Abstract
There are significant differences in the immune response and in the susceptibility to autoimmune diseases among rodent strains. It would thus be expected that the contribution of the immune response to cerebral ischemic injury would also differ among rodent strains. More importantly, there are significant differences between the immune responses of rodents and humans. All of these factors are likely to impact the successful translation of immunomodulatory therapies from experimental rodent models to patients with stroke.
Keywords: stroke, immunology, EAE, strain, immune response
There is variability among infarct size and stroke outcome in different strains of mice and rats subjected to similar ischemic insults [1-6]. Much of the literature suggests that there are differences in cerebrovascular anatomy/cerebral blood flow among these strains that that leads to the variability in infarct size [7-9, 2, 10]. Little attention has been given to systemic factors that might influence infarct development and functional recovery. Increasing data show that the immune system plays a critical role in the response to cerebral ischemic injury. Following the onset of cerebral ischemia, neutrophils, monocytes and lymphocytes infiltrate the brain [11, 12]. There is also a marked systemic inflammatory response immediately after stroke, which is paradoxically accompanied by a depression in cellular immune responses that predispose to infection [13, 14]. Despite the defect in lymphocyte responses, humoral and cellular immune responses to central nervous system (CNS) antigens can be detected in the weeks to months after stroke [15-24]. Understanding how the immune response affects stroke outcome and how it can be manipulated to improve outcome is an active area of research, and the results from this line of research would seemingly be dependent upon the immunologic background in which these phenomena are studied. This review will highlight differences in the immune response among rodent strains and discuss the implications for stroke research and its translation into the clinical arena.
Strain Differences – Lessons from EAE
Rats
Susceptibility to experimental allergic encephalomyelitis (EAE) by active immunization with myelin basic protein (MBP) differs significantly among rodent strains (Table). Lewis rats and Dark Agouti (DA) rats are highly susceptible to EAE while strains such as Fischer (F344), Brown Norway (BN), Wistar-Kyoto (WKY) and Piebald Virol Glaxo (PVG) are poorly susceptible [25-28]. Even among EAE susceptible Lewis rats, the relative ease of inducing EAE may differ between commercial vendors [29]. And while Wistar and Sprague-Dawley rats are capable of developing EAE, they are used less commonly than Lewis and DA rats to study this autoimmune disease as well as other immunologically mediated diseases [30-36]. The susceptibility of rats to autoimmune diseases of the peripheral nervous system parallels that of EAE, with Lewis rats being relatively susceptible to experimental allergic neuritis (EAN) and BN rats being relatively resistant [37, 38]. Wistar and Sprague-Dawley rats are also susceptible to EAN but develop less severe disease than Lewis rats [37]. Because Lewis and DA rats are more prone to develop EAE and EAN, as well as autoimmune diseases that do not affect the nervous system, it suggests that the mechanisms predisposing these strains to autoimmunity are systemic and not unique to the nervous system [39-42].
Table.
Relative propensities for developing inflammatory/autoimmune disease among commonly used rodent strains.
| Mouse Strains | Rat Strains | |
|---|---|---|
|
High
Susceptibility |
SJL/J C3H/He |
Lewis Dark Agouti (DA) |
|
Intermediate
Susceptibility |
C57BL/6 CBA |
Sprague-Dawley (SD) Wistar Buffalo |
|
Poor
Susceptibility |
DBA/2 B10.S BALB/c CD1 |
Fischer (F344) Brown Norway (BN) Wistar-Kyoto (WKY) Piebald Virol Glaxo (PVG) |
There appear to be fundamental differences in the immune response among rat strains that contribute to the differing susceptibilities to EAE. For instance, susceptible Lewis rats express more major histocompatibility complex (MHC) II on their astrocytes than resistant strains like BN [27, 43]. The relative numbers of leukocyte subsets also varies among strains; in comparison to BN rats, Lewis rats have more mast cells in the CNS (and PNS) [44]. And EAE resistant F344 rats have increased numbers of MBP specific CD8+ regulatory T cells compared to EAE susceptible Lewis rats [45]. Further, cytokine profiles differ among strains and correlate with the ability to induce EAE. Within astrocytes, gene expression for pro-inflammatory cytokines like tumor necrosis factor (TNF)-α is associated with increased susceptibility to EAE, while production of immunomodulatory cytokines like transforming growth factor (TGF)-β1 is associated with protection from EAE [46, 47]. EAE resistant and EAE susceptible rats also differ in the production of Th1 and Th17 cytokines, with susceptible rats expressing more interferon (IFN)-γ, IL-17 and IL-12 in the draining lymph nodes following immunization [48, 49]. Other factors may also contribute to the susceptibility or resistance to EAE, including IL-2 production (lower levels are protective) [50] and endogenous nitric oxide (higher levels are protective) [51].
Given the interplay between the brain, the immune system and the endocrine system, it is not surprising that there are differences in the neuroendocrine response to stress that influence the susceptibility to EAE. Among wild type rats, behavioral characteristics like aggression (“attack latency time”) predict susceptibility to EAE [52]. And among inbred rats, EAE resistant strains (PVG, F344 and BN) exhibit higher levels of endogenous steroid hormones, including corticosterone, than EAE susceptible strains [27, 53-55]. As might be expected, hormonal manipulations affect susceptibility to EAE, with adrenalectomy rendering the normally EAE resistant PVG rats susceptible to severe disease [55]. Further, blockade of endogenous glucocorticoids with RU-486 leads to worsened severity of EAE in strains that are already susceptible (ie. Lewis rats) [56]. EAE susceptible rat strains also differ with regards to the relative antigenicity of different myelin epitopes [57], and strains resistant to active induction of EAE (ie. by immunization) may be susceptible to EAE induction by adoptive transfer of lymphocytes [58]. Both of these factors (the antigen/epitope used to induce EAE and whether the disease was induced by active immunization or adoptive transfer) affect the clinical presentation and the neuropathology of the disease [59, 60, 58].
Mice
The SJL/J and C3H/He strains of mice are highly susceptible to EAE following immunization with MBP, while the DBA/2, B10.S and BALB/c strains are relatively resistant (Table 1) [61-63]. Like Lewis rats, SJL mice express more MHC II on their astrocytes than EAE resistant strains (BALB/c) [43]. That the differences in susceptibility are due (at least in part) to inherent differences in the immune response is demonstrated by the fact that the normally resistant BALB/c mice can be rendered susceptible to active induction of EAE by inhibition of cytotoxic T lymphocyte antigen-4 (CTLA-4), which prevents an inhibitory signal from be delivered to the T cell [64]. The cytokine profiles of mouse strains, like rat strains, also predict the susceptibility to EAE. In general, EAE susceptible mouse strains tend to have a Th1 type phenotype, while EAE resistant strains have a Th2/Treg phenotype [65-67]. And again, paralleling the situation in rats, neuroendocrine responses play a role in determining the susceptibility of mice to EAE. For example, EAE susceptible SJL/J mice, similar to Lewis rats, have a blunted response of the hypothalamic-pituitary-adrenal axis to stress, contributing to EAE susceptibility [68].
C57BL/6 mice are resistant to the active induction of EAE following immunization with MBP but are susceptible to EAE induced by the myelin oligodendrocyte glycoprotein 35–55 peptide (MOG35–55); CD1 mice, on the other hand, are resistant to EAE induced with MOG35–55 [69]. The difference in susceptibility to EAE following immunization with MOG35–55 in these strains appears to be related, in part, to the fact that C57BL/6 mice have a higher percentage of CD4+ T cells that produce IFN-γ and IL-17, as well as increased systemic IL-17 [69]. Increased numbers of regulatory B and T cells may also contribute to the resistance of CD1 mice to EAE [69].
EAE is a model of multiple sclerosis (MS). It is an imperfect model, but it has provided insights into both the systemic and CNS immune responses in rodents. Another rodent model of MS results from injection of Theiler’s murine encephalomyelitis virus (TMEV) into the brains of susceptible mouse strains. Resistant strains are able to clear the virus while susceptible strains become persistently infected. The persistent infection leads to a chronic demyelinating disease that is used as a surrogate of MS [70]. The susceptibility to TMEV among mouse strains resembles that of the susceptibility to EAE. The disease is most severe in SJL mice, of intermediate severity in CBA and C3H/He strains, and least severe in C57BL/6 mice [71]. BALB/c mice are resistant to the neuropathology of TMEV [72]. During CNS infection with TMEV, there are increases in Treg and CD45r(+) B cells, delays in viral elimination and increases in IL-10 mRNA in susceptible SJL mice compared to C57BL/6 mice [73]. Further, the astrocytes of susceptible strains appear to produce more IL-1α than the astrocytes of resistant strains [74]. And as is the case with EAE, the susceptibility to TMEV correlates with the ease of induction of MHC II on astrocytes [75].
