored adjuvant treatment without the potential adverse effects of complete lymphadenectomy, such as increased operative time, intraoperative bleeding, length of hospital stay, and morbidity (11).
We recently reported increased bilateral and overall detection rates with the use of ICG and NIR fluorescence imaging versus blue dye in endometrial and cervical cancer (7). We reproduced those results in this cohort of uterine cancer patients, which included a larger number of cases as a result of expanding the inclusion period. Our results also show that with increasing BMI, the rates of overall and bilateral SLN mapping decrease with the use of either ICG or blue dye. However, mapping rates were significantly improved with the use of ICG in obese and morbidly obese patients. This is reflected in the need for additional lymphadenectomy in 61% of the patients in the blue dye group compared with 39% in the ICG group (p<0.001). Our results demonstrate that ICG should be the dye of choice for this patient population, and that obese patients should be counseled preoperatively on their decreased mapping rates and subsequent increased likelihood of additional lymphadenectomy and its associated morbidities.
Our results show that there is a significant correlation between BMI and SLN time, with an increase in SLN time with increasing BMI (Table 2); unfortunately, we have a limited number of patients in the group of patients with a BMI ≥40. Our data did not reach significance in the subset analysis of blue dye versus ICG due to sample size limitation.
The reason for the superior overall and bilateral mapping rates in the ICG group may be explained by the improved visualization of SLNs and lymphatic channels with the use of ICG and NIR fluorescence imaging compared with blue dye or the naked eye. Subserosal or submucosal injections of ICG lead to uptake by the lymphatic vessels and binding to plasma proteins. It is exclusively removed by the liver and excreted through the bile without being metabolized. NIR fluorescence imaging is capable of a penetration depth of up to 1 cm in tissue (12). Therefore, a thorough dissection to reach the lymphatic tissue is required to identify lymph channels and nodes with dye uptake. The limited, 1-cm visualization may explain the decreased SLN mapping rates in obese cases. In our study, we were unable to identify a critical cut-off point for BMI at which the mapping rate changes abruptly. We did find a gradual decrease in mapping with increasing BMI for both dyes (Figure 2). This may be explained by the increasing difficulty in visualizing lymphatic tissue with increasing amounts of visceral adipose tissue.
Only 15% of our population was morbidly obese (BMI ≥40), and we realize some centers may see patients with significantly higher median BMIs than that reported in our study. For this subgroup, we achieved a bilateral mapping rate of 69% and an overall mapping rate of 86% when using ICG and NIR fluorescence imaging compared with 25% and 56%, respectively, when using blue dye. The morbidly obese population is at increased risk for intraoperative and postoperative complications associated with prolonged operative time from a surgical and anesthesia standpoint. The advantage of an SLN approach compared with a full lymph node dissection is of particular importance in the morbidly obese, and we should continue to strive to obtain even higher rates of successful SLN mapping in these patients.
Future directions of research should examine the use of other dyes that may allow for the identification of SLNs through adipose tissue. Preclinical dyes such as CW800, a derivative of ICG, has a fluorescent intensity many times stronger than ICG, providing improved visibility in adipose tissue. CW800 can facilitate the detection of SLNs in our obese population. SIDAG (1,1(')-bis-(4-sulfobutyl) indotricarbocyanine-5,5(')-dicarboxylic acid diglucamide monosodium salt), another ICG derivate, is a highly hydrophilic molecule, whereas ICG is rather lipophilic. SIDAG is subsequently mainly eliminated via the renal system. Due to its low plasma protein binding and its low molecular weight, SIDAG can diffuse rapidly through pores of altered tumor vessels and can reach deeper regions of tumor tissue in a shorter time in rat models (13). Grossly enlarged nodes should always be removed as per our SLN algorithm, as we know that high numbers of cancerous cells block the uptake of ICG (14). The incorporation of novel dyes such as CW800 and SIDAG is nevertheless promising in regards to identifying cancerous tissue as the field evolves.
In conclusion, ICG with NIR fluorescence imaging results in higher overall and bilateral detection of SLNs in patients with uterine cancer compared with blue dye. Successful mapping decreases with increasing BMI irrespective of dye used, but it is significantly improved with the use of ICG compared with blue dye. Based on our findings, we recommend the use of ICG for all patients with uterine cancer, particularly obese patients. As novel dyes are introduced in the evolving field of image-guided therapy, incorporating tagged molecules for the detection of tissue positive for cancer, one must ensure that dyes and molecules utilized in other patient populations such as melanoma and breast are suitable for retroperitoneal detection in the obese, as these women are highly represented in the endometrial cancer population.
