Abstract
Early childhood caries (ECC) is one of the most prevalent infectious diseases affecting children worldwide. ECC is an aggressive form of dental caries, which left untreated, can result in rapid and extensive cavitation in teeth (rampant caries) that is painful and costly to treat. Furthermore, it affects mostly children from impoverished background, and thus constitutes a major challenge in public health. The disease is a prime example of the consequences arising from complex, dynamic interactions between microorganisms, host and diet, leading to the establishment of highly pathogenic (cariogenic) biofilms. To date, there are no effective methods to identify those at risk of developing ECC or control the disease in affected children. Recent advances in deep-sequencing technologies, novel imaging methods and (meta)proteomics-metabolomics approaches provide an unparalleled potential to reveal new insights to illuminate our current understanding about the etiology and pathogenesis of the disease. In this concise review, we provide a broader perspective about the etiology and pathogenesis of ECC based on previous and current knowledge on biofilm matrix, microbial diversity and host-microbe interactions which could have direct implications for developing new approaches for improved risk assessment and prevention of this devastating and costly childhood health condition.
Keywords: Dental caries, biofilm, matrix, diet, saliva, Streptococcus, Candida, microbiome
Introduction
Early childhood caries (ECC) is one of the most prevalent biofilm-dependent infectious diseases in childhood worldwide. It afflicts 23% of the preschoolers (< 6 years of age) in the US, and can be observed in toddlers as young as 12 months of age (Dye et al., 2015). The disease most frequently targets children from poor socioeconomic families (>50%) and racial/ethnic minority backgrounds (Dye et al., 2015). Left untreated, ECC can result in rapid and extensive carious lesions and destruction of primary teeth causing painful pulpal and complicated systemic infections (Casamassimo et al., 2009). Even after removal or restoration of carious teeth, children remain at high risk for future recurrences despite of pharmacologic interventions, such as topical fluoride/antimicrobial applications, or recommendations to alter caries-promoting feeding behaviors (O’Sullivan & Tinanoff, 1996). Thus, ECC places enormous health and economic burdens most often on those least able to bear them. There is a clear need to enhance our understanding of the pathogenesis of this devastating disease, which could help to develop valid ECC risk assessment tools and lasting preventive therapies.
The etiology of ECC is multifactorial and complex involving environmental, behavioral, socioeconomic and biological factors (Fontana, 2015). In this review, we focused on the infectious aspects of ECC, highlighting the previous knowledge and recent advances on the microbial etiology of this disease. OMICS-based approaches, such as high-throughput (meta) genomic, transcriptomic and proteomics as well as metabolomics, have been providing important insights in deciphering the complex ecology and microbial interactions within biofilms (Nyvad et al., 2013). However, these powerful high-throughput analytical tools have limitations (e.g., overestimation of microbial diversity) and must be approached as a complement to the large amount of knowledge obtained from previous “classical” studies (Do et al., 2013). At the same time, such technologies can be integrated with in vivo models and longitudinal clinical studies to further enhance our understanding of the pathophysiology of the disease, which may lead to effective ways to assess ECC susceptibility in children and target intensive preventive measure on those who need them the most.
ECC is an Infectious, Diet-dependent and Biofilm-associated Disease
Dental caries is a dynamic pathological process that is primarily dependent on the development of virulent biofilms (known as plaque) as a result of complex interactions that occur on tooth surfaces between oral microbes (and their products), host salivary constituents and dietary carbohydrates (Paes-Leme et al., 2006; Takahashi & Nyvad, 2011). ECC is an aggressive form of dental caries that is characterized by a heavy mutans streptococci (MS) infection, which at times exceeds 30% of the cultivable plaque-biofilm flora (as reviewed by Parisotto et al, 2010). In general, two species of MS are found in human lesions, Streptococcus mutans (serotypes c, e, f and k) and less frequently Streptococcus sobrinus (serotypes d and g) (Mattos-Graner, 2014). However, MS level in plaque varies depending on the stage of caries development while other microorganism may be also associated with the disease (Tanner et al., 2011; Gross et al., 2012).
The dietary sugars are one of the most critical mediators in the pathogenesis of ECC. Among them, sucrose is the most cariogenic since it serves as substrate for the production of acid and exopolysaccharides by microorganisms facilitating the initiation and accumulation of cariogenic biofilms (Paes-Leme et al., 2006). The children who are afflicted with ECC have often a history of being allowed to indulge in protracted ingestion of dietary sugars (Berkowitz et al., 1984; Palmer et al., 2010). This includes practices such as placing sugary drinks (i.e., soft drinks and honey) in a sippy-cup for ingestion throughout the day, or in a nursing bottle that is left undisturbed in the infant’s mouth during the night, thus promoting the rapid onset and progression of carious lesions.
Dental caries is not just cavitation in teeth; it is a pathologic process where accumulation of biofilms is usually the first manifestation of the disease (Bowen, 2015). Although an acidic pH is undeniably the immediate cause of tooth enamel dissolution, the environment within which acid is produced and maintained at the tooth surface, i.e. the biofilm matrix, is as critical, particularly when there is sufficient buffering saliva capable of neutralizing acids in the mouth.
ECC and the biofilm matrix
The matrix is the extracellular milieu that holds the microbial cells together within biofilms, provides bulk and keeps them firmly attached to the tooth surface. Importantly, it also affects diffusion into and out of the plaque affecting bacterial metabolism and promoting acidification at the biofilm-tooth interface (as reviewed by Bowen & Koo, 2011). The exopolysaccharides (EPS) are the prime biofilm building blocks, which form the core of the matrix. The EPS are comprised primarily of soluble and insoluble glucans and to a lesser extent fructans, but the structure and composition varies depending on the interval since the last intake of dietary sugars (Bowen & Koo, 2011). EPS are chiefly produced by bacterial exoenzymes (e.g., glucosyltransferases) at the biofilm-tooth interface utilizing dietary sucrose and starch as reviewed previously (Paes-Leme et al., 2006; Bowen & Koo, 2011). MS, particularly S. mutans, appear to be the main organisms associated with the production of the insoluble EPS matrix, although other streptococci and Lactobacillus reuteri can synthesize exopolysaccharides (as reviewed by Klein et al., 2013). Whether other species detected in ECC biofilm can contribute to the assembly of the insoluble EPS matrix remain to be thoroughly investigated.