Age and Gender Considerations in EAE
The susceptibility to both EAE and TMEV decreases with age [76-81]. The age related decline in susceptibility to EAE and TMEV is related to the senescence of the immune response that occurs with aging [80, 82]. Sex and sex hormones also affect the susceptibility to EAE, TMEV and other autoimmune diseases [83-88]. These facts highlight the need for using age and sex appropriate models when studying the immune response and also suggest that the relative benefits of immunomodulatory therapies are likely to vary depending on age and sex.
Psychiatric Disorders: Immune and Strain Related Issues
Numerous psychiatric disorders are posited to have an immunologic basis. Chief among these disorders is depression [89]. Given strain related differences in the immune response, it is reasonable to assume that there would be strain related differences in the modeling of psychiatric disorders. For instance, administration of LPS leads to long-term depressive like behavior in C57BL/6 mice but not in CD1 mice [90]. And in comparison to C57BL/6 mice, BALB/c mice have increased immune activation following LPS injection with an increase in depressive behavior [91]. And following an LPS challenge, the microglia of high anxiety inbred mice (DBA/2J and 129S2/Sv) are polarized to an M1 phenotype relative to mice without anxiety [92]. We also showed that were strain related differences in “fatigue” and “depression” after stroke with Lewis rats displayed depressive-like behavior while Wistar and SD rats displayed fatigue like behavior [93]. These examples just skim the surface of a robust literature showing an association between the immune response and psychiatric disease, and thus, the relationship between animal strains and the modeling of psychiatric disorders.
The Immunology of Stroke: Strain Related Issues
Based on the literature that shows fundamental differences in the immune response among rodent strains, one would expect that the immune response following ischemic brain injury would also differ among strains. The relative benefit of immunomodulatory interventions for the treatment of stroke would thus be expected to differ as well. When studying immune mechanisms in stroke, one should ideally evaluate both EAE susceptible and EAE resistant strains to best understand the true contribution of the immune response to stroke outcome and the likelihood that modulating that response would be of benefit. For instance, we showed that the long-term behavioral outcomes among Lewis, Sprague Dawley and Wistar rats were quite different despite similar infarct volumes at 24 hours after middle cerebral artery occlusion (MCAO) [94]. At one month after MCAO, the cytokine profiles among these three strains differed with less circulating IL-1α in Sprague-Dawley rats and more circulating IL-10 in Lewis rats [93]; many of the behaviors assessed at this time point correlated significantly with these cytokine levels.
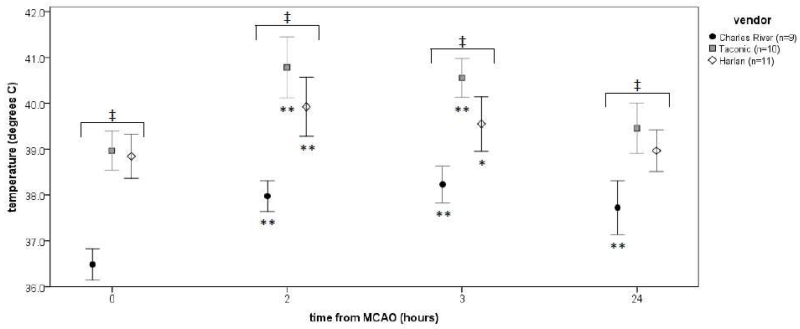
Wistar and Sprague Dawley rats, strains with intermediate susceptibility to EAE, are arguably the most common rat strains used in stroke research. Very few stroke studies are done in EAE resistant F344 rats (with some authors reporting that the strain is unsuitable for the filament model of MCAO) [95]. Our group has used EAE susceptible Lewis rats almost exclusively for studies that address the post-ischemic immune response. Upon noticing variation in stroke outcomes after switching vendors, we began to take note of differences in what is supposed to be an inbred rat strain. For instance, there were significant differences in baseline temperatures as well as differences in the magnitude of temperature changes following MCAO (Figure 1). At least one plausible explanation for such differences among inbred animals between vendors is that of differences in the gut microbiome [96]. Given that the gut microbiome plays an important role in driving the immune response and triggering autoimmune disease [97-101], vendor differences in diet and the microbiological milieu may significantly affect the post-ischemic immune response as well as responses to immunomodulatory therapy.
Figure 1.
Temperatures after MCAO. Data are plotted as the mean (± standard deviation). Differences in temperature between vendors is assessed by ANOVA and noted by ‡ (P<0.001). Differences from baseline temperatures after MCAO among animals from a single vendor is assessed by paired t-test and noted by *(P<0.05) and **(P<0.01).
With limited exceptions, virtually all of the studies examining immunologic changes after stroke in mice, as well as the response to immunologic therapies, have been done in the C57BL/6 strain, which has intermediate susceptibility to EAE. One of the obvious reasons for using C57BL/6 mice is the ability to create transgenic animals from this genetic background. What this means practically, however, is that almost all of what we know about the role of the immune system in stroke is derived from a single line of inbred mice. And a recent study showed that despite similar infarct volumes after MCAO in C57BL/6 and FVB mice (in which EAE can be reproducibly induced [102]), there were significant differences in neurological outcome and the immune response between these strains [103]. That the infarct volumes were similar between these strains is important, given that the volume of ischemic tissue injury will undoubtedly affect the nature and strength of the immune response. Relative to FVB mice, C57BL/6 mice had a more robust increase in leukocytes within the brain at 24 hours after MCAO. And within C57BL/6 mice the post-ischemic leukocyte infiltration of the brain was composed mostly of lymphocytes, while in FVB mice it was composed mostly of neutrophils and myeloid cells. In addition, the systemic effects of stroke differed by strain, with a decrease in splenic T cells in FVB mice, but not C57BL/6 mice, 24 hours after MCAO. These data again highlight the need to evaluate different strains of mice when studying the contribution of the immune response to ischemic brain injury.
Susceptibility to Infection
In a mouse model of stroke, sympathetically mediated suppression of Th1 type immune responses was associated with a predisposition to infection [104]. These data were generated in SV129/J and C57BL/6J mice. Subsequent studies showed that SV129 mice are much more susceptible to infection following MCAO than either C57BL/6 or BALB/C mice [105]. As it turns out, mouse strains differ markedly in their resistance and response to infection in general, independent of stroke. For instance, given identical pneumococcal infections of the respiratory tract, BALB/c mice demonstrate no bacteremia or death, CBA/Ca, C3H/He and SJL mice develop severe bacteremia with 100% mortality, and C57BL/6 and DBA/2 strains develop only modest bacteremia with approximately 50% mortality [106]. One would thus expect that not only the susceptibility to infection following stroke would differ among different strains, but that the clinical manifestation of those infections might differ as well.
As mentioned, the increased risk of infection following stroke appears to be, at least in part, mediated by the sympathetic nervous system (SNS) [104, 107]. That the SNS can affect the immune response has been appreciated for some time [108, 109]. The effect of sympathetic activation on the immune response also appears to be strain dependent in that chemical sympathectomy increases mitogen-induced lymphocyte proliferation in EAE resistant DBA/2 mice but not in EAE susceptible C57BL/6 mice [110]. Further, systemic epinephrine levels differ with the strain as well as the age of rat, suggesting that modulation of the sympathetic response may affect the immune response, the susceptibility to infection, and the chance of developing autoimmunity/sustained inflammation after stroke in a strain and age dependent fashion [111, 112].
