Participation in physical activities declines with age (Hunter, Thompson, & Adams, 2000; Westerterp & Meijer, 2001). This decline is unfortunate because physical activity has been shown to benefit cognitive functioning in older adults. In cross-sectional studies, older adults who engaged in aerobic activities performed better on measures of working memory and fluid intelligence compared to those engaged in sedentary activities (Lochbaum, Karoly, & Landers, 2002; van Boxtel, Langerak, Houx, & Jolles, 1996). In longitudinal studies, older sedentary adults who began and maintained an aerobic exercise program for at least 6 months performed better on measures of processing speed, executive functioning, and memory (Colcombe & Kramer, 2003). Based upon these studies, it is clear that engaging in physical activity may lead to cognitive benefits, which is one of the vital components necessary for successful aging (Rowe & Kahn, 1997).
Two mechanisms by which physical activity may promote successful cognitive aging are the depression-reduction hypothesis and the social-stimulation hypothesis. The depression reduction hypothesis asserts that depression interferes with normal cognitive functioning; therefore, successful strategies to reduce depression will automatically improve cognitive functioning. This hypothesis is based upon two facts. First, depressive symptoms are correlated with poor cognitive functioning in older adults (La Rue, Swan, & Carmelli, 1995; Potter & Steffens, 2007; Rabbitt, Donlan, Watson, McInnes, & Bent, 1995). This phenomenon can be observed in longitudinal studies as well. In a sample of 641 older adults, Comijs, Jonker, Beekman, and Deeg (2001) found that over a 3-year period, depressive symptoms were associated with declines in processing speed. Second, studies show that older depressed adults who engaged in physical activity actually experience declines in depressive symptomatology and improvements on measures of cognitive functioning. In a sample of 84 older clinically depressed adults, Khatri and colleagues (2001) randomized half to receive a four-month exercise program and half to receive antidepressant medications. These researchers found that those assigned to the exercise program improved on measures of executive functioning and memory, and such improvements corresponded to decreases in depressive symptomatology. Other studies have found similar effects (Bartholomew & Ciccolo, 2008). However, in interpreting these results, others have warned that depression may also reduce the motivation with which cognitive tests are taken, resulting in poorer performance (Brebion et al., 2000; Powell, al-Adawi, Morgan, & Greenwood, 1996).
The social-stimulation hypothesis asserts that engaging in social activities can promote or maintain cognitive functioning. As such, many physical activities (i.e., bowling, dancing) are social activities (King et al., 1992; O'Brien, 1995; Oka, King, & Young, 1995; Wolinsky, Stump, & Clark, 1995; Young & King, 1995). This hypothesis is supported from the enriched environmental paradigm in which rats placed in environments with other rats exhibited increased synaptic density, increased thickness of the cerebral cortex, and better maze performance, which approximated cognitive ability, than rats placed in isolation (Diamond, 1993; Diamond, Johnson, Protti, Ott, & Kajisa, 1985; Diamond, Krech, & Rosenzweig, 1964; Sirevaag & Greenough, 1987). Some studies in humans also support this hypothesis. For instance, in a sample of 755 older adults, Newson and Kemps (2005) found that those with a higher level of activities, including social activities, experienced less cognitive declines. Also, Dufouil and Alperovitch (2000) found that having an intelligent spouse was protective against cognitive loss in older adults. Yet, in examining relationships among physical activity, social networks, and cognitive function, one must be mindful that causal directionality is not always clear. Most researchers endorse the idea that social networks facilitate physical activity, rather than the reverse (Spanier & Allison, 2001; Stahl et al., 2001; Treiber et al., 1991). However, with respect to cognitive function, the social stimulation hypothesis proposes that given the social component that often accompanies physical activity (Booth, Owen, Bauman, Clavisi, & Leslie, 2000; Calfas, Sallis, Oldenburg, & Ffrench, 1997; Duncan & Stoolmiller, 1993), the social environment and social stimulation may facilitate some of the cognitive gains as observed in rat studies, instead of, or in addition to, the physical activity itself.
Testing these two hypotheses in a sample of 158 cognitively-intact, community-dwelling older adults, Vance, Wadley, Ball, Roenker, and Rizzo (2005), used structural equation modeling to examine the depression-reduction hypothesis and the social-stimulation hypothesis. The model proposed fit the data well, and partial support for both hypotheses was found. A significant path emerged leading from physical activity to social networks to depressive symptomatology to cognition. Specifically, those who engaged in more physical activity had bigger social networks; bigger social networks were predictive of less depression; and less depression was predictive of better cognitive functioning. Meanwhile, a direct path from physical activity to cognition indicated that those who engaged in more physical activity had better cognitive functioning.
This prior study also included an indicator variable for lack of physical activity, called sedentary behavior. With this variable, a significant path emerged leading from sedentary behavior to depressive symptomatology to cognition. Specifically, those who spent more time being sedentary were more depressed, and more depression was predictive of poorer cognitive functioning. However, a direct path between sedentary behavior and cognition indicated that those who engaged in more sedentary behavior had better cognitive functioning, which seems to contradict the finding that sedentary behavior predicts depression which is related to poorer cognitive functioning (DiPietro, 2001; Epstein & Hundert, 2002). Although sedentary behavior may be associated with depressive symptoms that hamper cognitive functioning, it is also known that an adequate amount of sleep is necessary for good cognitive functioning (Gale & Martyn, 1998; Hauri, 1997; Vance, Heaton, Eaves, & Fazeli, 2011; Yaffe, Blackwell, Barnes, Ancoli-Israel, & Stone, 2007). Unfortunately, this previous measure of sedentary behavior combined sleeping with sedentary activity during waking hours, and these activities could not be separated.
Finally, this prior study recruited older adults “operating within a generally intact range of cognition” (p. 207). In this prior study, these hypotheses have not been examined among older adults with a wider range of cognitive abilities. Edwards, Linquist, and Yaffe (2004) suggested that cognitive aging ranges from normal functioning, to mild cognitive impairment (MCI), to dementia. Unverzagt and colleagues (2001) found that the prevalence of MCI increases with age. In a large sample of older African Americans, the average prevalence of MCI was 23.4% and grew from 19.2% for those between 65 to 74 years of age to 38% for those 85 years of age or older. These researchers reported that these findings were comparable to MCI rates in European countries. In the United States, by 2030 there will be nearly 70 million adults 65 years of age and older; of that, nearly a quarter (17.5 million) will meet criteria for MCI (Aging, 2004). This projection is startling given the high risk for conversion from MCI to dementia (Edwards et al., 2004). Therefore, it is important to understand mechanisms that support cognitive functioning in older adults with a wide range of cognitive abilities.