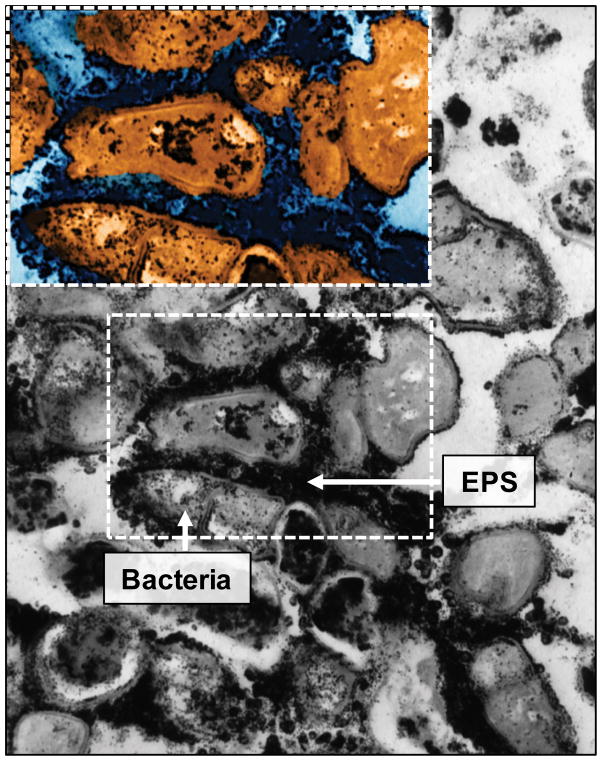
S. mutans-released glucosyltransferases (Gtfs) become constituents of the pellicle and remain active despite major conformational changes (Fears et al., 2015), producing large amounts of glucans in situ upon sucrose exposure (Bowen & Koo, 2011). The glucans formed on pellicle provide new microbial binding sites that promote local colonization of S. mutans and other microorganisms (Bowen & Koo, 2011). Gtfs also bind to the surface of other bacteria converting them into glucan producers. Thus, the EPS formed in situ enhances local accumulation of microbes on teeth while embedding them in a diffusion-limiting matrix. These observations could explain microscopic images of plaque collected from caries-active children, which reveal bacteria enmeshed in EPS (Fig. 1). The increased formation of biofilm biomass or “visible plaque” often on the smooth-surfaces of the children at risk of ECC (Karjalainen et al., 2001) highlights the importance of EPS in the pathological process (Parisotto et al., 2015). However, other salivary and bacterially-derived constituents, including proteins, lipoteichoic acid and eDNA have been also identified in the matrix, which could contribute to its structural organization and diffusion properties (as reviewed by Klein et al., 2015).
Figure 1.
The metabolic activity of S. mutans and other acidogenic organisms in the EPS-rich matrix facilitate the creation of acidic microenvironments within the biofilm. The low-pH niches promote EPS glucan production (e.g., enhancing expression of gtf genes), ensuring continuous biofilm accretion. Different glucans present in the matrix of plaque offer binding sites for additional organisms while aciduric and acidogenic microbiota prosper within the acidic milieu, greatly modifying the biofilm microbial complexity (Lemos & Burne, 2008; Bowen & Koo, 2011). The acidic microenvironment may benefit not only aciduric species but also those that use lactate as a carbon source (e.g., Veillonella ssp) while others that are acid sensitive or that cannot metabolize the acids present in its surroundings may perish. As the environmental acidic stress increases, the microbial diversity is further reduced in favor of a highly acid-tolerant and acidogenic microbiota (Takahashi & Nyvad, 2011).
The creation of localized microenvironments, delineated by a diffusion-limiting matrix, has profound effects on the architecture, metabolism and expression of virulence of biofilm as a whole (Xiao et al., 2012). Although the immediate cause of enamel dissolution is certainly acid production, the presence of the ‘sheltering’ effect of the biofilm matrix would minimize the ability of acids to demineralize in the presence of the saliva. Further in vivo studies shall elucidate the dynamic structural changes of the plaque matrix and how it modulates development of the acidic milieu.
ECC and microbiome: the role of MS and acidogenic-aciduric microbiota
Results from animal studies, systematic reviews and microbiome-based approaches revealed a clear role of MS in the etiology and pathogenesis of dental caries, and their association with ECC (Tanzer, 1995; Becker et al., 2002; Kanasi et al., 2010; Parisotto et al., 2010; Tanner et al., 2011; Gross et al., 2012). MS possess an extraordinary ability to infect and colonize teeth, and promote the development of cariogenic biofilms in the presence of sucrose (Paes Leme et al., 2006). Infants acquire MS through vertical transmission from the oral cavity of their primary care givers, but also through horizontal transmission, from other individuals in their immediate environment (Caufield et al., 2005; Lapirattanakul & Nakano, 2014). Interestingly, early acquisition and colonization of MS (i.e. before 3 years of age) resulted in higher levels of oral MS and decayed-missing-filed (DMF) index at the age of 19 years (Kohler & Andreen, 2012), indicating the importance of controlling the infection by these bacteria in the oral cavity of young children.