Of Mice and Men
Highly conserved molecular motifs in pathogens, termed pathogen associated molecular patterns (PAMPs), activate the innate immune response through toll-like receptors (TLRs). TLR-4, for example, is activated by endotoxin/lipopolysaccharide (LPS), a component of the Gram-negative bacterial cell wall. The sensitivity of TLR4 to endotoxin/LPS is vastly different among rodents and humans, with the immune system of rodents favoring “tolerance” to immunologic threats while that of humans favors “resistance” to similar threats [113, 114]. For example, administration of endotoxin intravenously at doses of 15 μg/kg leads to a severe systemic response with shock in humans [115, 116]. In mice, on the other hand, the median lethal dose of endotoxin/LPS is in the range of 10-12 mg/kg [117, 118]. And the dose endotoxin/LPS needed to elicit similar systemic levels of IL-6 is about 200 fold higher in mice than in humans [119]. Not surprisingly, the response to endotoxin in rodents is also strain dependent [120]. Similarly, genetic variations in humans (like single nucleotide polymorphisms [SNPs]) modulate the responsiveness of TLR4 to endotoxin and the course of infection/sepsis [121-124].
TLRs are also activated by danger associated molecular patterns (DAMPs) released from injured cells. The post-ischemic inflammatory response is a sterile response that is undoubtedly set in motion by DAMPs from necrotic tissue. These DAMPs include high-mobility group box (HMGB)-1, adenosine, ATP, uric acid, heat-shock proteins, heparin sulfate, hyaluronan fragments and DNA [125, 126]. Based on what is known about the differences in the responsiveness to PAMPs, it would not be surprising if there were similar differences in the relative responsiveness to DAMPs between rodents and humans as well as among rodent strains. Additionally, genetic variation among humans would likely influence the responsiveness of the innate immune system to these endogenous DAMPs.
The dissimilarity in the response to sterile injury between rodents and humans is highlighted by the fact there is essentially no correlation between the genomic profiles of mice and humans subjected to blunt force trauma or burn injury [127]. Likewise, the correlation between gene expression in human volunteers treated with LPS and mice treated with LPS is essentially random [127]. These observations highlight the fact that the response to both PAMPs and DAMPs differ significantly between humans and rodents.
Another important difference between the immune system of homo sapiens and rodents is illustrated by the difference in the composition of circulating leukocytes. In C57BL/6 mice, for instance, the majority of circulating cells are lymphocytes (75-90%) while only 20-40% of circulating leukocytes in humans are lymphocytes [128, 129]. In humans, most WBCs in circulation are neutrophils (50-70%), and these neutrophils are not only more numerous that in rodents but also have different properties than rodent neutrophils [114].
Inhibition of α-4 for the treatment of stroke
Lymphocytes and monocytes express the integrins α4β1 (CD49d/CD29) and α4β7 (CD49d/CD103), both of which are important for cell trafficking [130]. CD49d/CD29 is also known as very late activation antigen-4 (VLA-4), and CD49d/CD103 is also known as lymphocyte-Peyer’s patch adhesion molecule-1 (LPAM-1). Binding of lymphocytes and monocytes to the endothelium occurs through the interaction of the α4 integrins with either vascular cell adhesion molecule-1 (VCAM-1) or mucosal addressin cell adhesion molecule-1 (MAdCAM-1). In 2001 we showed that inhibition of the α4 integrin with blocking antibodies (TA-2) decreased infarct volume and improved outcome at 48 hours after MCAO in Lewis rats [131]. Treatment with the same antibody was shown to decrease infarct volume at 24 hours after MCAO in both Sprague-Dawley and spontaneously hypertensive (SHR) rats [132]. Subsequent studies showed that inhibition of VLA-4 (with a different antibody) in C57BL/6J mice improved outcome in moderately severe stroke [133], although subsequent studies in C57BL/6 mice with the same antibody showed no benefit [134]. While the difference in outcomes in these studies may have been due chance alone, it is also clear that not all C57BL/6 mice are the same and there may be important genetic and behavioral differences between substrains [135-137]. In a recent “multi-center study” using young male C57BL/6 mice, inhibition of CD49d was found to decrease infarct volume and improve outcome in mild stroke (permanent cortical ischemia induced by electrocoagulation) but not in severe stroke (transient MCAO induced by an intraluminal filament) [138]. Based on inherent differences in the immune response within a species (not to mention between species), the outcomes of this study may have been different if the strokes were performed in different mouse strains (ones more predisposed to inflammation/autoimmune disease), mice with a different gut microbiome, older mice, or female mice. In particular, female mice are noted to express less VLA-4 in the spleen and brain after MCAO compared to male rats [139].
Of note, rodents are known to express VLA-4 on neutrophils as well as on monocytes and lymphocytes; inhibition of α4 thus blocks the migration of all of these cell types in rodents [140, 141]. The influx of PMNs (as well as lymphocytes and monocytes) into the rodent brain following stroke would thus inhibited by antibodies that block VLA-4 [142].
In contrast to rodents, there are relatively more circulating PMNs in humans, yet these PMNs don’t express appreciable α4 [143, 144]. Inhibition of α4 may therefore not prevent the influx of PMNs into the human brain after stroke, suggesting that the effect/benefit of VLA-4 inhibition might differ in rodents and humans.
Summary
Characterizing the immunology of ischemic stroke in a single rodent strain provides information only about the immunologic consequences of stroke in that rodent strain. Predicting the clinical response of patients to an immunological intervention from studies done in a single strain of inbred mice or rats would therefore seem destined to fail. Not only are there critical differences between the immune responses of different rodent strains, there are even larger differences between the immune response of rodents and homo sapiens [145-147, 114]. And as opposed to inbred rodent strains, humans are genetically distinct from each other and have a wide range of genetic polymorphisms that influence the immune response, which suggests that the efficacy of a given intervention might differ from person to person. In summary, these observations suggest that the use of animal models for understanding the development of the post-ischemic inflammatory response has serious limitations and that translation of effective immunomodulatory therapies from rodents to humans is likely to be unreliable. While rodent models of stroke may never adequately address what happens in humans, it would seem that, at a minimum, studies of immunologic therapies for stroke be conducted in strains with different propensities for the development of inflammatory/immunologic diseases.