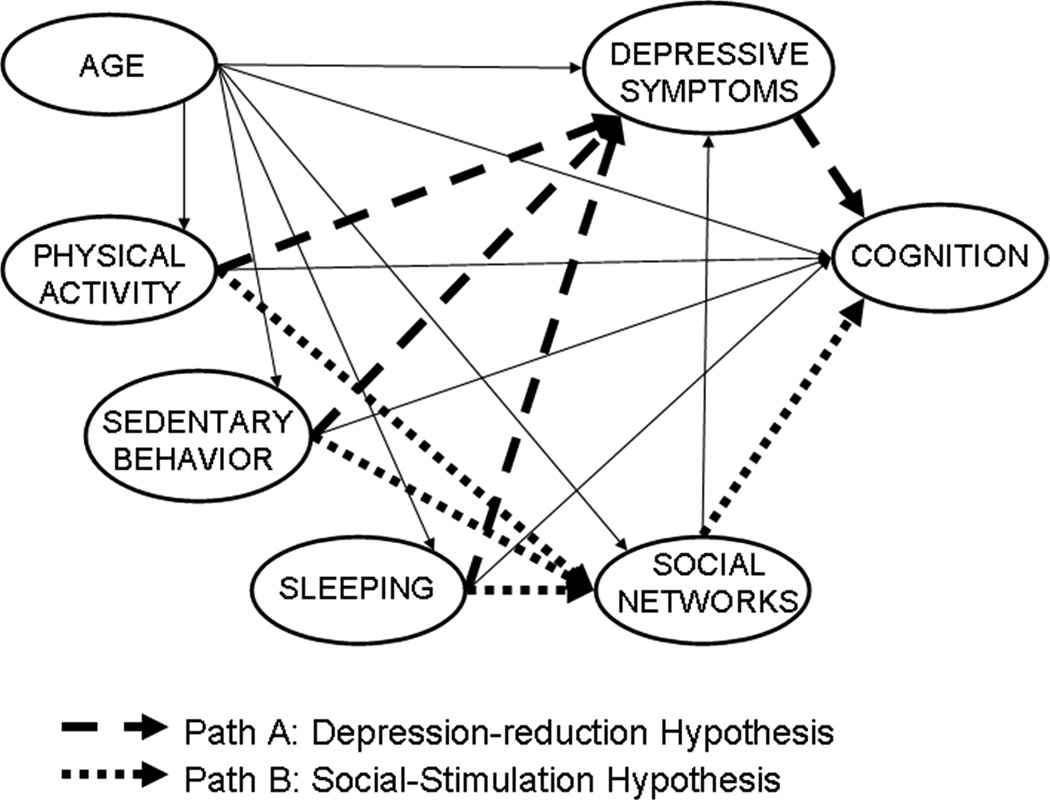
The purpose of this study is to not only replicate the previous study from Vance et al., (2005) but to improve it in two important ways. First, the role of duration of sedentary behavior and sleeping were examined separately in order to gain a better understanding of their individual contributions within the depression-reduction hypothesis (Path A) and the social-stimulation hypothesis (Path B) as they relate to physical activity and cognitive functioning in older adults (See Figure 1). Second, the previous study included only cognitively intact, older adults; this current study included individuals with a wider range of cognitive abilities, including individuals with MCI, which allowed more variance to be examined in relationship to these hypotheses.
Figure 1. Hypothesized Paths of Cognitive Aging.
Solid lines represent hypothesized paths. Path A = Depression Reduction Hypothesis (Physical Activity/Sedentary Behavior/Sleeping → Social Networks → Cognition); Path B = Social Stimulation Hypothesis (Physical Activity/Sedentary Behavior/Sleeping → Depression → Cognition). Sedentary Behavior and Sleeping are predicted to operate through the same paths as Physical Activity.
Method
Participants
One-hundred and twenty-two community-dwelling older adults were recruited from the Birmingham, Alabama metropolitan area for the Measuring Independent Living in the Elderly Study (MILES) of cognitive, functional (e.g., driving ability), and psychosocial differences among adults with and without mild cognitive impairment (MCI). This study (MILES) was a funded project within the University of Alabama at Birmingham (UAB) Alzheimer’s Disease Research Center (ADRC). All participants completed a neurological examination and neuropsychological testing in order for diagnoses to be determined in ADRC consensus conferences according to Petersen/Mayo criteria for MCI (Petersen, 2004). Sixty-two (50.82%) participants were diagnosed with MCI and 60 (49.18%) participants were determined to be neurocognitively normal controls. Participants provided informed consent prior to enrollment in this IRB-approved study.
Measures
Physical Activity Questionnaire
This instrument was adapted from the National Cardiovascular Health Study (NCHS) Health Interview Survey: Health Promotion and Disease Prevention Supplement and the Paffenbarger Questionnaire (Cardiovascular_Health_Study, 1989). Physical activity is measured by participation in several specific activities, frequency of participation, and average duration of participation. This measure is unique in that the physical activity questions focus on what participants have actually done in the past two weeks, rather than what they typically do or what they would like to do. The specific physical activities included in this measure are housework, yard work or gardening, walking for exercise, jogging, riding a bike, stationary cycle, dancing, bowling, golf, exercise calisthenics, and swimming. Participants were also asked if they had engaged in any other physical activities in the past two weeks, and if so, how frequently and for how much time. No assessment of physical intensity is included. Based on the conceptual framework, the questionnaire items were divided into three mutually exclusive domains: duration of sleeping in a typical 24-hour period behavior, duration of sedentary behavior in a typical 24-hour period, and amount of physical activity over the previous two weeks.
For ease of interpretation, all physical activity data were transformed to reflect the total time spent in physical activity during one week instead of two weeks. The reported average amount of time per session was multiplied by the number of sessions in which they engaged in the given activity; each of these products was then summed across all activities to derive the total amount of time engaged in physical activity over two weeks. This total was divided by two in order to obtain the time spent in the activity during one week.