Successful establishment of MS infection depend on several factors as recently reviewed (Mattos-Graner et al., 2014), which include MS carriage in caregivers, the virulence traits of MS strains, a competitive microbiota, the diet, genetic constitution, behavior and immunity of the host. While there is some evidence for innate immunity influences on ECC development, the role of adaptive immunity remains unclear. Most children become orally immunocompetent soon after birth and their serum and saliva antibodies levels against S. mutans increase with age. Salivary antibodies (IgA, IgG) could influence colonization of MS either directly or through their interaction with other enzymes or complement activation and opsonization (Nogueira et al., 2005). However, levels of anti-MS immunoglobulins have not been shown to be consistently associated with caries experience. In addition to the immune responses, it was shown that the oral microbiota differs between breastfed and formula-fed infants, with a potentially more health-associated oral flora in breast-fed infants (Holgerson et al., 2013). Moreover, the acquisition and maturation of the oral microbiota can be affected by the competitive and antagonistic interactions between microorganism, which can modulate the profiles of dental biofilm communities in the oral cavity of infants (Caufield et al., 2005; Cephas et al., 2011). Nevertheless, once transmitted and colonized, MS can effectively orchestrate the development of cariogenic biofilms, even if in low numbers, when frequent exposure of dietary sugars occurs.
Streptococcus mutans effectively utilizes dietary sucrose (and possibly starch) to rapidly synthesize EPS (especially insoluble glucans) and produce organic acids (Bowen & Koo, 2011). S. mutans adapts to acidic pH and other environmental stresses efficiently, and some of its strains are capable of genetic transformation and bacteriocin production; all of these properties contribute to its ability to compete with other oral bacteria (such as the H2O2 producers, e.g. S. gordonii and S. sanguinis) and initiate cariogenic biofilm development (Kreth et al., 2008; Lemos & Burne, 2008; Merritt & Qi, 2012). However, the high genetic and phenotypic diversity of S. mutans can impact these virulence factors, which may influence its cariogenic activity in the setting of ECC (Mattos-Graner et al., 2014).
Within the highly acidic dental plaque of children with ECC, besides MS, other acidogenic and acid tolerant bacteria are detected. Such bacteria include non-mutans streptococci, actinomyces, lactobacilli, bifidobacterieae and Scardovia species, which could contribute to the pathogenesis of the disease by enhancing acidification of the biofilm milieu (Becker et al., 2002; Tanner et al., 2011; Gross et al., 2012; Jiang et al., 2014; Simón-Soro & Mira, 2015). Interestingly, relatively few of these bacteria can produce insoluble glucans and thereby may not contribute to the assembly of the cariogenic biofilm matrix. A recent review (Klein et al., 2013), including searches at the Human Oral Microbiome Database, points out that the majority of species associated with insoluble glucans synthesis are MS. Thus, it appears that in the etiology of dental caries the major role of S. mutans is to provide the matrix of biofilms, thereby protecting the acid milieus within which acidogenic and aciduric organisms thrive. It has been noted in other biofilms that the organism that initiates formation is frequently found in low numbers and even absent in mature biofilms (Flemming & Wingender, 2010), which could explain the variable levels of S. mutans in the plaque (Gross et al., 2012). S. mutans may well be a biofim initiator (and also a potent acid producer) that paves the way for other cariogenic bacteria to become dominant, possibly at the expense of S. mutans itself, as the biofilm matures.
The role of other bacteria
In contrast to ECC linked acidogens, other bacterial species found in plaque-biofilms may counter the deleterious effects of acidification by producing alkali that can neutralize the acids and therefore influence caries susceptibility in children. Such organisms are capable of ammonia production, using salivary substrates such as arginine (S. gordonii and S. sanguinis) via the arginine deiminase system (ADS) and urea (S. salivarius and A. naeslundii) by urease enzymes (Liu et al., 2012; Nascimento et al., 2013). Presumably, the alkali-producing bacteria protect against plaque acidification and further growth/dominance of cariogenic bacteria that thrive in acidic conditions, while helping prevent the damaging effects of demineralization. Interestingly, ADS and urease expression/activity is enhanced in response to increased arginine or urea levels, lowered pH values and even interspecies interactions (such as between S. gordonii with A. naelundii) (Jakubovics et al., 2008; Liu et al., 2012). These mechanisms can provide alkali-producing bacteria a competitive advantage when biofilm environments become acidic, potentially resulting in lowered caries risk. In this regard, enhanced arginine metabolism associated with use of arginine-containing toothpaste may exert caries inhibiting effect clinically (Nascimento et al., 2014). Interestingly, the species that have ADS and urease system are also acidogenic and aciduric. Whether the use of arginine and urea is only to neutralize the acidic environment in biofilms or whether these systems are activated to out-compete other aciduric species and affect biofilm matrix assembly remains to be elucidated.
Some of the bacteria found in the ECC-plaque do not fit the classical profile of cariogenic organisms being acid tolerant but not acidogenic or even acid sensitive (Simón-Soro & Mira, 2015). Whether they are just bystanders or play an active role in cariogenesis needs to be explored. For example, weak or non-acidogenic but proteolytic Gram negative bacteria such as Prevotella species have been detected in dental plaque of children with severe-ECC and associated with caries progression into the dentin for which proteolysis of proteins denatured by acidic species is necessary (Chalmers et al., 2015). Similarly, Veillonella species are frequently detected in severe form of ECC lesions and believed to be involved in rapid extension of lesions deep into dentine. Although they are not acidogenic, Veillonella utilize lactate produced by several acidogenic species as a carbon source, which may support the growth or survival of cariogenic species (Liu et al., 2011).
Candida and ECC
Intriguingly, results from several clinical studies reveal that, in addition to MS infection, the fungus Candida albicans is frequently detected in high numbers in plaque-biofilms from toddlers with ECC (de Carvalho et al., 2006; Raja et al., 2010; Yang et al., 2012). Other Candida species (e.g., C. tropicalis, C. krusei, and C. glabrata) are also detected, but not as frequently or as numerous as C. albicans (de Carvalho et al., 2006). In contrast, C. albicans is either absent or detected sporadically in the plaque-biofilms from healthy, ECC-free children (de Carvalho et al., 2006; Raja et al., 2010; Yang et al., 2012).