References
- 1.Lartaud I, Bray-des-Boscs L, Chillon JM, Atkinson J, Capdeville-Atkinson C. In vivo cerebrovascular reactivity in Wistar and Fischer 344 rat strains during aging. Am J Physiol. 1993;264(3 Pt 2):H851–8. doi: 10.1152/ajpheart.1993.264.3.H851. [DOI] [PubMed] [Google Scholar]
- 2.Prieto R, Carceller F, Roda JM, Avendano C. The intraluminal thread model revisited: rat strain differences in local cerebral blood flow. Neurol Res. 2005;27(1):47–52. doi: 10.1179/016164105X18214. [DOI] [PubMed] [Google Scholar]
- 3.Connolly ES, Jr., Winfree CJ, Stern DM, Solomon RA, Pinsky DJ. Procedural and strain-related variables significantly affect outcome in a murine model of focal cerebral ischemia. Neurosurgery. 1996;38(3):523–31. doi: 10.1097/00006123-199603000-00021. [DOI] [PubMed] [Google Scholar]
- 4.Oliff HS, Weber E, Eilon G, Marek P. The role of strain/vendor differences on the outcome of focal ischemia induced by intraluminal middle cerebral artery occlusion in the rat. Brain Res. 1995;675(1-2):20–6. doi: 10.1016/0006-8993(95)00033-m. [DOI] [PubMed] [Google Scholar]
- 5.Oliff HS, Weber E, Miyazaki B, Marek P. Infarct volume varies with rat strain and vendor in focal cerebral ischemia induced by transcranial middle cerebral artery occlusion. Brain Res. 1995;699(2):329–31. doi: 10.1016/0006-8993(95)01045-w. [DOI] [PubMed] [Google Scholar]
- 6.Doyle KP, Buckwalter MS. A mouse model of permanent focal ischemia: distal middle cerebral artery occlusion. Methods Mol Biol. 2014;1135:103–10. doi: 10.1007/978-1-4939-0320-7_9. [DOI] [PubMed] [Google Scholar]
- 7.Barone FC, Knudsen DJ, Nelson AH, Feuerstein GZ, Willette RN. Mouse strain differences in susceptibility to cerebral ischemia are related to cerebral vascular anatomy. J Cereb Blood Flow Metab. 1993;13(4):683–92. doi: 10.1038/jcbfm.1993.87. [DOI] [PubMed] [Google Scholar]
- 8.Wellons JC, 3rd, Sheng H, Laskowitz DT, Mackensen GB, Pearlstein RD, Warner DS. A comparison of strain-related susceptibility in two murine recovery models of global cerebral ischemia. Brain Res. 2000;868(1):14–21. doi: 10.1016/s0006-8993(00)02216-2. [DOI] [PubMed] [Google Scholar]
- 9.Fujii M, Hara H, Meng W, Vonsattel JP, Huang Z, Moskowitz MA. Strain-related differences in susceptibility to transient forebrain ischemia in SV-129 and C57black/6 mice. Stroke. 1997;28(9):1805–10. doi: 10.1161/01.str.28.9.1805. [DOI] [PubMed] [Google Scholar]
- 10.Oliff HS, Coyle P, Weber E. Rat strain and vendor differences in collateral anastomoses. J Cereb Blood Flow Metab. 1997;17(5):571–6. doi: 10.1097/00004647-199705000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Schroeter M, Jander S, Witte OW, Stoll G. Local immune responses in the rat cerebral cortex after middle cerebral artery occlusion. J Neuroimmunol. 1994;55(2):195–203. doi: 10.1016/0165-5728(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 12.Stevens SL, Bao J, Hollis J, Lessov NS, Clark WM, Stenzel-Poore MP. The use of flow cytometry to evaluate temporal changes in inflammatory cells following focal cerebral ischemia in mice. Brain Res. 2002;932(1-2):110–9. doi: 10.1016/s0006-8993(02)02292-8. [DOI] [PubMed] [Google Scholar]
- 13.Vogelgesang A, Becker KJ, Dressel A. Immunological consequences of ischemic stroke. Acta Neurol Scand. 2014;129(1):1–12. doi: 10.1111/ane.12165. [DOI] [PubMed] [Google Scholar]
- 14.Courties G, Moskowitz MA, Nahrendorf M. The innate immune system after ischemic injury: lessons to be learned from the heart and brain. JAMA Neurol. 2014;71(2):233–6. doi: 10.1001/jamaneurol.2013.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Becker KJ, Kindrick DL, Lester MP, Shea C, Ye ZC. Sensitization to brain antigens after stroke is augmented by lipopolysaccharide. J Cereb Blood Flow Metab. 2005;25(12):1634–44. doi: 10.1038/sj.jcbfm.9600160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Becker KJ, Kalil AJ, Tanzi P, Zierath DK, Savos AV, Gee JM, et al. Autoimmune responses to the brain after stroke are associated with worse outcome. Stroke. 2011;42(10):2763–9. doi: 10.1161/STROKEAHA.111.619593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shibata D, Cain K, Tanzi P, Zierath D, Becker K. Myelin basic protein autoantibodies, white matter disease and stroke outcome. J Neuroimmunol. 2012;252(1-2):106–12. doi: 10.1016/j.jneuroim.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalev-Zylinska ML, Symes W, Little KC, Sun P, Wen D, Qiao L, et al. Stroke patients develop antibodies that react with components of N-methyl-D-aspartate receptor subunit 1 in proportion to lesion size. Stroke. 2013;44(8):2212–9. doi: 10.1161/STROKEAHA.113.001235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bornstein NM, Aronovich B, Korczyn AD, Shavit S, Michaelson DM, Chapman J. Antibodies to brain antigens following stroke. Neurology. 2001;56(4):529–30. doi: 10.1212/wnl.56.4.529. [DOI] [PubMed] [Google Scholar]
- 20.Dambinova SA, Khounteev GA, Izykenova GA, Zavolokov IG, Ilyukhina AY, Skoromets AA. Blood test detecting autoantibodies to N-methyl-D-aspartate neuroreceptors for evaluation of patients with transient ischemic attack and stroke. Clin Chem. 2003;49(10):1752–62. doi: 10.1373/49.10.1752. [DOI] [PubMed] [Google Scholar]
- 21.Youngchaiyud U, Coates AS, Whittingham S, Mackay IR. Cellular-immune response to myelin protein: absence in multiple sclerosis and presence in cerebrovascular accidents. Aust N Z J Med. 1974;4(6):535–8. doi: 10.1111/j.1445-5994.1974.tb03233.x. [DOI] [PubMed] [Google Scholar]
- 22.Kallen B, Nilsson O, Thelin C. Effect of encephalitogenic protein on migration in agarose of leukoytes from patients with multiple sclerosis. A longitudinal study of patients with relapsing multiple sclerosis or with cerebral infarction. Acta Neurol Scand. 1977;55(1):47–56. [PubMed] [Google Scholar]
- 23.Wang WZ, Olsson T, Kostulas V, Hojeberg B, Ekre HP, Link H. Myelin antigen reactive T cells in cerebrovascular diseases. Clin Exp Immunol. 1992;88(1):157–62. doi: 10.1111/j.1365-2249.1992.tb03056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rocklin RE, Sheremata WA, Feldman RG, Kies MW, David JR. The Guillain-Barre syndrome and multiple sclerosis. In vitro cellular responses to nervous-tissue antigens. N Engl J Med. 1971;284(15):803–8. doi: 10.1056/NEJM197104152841501. [DOI] [PubMed] [Google Scholar]
- 25.Gasser DL, Newlin CM, Palm J, Gonatas NK. Genetic control of susceptibility to experimental allergic encephalomyelitis in rats. Science. 1973;181(4102):872–3. doi: 10.1126/science.181.4102.872. [DOI] [PubMed] [Google Scholar]
- 26.Levine S, Sowinski R. Allergic encephalomyelitis in the reputedly resistant Brown Norway strain of rats. J Immunol. 1975;114(2 Pt 1):597–601. [PubMed] [Google Scholar]
- 27.Gunther E, Odenthal H, Wechsler W. Association between susceptibility to experimental allergic encephalomyelitis and the major histocompatibility system in congenic rat strains. Clin Exp Immunol. 1978;32(3):429–34. [PMC free article] [PubMed] [Google Scholar]
- 28.Stevens DB, Gold DP, Sercarz EE, Moudgil KD. The Wistar Kyoto (RT1(l)) rat is resistant to myelin basic protein-induced experimental autoimmune encephalomyelitis: comparison with the susceptible Lewis (RT1(l)) strain with regard to the MBP-directed CD4+ T cell repertoire and its regulation. J Neuroimmunol. 2002;126(1-2):25–36. doi: 10.1016/s0165-5728(02)00045-0. [DOI] [PubMed] [Google Scholar]
- 29.Gould KE, Stepaniak JA, Swanborg RH. Variable susceptibility of Lewis rats to experimental autoimmune encephalomyelitis. J Neuroimmunol. 1994;54(1-2):145–6. doi: 10.1016/0165-5728(94)90240-2. [DOI] [PubMed] [Google Scholar]
