Abstract
Background & Aims
Eosinophilic esophagitis (EoE) is an immune-mediated allergic disease characterized by progressive esophageal dysmotility and fibrotic stricture associated with chronic esophageal fibroblast activation. It remains unknown how esophageal fibroblasts respond to EoE-relevant matrix stiffness or inflammatory cytokines.
Methods
Immunofluorescence was used to evaluate α-smooth muscle actin (α-SMA) expression in endoscopic esophageal biopsies. Primary esophageal fibroblasts from adult and pediatric patients with or without EoE were exposed to TGFβ to determine gene expression, collagen-matrix contractility, and cytoskeletal organization. The influence of matrix stiffness upon fibroblast behavior was assessed on the engineered surface of polyacrylamide gels with varying stiffness. Fibroblast traction forces were measured using microfabricated-Post-Array-Detectors.
Results
EoE esophageal fibroblasts had enhanced α-SMA expression. TGFβ stimulated not only enhanced fibroblast-specific gene expression, but also promoted fibroblast-mediated collagen-matrix contraction, despite disease state or age of patients as the origin of cells. Unlike conventional monolayer cell culture conditions using plastic surface (1 GPa) that activate fibroblasts constitutively, our engineered platforms recapitulating physiologically relevant stiffness (1-20 kPa) revealed that matrix stiffness defines the extent of α-SMA expression, intracellular collagen fibril organization, SMAD3 phosphorylation and fibroblast traction force.
Conclusions
Matrix stiffness may critically influence TGFβ-mediated gene expression and functions of esophageal fibroblasts ex vivo independent of age and disease conditions. These findings provide a novel insight into the pathogenesis of fibrostenotic disease in EoE.
Introduction
Eosinophilic esophagitis (EoE) is a chronic inflammatory disorder that affects people of all ages. It is presumed that exposure to food antigens and seasonal allergens produce an inflammatory response within the esophagus characterized by infiltrating immune cells including eosinophils, mast cells, and basophils 1,2. This robust immune response underlies tissue remodeling in the subepithelial matrix compartment, leading to fibrosis and progressive esophageal dysfunction manifested by dysphagia, recurrent food impactions, and esophageal stricture 3,4,5.
Interestingly, the symptoms of EoE depend on the age at diagnosis. In the pediatric population, younger children usually present with feeding aversion and vomiting, whereas teenagers are more likely to present with food impaction and dysphagia6. The duration of symptoms prior to diagnosis correlates strongly with stricture formation7. Patients with a shorter duration of disease prior to diagnosis are more likely to have mucosal findings consistent with inflammatory changes such as furrows, edema and exudates. Those with long-standing untreated disease have a significantly increased likelihood of stricture.
Fibroblast behavior changes during maturation. In vivo studies have shown that early fetal wounds heal without scar formation while late fetal wounds heal with scars8,9,10,11. Furthermore, fetal fibroblasts have enhanced migratory behaviors and decreased cytokine production compared to adult fibroblasts in vitro. There have been no studies examining fibroblast activation and myofibroblast transdifferentiation during the transition from childhood to adulthood, and how this transition is affected by the disease state is completely unknown.
Esophageal stricture is the most devastating complication of EoE resulting from esophageal fibrosis. Fibrosis in the healthy state allows for tissue homeostasis and wound healing; however, in the pathologic state, excessive extracellular matrix deposition leads to organ stiffness and dysfunction. The key effector cells in fibrosis are activated myofibroblasts. These cells, defined by the de novo expression of α-smooth muscle actin (α-SMA), contract and produce collagens, elastins, and fibronectin, which contribute to overall stromal stiffness.
The tissue microenvironment influences fibroblast activation. Transforming growth factor-β (TGFβ) is a key pro-fibrotic cytokine. Importantly, TGFβ has been proposed as the major driving force behind fibroblast activation and tissue remodeling in EoE. In patients with EoE, there is a simultaneous increase in collagen deposition and expression of TGFβ and its downstream transcription factor pSMAD3 in the lamina propria 12. Furthermore, pSMAD3 deficient mice with EoE have attenuated fibrosis, reinforcing the importance of the canonical TGFβ pathway in EoE disease progression 13.
Little is known about how the mechanical environment affects fibroblast activation in the esophagus. EoE patients with food impactions have decreased esophageal distensibility, suggesting increased tissue stiffness14. Crohn-associated strictures in the small intestine have been shown to have a 6-fold increase in tissue stiffness compared with normal tissue15. Fibroblasts in vitro have been shown to be activated in a stiff environment16,17. Substrate stiffness leads to increased spindle morphology as well as increased collagen and α-SMA production even in the absence of proinflammatory cytokines. Thus it is critical to characterize the differences in esophageal fibroblast activities with both the chemical and mechanical stressors associated with EoE.