Bacterial-fungal interactions commonly occur in humans, frequently influencing the transition from a healthy to a diseased state within a specific host niche (Peleg et al., 2010). In the mouth, C. albicans, a major component of the oral fungal microbiome, is known to form mixed microbial communities on mucosal and prosthetic surfaces (Diaz et al., 2012). However, previous in vitro observations (Pereira-Cenci et al., 2008; Gregoire et al., 2011; Metwalli et al., 2013) and recent in vivo (Falsetta et al., 2014) data show that C. albicans interactions with S. mutans also occur on tooth surfaces in the presence of sucrose. Specifically, C. albicans and S. mutans develop a symbiotic interaction mediated through the influence of Gtfs exoenzymes, particularly GtfB. GtfB binds with exceptional avidity to the surface of C. albicans cells even when they are in hyphal form (Hwang et al., 2015), producing large amounts of glucans on Candida surface when sucrose is available (Gregoire et al., 2011; Falsetta et al., 2014). Furthermore, the presence of C. albicans within mixed-species biofilms induces S. mutans gtfs expression. This unique interaction boosts the ability of both organisms to colonize teeth, dramatically increases the amount of EPS in the biofilm matrix, and synergistically enhances virulence leading to aggressive onset of rampant dental caries (similar to lesions in ECC) in an animal model of the disease (Falsetta et al., 2014). Further investigation on the mechanisms by which EPS-matrix is enhanced and other metabolic pathways that are influenced when these species are together may offer additional insights into the disease process.
It is readily apparent that the virulence of biofilm in ECC is enhanced as a consequence of overexposure to sugars and complex interspecies as well as cross-kingdom interactions, leading to the development of an EPS-rich matrix and highly acidic milieu. These observations may help explain why the current standard for risk assessment based on single species identification or counting (e.g., S. mutans levels in saliva) has not been found to be an accurate method for identifying children at risk for caries. How these ECC-associated microbes interact to each other and contribute to the biofilm matrix and how they modulate the development of a cariogenic milieu should be investigated in-depth. The use of in vivo models of the disease taking full advantage of the current OMICS-based and imaging approaches (e.g., biophotonics; Merritt et al., 2015) would increase our understanding of their role in the pathogenesis, and lead to new ways to prevent the disease.
Complex Microbe and Host Saliva Interactions: ECC Biological Markers
Microorganisms are not by themselves in the mouth but constantly interacting with salivary host and microbial biomolecules, ranging from high molecular weight proteins to small peptides and even single amino acids (Oppenheim et al., 2007). Many salivary proteins, such as acidic and basic proline-rich glycoproteins (PRPs), mucins, immunoglobulins, agglutinins, lactoferrin, cystatins and lysozyme are thought to be important modulators of oral health but their exact role and significance in caries development or in ECC have not been easy to demonstrate (Guo & Shi, 2013; Martins et al., 2013). This is due in part to the multifactorial etiology of ECC, but also the individual variability of the concentration and composition of salivary proteome, the maturation phase of the child’s immune system, the timing of sample collection, and the type of saliva (Martins et al., 2013). It is also possible that the difficulties of correlation may be related to redundant functions and synergism of individual salivary components, as well as functional changes promoted by intermolecular interactions. Moreover, saliva may not reflect the bacterial composition or metabolic activity found in plaque of the diseased sites (Simón-Soro & Mira, 2015). Nevertheless, there are differences in the saliva metaproteomic profile between ECC and caries free children (Hart et al., 2011).
Salivary constituents form a pellicle on teeth that directly mediates selective bacterial adhesion and also bind to bacterial surfaces (or can be utilized by them), thereby influencing cariogenic biofilm formation (Oppenheim et al., 2007; Nobbs et al., 2011). For example, acidic PRPs bind strongly to teeth (as part of the pellicle), which in turn influence the adherence of bacteria to tooth surfaces. On the other hand, basic PRPs bind to oral streptococci, and their arginine and lysine residues can be utilized by alkali-producing bacteria (e.g., S. gordonii) via ADS to produce ammonia which neutralizes biofilm acids. The absence of some of the basic PRP allelic phenotypes have been associated with ECC (Levine, 2011), although there are contradictory results regarding the role of PRPs in caries prevalence (Guo & Shi, 2013). Similarly, the host salivary protein CSP-1 binds to S. mutans cells and may influence the initial colonization of this pathogenic bacterium onto the tooth surface, by promoting its adhesion to the pellicle and to glucans (Ambatipudi et al., 2010). Besides proteins, antibacterial arginine-rich peptides such as the salivary defensins HNP1-3 (α-defensin 3) and HBD-3 (β-defensin 3) in oral tissue and gingival crevicular fluid appear to lower ECC susceptibility in children. Presumably, cleavage of these peptides results in free arginine in saliva followed by increased production of NH4+, which increases the plaque pH thus contributing to reduction in caries susceptibility (Ribeiro et al., 2013).
Likewise, elevated levels of bacterial products (such as GtfB from S. mutans) present in saliva (Vacca-Smith et al., 2007) or reduced activity of bacterial arginine deiminase in plaque have been linked with enhanced caries activity in children (Nascimento et al., 2013). Along these findings, insoluble EPS analysis (mainly produced by S. mutans Gtfs) combined with diet (solid sugar/sucrose) and cariogenic bacteria could be used to predict caries development in ECC (Parisotto et al., 2015). Recently, (meta)proteomic data from biofilms revealed potential biomarkers for healthy status or caries disease, including six bacterial- (e.g., glucose PTS system) and four host-derived (e.g., protein S100-A9) proteins (Belda-Ferre et al., 2015) as well as unusual microbial peptides (Si et al., 2015). However, further research is required to demonstrate whether these biomolecules can be indeed used as biomarkers of dental caries, particularly to predict ECC risk.
It is possible that many yet to be discovered compounds in saliva or plaque may play a role in either protecting from or increasing the susceptibility of a child to develop ECC. Further exploration of oral microbiome metabolism and host-microbe interactions using (meta)transcriptomics/proteomics and metabolomics together with biofilm matrix analysis could identify additional biomolecules associated with ECC (Bowen & Koo, 2011; Nyvad et al., 2013). Taking into consideration the dynamic nature of the disease, in vivo caries models combined with longitudinal clinical studies would be required to validate “putative” markers and establish a causal relationship between the discovered biomolecules and ECC.