Lubben Social Network Scale (LSNS)
The LSNS (Giranda, Lubben, & Atchison, 1999; Lubben, 1988; Rubinstein, Lubben, & Mintzer, 1994) is a measure of social contact and social isolation. The scale consists of a total of 10 items. Six items measure the frequency and intimacy of social contact with friends and family. Responses to each of these questions are based on a six-point Likert scale ranging from 0 to 5. Two questions assess the availability of confidant relationships, such as, “When you have an important decision to make, do you have someone you can talk to about it?” Responses to these questions are based on a six-point Likert scale ranging from 0 to 5. Two additional items pertain to living arrangements and the extent to which the participants help others with activities like shopping, childcare, repair work, etc., coded on a scale ranging from 0 to 5 The final question assesses the living arrangement of the participant (i.e., 0 = lives alone, 5 = lives with spouse). The range of possible total scores is from 0 to 50, with higher scores indicating more social contact. Lubben (1988) suggested that a score below 20 serves as a clinical cut point for social isolation. Others have suggested four levels of isolation: isolated (≤20), high risk for isolation (21–25), moderate risk for isolation (26–30), and low risk for isolation (≥31) (Rubinstein et al., 1994). Because inter-item consistency was acceptable (Cronbach’s alpha = .70) in this sample, scores were used continuously in the present analyses.
Geriatric Depression Scale (GDS)
The GDS provides a quick screening of depressive symptoms experienced over the past week (Yesavage, 1983). The scale includes 30 yes-no statements, framed both positively and negatively (e.g., “Do you feel your life is empty?” and “Do you feel happy most of the time?”). Therefore, some items are reverse-scored. Higher scores indicate more depressive symptoms. This scale has a high test-retest reliability (r = .85) and has been used extensively for screening depressive symptoms in community-dwelling elderly persons (Yesavage, 1983). Inter-item consistency on this measure was very high in this sample (Cronbach’s alpha = .99).
Cognitive Outcomes
Four instruments were used to assess cognition in the domains of executive functioning, memory, and attention.
Animal Fluency
This category fluency test is a measure of semantic fluency and executive functioning. In this test, participants are instructed to name as many items as they can that belong to a specified category within one minute, in this case animals. This test is sensitive to temporal and parietal lobe damage in addition to frontal-subcortical circuitry which are associated with executive functioning. The category of “animals” is the most commonly used and is well normed. In this study, the raw score is the total number of animals generated, excluding intrusions (non-animals) and repetitions (Spreen & Strauss, 1998).
California Verbal Learning Test (CVLT)-II Long Delay Free Recall
The CVLT-II is a measure of verbal memory. In this test, participants are read aloud a list of 16 words from 4 categories (e.g., spices, fruits). Participants are asked to recall the maximum number of words they can remember after several learning trials. Then, participants are given another set of words to remember and recall. After a 20-min delay, participants are instructed to recall the maximum number of words from the first list (Delis, Kramer, Kaplan, & Ober, 1987). Although there are other components of this test, only the number of recalled words after the long delay was used in this study.
Trails A
Trails A is a commonly used measure of attention, visuospatial tracking, and perceptual motor speed (R. Reitan, 1958; R. M. Reitan, 1979). This reliable measure possesses good sensitivity to age-related cognitive declines (Spreen & Strauss, 1998). In this paper-and-pencil test, participants connect randomly scattered numbers on a page with a pencil in sequence (e.g., 1-2-3-4). Performance is determined by how much time (sec) participants need to complete the task; lower scores indicate better cognitive functioning.
Trails B
Trails B is a commonly used measure of executive functioning as well as attention, visuospatial tracking, and perceptual motor speed (R. Reitan, 1958; R. M. Reitan, 1979). This reliable measure also possesses good sensitivity to age-related cognitive declines (Spreen & Strauss, 1998). In this paper-and-pencil test, participants connect randomly scattered numbers and letters on a page with a pencil in alternating sequence (e.g., 1-A-2-B-3-C). Performance is determined by how much time (sec) participants need to complete the task; lower scores indicate better cognitive functioning.
Procedure
Data collection was completed during two separate sessions. The Physical Activity Questionnaire, LSNS, and GDS were completed as part of the MILES study visit at the UAB Center for Translational Research on Aging and Mobility. The cognitive measures were completed at the UAB ADRC. Other neuropsychological and functional data not used in these analyses were collected during these two sessions as well.
Results
Sample characteristics are displayed in Table 1. Table 2 displays the correlation matrix for the variables used in the structural equation model. Pearson’s correlations were calculated between pairs of continuous variables. Violations of multivariate normality were examined. Kurtosis and skewness were detected; therefore, the data were normalized using the LISREL transformation option (Jöreskog & Sörbom, 1993). Furthermore, maximum likelihood estimation was used because it allows for all fit indices to be produced (Hoyle & Panter, 1995; Jöreskog & Sörbom, 1993; Kelloway, 1998). Finally, a covariance matrix produced from this procedure was used for structural equation modeling analysis.
Table 1.
Demographic and Overall Test Scores of Participants (N = 122)
| Variable | Number (%) | M (SD) | Range |
|---|---|---|---|
| Gender | |||
| Female | 70 (57.38) | ||
| Male | 52 (42.62) | ||
| Ethnicity | |||
| African American | 26 (21.31) | ||
| White | 96 (78.69) | ||
| Age in years | 70.45 (7.16) | 55.52 – 88.31 | |
| Education in years | 14.99 (2.77) | 8 – 20 | |
| Duration of Physical Activity (hr) in One Week | 7.07 (9.95) | 0 – 70.13 | |
| Duration of Sedentary Behavior (hr) in One Week | 48.76 (27.83) | 7 – 126 | |
| Duration of Sleeping (hr) in One Week | 52.17 (8.69) | 35 – 84 | |
| LSNS | 36.68 (7.22) | 18 – 50 | |
| GDS | 6.29 (5.30) | 0 – 22 | |
| Animal Fluency (# correct) | 17.93 (5.22) | 7 – 30 | |
| CVLT-II Long Delay Free Recall (# correct) | 7.41 (4.18) | 0 – 15 | |
| Trails A (sec.) | 37.98 (15.31) | 19 – 87 | |
| Trails B (sec.) | 1076.89 (68.64) | 12 – 480 |
Note.
CVLT-II = California Verbal Learning Test; GDS = Geriatric Depression Scale; LSSN = Lubben Social Network Scale.
Table 2.