- 30.Rivero VE, Riera CM, Roth GA. Humoral response against myelin antigens in two strains of rats with different susceptibility to experimental allergic encephalomyelitis (EAE) Autoimmunity. 1999;29(2):129–37. doi: 10.3109/08916939908995382. [DOI] [PubMed] [Google Scholar]
- 31.Maccioni M, Riera CM, Rivero VE. Peritoneal antigen-presenting cells pulsed in vivo with myelin basic protein induce the suppression of experimental autoimmune encephalomyelitis (EAE) in Wistar rats. J Neuroimmunol. 1999;96(1):46–56. doi: 10.1016/s0165-5728(99)00013-2. [DOI] [PubMed] [Google Scholar]
- 32.Correa SG, Rodriguez-Galan MC, Rivero VE, Riera CM. Chronic varied stress modulates experimental autoimmune encephalomyelitis in Wistar rats. Brain Behav Immun. 1998;12(2):134–48. doi: 10.1006/brbi.1998.0519. [DOI] [PubMed] [Google Scholar]
- 33.Ljubisavljevic S, Stojanovic I, Pavlovic D, Milojkovic M, Sokolovic D, Stevanovic I, et al. Suppression of the lipid peroxidation process in the CNS reduces neurological expression of experimentally induced autoimmune encephalomyelitis. Folia Neuropathol. 2013;51(1):51–7. doi: 10.5114/fn.2013.34196. [DOI] [PubMed] [Google Scholar]
- 34.Wang ZW, Wang P, Lin FH, Li XL, Li XF, O’Byrne KT, et al. Early-life exposure to lipopolysaccharide reduces the severity of experimental autoimmune encephalomyelitis in adulthood and correlated with increased urine corticosterone and apoptotic CD4+ T cells. Neuroscience. 2011;193:283–90. doi: 10.1016/j.neuroscience.2011.07.047. [DOI] [PubMed] [Google Scholar]
- 35.Li XL, Lv J, Xi NN, Wang T, Shang XF, Xu HQ, et al. Neonatal endotoxin exposure suppresses experimental autoimmune encephalomyelitis through regulating the immune cells responsivity in the central nervous system of adult rats. Biochem Biophys Res Commun. 2010;398(2):302–8. doi: 10.1016/j.bbrc.2010.06.086. [DOI] [PubMed] [Google Scholar]
- 36.Selivonchick DP, Johnston PV. Fat deficiency in rats during development of the central nervous system and susceptibility to experimental allergic encephalomyelitis. J Nutr. 1975;105(3):288–300. doi: 10.1093/jn/105.3.288. [DOI] [PubMed] [Google Scholar]
- 37.Hoffman PM, Powers JM, Weise MJ, Brostoff SW. Experimental allergic neuritis. I. Rat strain differences in the response to bovine myelin antigens. Brain Res. 1980;195(2):355–62. doi: 10.1016/0006-8993(80)90071-2. [DOI] [PubMed] [Google Scholar]
- 38.Zhu J, Zou LP, Bakhiet M, Mix E. Resistance and susceptibility to experimental autoimmune neuritis in Sprague-Dawley and Lewis rats correlate with different levels of autoreactive T and B cell responses to myelin antigens. J Neurosci Res. 1998;54(3):373–81. doi: 10.1002/(SICI)1097-4547(19981101)54:3<373::AID-JNR8>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 39.Lipscomb HL, Gardner PJ, Sharp JG. The effect of neonatal thymectomy on the induction of autoimmune orchitis in rats. J Reprod Immunol. 1979;1(4):209–17. doi: 10.1016/0165-0378(79)90001-9. [DOI] [PubMed] [Google Scholar]
- 40.Piatier-Tonneau D, Mach PS, Kahan A, Delbarre F. T suppressor lymphocytes regulation of adjuvant arthritis in two inbred strains of rats. Clin Exp Immunol. 1982;49(3):645–51. [PMC free article] [PubMed] [Google Scholar]
- 41.Sado Y, Naito I, Akita M, Okigaki T. Strain specific responses of inbred rats on the severity of experimental autoimmune glomerulonephritis. J Clin Lab Immunol. 1986;19(4):193–9. [PubMed] [Google Scholar]
- 42.Rintisch C, Holmdahl R. DA rats from two colonies differ genetically and in their arthritis susceptibility. Mamm Genome. 2008;19(6):420–8. doi: 10.1007/s00335-008-9125-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Massa PT, ter Meulen V, Fontana A. Hyperinducibility of Ia antigen on astrocytes correlates with strain-specific susceptibility to experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 1987;84(12):4219–23. doi: 10.1073/pnas.84.12.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson D, Yasui D, Seeldrayers P. An analysis of mast cell frequency in the rodent nervous system: numbers vary between different strains and can be reconstituted in mast cell-deficient mice. J Neuropathol Exp Neurol. 1991;50(3):227–34. doi: 10.1097/00005072-199105000-00005. [DOI] [PubMed] [Google Scholar]
- 45.Sun D, Whitaker JN, Wilson DB. Regulatory T cells in experimental allergic encephalomyelitis. III. Comparison of disease resistance in Lewis and Fischer 344 rats. Eur J Immunol. 1999;29(4):1101–6. doi: 10.1002/(SICI)1521-4141(199904)29:04<1101::AID-IMMU1101>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 46.Chung IY, Norris JG, Benveniste EN. Differential tumor necrosis factor alpha expression by astrocytes from experimental allergic encephalomyelitis-susceptible and -resistant rat strains. J Exp Med. 1991;173(4):801–11. doi: 10.1084/jem.173.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cautain B, Damoiseaux J, Bernard I, van Straaten H, van Breda Vriesman P, Boneu B, et al. Essential role of TGF-beta in the natural resistance to experimental allergic encephalomyelitis in rats. Eur J Immunol. 2001;31(4):1132–40. [PubMed] [Google Scholar]
- 48.Markovic M, Miljkovic D, Momcilovic M, Popadic D, Miljkovic Z, Savic E, et al. Strain difference in susceptibility to experimental autoimmune encephalomyelitis in rats correlates with T(H)1 and T(H)17-inducing cytokine profiles. Mol Immunol. 2009;47(1):141–6. doi: 10.1016/j.molimm.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 49.Miljkovic D, Stosic-Grujicic S, Markovic M, Momcilovic M, Ramic Z, Maksimovic-Ivanic D, et al. Strain difference in susceptibility to experimental autoimmune encephalomyelitis between Albino Oxford and Dark Agouti rats correlates with disparity in production of IL-17, but not nitric oxide. J Neurosci Res. 2006;84(2):379–88. doi: 10.1002/jnr.20883. [DOI] [PubMed] [Google Scholar]
- 50.Vukmanovic S, Mostarica Stojkovic M, Lukic ML. Experimental autoimmune encephalomyelitis in “low” and “high” interleukin 2 producer rats. I. Cellular basis of induction. Cell Immunol. 1989;121(2):237–46. doi: 10.1016/0008-8749(89)90022-1. [DOI] [PubMed] [Google Scholar]
- 51.Cowden WB, Cullen FA, Staykova MA, Willenborg DO. Nitric oxide is a potential down-regulating molecule in autoimmune disease: inhibition of nitric oxide production renders PVG rats highly susceptible to EAE. J Neuroimmunol. 1998;88(1-2):1–8. doi: 10.1016/s0165-5728(98)00040-x. [DOI] [PubMed] [Google Scholar]
- 52.Kavelaars A, Heijnen CJ, Tennekes R, Bruggink JE, Koolhaas JM. Individual behavioral characteristics of wild-type rats predict susceptibility to experimental autoimmune encephalomyelitis. Brain Behav Immun. 1999;13(4):279–86. doi: 10.1006/brbi.1998.0534. [DOI] [PubMed] [Google Scholar]
- 53.Villas PA, Dronsfield MJ, Blankenhorn EP. Experimental allergic encephalomyelitis and corticosterone studies in resistant and susceptible rat strains. Clin Immunol Immunopathol. 1991;61(1):29–40. doi: 10.1016/s0090-1229(06)80005-x. [DOI] [PubMed] [Google Scholar]
- 54.Staykova MA, Cowden W, Willenborg DO. Macrophages and nitric oxide as the possible cellular and molecular basis for strain and gender differences in susceptibility to autoimmune central nervous system inflammation. Immunol Cell Biol. 2002;80(2):188–97. doi: 10.1046/j.1440-1711.2002.01072.x. [DOI] [PubMed] [Google Scholar]
- 55.Mason D, MacPhee I, Antoni F. The role of the neuroendocrine system in determining genetic susceptibility to experimental allergic encephalomyelitis in the rat. Immunology. 1990;70(1):1–5. [PMC free article] [PubMed] [Google Scholar]