New detection technologies
While enhanced knowledge in the pathogenesis of ECC have revealed the complex relationship between the host and caries-associated microbes, recent technological advancements have enhanced our ability to detect and analyze them. High-throughput methodologies are capable of characterizing both the biomolecules and microbes in saliva or plaque samples which may lead to the development of new tools that can help predict ECC risk. In this regard, the advent of nanotechnology, such as ultrasensitive nanomaterials or nanostructured sensors, combined with microfluidics devices has revolutionized biomarker analysis and molecular diagnostics. These technologies are capable of rapid, portable, accurate and inexpensive detection of biomarkers. For example, nanomaterial-enhanced surface plasmon resonance or nanoplasmonic sensors (based on optical spectroscopy) are able to detect molecules at femtomole (or even lower) concentrations (Ninno et al., 2015). The multiplexing capacity of microfluidics offers the potential for improving sensitivity and specificity by combining several markers for analysis into a single device. Low sample and reagent requirements, fast analytical times and low cost production make these technologies well-suited for ECC-biomarker analysis and development of a single, point-of-care platform to identify children at-risk for ECC.
Nie et al. (2014) have already developed an automated, portable microfluidics-based platform for rapid multiplexed protein profiling (within 70 min) using as little as 10 μL of human saliva for in-office diagnostics of respiratory diseases such as asthma. This platform can be adapted for multi-microbe detection, which would allow simultaneous analysis of salivary and microbial biomarkers associated with the disease. Such comprehensive approach is in line with the complex and multifactorial biology of ECC and can provide robust scientific data to evaluate and validate biomarkers for ECC risk in longitudinal clinical studies, which ultimately can improve the current screening tools for ECC-risk assessment. Given that ECC affects very young children, such diagnostic tools could be implemented in the pediatric offices (Khanna & Walt, 2015) where child visit during the first years of life occur more frequently than the dental visits. Early monitoring for ECC risk could guide educative programs for the caregivers and early dental referrals for targeted preventative therapies.
Conclusions and Perspectives
Cavitation or carious lesions is a late manifestation of ECC, a highly dynamic pathological process involving complex host-diet-microbe interactions that initiates with the formation of a virulent biofilm on teeth.
The biofilm matrix plays a key role in the pathogenesis of dental caries, particularly when conditions (e.g., sugar-laded dietary behavior) are conducive to the development of ECC. The matrix provides an essential physical-scaffold that facilitate microbial accumulation and adherence onto teeth while providing a diffusion-limiting milieu that help to create low pH microenvironments at the biofilm/tooth surface interface.
MS, particularly S. mutans, are major organisms involved in the etiology and pathogenesis of ECC. However, the precise role of MS in the etiopathogenesis needs reevaluation using mixed-species biofilm models that mimic cariogenic challenges found in ECC.
Besides MS, other microbes (including C. albicans) have been clearly detected in the complex plaque-biofilm microbiome of ECC, but additional in-depth studies using in vivo models of dental caries are needed to elucidate their specific role in the initiation and progression of dental caries.
Host and bacterial-derived molecules that either protect from or increase the susceptibility of a child to dental caries are being identified. However, further validation through carefully designed longitudinal clinical studies that takes into consideration the dynamic nature of the disease is required. Identification of biomolecules associated with ECC that can be detected prior to the onset of cavitation would have higher predictive value to assess disease activity.
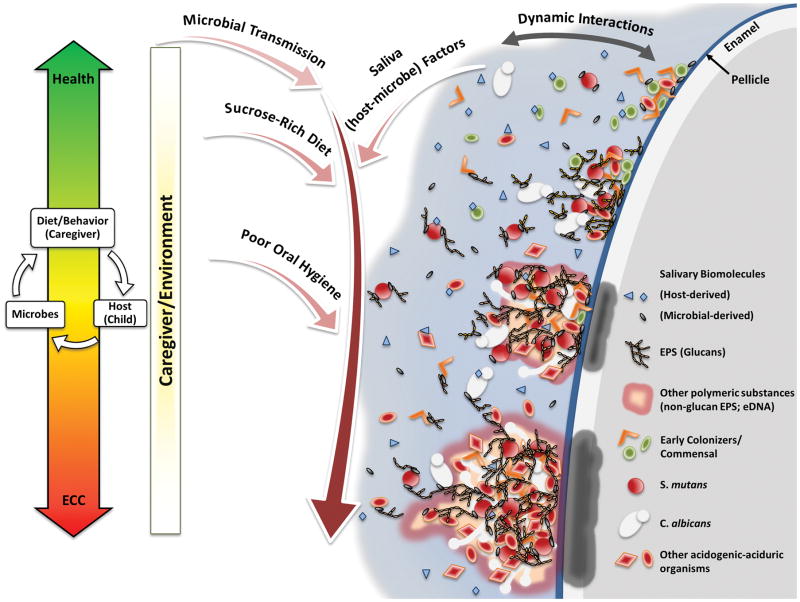
Although ECC is a highly complex disease (as summarized above and depicted in Fig. 2), emerging (nano)technologies make identification of biomarkers and development of biology-based analytical tools or devices for caries risk assessment feasible in the near future.
Figure 2.
In summary, the enhanced knowledge about the pathogenesis of ECC and technological advancements point to exciting new research directions. Microbiome and salivary biomolecules analyses could be combined with behavioral-risk assessments to improve the accuracy of the existing ECC-risk screening methods, and provide greater predictability of ECC development. This combined approach may help us accurately identify children who have not manifested the clinical signs of cavitation but have the behavioral traits and the microbial and salivary biomarkers at a level that put them at high-risk to develop ECC. This personalized approach once validated could lead to implementation of targeted early intervention and enhanced preventive care for the susceptible children, thus reducing the economic burdens and painful consequences of the progressed stages of the disease.