Correlation Matrix Using Defined Variables from SPSS (N = 122)
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| R | Age | Duration of Physical Activity |
Duration of Sedentary Behavior |
Duration of Sleeping |
LSNS | GDS | Animal Fluency |
CVLT-II Long Delay Free Recall |
Trails A | Trails B |
| 1 | 1.00 | |||||||||
| 2 | .03 | 1.00 | ||||||||
| 3 | −.10 | −.20† | 1.00 | |||||||
| 4 | −.16 | −.08 | .00 | 1.00 | ||||||
| 5 | −.04 | .01 | −.25‡ | −.08 | 1.00 | |||||
| 6 | −.04 | −.21† | .08 | .17 | −.05 | 1.00 | ||||
| 7 | −.21† | .13 | −.09 | .01 | .02 | −.26‡ | 1.00 | |||
| 8 | −.21† | .21† | .00 | .01 | .02 | −.24‡ | .53‡ | 1.00 | ||
| 9 | .36‡ | −.05 | .10 | .09 | −.16 | .22† | −.44‡ | −.39‡ | 1.00 | |
| 10 | .24‡ | −.07 | −.03 | .02 | −.06 | .14 | −.51‡ | −.55‡ | .66‡ | 1.00 |
Note.
CVLT-II = California Verbal Learning Test; GDS = Geriatric Depression Scale; LSSN = Lubben Social Network Scale. Scale.
p < .05.
p < .01.
Anderson and Gerbing (1988) recommended that structural equation modeling must proceed sequentially, which allows different models to be compared to one another. Thus, a baseline model is created first which allows the latent variables to correlate to each other. Then the full causal model (Figure 1) is created which reflects the full hypothesized causal model. Finally, the trimmed model is created by eliminating each nonsignificant path in the model and reanalyzing the model until only significant paths in the model remain (Figure 2). For this analysis, a baseline model was created incorporating age as well as the six conceptual variables –physical activity, sedentary behavior, sleeping, social networks, depressive symptoms, and cognition. Cognition was a latent variable with scores on Animal Fluency, CVLT-II Long Delay Free Recall, Trails A, and Trails B used as indicators. Trails A served as the reference variable for this latent variable. The other conceptual variables had only one indicator variable, using either summary scores such as depressive symptoms (i.e., GDS total) or direct measures such as sedentary behavior (i.e., amount of time sitting, which was assessed with a single item of the Physical Activity Questionnaire). In all models, social networks and depressive symptoms were assessed as mediators of the effects of physical activity on cognition.
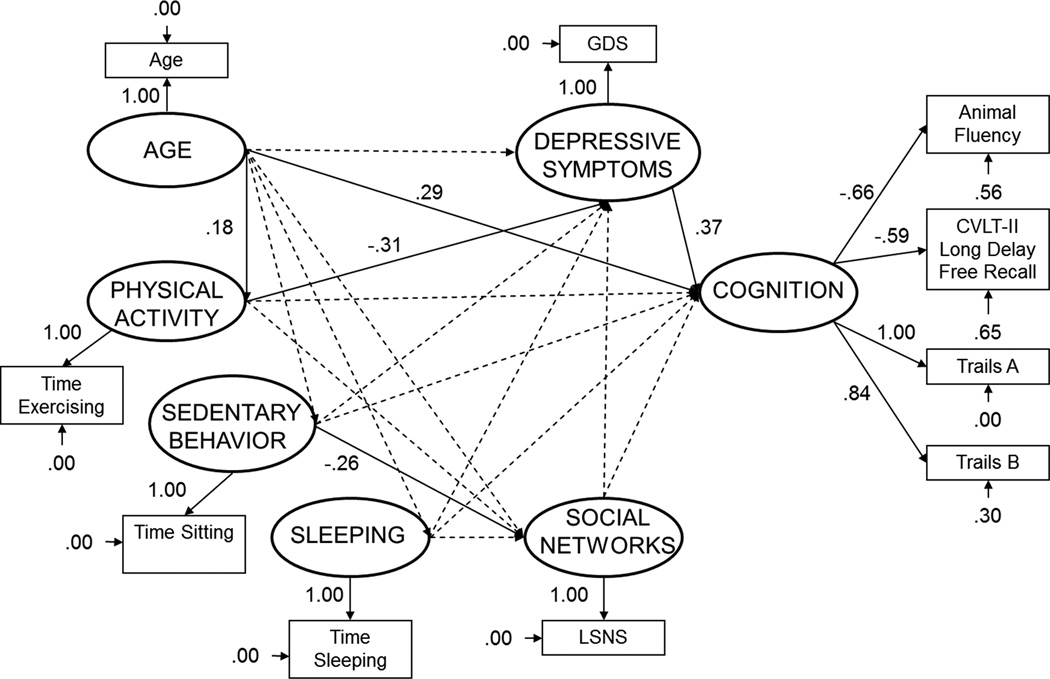
Figure 2. Trimmed structural equation model predicting cognitive ability (standardized solution).
All solid lines represent significant paths (p < 0.05). CVLT-II = California Verbal Learning Test; GDS = Geriatric Depression Scale; LSNS = Lubben Social Network Scale.
The baseline model was constructed first to test the stability of the latent variables. All latent variables were free to correlate and had significant loadings, signifying that the variables were stable and appropriate for use in subsequent analyses. In addition, as viewed in Table 3, the baseline model fit the data well, permitting further analysis to continue.
Table 3.
Fit Measures of Baseline, Causal, and Trimmed Models
| X2(df) | GFI | AGFI | PGFI | RMR | RMSEA | NNFI | CFI | |
|---|---|---|---|---|---|---|---|---|
| Baseline Model | 21.11 (19) | .97 | .91 | .33 | .04 | .03 | .98 | .99 |
| Full Causal Model | 44.42 (22) | .95 | .89 | .38 | .06 | .05 | .93 | .97 |
| Trimmed Model | 44.29 (36) | .93 | .90 | .61 | .07 | .06 | .96 | .97 |
Note.
AGFI = Adjusted Goodness-of-Fit Index; CFI = Comparative Fit Index; df = degrees of freedom; GFI = Goodness-of-Fit Index; NNFI = Non-Normed Fit Index; PGFI = Parsimony Goodness-of-Fit Index; RMR = Root Mean Residual; RMSEA = Root Mean Square Error of Approximation; χ2 = Chi-Square.