- 56.Reder AT, Thapar M, Jensen MA. A reduction in serum glucocorticoids provokes experimental allergic encephalomyelitis: implications for treatment of inflammatory brain disease. Neurology. 1994;44(12):2289–94. doi: 10.1212/wnl.44.12.2289. [DOI] [PubMed] [Google Scholar]
- 57.Stepaniak JA, Gould KE, Sun D, Swanborg RH. A comparative study of experimental autoimmune encephalomyelitis in Lewis and DA rats. J Immunol. 1995;155(5):2762–9. [PubMed] [Google Scholar]
- 58.Weissert R. Actively Induced Experimental Autoimmune Encephalomyelitis in Rats. Methods Mol Biol. 2015 doi: 10.1007/7651_2014_177. [DOI] [PubMed] [Google Scholar]
- 59.Stromnes IM, Goverman JM. Passive induction of experimental allergic encephalomyelitis. Nat Protoc. 2006;1(4):1952–60. doi: 10.1038/nprot.2006.284. [DOI] [PubMed] [Google Scholar]
- 60.Stromnes IM, Goverman JM. Active induction of experimental allergic encephalomyelitis. Nat Protoc. 2006;1(4):1810–9. doi: 10.1038/nprot.2006.285. [DOI] [PubMed] [Google Scholar]
- 61.Yasuda T, Tsumita T, Nagai Y, Mitsuzawa E, Ohtani S. Experimental allergic encephalomyelitis (EAE) in mice. I. Induction of EAE with mouse spinal cord homogenate and myelin basic protein. Jpn J Exp Med. 1975;45(5):423–7. [PubMed] [Google Scholar]
- 62.Binder TA, Greiner DL, Grunnet M, Goldschneider I. Relative susceptibility of SJL/J and B10.S mice to experimental allergic encephalomyelitis (EAE) is determined by the ability of prethymic cells in bone marrow to develop into EAE effector T cells. J Neuroimmunol. 1993;42(1):23–32. doi: 10.1016/0165-5728(93)90208-g. [DOI] [PubMed] [Google Scholar]
- 63.Tuohy VK, Sobel RA, Lees MB. Myelin proteolipid protein-induced experimental allergic encephalomyelitis. Variations of disease expression in different strains of mice. J Immunol. 1988;140(6):1868–73. [PubMed] [Google Scholar]
- 64.Hurwitz AA, Sullivan TJ, Sobel RA, Allison JP. Cytotoxic T lymphocyte antigen-4 (CTLA-4) limits the expansion of encephalitogenic T cells in experimental autoimmune encephalomyelitis (EAE)-resistant BALB/c mice. Proc Natl Acad Sci U S A. 2002;99(5):3013–7. doi: 10.1073/pnas.042684699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maron R, Hancock WW, Slavin A, Hattori M, Kuchroo V, Weiner HL. Genetic susceptibility or resistance to autoimmune encephalomyelitis in MHC congenic mice is associated with differential production of pro- and anti-inflammatory cytokines. Int Immunol. 1999;11(9):1573–80. doi: 10.1093/intimm/11.9.1573. [DOI] [PubMed] [Google Scholar]
- 66.Charles PC, Weber KS, Cipriani B, Brosnan CF. Cytokine, chemokine and chemokine receptor mRNA expression in different strains of normal mice: implications for establishment of a Th1/Th2 bias. J Neuroimmunol. 1999;100(1-2):64–73. doi: 10.1016/s0165-5728(99)00189-7. [DOI] [PubMed] [Google Scholar]
- 67.Watanabe H, Numata K, Ito T, Takagi K, Matsukawa A. Innate immune response in Th1- and Th2-dominant mouse strains. Shock. 2004;22(5):460–6. doi: 10.1097/01.shk.0000142249.08135.e9. [DOI] [PubMed] [Google Scholar]
- 68.Andreini I, Getuli C, Pacelli V, Manno R, Ragazzoni E, Nunziata A, et al. Function of the hypothalamo-pituitary-adrenal axis and humoral immune mechanisms during experimental allergic encephalomyelitis in SJL/J mice. Neuroimmunomodulation. 2002;10(1):9–16. doi: 10.1159/000064410. [DOI] [PubMed] [Google Scholar]
- 69.Marin N, Mecha M, Espejo C, Mestre L, Eixarch H, Montalban X, et al. Regulatory lymphocytes are key factors in MHC-independent resistance to EAE. J Immunol Res. 2014;2014:156380. doi: 10.1155/2014/156380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dal Canto MC, Kim BS, Miller SD, Melvold RW. Theiler’s Murine Encephalomyelitis Virus (TMEV)-Induced Demyelination: A Model for Human Multiple Sclerosis. Methods. 1996;10(3):453–61. doi: 10.1006/meth.1996.0123. [DOI] [PubMed] [Google Scholar]
- 71.Lipton HL, Dal Canto MC. Susceptibility of inbred mice to chronic central nervous system infection by Theiler’s murine encephalomyelitis virus. Infect Immun. 1979;26(1):369–74. doi: 10.1128/iai.26.1.369-374.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Melvold RW, Jokinen DM, Knobler RL, Lipton HL. Variations in genetic control of susceptibility to Theiler’s murine encephalomyelitis virus (TMEV)-induced demyelinating disease. I. Differences between susceptible SJL/J and resistant BALB/c strains map near the T cell beta-chain constant gene on chromosome 6. J Immunol. 1987;138(5):1429–33. [PubMed] [Google Scholar]
- 73.Herder V, Gerhauser I, Klein SK, Almeida P, Kummerfeld M, Ulrich R, et al. Interleukin-10 expression during the acute phase is a putative prerequisite for delayed viral elimination in a murine model for multiple sclerosis. J Neuroimmunol. 2012;249(1-2):27–39. doi: 10.1016/j.jneuroim.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 74.Rubio N, Capa L. Differential IL-1 synthesis by astrocytes from Theiler’s murine encephalomyelitis virus-susceptible and -resistant strains of mice. Cell Immunol. 1993;149(2):237–47. doi: 10.1006/cimm.1993.1151. [DOI] [PubMed] [Google Scholar]
- 75.Borrow P, Nash AA. Susceptibility to Theiler’s virus-induced demyelinating disease correlates with astrocyte class II induction and antigen presentation. Immunology. 1992;76(1):133–9. [PMC free article] [PubMed] [Google Scholar]
- 76.Smith ME, Eller NL, McFarland HF, Racke MK, Raine CS. Age dependence of clinical and pathological manifestations of autoimmune demyelination. Implications for multiple sclerosis. Am J Pathol. 1999;155(4):1147–61. doi: 10.1016/S0002-9440(10)65218-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huseby ES, Sather B, Huseby PG, Goverman J. Age-dependent T cell tolerance and autoimmunity to myelin basic protein. Immunity. 2001;14(4):471–81. doi: 10.1016/s1074-7613(01)00127-3. [DOI] [PubMed] [Google Scholar]
- 78.Djikic J, Nacka-Aleksic M, Pilipovic I, Kosec D, Arsenovic-Ranin N, Stojic-Vukanic Z, et al. Age-related changes in spleen of Dark Agouti rats immunized for experimental autoimmune encephalomyelitis. J Neuroimmunol. 2015;278:123–35. doi: 10.1016/j.jneuroim.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 79.Ditamo Y, Degano AL, Maccio DR, Pistoresi-Palencia MC, Roth GA. Age-related changes in the development of experimental autoimmune encephalomyelitis. Immunol Cell Biol. 2005;83(1):75–82. doi: 10.1111/j.1440-1711.2004.01294.x. [DOI] [PubMed] [Google Scholar]
- 80.Endoh M, Rapoport SI, Tabira T. Studies of experimental allergic encephalomyelitis in old mice. J Neuroimmunol. 1990;29(1-3):21–31. doi: 10.1016/0165-5728(90)90144-c. [DOI] [PubMed] [Google Scholar]
- 81.Steiner CM, Rozhon EJ, Lipton HL. Relationship between host age and persistence of Theiler’s virus in the central nervous system of mice. Infect Immun. 1984;43(1):432–4. doi: 10.1128/iai.43.1.432-434.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Djikic J, Nacka-Aleksic M, Pilipovic I, Stojic-Vukanic Z, Bufan B, Kosec D, et al. Age-associated changes in rat immune system: lessons learned from experimental autoimmune encephalomyelitis. Exp Gerontol. 2014;58:179–97. doi: 10.1016/j.exger.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 83.Spach KM, Blake M, Bunn JY, McElvany B, Noubade R, Blankenhorn EP, et al. Cutting edge: the Y chromosome controls the age-dependent experimental allergic encephalomyelitis sexual dimorphism in SJL/J mice. J Immunol. 2009;182(4):1789–93. doi: 10.4049/jimmunol.0803200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dalal M, Kim S, Voskuhl RR. Testosterone therapy ameliorates experimental autoimmune encephalomyelitis and induces a T helper 2 bias in the autoantigen-specific T lymphocyte response. J Immunol. 1997;159(1):3–6. [PubMed] [Google Scholar]