Acknowledgments
The authors thank Guillaume Jouanny and Dr. Geelsu Hwang for helping with the preparation of the figures. The authors’ research in this area was supported in part by research grant 1R01DE025220-01 from the National Institute for Dental and Craniofacial Research, National Institutes of Health (NIDCR, NIH). São Paulo Research Foundation (FAPESP) provided additional funding to M.I.K. (grant #2014/05423-0).
Footnotes
The authors declare no conflicts of interest with respect to the authorship and/or publication of this article.
References
- 1.Ambatipudi KS, Hagen FK, Delahunty CM, et al. Human common salivary protein 1 (CSP-1) promotes binding of Streptococcus mutans to experimental salivary pellicle and glucans formed on hydroxyapatite surface. J Proteome Res. 2010;9:6605–6614. doi: 10.1021/pr100786y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becker MR, Paster BJ, Leys EJ, et al. Molecular analysis of bacterial species associated with childhood caries. J Clin Microbiol. 2002;40:1001–1009. doi: 10.1128/JCM.40.3.1001-1009.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belda-Ferre P, Williamson J, Simón-Soro Á, Artacho A, Jensen ON, Mira A. The human oral metaproteome reveals potential biomarkers for caries disease. Proteomics. 2015;15:3497–3507. doi: 10.1002/pmic.201400600. [DOI] [PubMed] [Google Scholar]
- 4.Berkowitz RJ, Turner J, Hughes C. Microbial characteristics of the human dental caries associated with prolonged bottle-feeding. Arch Oral Biol. 1984;29:949–951. doi: 10.1016/0003-9969(84)90097-9. [DOI] [PubMed] [Google Scholar]
- 5.Bowen WH, Koo H. Biology of Streptococcus mutans-derived glucosyltransferases: role in extracellular matrix formation of cariogenic biofilms. Caries Res. 2011;45:69–86. doi: 10.1159/000324598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowen WH. Dental caries – not just holes in teeth! A perspective. Mol Oral Microbiol. 2015 doi: 10.1111/omi.12132. [DOI] [PubMed] [Google Scholar]
- 7.Casamassimo PS, Thikkurissy S, Edelstein BL, Maiorini E. Beyond the dmft: the human and economic cost of early childhood caries. J Am Dent Assoc. 2009;140:650–657. doi: 10.14219/jada.archive.2009.0250. [DOI] [PubMed] [Google Scholar]
- 8.Caufield PW, Li Y, Dasanayake A. Dental caries: an infectious and transmissible disease. Compend Contin Educ Dent. 2005;26:10–16. [PubMed] [Google Scholar]
- 9.Cephas KD, Kim J, Mathai RA, et al. Comparative analysis of salivary bacterial microbiome diversity in edentulous infants and their mothers or primary care givers using pyrosequencing. PLoS One. 2011;6:e23503. doi: 10.1371/journal.pone.0023503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chalmers NI, Oh K, Hughes CV, et al. Pulp and plaque microbiotas of children with severe early childhood caries. J Oral Microbiol. 2015;7:25951. doi: 10.3402/jom.v7.25951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Carvalho FG, Silva DS, Hebling J, Spolidorio LC, Spolidorio DM. Presence of mutans streptococci and Candida spp. in dental plaque/dentine of carious teeth and early childhood caries. Arch Oral Biol. 2006;51:1024–1028. doi: 10.1016/j.archoralbio.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Diaz PI, Xie Z, Sobue T, et al. Synergistic interaction between Candida albicans and commensal oral streptococci in a novel in vitro mucosal model. Infect Immun. 2012;80:620–632. doi: 10.1128/IAI.05896-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Do T, Devine D, Marsh PD. Oral biofilms: molecular analysis, challenges, and future prospects in dental diagnostics. Clin Cosmet Investig Dent. 2013;5:11–19. doi: 10.2147/CCIDE.S31005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dye BA, Hsu KL, Afful J. Prevalence and Measurement of Dental Caries in Young Children. Pediatr Dent. 2015;37:200–216. [PubMed] [Google Scholar]
- 15.Falsetta ML, Klein MI, Colonne PM, et al. Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes virulence of plaque biofilms in vivo. Infect Immun. 2014;82:1968–1981. doi: 10.1128/IAI.00087-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fears KP, Gonzalez-Begne M, Love CT, Day DE, Koo H. Surface-induced changes in the conformation and glucan production of glucosyltransferase adsorbed on saliva-coated hydroxyapatite. Langmuir. 2015;31:4654–4662. doi: 10.1021/la504461h. [DOI] [PubMed] [Google Scholar]
- 17.Flemming HC, Wingender J. The biofilm matrix. Nat Rev Microbiol. 2010;8:623–633. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- 18.Fontana M. The Clinical, Environmental, and Behavioral Factors That Foster Early Childhood Caries: Evidence for Caries Risk Assessment. Pediatr Dent. 2015;37:217–225. [PubMed] [Google Scholar]
- 19.Gregoire S, Xiao J, Silva BB, et al. Role of glucosyltransferase B in interactions of Candida albicans with Streptococcus mutans and with an experimental pellicle on hydroxyapatite surfaces. Appl Environ Microbiol. 2011;77:6357–6367. doi: 10.1128/AEM.05203-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gross EL, Beall CJ, Kutsch SR, Firestone ND, Leys EJ, Griffen AL. Beyond Streptococcus mutans: dental caries onset linked to multiple species by 16S rRNA community analysis. PLoS One. 2012;7:e47722. doi: 10.1371/journal.pone.0047722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo L, Shi W. Salivary biomarkers for caries risk assessment. J Calif Dent Assoc. 2013;41:107–118. [PMC free article] [PubMed] [Google Scholar]