Baseline and full causal models were compared to an independent model in which all the variables are uncorrelated. For the baseline model, a chi-square difference test revealed that there was a significant improvement in fit of the baseline model relative to the independent model, χ2(45, N = 122) = 303.97, p < 0.001. Next the full causal model was tested (Figure 1). A chi-square difference test revealed a significant improvement in the fit of the full causal model relative to the independent model, χ2diff (23, N = 122) = 273.11, p < 0.001. Because many of the model estimates were nonsignificant, a trimmed model was constructed by sequentially removing nonsignficant paths and re-evaluating model fit. The least significant paths (based on the lowest t-value) were dropped one at a time and model estimates were then recalculated each time. This process continued until only statistically significant causal paths remained in the model (p < 0.05).
Figure 2 shows the trimmed model including coefficients in standardized form. A chi-square difference test between the full causal model and the trimmed model revealed no significant difference, χ2diff (14, N = 122) = 13.43, p > 0.25, indicating that the trimmed model fit as well as the full causal model. Standard fit indices for the baseline model and the trimmed model are displayed in Table 3; the trimmed model fit the data very well (e.g., GFI = 0.93, CFI = 0.97); values of .90 or higher on these measures are considered excellent (Kelloway, 1998). Post-hoc model modifications were conducted; therefore, a correlation was calculated between the causal model estimates and the trimmed model estimates (Ullman, 1996). A high correlation was observed (r = 0.99) which indicates that the parameter estimates for the statistically significant paths were unchanged after trimming the nonsignificant paths from the model.
The final trimmed model shows that physical activity, depressive symptomatology, and age all contribute either directly or indirectly to cognition. The trimmed causal model explained 22% of the variance in cognition. Sedentary behavior (time spent sitting) was directly related to social networks. Physical activity (standardized coefficient = −0.31) exerted a direct influence on depressive symptomatology, and depressive symptomatology (standardized coefficient = 0.37) exerted a direct influence on cognition in the predicted directions. Specifically, those who engaged in more physical activity exhibited less depressive symptomatology. In turn, those who reported more depressive symptomatology displayed poorer cognitive functioning. Likewise, age (standardized coefficient = .29) exerted a direct influence on cognition; specifically, those who were older exhibited poorer cognitive functioning. Finally, sedentary behavior (standardized coefficient = −.26) exerted a direct influence on social networks; specifically, those who reported more sedentary behavior reported a smaller social network. Indirect relationships also were observed. The indirect causal path from physical activity to depressive symptomatology to cognition (standardized coefficient = −.10) was significant. Sleep was not significantly related to social networks, depressive symptoms, or cognitive functioning.
Discussion
The purpose of this study was to examine the depression-reduction hypothesis and the social-stimulation hypothesis in a cognitively diverse sample of older adults. By using structural equation modeling techniques, the unique roles of age, sedentary behavior, and sleep on the variables of interest were examined. A path was observed from physical activity to depressive symptoms to cognition, showing that those who engaged in more physical activity had less depressive symptoms, and having less depressive symptoms was related to better cognitive ability. Thus, support was found for the depression-reduction hypothesis.
Other relationships were observed in this model. A path from sedentary behavior to social networks was observed showing that higher levels of sedentary behavior were associated with reduced social networks. This finding is somewhat consistent with the social-stimulation hypothesis. Specifically, as sedentary behavior is a negative indicator of physical activity, its negative relationship to social networks would be expected; however, social networks were not directly related to cognition. This finding was somewhat surprising given that social interaction has been found to be associated with greater cognitive functioning (Green, Rebok, & Lyketsos, 2008; Moyle, Kellett, Ballantyne, & Gracia, 2011; Vance, Fazeli, Ross, Wadley, & Ball, 2012).
A path from age to physical activity was observed; specifically, those who were older reported being engaged in more physical activity. This finding was surprising; however, older adults may have more time to engage in such activities and may view engaging in physical activity as a way to facilitate their ability to age successfully (Depp, Vahia, & Jeste, 2010).
Finally, a path from age to cognition was observed; specifically, being older was associated with poorer cognitive functioning. This finding was not surprising given the general findings in the literature between cognition and age in both normal adults and adults with MC (Ball, Vance, Edwards, & Wadley, 2004; Unverzagt et al., 2001; Vance et al., 2012).
Of note, the final trimmed model accounted for 22% of the variance in cognition. Therefore, other factors not included in the model might account for the remaining variance in cognitive function. Candidates for inclusion in future studies include physical health status, education, leisure time activities, occupational status over the life course, and cognitive reserve (Vance et al., 2012).
Several strengths and limitations of this study are noted. First, a strength of this study was that a unique sample of older adults with a more varied range in cognitive ability was used which may more accurately reflect the cognitive functioning of older adults in general. In order to determine a diagnosis of MCI, participants were screened and assessed through the university’s ADRC and confirmed through clinical consensus. Second, all of the cognitive and psychosocial measures were well accepted and valid measures. Unfortunately, the measures for sedentary behavior and sleep, although separate variables for this study as opposed to the previous study, there are single self-report indicators and may not be sufficient to represent these constructs (Vance et al., 2005). More validated measures of sedentary behavior and sleep hygiene, such as the Pittsburgh Sleep Quality Index, should be incorporated in future studies (Buysse, Reynolds, Monk, Berman, & Kupfer, 1989). Finally, in examining the social-stimulation hypothesis, a different measure of social networks or support may be incorporated. While the LSNS is measure of the size of the social network, it does not account for the quality of such networks. In other words, someone may have a larger social network but it may not provide the enjoyment or intellectual and emotional stimulation necessary to foster physical activity or facilitate cognition.
In conclusion, the final trimmed model shows support for the idea that physical activity may reduce depressive symptomatology which can support cognitive functioning in older adults with varying levels of cognitive functioning. This finding may be extrapolated and paired with other interventions to support cognitive functioning. For example, Vance and colleagues (2007) used speed of processing training to improve visual processing speed in older adults. Others have found that speed of processing training can be protective against depression in older adults as well (Wolinsky et al., 2009). Thus, speed of processing training may be combined with physical exercise to improve cognitive functioning, with the expectation that those who receive both physical exercise and cognitive remediation therapy in the form of speed of processing training will experience the most gain in their cognitive functioning. The findings of the present article suggest that the reduction of depressive symptomatology should also be examined as a mechanism by which such cognitive gains occur. Hopefully, many other studies such as this will incorporate the role of physical exercise and depression reduction in examining the process of cognitive aging.