Acknowledgments
Funding Source: Funded in part by the cancer center core grant P30 CA008748. The core grant provides funding to institutional cores, such as Biostatistics and Pathology, which were used in this study.
References
- 1.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Uterine Neoplasms. [accessed June 2, 2015];Version 2.2015. Available: http://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf. [Google Scholar]
- 2.Khoury-Collado F, Abu-Rustum NR. Lymphatic mapping in endometrial cancer: a literature review of current techniques and results. Int J Gynecol Cancer. 2008;18:1163–1168. doi: 10.1111/j.1525-1438.2007.01188.x. [DOI] [PubMed] [Google Scholar]
- 3.Barlin JN, Khoury-Collado F, Kim CH, et al. The importance of applying a sentinel lymph node mapping algorithm in endometrial cancer staging: Beyond removal of blue nodes. Gynecol Oncol. 2012;125:531–535. doi: 10.1016/j.ygyno.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 4.Ballester M, Dubernard G, Lécuru F, et al. Detection rate and diagnostic accuracy of sentinel-node biopsy in early stage endometrial cancer: a prospective multicenter study (SENTI-ENDO) Lancet Oncol. 2011;12:469–476. doi: 10.1016/S1470-2045(11)70070-5. [DOI] [PubMed] [Google Scholar]
- 5.Speich Saesseli B, Hoffmann U, Neftel KA, Reichen J. Anaphylactoid reactions after indocyanine-green administration. Ann Intern Med. 1988;109:345–346. doi: 10.7326/0003-4819-109-4-345_2. [DOI] [PubMed] [Google Scholar]
- 6.Sinno AK, Fader AN, Roche KL, Giuntoli RL, 2nd, Tanner EJ. A comparison of colorimetric versus fluorometric sentinel lymph node mapping during robotic surgery for endometrial cancer. Gynecol Oncol. 2014;134:281–286. doi: 10.1016/j.ygyno.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 7.Jewell EL, Huang JJ, Abu-Rustum NR, et al. Detection of sentinel lymph nodes in minimally invasive surgery using indocyanine green and near-infrared fluorescence imaging for uterine and cervical malignancies. Gynecol Oncol. 2014;133:274–277. doi: 10.1016/j.ygyno.2014.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khoury-Collado F, Glaser GE, Zivanovic O, et al. Improving sentinel lymph node detection rates in endometrial cancer: How many cases are needed? Gynecol Oncol. 2009;115:453–455. doi: 10.1016/j.ygyno.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 9.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 10.Kim CH, Soslow RA, Park KJ, et al. Pathologic ultrastaging improves micrometastasis detection in sentinel lymph nodes during endometrial cancer staging. Int J Gynecol Cancer. 2013;23:964–970. doi: 10.1097/IGC.0b013e3182954da8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dowdy SC, Borah BJ, Bakkum-Gamez JN, et al. Prospective assessment of survival, morbidity, and cost associated with lymphadenectomy in low-risk endometrial cancer. Gynecol Oncol. 2012;127:5–10. doi: 10.1016/j.ygyno.2012.06.035. [DOI] [PubMed] [Google Scholar]
- 12.Schols RM, Connell NJ, Stassen LP. Near-infrared fluorescence imaging for real-time intraoperative anatomical guidance in minimally invasive surgery: a systematic review of the literature. World J Surg. 2015;39:1069–1079. doi: 10.1007/s00268-014-2911-6. [DOI] [PubMed] [Google Scholar]
- 13.Ebert B, Riefke B, Sukowski U, Licha K. Cyanine dyes as contrast agents for near-infrared imaging in vivo: acute tolerance, pharmacokinetics, and fluorescence imaging. J Biomed Opt. 2011;16:066003. doi: 10.1117/1.3585678. [DOI] [PubMed] [Google Scholar]
- 14.Kusano M, Tajima Y, Yamazaki K, Kato M, Watanabe M, Miwa M. Sentinel node mapping guided by indocyanine green fluorescence imaging: a new method for sentinel node navigation surgery in gastrointestinal cancer. Dig Surg. 2008;25:103–108. doi: 10.1159/000121905. [DOI] [PubMed] [Google Scholar]