- 85.Bebo BF, Jr., Fyfe-Johnson A, Adlard K, Beam AG, Vandenbark AA, Offner H. Low-dose estrogen therapy ameliorates experimental autoimmune encephalomyelitis in two different inbred mouse strains. J Immunol. 2001;166(3):2080–9. doi: 10.4049/jimmunol.166.3.2080. [DOI] [PubMed] [Google Scholar]
- 86.Jansson L, Holmdahl R. Estrogen-mediated immunosuppression in autoimmune diseases. Inflamm Res. 1998;47(7):290–301. doi: 10.1007/s000110050332. [DOI] [PubMed] [Google Scholar]
- 87.Kappel CA, Melvold RW, Kim BS. Influence of sex on susceptibility in the Theiler’s murine encephalomyelitis virus model for multiple sclerosis. J Neuroimmunol. 1990;29(1-3):15–9. doi: 10.1016/0165-5728(90)90143-b. [DOI] [PubMed] [Google Scholar]
- 88.Hill KE, Pigmans M, Fujinami RS, Rose JW. Gender variations in early Theiler’s virus induced demyelinating disease: differential susceptibility and effects of IL-4, IL-10 and combined IL-4 with IL-10. J Neuroimmunol. 1998;85(1):44–51. doi: 10.1016/s0165-5728(97)00263-4. [DOI] [PubMed] [Google Scholar]
- 89.Hodes GE, Kana V, Menard C, Merad M, Russo SJ. Neuroimmune mechanisms of depression. Nat Neurosci. 2015;18(10):1386–93. doi: 10.1038/nn.4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Painsipp E, Kofer MJ, Sinner F, Holzer P. Prolonged depression-like behavior caused by immune challenge: influence of mouse strain and social environment. PLoS ONE. 2011;6(6):e20719. doi: 10.1371/journal.pone.0020719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Browne CA, O’Brien FE, Connor TJ, Dinan TG, Cryan JF. Differential lipopolysaccharide-induced immune alterations in the hippocampus of two mouse strains: effects of stress. Neuroscience. 2012;225:237–48. doi: 10.1016/j.neuroscience.2012.08.031. [DOI] [PubMed] [Google Scholar]
- 92.Li Z, Ma L, Kulesskaya N, Voikar V, Tian L. Microglia are polarized to M1 type in high-anxiety inbred mice in response to lipopolysaccharide challenge. Brain Behav Immun. 2014;38:237–48. doi: 10.1016/j.bbi.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 93.Kunze A, Zierath D, Drogomiretskiy O, Becker K. Strain differences in fatigue and depression after experimental stroke. Transl Stroke Res. 2014;5(5):604–11. doi: 10.1007/s12975-014-0350-1. [DOI] [PubMed] [Google Scholar]
- 94.Kunze A, Zierath D, Drogomiretskiy O, Becker K. Variation in behavioral deficits and patterns of recovery after stroke among different rat strains. Transl Stroke Res. 2014;5(5):569–76. doi: 10.1007/s12975-014-0337-y. [DOI] [PubMed] [Google Scholar]
- 95.Dittmar MS, Vatankhah B, Fehm NP, Schuierer G, Bogdahn U, Horn M, et al. Fischer-344 rats are unsuitable for the MCAO filament model due to their cerebrovascular anatomy. J Neurosci Methods. 2006;156(1-2):50–4. doi: 10.1016/j.jneumeth.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 96.Ericsson AC, Davis JW, Spollen W, Bivens N, Givan S, Hagan CE, et al. Effects of vendor and genetic background on the composition of the fecal microbiota of inbred mice. PLoS ONE. 2015;10(2):e0116704. doi: 10.1371/journal.pone.0116704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4615–22. doi: 10.1073/pnas.1000082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139(3):485–98. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci U S A. 2010;107(27):12204–9. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wu HJ, Ivanov II, Darce J, Hattori K, Shima T, Umesaki Y, et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32(6):815–27. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Berer K, Mues M, Koutrolos M, Rasbi ZA, Boziki M, Johner C, et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 2011;479(7374):538–41. doi: 10.1038/nature10554. [DOI] [PubMed] [Google Scholar]
- 102.Baker AM, Grekova MC, Richert JR. EAE susceptibility in FVB mice. J Neurosci Res. 2000;61(2):140–5. doi: 10.1002/1097-4547(20000715)61:2<140::AID-JNR4>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 103.Kim HA, Whittle SC, Lee S, Chu HX, Zhang SR, Wei Z, et al. Brain immune cell composition and functional outcome after cerebral ischemia: comparison of two mouse strains. Front Cell Neurosci. 2014;8:365. doi: 10.3389/fncel.2014.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Prass K, Meisel C, Hoflich C, Braun J, Halle E, Wolf T, et al. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J Exp Med. 2003;198(5):725–36. doi: 10.1084/jem.20021098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schulte-Herbruggen O, Klehmet J, Quarcoo D, Meisel C, Meisel A. Mouse strains differ in their susceptibility to poststroke infections. Neuroimmunomodulation. 2006;13(1):13–8. doi: 10.1159/000092109. [DOI] [PubMed] [Google Scholar]
- 106.Gingles NA, Alexander JE, Kadioglu A, Andrew PW, Kerr A, Mitchell TJ, et al. Role of genetic resistance in invasive pneumococcal infection: identification and study of susceptibility and resistance in inbred mouse strains. Infect Immun. 2001;69(1):426–34. doi: 10.1128/IAI.69.1.426-434.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wong CH, Jenne CN, Lee WY, Leger C, Kubes P. Functional innervation of hepatic iNKT cells is immunosuppressive following stroke. Science. 2011;334(6052):101–5. doi: 10.1126/science.1210301. [DOI] [PubMed] [Google Scholar]
- 108.Paegelow I, Werner H. Influence of adrenergic agonists and antagonists on lymphokine secretion in vitro. Int J Immunopharmacol. 1987;9(7):761–8. doi: 10.1016/0192-0561(87)90071-3. [DOI] [PubMed] [Google Scholar]
- 109.Ottaway CA, Husband AJ. Central nervous system influences on lymphocyte migration. Brain Behav Immun. 1992;6(2):97–116. doi: 10.1016/0889-1591(92)90011-c. [DOI] [PubMed] [Google Scholar]
- 110.Lyte M, Ernst S, Driemeyer J, Baissa B. Strain-specific enhancement of splenic T cell mitogenesis and macrophage phagocytosis following peripheral axotomy. J Neuroimmunol. 1991;31(1):1–8. doi: 10.1016/0165-5728(91)90080-q. [DOI] [PubMed] [Google Scholar]
- 111.Avakian EV, Horvath SM, Colburn RW. Influence of age and cold stress on plasma catecholamine levels in rats. J Auton Nerv Syst. 1984;10(2):127–33. doi: 10.1016/0165-1838(84)90051-1. [DOI] [PubMed] [Google Scholar]
- 112.McCarty R, Kirby RF, Garn PG. Strain differences in sympathetic-adrenal medullary responsiveness and behavior. Behav Neural Biol. 1984;40(1):98–113. doi: 10.1016/s0163-1047(84)90206-1. [DOI] [PubMed] [Google Scholar]
- 113.Warren HS, Fitting C, Hoff E, Adib-Conquy M, Beasley-Topliffe L, Tesini B, et al. Resilience to bacterial infection: difference between species could be due to proteins in serum. J Infect Dis. 2010;201(2):223–32. doi: 10.1086/649557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zschaler J, Schlorke D, Arnhold J. Differences in innate immune response between man and mouse. Crit Rev Immunol. 2014;34(5):433–54. [PubMed] [Google Scholar]
- 115.Taveira da Silva AM, Kaulbach HC, Chuidian FS, Lambert DR, Suffredini AF, Danner RL. Brief report: shock and multiple-organ dysfunction after self-administration of Salmonella endotoxin. N Engl J Med. 1993;328(20):1457–60. doi: 10.1056/NEJM199305203282005. [DOI] [PubMed] [Google Scholar]
- 116.Sauter C, Wolfensberger C. Interferon in human serum after injection of endotoxin. Lancet. 1980;2(8199):852–3. doi: 10.1016/s0140-6736(80)90189-0. [DOI] [PubMed] [Google Scholar]