Nursing Implications
Detecting depression in older adults can be challenging. In fact, depression is usually undiagnosed because clinical symptoms of depression in older adults are often atypical (e.g., pain, excessive sleeping, forgetfulness) and may mimic characteristics of chronic diseases such as dementia (Acharya, 2004). Therefore, it is necessary for nurses to perform routine psychosocial assessments to detect early signs of depression and incorporate cognitive interventions that promote optimal function. Findings in this study suggest that nurses should encourage neuroprotective health behaviors such as engaging in active leisure and social activities. Nurses have an important role in teaching patients the benefits of staying physically and cognitively fit.
Caregivers and healthcare providers must understand the unique needs of the aging population. Some older adults may feel they no longer have a purpose after retirement, which can be a loss in itself and lead to sedentary behaviors. Also, older adults often experience loneliness as they survive the loss of many of their loved ones. These stressors along with age-related illnesses can impact cognitive functioning and hinder healthy aging (Aggarwal et al., 2014). In these cases, healthcare providers can recommend support groups and encourage participation in cognitive stimulating group activities at gymnasiums, community centers, churches, or senior citizens centers. Given that studies have shown social stimulation and physical exercise improve cognitive function, nurses will need to promote a balance of social and physical activity in conjunction with other healthy behaviors (e.g., proper diet, adequate sleep) (Vance, Eagerton, Harnish, McKie-Bell, & Fazeli, 2011). These lifestyle changes and cognitive remediation therapies such as speed of processing training may slow cognitive aging and allow older adults to function independently for years beyond that expected (Rebok et al., 2014).
These findings also provide partial support to the framework of positive and negative neuroplasticity which either builds or detracts from cognitive reserve; this framework can also help guide patient care for maintaining cognitive reserve and optimal cognitive functioning (Vance & Wright, 2009). There are several factors that can encourage positive neuroplasticity which is necessary to support cognitive reserve; these include physical activity, good mood support, stimulating and mentally challenging activities, and social interaction. In fact, even employment and volunteer work has been suggested recently as being an important vector for maintaining optimal cognitive functioning as people age (Vance, Cody, Yoo-Jeong, Jones, & Nicholson, 2015). Likewise, there are several factors that likewise promote negative neuroplasticity and reduce cognitive reserve, thus making people more vulnerable for age-related cognitive declines; these include depression and anxiety, loneliness, poor health, substance abuse, and lacking stimulating activities. As such, a tailored individualized program called Cognitive Prescriptions can be developed by nurses to increase or decrease a combination of such factors to encourage a healthier, more robust brain (Vance, Eagerton et al., 2011). Thus, it is important to glean from this study and the supporting literature that there are many modifiable factors that may interact to promote cognitive reserve, resulting in better overall cognitive functioning.
References
- Acharya A. Depression in older people: A point to remember in all specialties. Journal of the Indian Medical Association. 2004;102(10):559–561. [PubMed] [Google Scholar]
- Aggarwal NT, Wilson RS, Beck TL, Rajan KB, Mendes de Leon CF, Evans DA, Everson-Rose SA. Perceived stress and change in cognitive function among adults 65 years and older. Psychosomatic Medicine. 2014;76(1):80–85. doi: 10.1097/PSY.0000000000000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aging, Ao. Aging into the 21st Century. [Retrieved February 12, 2007];2004 from http://www.aoa.gov/prof/statistics/future_growth/aging21/demography.asp.
- Anderson JC, Gerbing DW. Structural equation modeling in practice: A review and recommended two-step approach. Psychological Bulletin. 1988;103(3):411–423. [Google Scholar]
- Ball K, Vance D, Edwards J, Wadley V. Aging and the brain. Principles and practice of behavioral neurology and neuropsychology. 2004:795–809. [Google Scholar]
- Bartholomew JB, Ciccolo JT. Exercise, depression, and cognition. Exercise and its mediating effects on cognition. 2008;2 [Google Scholar]
- Booth ML, Owen N, Bauman A, Clavisi O, Leslie E. Social-cognitive and perceived environment influences associated with physical activity in older Australians. Preventive Medicine. 2000;31(1):15–22. doi: 10.1006/pmed.2000.0661. [DOI] [PubMed] [Google Scholar]
- Brebion G, Smith MJ, Gorman JM, Malaspina D, Sharif Z, Amador X. Memory and schizophrenia: Differential link of processing speed and selective attention with two levels of encoding. Journal of Psychiatric Research. 2000;34(2):121–127. doi: 10.1016/s0022-3956(99)00050-3. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Calfas KJ, Sallis JF, Oldenburg B, Ffrench M. Mediators of change in physical activity following an intervention in primary care: PACE. Preventive Medicine. 1997;26(3):297–304. doi: 10.1006/pmed.1997.0141. [DOI] [PubMed] [Google Scholar]
- Cardiovascular_Health_Study. Manual of Operations. Seattle: University of Washington Coordinating Center; 1989. [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychological Science. 2003;14(2):125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Comijs HC, Jonker C, Beekman AT, Deeg DJ. The association between depressive symptoms and cognitive decline in community-dwelling elderly persons. International Journal of Geriatric Psychiatry. 2001;16(4):361–367. doi: 10.1002/gps.343. [DOI] [PubMed] [Google Scholar]
- Delis D, Kramer J, Kaplan E, Ober B. The California Verbal Learning Test. New York: Psychological Corporation; 1987. [Google Scholar]
- Depp C, Vahia IV, Jeste D. Successful aging: Focus on cognitive and emotional health. Annual Review of Clinical Psychology. 2010;6:527–550. doi: 10.1146/annurev.clinpsy.121208.131449. [DOI] [PubMed] [Google Scholar]