- 22.Hart TC, Corby PM, Hauskrecht M, et al. Identification of microbial and proteomic biomarkers in early childhood caries. Int J Dent. 2011;2011:196721. doi: 10.1155/2011/196721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holgerson PL, Vestman NR, Claesson R, et al. Oral microbial profile discriminates breast-fed from formula-fed infants. J Pediatr Gastroenterol Nutr. 2013;56:127–136. doi: 10.1097/MPG.0b013e31826f2bc6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hwang G, Marsh G, Gao L, Waugh R, Koo H. Binding Force Dynamics of Streptococcus mutans-glucosyltransferase B to Candida albicans. J Dent Res. 2015;94:1310–1317. doi: 10.1177/0022034515592859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jakubovics NS, Gill SR, Iobst SE, Vickerman MM, Kolenbrander PE. Regulation of gene expression in a mixed-genus community: stabilized arginine biosynthesis in Streptococcus gordonii by coaggregation with Actinomyces naeslundii. J Bacteriol. 2008;190:3646–3657. doi: 10.1128/JB.00088-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang EM, Lo EC, Chu CH, Wong MC. Prevention of early childhood caries (ECC) through parental toothbrushing training and fluoride varnish application: a 24-month randomized controlled trial. J Dent. 2014;42:1543–1550. doi: 10.1016/j.jdent.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Kanasi E, Dewhirst FE, Chalmers NI, et al. Clonal analysis of the microbiota of severe early childhood caries. Caries Res. 2010;44:485–497. doi: 10.1159/000320158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karjalainen S, Soderling E, Sewon L, Lapinleimu H, Simell O. A prospective study on sucrose consumption, visible plaque and caries in children from 3 to 6 years of age. Community Dent Oral Epidemiol. 2001;29:136–142. doi: 10.1034/j.1600-0528.2001.290208.x. [DOI] [PubMed] [Google Scholar]
- 29.Khanna P, Walt DR. Salivary diagnostics using a portable point-of-service platform: a review. Clin Ther. 2015;37:498–504. doi: 10.1016/j.clinthera.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein MI, Hwang G, Santos PHS, Campanella OH, Koo H. Streptococcus mutans-derived extracellular matrix in cariogenic oral biofilms. Front Cell Infect Microbiol. 2015;5:10. doi: 10.3389/fcimb.2015.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klein MI, Falsetta ML, Xiao J, Bowen WH, Koo H. The role of extracellular polysaccharides matrix in virulent oral biofilms. In: Jakubovics NS, Palmer RJ Jr, editors. Oral Microbial Ecology: Current Research and New Perspectives. Norfolk, UK: Caister Academic Press; 2013. pp. 63–83. [Google Scholar]
- 32.Kohler B, Andreen I. Mutans streptococci and caries prevalence in children after early maternal caries prevention: a follow-up at 19 years of age. Caries Res. 2012;46:474–480. doi: 10.1159/000339665. [DOI] [PubMed] [Google Scholar]
- 33.Kreth J, Zhang Y, Herzberg MC. Streptococcal antagonism in oral biofilms: Streptococcus sanguinis and Streptococcus gordonii interference with Streptococcus mutans. J Bacteriol. 2008;190:4632–4640. doi: 10.1128/JB.00276-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lapirattanakul J, Nakano K. Mother-to-child transmission of mutans streptococci. Future Microbiol. 2014;9:807–823. doi: 10.2217/fmb.14.37. [DOI] [PubMed] [Google Scholar]
- 35.Lemos JA, Burne RA. A model of efficiency: stress tolerance by Streptococcus mutans. Microbiology. 2008;154:3247–3255. doi: 10.1099/mic.0.2008/023770-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levine M. Susceptibility to dental caries and the salivary proline-rich proteins. Int J Dent. 2011;2011:953412. doi: 10.1155/2011/953412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu J, Wu C, Huang IH, Merritt J, Qi F. Differential response of Streptococcus mutans towards friend and foe in mixed-species cultures. Microbiology. 2011;157:2433–2444. doi: 10.1099/mic.0.048314-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu YL, Nascimento M, Burne RA. Progress toward understanding the contribution of alkali generation in dental biofilms to inhibition of dental caries. Int J Oral Sci. 2012;4:135–140. doi: 10.1038/ijos.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martins C, Buczynski AK, Maia LC, Siqueira WL, Castro GF. Salivary proteins as a biomarker for dental caries--a systematic review. J Dent. 2013;41:2–8. doi: 10.1016/j.jdent.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 40.Mattos-Graner RO, Klein MI, Smith DJ. Lessons Learned from Clinical Studies: Roles of Mutans Streptococci in the Pathogenesis of Dental Caries. Curr Oral Health Rep. 2014;1:70–78. [Google Scholar]
- 41.Merritt J, Qi F. The mutacins of Streptococcus mutans: regulation and ecology. Mol Oral Microbiol. 2012;27:57–69. doi: 10.1111/j.2041-1014.2011.00634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Merritt J, Senpuku H, Kreth J. Let there be bioluminescence: development of a biophotonic imaging platform for in situ analyses of oral biofilms in animal models. Environ Microbiol. 2015 doi: 10.1111/1462-2920.12953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Metwalli KH, Khan SA, Krom BP, Jabra-Rizk MA. Streptococcus mutans, Candida albicans, and the Human Mouth: A Sticky Situation. PLoS Pathog. 2013;9:e1003616. doi: 10.1371/journal.ppat.1003616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nascimento MM, Browngardt C, Xiaohui X, Klepac-Ceraj V, Paster BJ, Burne RA. The effect of arginine on oral biofilm communities. Mol Oral Microbiol. 2014;29:45–54. doi: 10.1111/omi.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nascimento MM, Liu Y, Kalra R, et al. Oral arginine metabolism may decrease the risk for dental caries in children. J Dent Res. 2013;92:604–608. doi: 10.1177/0022034513487907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nie S, Henley WH, Miller SE, et al. An automated integrated platform for rapid and sensitive multiplexed protein profiling using human saliva samples. Lab Chip. 2014;14:1087–1098. doi: 10.1039/c3lc51303c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ninno AD, Ciasca G, Gerardino A, et al. An integrated superhydrophobic-plasmonic biosensor for mid-infrared protein detection at the femtomole level. Phys Chem Chem Phys. 2015;17:21337–21342. doi: 10.1039/c4cp05023a. [DOI] [PubMed] [Google Scholar]