- 117.Rose WC, Bradley SG. Enhanced toxicity for mice of combinations of antibiotics with Escherichia coli cells or Salmonella typhosa endotoxin. Infect Immun. 1971;4(5):550–5. doi: 10.1128/iai.4.5.550-555.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Glode LM, Mergenhagen SE, Rosenstreich DL. Significant contribution of spleen cells in mediating the lethal effects of endotoxin in vivo. Infect Immun. 1976;14(3):626–30. doi: 10.1128/iai.14.3.626-630.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Copeland S, Warren HS, Lowry SF, Calvano SE, Remick D. Acute inflammatory response to endotoxin in mice and humans. Clin Diagn Lab Immunol. 2005;12(1):60–7. doi: 10.1128/CDLI.12.1.60-67.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Moeller GR, Terry L, Snyderman R. The inflammatory response and resistance to endotoxin in mice. J Immunol. 1978;120(1):116–23. [PubMed] [Google Scholar]
- 121.Arbour NC, Lorenz E, Schutte BC, Zabner J, Kline JN, Jones M, et al. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat Genet. 2000;25(2):187–91. doi: 10.1038/76048. [DOI] [PubMed] [Google Scholar]
- 122.Henckaerts L, Nielsen KR, Steffensen R, Van Steen K, Mathieu C, Giulietti A, et al. Polymorphisms in innate immunity genes predispose to bacteremia and death in the medical intensive care unit. Crit Care Med. 2009;37(1):192–201. e1–3. doi: 10.1097/CCM.0b013e31819263d8. [DOI] [PubMed] [Google Scholar]
- 123.Kumpf O, Giamarellos-Bourboulis EJ, Koch A, Hamann L, Mouktaroudi M, Oh DY, et al. Influence of genetic variations in TLR4 and TIRAP/Mal on the course of sepsis and pneumonia and cytokine release: an observational study in three cohorts. Crit Care. 2010;14(3):R103. doi: 10.1186/cc9047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ferwerda B, McCall MB, Verheijen K, Kullberg BJ, van der Ven AJ, Van der Meer JW, et al. Functional consequences of toll-like receptor 4 polymorphisms. Mol Med. 2008;14(5-6):346–52. doi: 10.2119/2007-00135.Ferwerda. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Miyake Y, Yamasaki S. Sensing necrotic cells. Adv Exp Med Biol. 2012;738:144–52. doi: 10.1007/978-1-4614-1680-7_9. [DOI] [PubMed] [Google Scholar]
- 126.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81(1):1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 127.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2013;110(9):3507–12. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Doeing DC, Borowicz JL, Crockett ET. Gender dimorphism in differential peripheral blood leukocyte counts in mice using cardiac, tail, foot, and saphenous vein puncture methods. BMC Clin Pathol. 2003;3(1):3. doi: 10.1186/1472-6890-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Haley PJ. Species differences in the structure and function of the immune system. Toxicology. 2003;188(1):49–71. doi: 10.1016/s0300-483x(03)00043-x. [DOI] [PubMed] [Google Scholar]
- 130.Sharar SR, Winn RK, Harlan JM. The adhesion cascade and anti-adhesion therapy: an overview. Springer Semin Immunopathol. 1995;16(4):359–78. doi: 10.1007/BF00196093. [DOI] [PubMed] [Google Scholar]
- 131.Becker K, Kindrick D, Relton J, Harlan J, Winn R. Antibody to the alpha4 integrin decreases infarct size in transient focal cerebral ischemia in rats. Stroke. 2001;32(1):206–11. doi: 10.1161/01.str.32.1.206. [DOI] [PubMed] [Google Scholar]
- 132.Relton JK, Sloan KE, Frew EM, Whalley ET, Adams SP, Lobb RR. Inhibition of alpha4 integrin protects against transient focal cerebral ischemia in normotensive and hypertensive rats. Stroke. 2001;32(1):199–205. doi: 10.1161/01.str.32.1.199. [DOI] [PubMed] [Google Scholar]
- 133.Liesz A, Zhou W, Mracsko E, Karcher S, Bauer H, Schwarting S, et al. Inhibition of lymphocyte trafficking shields the brain against deleterious neuroinflammation after stroke. Brain. 2011;134(Pt 3):704–20. doi: 10.1093/brain/awr008. [DOI] [PubMed] [Google Scholar]
- 134.Langhauser F, Kraft P, Gob E, Leinweber J, Schuhmann MK, Lorenz K, et al. Blocking of alpha4 integrin does not protect from acute ischemic stroke in mice. Stroke. 2014;45(6):1799–806. doi: 10.1161/STROKEAHA.114.005000. [DOI] [PubMed] [Google Scholar]
- 135.Kiselycznyk C, Holmes A. All (C57BL/6) Mice are not Created Equal. Front Neurosci. 2011;5:10. doi: 10.3389/fnins.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mekada K, Abe K, Murakami A, Nakamura S, Nakata H, Moriwaki K, et al. Genetic differences among C57BL/6 substrains. Exp Anim. 2009;58(2):141–9. doi: 10.1538/expanim.58.141. [DOI] [PubMed] [Google Scholar]
- 137.Matsuo N, Takao K, Nakanishi K, Yamasaki N, Tanda K, Miyakawa T. Behavioral profiles of three C57BL/6 substrains. Front Behav Neurosci. 2010;4:29. doi: 10.3389/fnbeh.2010.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Llovera G, Hofmann K, Roth S, Salas-Perdomo A, Ferrer-Ferrer M, Perego C, et al. Results of a preclinical randomized controlled multicenter trial (pRCT): Anti-CD49d treatment for acute brain ischemia. Sci Transl Med. 2015;7(299):299ra121. doi: 10.1126/scitranslmed.aaa9853. [DOI] [PubMed] [Google Scholar]
- 139.Banerjee A, Wang J, Bodhankar S, Vandenbark AA, Murphy SJ, Offner H. Phenotypic changes in immune cell subsets reflect increased infarct volume in male vs. female mice. Transl Stroke Res. 2013;4(5):554–63. doi: 10.1007/s12975-013-0268-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Issekutz TB, Miyasaka M, Issekutz AC. Rat blood neutrophils express very late antigen 4 and it mediates migration to arthritic joint and dermal inflammation. J Exp Med. 1996;183(5):2175–84. doi: 10.1084/jem.183.5.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pereira S, Zhou M, Mocsai A, Lowell C. Resting Murine Neutrophils Express Functional alpha(4) Integrins that Signal Through Src Family Kinases. J Immunol. 2001;166(6):4115–23. doi: 10.4049/jimmunol.166.6.4115. [DOI] [PubMed] [Google Scholar]
- 142.Neumann J, Riek-Burchardt M, Herz J, Doeppner TR, Konig R, Hutten H, et al. Very-late-antigen-4 (VLA-4)-mediated brain invasion by neutrophils leads to interactions with microglia, increased ischemic injury and impaired behavior in experimental stroke. Acta Neuropathol. 2015;129(2):259–77. doi: 10.1007/s00401-014-1355-2. [DOI] [PubMed] [Google Scholar]
- 143.Walsh GM, Mermod JJ, Hartnell A, Kay AB, Wardlaw AJ. Human eosinophil, but not neutrophil, adherence to IL-1-stimulated human umbilical vascular endothelial cells is alpha 4 beta 1 (very late antigen-4) dependent. J Immunol. 1991;146(10):3419–23. [PubMed] [Google Scholar]
- 144.Bochner BS, Luscinskas FW, Gimbrone MA, Jr., Newman W, Sterbinsky SA, Derse-Anthony CP, et al. Adhesion of human basophils, eosinophils, and neutrophils to interleukin 1-activated human vascular endothelial cells: contributions of endothelial cell adhesion molecules. J Exp Med. 1991;173(6):1553–7. doi: 10.1084/jem.173.6.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172(5):2731–8. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 146.Mann ER, Landy JD, Bernardo D, Peake ST, Hart AL, Al-Hassi HO, et al. Intestinal dendritic cells: their role in intestinal inflammation, manipulation by the gut microbiota and differences between mice and men. Immunol Lett. 2013;150(1-2):30–40. doi: 10.1016/j.imlet.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 147.Sharp FR, Jickling GC. Modeling immunity and inflammation in stroke: differences between rodents and humans? Stroke. 2014;45(9):e179–80. doi: 10.1161/STROKEAHA.114.005639. [DOI] [PMC free article] [PubMed] [Google Scholar]