- Diamond MC. An optimistic view of aging. Generations. 1993;17:31–33. [Google Scholar]
- Diamond MC, Johnson RE, Protti AM, Ott C, Kajisa L. Plasticity in the 904-day-old male rate cerebral cortex. Experimental Neurology. 1985;87(2):309–317. doi: 10.1016/0014-4886(85)90221-3. [DOI] [PubMed] [Google Scholar]
- Diamond MC, Krech D, Rosenzweig MR. The effects of an enriched environment on the histology of the rat cerbral cortex. Journal of Comparative Neurology. 1964;123:111–120. doi: 10.1002/cne.901230110. [DOI] [PubMed] [Google Scholar]
- DiPietro L. Physical activity in aging changes in patterns and their relationship to health and function. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2001;56(suppl 2):13–22. doi: 10.1093/gerona/56.suppl_2.13. [DOI] [PubMed] [Google Scholar]
- Dufouil C, Alpérovitch A. Couple similarities for cognitive functions and psychological health. Journal of Clinical Epidemiology. 2000;53(6):589–593. doi: 10.1016/s0895-4356(99)00189-4. [DOI] [PubMed] [Google Scholar]
- Duncan TE, Stoolmiller M. Modeling social and psychological determinants of exercise behaviors via structural equation systems. Research Quarterly for Exercise and Sport. 1993;64(1):1–16. doi: 10.1080/02701367.1993.10608773. [DOI] [PubMed] [Google Scholar]
- Edwards ER, Lindquist K, Yaffe K. Clinical profile and course of cognitively normal patients evaluated in memory disorders clinics. Neurology. 2004;62(9):1639–1642. doi: 10.1212/01.wnl.0000123350.61053.15. [DOI] [PubMed] [Google Scholar]
- Epstein RM, Hundert EM. Defining and assessing professional competence. JAMA. 2002;287(2):226–235. doi: 10.1001/jama.287.2.226. [DOI] [PubMed] [Google Scholar]
- Gale C, Martyn C. Larks and owls and health, wealth, and wisdom. British Medical Journal. 1998;317(7174):1675–1677. doi: 10.1136/bmj.317.7174.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giranda M, Lubben JE, Atchison KA. Social networks of elders without children. Journal of Gerontological Social Work. 1999;31(3–4):197. [Google Scholar]
- Green AF, Rebok G, Lyketsos CG. Influence of social network characteristics on cognition and functional status with aging. International Journal of Geriatric Psychiatry. 2008;23(9):972–978. doi: 10.1002/gps.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauri P. Cognitive deficits in insomnia patients. Acta Neurologica Belgica. 1997;97(2):113–117. [PubMed] [Google Scholar]
- Hoyle RH, Panter AT. Writing about structural equation models. In: Hoyle RH, editor. Structural equation modeling: Concepts, issues, and applications. Thousand Oaks, CA: Sage; 1995. pp. 158–176. [Google Scholar]
- Hunter SK, Thompson MW, Adams RD. Relationships among age-associated strength changes and physical activity level, limb dominance, and muscle group in women. Journals of Gerontology. Series A: Biological Sciences and Medical Sciences. 2000;55(6):B264–B273. doi: 10.1093/gerona/55.6.b264. [DOI] [PubMed] [Google Scholar]
- Jöreskog KG, Sörbom D. LISREL 8: The SIMPLIS command language. Chicago: SPSS; 1993. [Google Scholar]
- Kelloway EK. Using LISREL for structural equation modeling: A researcher's guide. Thousand Oaks, CA: Sage; 1998. [Google Scholar]
- Khatri P, Blmnenthal A, Babyak MA, Craighead WE, Herman S, Balriewicz T, Krishnan KR. Effects of exercise training on cognitive functioning among depressed older men and women. Journal of Aging and Ph_vst'ca'. J'Actit-if_t'. 200i. 2001;9(4357):2001. [Google Scholar]
- King AC, Blair SN, Bild DE, Dishman RK, Dubbert PM, Marcus BH, Yeager KK. Determinants of physical activity and interventions in adults. Medicine and Science in Sports and Exercise. 1992;24(6 Suppl):S221–S236. [PubMed] [Google Scholar]
- La Rue A, Swan GE, Carmelli D. Cognition and depression in a cohort of aging men: Results from the Western Collaborative Group Study. Psychology and Aging. 1995;10(1):30. doi: 10.1037//0882-7974.10.1.30. [DOI] [PubMed] [Google Scholar]
- Lochbaum MR, Karoly P, Landers DM. Evidence for the importance of openness to experience on performance of a fluid intelligence task by physically active and inactive participants. Research Quarterly for Exercise and Sport. 2002;73(4):437–444. doi: 10.1080/02701367.2002.10609043. [DOI] [PubMed] [Google Scholar]
- Lubben JE. Assessing social networks among elderly populations. Family and Community Health. 1988;11(3):42–52. [Google Scholar]
- Moyle W, Kellett U, Ballantyne A, Gracia N. Dementia and loneliness: an Australian perspective. Journal of Clinical Nursing. 2011;20(9–10):1445–1453. doi: 10.1111/j.1365-2702.2010.03549.x. [DOI] [PubMed] [Google Scholar]
- Newson RS, Kemps EB. General lifestyle activities as a predictor of current cognition and cognitive change in older adults: A cross-sectional and longitudinal examination. Journals of Gerontology. Series B: Psychological Sciences and Social Sciences. 2005;60(3):P113–P120. doi: 10.1093/geronb/60.3.p113. [DOI] [PubMed] [Google Scholar]
- O'Brien CS. Social support for exercise among elderly women in Canada. Health Promotion International. 1995;10:273–282. [Google Scholar]
- Oka RK, King AC, Young DR. Sources of social support as predictors of exercise adherence in women and men ages 50 to 65 years. Women's Health. 1995;1:161–175. [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Potter GG, Steffens DC. Contribution of depression to cognitive impairment and dementia in older adults. Neurologist. 2007;13(3):105–117. doi: 10.1097/01.nrl.0000252947.15389.a9. [DOI] [PubMed] [Google Scholar]
- Powell JH, al-Adawi S, Morgan J, Greenwood RJ. Motivational deficits after brain injury: Effects of bromocriptine in 11 patients. Journal of Neurology, Neurosurgery and Psychiatry. 1996;60(4):416–421. doi: 10.1136/jnnp.60.4.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbitt P, Donlan C, Watson P, McInnes L, Bent N. Unique and interactive effects of depression, age, socioeconomic advantage, and gender on cognitive performance of normal healthy older people. Psychology and Aging. 1995;10(3):307–313. doi: 10.1037//0882-7974.10.3.307. [DOI] [PubMed] [Google Scholar]