- 48.Nobbs AH, Jenkinson HF, Jakubovics NS. Stick to your gums: mechanisms of oral microbial adherence. J Dent Res. 2011;90:1271–1278. doi: 10.1177/0022034511399096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nogueira RD, Alves AC, Napimoga MH, Smith DJ, Mattos-Graner RO. Characterization of salivary immunoglobulin A responses in children heavily exposed to the oral bacterium Streptococcus mutans: influence of specific antigen recognition in infection. Infect Immun. 2005;73:5675–5684. doi: 10.1128/IAI.73.9.5675-5684.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nyvad B, Crielaard W, Mira A, Takahashi N, Beighton D. Dental caries from a molecular microbiological perspective. Caries Res. 2013;47:89–102. doi: 10.1159/000345367. [DOI] [PubMed] [Google Scholar]
- 51.Oppenheim FG, Salih E, Siqueira WL, Zhang W, Helmerhorst EJ. Salivary proteome and its genetic polymorphisms. Ann N Y Acad Sci. 2007;1098:22–50. doi: 10.1196/annals.1384.030. [DOI] [PubMed] [Google Scholar]
- 52.O’Sullivan DM, Tinanoff N. The association of early childhood caries patterns with caries incidence in pre-school children. J Public Health Dent. 1996;56:81–83. doi: 10.1111/j.1752-7325.1996.tb02401.x. [DOI] [PubMed] [Google Scholar]
- 53.Paes Leme AF, Koo H, Bellato CM, Bedi G, Cury JA. The role of sucrose in cariogenic dental biofilm formation--new insight. J Dent Res. 2006;85:878–887. doi: 10.1177/154405910608501002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Palmer CA, Kent R, Jr, Loo CY, et al. Diet and caries-associated bacteria in severe early childhood caries. J Dent Res. 2010;89:1224–1229. doi: 10.1177/0022034510376543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parisotto TM, Steiner-Oliveira C, Silva CM, Rodrigues LK, Nobre-dos-Santos M. Early childhood caries and mutans streptococci: a systematic review. Oral Health Prev Dent. 2010;8:59–70. [PubMed] [Google Scholar]
- 56.Parisotto TM, Stipp R, Rodrigues LK, Mattos-Graner RO, Costa LS, Nobre-Dos-Santos M. Can insoluble polysaccharide concentration in dental plaque, sugar exposure and cariogenic microorganisms predict early childhood caries? A follow-up study. Arch Oral Biol. 2015;60:1091–1097. doi: 10.1016/j.archoralbio.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 57.Pereira-Cenci T, Deng DM, Kraneveld EA, et al. The effect of Streptococcus mutans and Candida glabrata on Candida albicans biofilms formed on different surfaces. Arch Oral Biol. 2008;53:755–764. doi: 10.1016/j.archoralbio.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 58.Peleg AY, Hogan DA, Mylonakis E. Medically important bacterial-fungal interactions. Nat Rev Microbiol. 2010;8:340–349. doi: 10.1038/nrmicro2313. [DOI] [PubMed] [Google Scholar]
- 59.Raja M, Hannan A, Ali K. Association of oral candidal carriage with dental caries in children. Caries Res. 2010;44:272–276. doi: 10.1159/000314675. [DOI] [PubMed] [Google Scholar]
- 60.Ribeiro TR, Dria KJ, de Carvalho CB, et al. Salivary peptide profile and its association with early childhood caries. Int J Paediatr Dent. 2013;23:225–234. doi: 10.1111/j.1365-263X.2012.01258.x. [DOI] [PubMed] [Google Scholar]
- 61.Si Y, Ao S, Wang W, Chen F, Zheng S. Magnetic bead-based salivary peptidome profiling analysis for severe early childhood caries. Caries Res. 2015;49:63–69. doi: 10.1159/000360868. [DOI] [PubMed] [Google Scholar]
- 62.Simón-Soro A, Mira A. Solving the etiology of dental caries. Trends Microbiol. 2015;23:76–82. doi: 10.1016/j.tim.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 63.Takahashi N, Nyvad B. The role of bacteria in the caries process: ecological perspectives. J Dent Res. 2011;90:294–303. doi: 10.1177/0022034510379602. [DOI] [PubMed] [Google Scholar]
- 64.Tanner AC, Mathney JM, Kent RL, et al. Cultivable anaerobic microbiota of severe early childhood caries. J Clin Microbiol. 2011;49:1464–1474. doi: 10.1128/JCM.02427-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tanzer JM. Dental caries is a transmissible infectious disease: the Keyes and Fitzgerald revolution. J Dent Res. 1995;74:1536–1542. doi: 10.1177/00220345950740090601. [DOI] [PubMed] [Google Scholar]
- 66.Vacca-Smith AM, Scott-Anne KM, Whelehan MT, Berkowitz RJ, Feng C, Bowen WH. Salivary glucosyltransferase B as a possible marker for caries activity. Caries Res. 2007;41:445–450. doi: 10.1159/000107930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xiao J, Klein MI, Delahunty CM, et al. The exopolysaccharide matrix modulates the interaction between 3D architecture and virulence of a mixed-species oral biofilm. PLoS Pathog. 2012;8:e1002623. doi: 10.1371/journal.ppat.1002623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang XQ, Zhang Q, Lu LY, Yang R, Liu Y, Zou J. Genotypic distribution of Candida albicans in dental biofilm of Chinese children associated with severe early childhood caries. Arch Oral Biol. 2012;57:1048–1053. doi: 10.1016/j.archoralbio.2012.05.012. [DOI] [PubMed] [Google Scholar]