- Rebok GW, Ball K, Guey LT, Jones RN, Kim HY, King JW, Willis SL. Ten-year effecs of the Advanced Cognitive Training for Independent and Vital Elderly Cognitive Training Trial on cognition and everyday functioning in older adults. Journal of the American Geriatric Society. 2014;62:16–24. doi: 10.1111/jgs.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan R. Instructions and procedures for administering the psychological test battery used at the Neuropsychology Laboratory. Indianapolis, IN: Indiana University Medical Center; 1958. [Google Scholar]
- Reitan RM. Manual for administration of neuropsychological test batteries for adults and children: Neuropsychology Laboratory. Indiana University Medical Center; 1979. [Google Scholar]
- Rowe JW, Kahn RL. Successful aging. Gerontologist. 1997;37(4):433–440. doi: 10.1093/geront/37.4.433. [DOI] [PubMed] [Google Scholar]
- Rubinstein RL, Lubben JE, Mintzer JE. Social isolation and social support: An applied perspective. Journal of Applied Gerontology. 1994;13(1):58–72. [Google Scholar]
- Sirevaag AM, Greenough WT. Differential rearing effects on rat visual cortex synapses. II. Neuronal and glial nuclei, bouton, dendrites and capillaries. Brain Research. 1987;424(320–332) doi: 10.1016/0006-8993(87)91477-6. [DOI] [PubMed] [Google Scholar]
- Spanier PA, Allison KR. General social support and physical activity: An analysis of the Ontario Health Survey. Canadian Journal of Public Health. 2001;92(3):210–213. doi: 10.1007/BF03404308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreen O, Strauss E. A compendium of neuropsychological tests, 1991. Controlled oral word association (FAS). New York. 1998:447–464. [Google Scholar]
- Stahl T, Rutten A, Nutbeam D, Bauman A, Kannas L, Abel T, Van Der Zee J. The importance of the social environment for physically active lifestyle--results from an international study. Social Science and Medicine. 2001;52(1):1–10. doi: 10.1016/s0277-9536(00)00116-7. [DOI] [PubMed] [Google Scholar]
- Treiber FA, Baranowski T, Braden DS, Strong WB, Levy M, Knox W. Social support for exercise: Relationship to physical activity in young adults. Preventive Medicine. 1991;20(6):737–750. doi: 10.1016/0091-7435(91)90068-f. [DOI] [PubMed] [Google Scholar]
- Ullman JB. Structural equation modeling. In: Tabachnick BG, Fidell LS, editors. Using Multivariate Statistics. New York: HarperCollings; 1996. [Google Scholar]
- Unverzagt FW, Gao S, Baiyewu O, Ogunniyi AO, Gureje O, Perkins A, Hendrie HC. Prevalence of cognitive impairment: Data from the Indianapolis Study of Health and Aging. Neurology. 2001;57(9):1655–1662. doi: 10.1212/wnl.57.9.1655. [DOI] [PubMed] [Google Scholar]
- van Boxtel MP, Langerak K, Houx PJ, Jolles J. Self-reported physical activity, subjective health, and cognitive performance in older adults. Experimental Aging Research. 1996;22(4):363–379. doi: 10.1080/03610739608254017. [DOI] [PubMed] [Google Scholar]
- Vance DE, Cody SL, Yoo-Jeong M, Jones GD, Nicholson WC. The role of employment on neurocognitive reserve in adults with HIV: A review of the literature. Journal of the Association of Nurses in AIDS Care. 2015;26(4):316–329. doi: 10.1016/j.jana.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance D, Dawson J, Wadley V, Edwards J, Roenker D, Rizzo M, Ball K. The accelerate study: The longitudinal effect of speed of processing training on cognitive performance of older adults. Rehabilitation Psychology. 2007;52(1):89–96. [Google Scholar]
- Vance DE, Eagerton G, Harnish B, McKie-Bell P, Fazeli P. Cognitive prescription acros the lifespan: A nursing approach to increasing cognitive reserve. Journal of Gerontological Nursing. 2011;37(4):22–29. doi: 10.3928/00989134-20101202-03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance DE, Fazeli PL, Ross LA, Wadley VG, Ball KK. Speed of processing training with middle-age and older adults with HIV: A pilot study. Journal of the Association of Nurses in AIDS Care. 2012;23(6):500–510. doi: 10.1016/j.jana.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance DE, Heaton K, Eaves Y, Fazeli PL. Sleep and cognition on everyday functioning in older adults: Implications for nursing practice and research. Journal of Neuroscience Nursing. 2011;43(5):261–271. doi: 10.1097/JNN.0b013e318227efb2. [DOI] [PubMed] [Google Scholar]
- Vance DE, Wadley VG, Ball KK, Roenker DL, Rizzo M. The effects of physical activity and sedentary behavior on cognitive health in older adults. Journal of Aging and Physical Activity. 2005;13(3):294–313. doi: 10.1123/japa.13.3.294. [DOI] [PubMed] [Google Scholar]
- Vance DE, Wright MA. Positive and negative neuroplasticity: Implications for promotion of cognitive health in aging. Journal of Gerontological Nursing. 2009;35(6):11–17. doi: 10.3928/00989134-20090428-02. [DOI] [PubMed] [Google Scholar]
- Westerterp KR, Meijer EP. Physical activity and parameters of aging: A physiological perspective. Journals of Gerontology. Series A: Biological Sciences and Medical Sciences. 2001;56(Suppl 2):7–12. doi: 10.1093/gerona/56.suppl_2.7. [DOI] [PubMed] [Google Scholar]
- Wolinsky FD, Stump TE, Clark DO. Antecedents and consequences of physical activity and exercise among older adults. The Gerontologist. 1995;35(451–462) doi: 10.1093/geront/35.4.451. [DOI] [PubMed] [Google Scholar]
- Wolinsky FD, Vander Weg MW, Martin R, Unverzagt FW, Ball KK, Jones RN, Tennstedt SL. The effect of speed-of-processing training on depressive symptoms in ACTIVE. Journals of Gerontology. Series A: Biological Sciences and Medical Sciences. 2009;64(4):468–472. doi: 10.1093/gerona/gln044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Blackwell T, Barnes DE, Ancoli-Israel S, Stone KL. Preclinical cognitive decline and subsequent sleep disturbance in older women. Neurology. 2007;69(3):237–242. doi: 10.1212/01.wnl.0000265814.69163.da. [DOI] [PubMed] [Google Scholar]
- Yesavage JA. Development and validation of a geriatric depression screening scale: A primary report. Journal of Psychiatric Research. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Young DR, King AC. Exercise adherence: Determinants of physical activity and applications of health behavior change theories. Medicine, Exercise, and Nutritional Health. 1995;4:335–348. [Google Scholar]