We investigated how esophageal fibroblasts isolated from pediatric and adult subjects with or without EoE respond to TGFβ stimulation and a stiff environment. We identified matrix stiffness as a key determinant of TGFβ mediated fibroblast activation in vitro despite age and disease state.
Materials and Methods
Human subjects
Following informed consent, esophageal pinch biopsies were obtained from the mid-distal esophagus at The Children's Hospital of Philadelphia (pediatric patients) and the Hospital of the University of Pennsylvania (adult patients)(Table 1) during routine diagnostic esophagogastroduodenoscopy (EGD) under human subjects protocols approved by each institute's respective Institutional Review Board. Active EoE was diagnosed histologically by the presence of 15 or more esophageal eosinophils per high-powered field (hpf) and the absence of tissue eosinophilia in the distal gastrointestinal tract. Subjects defined as ‘normal’ were those undergoing EGD for symptoms of abdominal pain, reflux, and dysphagia who had no histopathologic findings. Subjects defined as inactive EoE were previously diagnosed with EoE according to the most recent consensus guidlelines and then on a subsequent scope were found to have <15 eosinophils per high power field. All subjects were on high-dose proton pump inhibitor (PPI) therapy for at least 6 weeks prior to biopsy.
Table 1. Profile of patients from whom primary fibroblasts were derived.
| Pediatric (n=14) | Adult (n=16) | |||
|---|---|---|---|---|
| Normal (n=6) | EoE (n=8) | Normal (n=4) | EoE (n=12) | |
| Age (mean ± SD) * | 10.9 ± 1.6 | 8.9 ± 1.0 | 35.5 ± 8.7 | 31.0 ± 3.1 |
| Male (n, percent) | 3 (50%) | 7 (88%) | 2 (50%) | 8 (67%) |
| Sx dysphagia (n, %) | 4 (57%) | 2 (25%) | 1 (25%) | 6 (67%) |
| Sx regurgitation (n, %) | 1 (14%) | 3 (38%) | 0 (0%) | 0 (0%) |
| Hx impaction (n, %) | 1 (14%) | 2 (25%) | 0 (0%) | 7 (78%) |
| Hx stricture (n, %) | 0 (0%) | 1 (13%) | 0 (0%) | 4 (44%) |
| EGD furrow (n, %) | 1 (14%) | 2 (25%) | 0 (0%) | 5 (56%) |
| EGD rings (n, %) | 0 (0%) | 0 (0%) | 0 (0%) | 5 (56%)* |
| EGD microabcesses (n, %) | 0 (0%) | 1 (13%) | 0 (0%) | 4 (44%) |
p<0.05
Primary esophageal fibroblast culture
To isolate esophageal fibroblasts, one endoscopic biopsy per patient was collected in full Dulbecco's Minimum Essential Media (DMEM) containing 10% fetal bovine serum (FBS) and penicillin (100 units/mL) and streptomycin (100ug/mL)(Life Technologies, Carlsbad, CA). Within 30 min, the tissue was transferred into Dulbecco's Phosphate-Buffered Saline (DPBS)(Life Technologies) containing 50 U/ml Dispase (BD Biosciences, San Jose, CA) and incubated at 37°C for 20 min without agitation. Tissues were then transferred into 3 ml of 0.05% Trypsin-EDTA (Life Technologies) and incubated at 37°C for 10 min with gentle manual shaking every 2 min. After adding an equal volume of soybean trypsin inhibitor (Sigma-Aldrich, St. Louis, MO), tissues were further dissociated by 10 strokes of gentle pipetting and centrifuged at 1,200 rpm at 4°C for 5 min to collect cell pellets. Cells were resuspended in full DMEM containing Fungizone® (1:500)(Life Technologies), seeded into a single well (per biopsy) of a 6-well dish (Thermo Fisher Scientific, Philadelphia, PA), and placed in a humidified incubator (Thermo Fisher Scientific) at 37°C and 5% CO2. 24 hours later, medium was replaced with full DMEM without Fungizone® and cells were grown for 7-14 days until cell density reached 70-80% confluence. Cells were then split into two 75-cm2 cell culture flasks (Passage 1). A subset of cells at passage 1 were stained with vimentin, to ensure that there was no epithelial cell contamination (data not shown). All experiments were performed using cells between passages 2-8.
RNA isolation and quantitative RT-PCR
Cells were seeded in 24-well plates and stimulated in triplicate with or without 10 ng/mL recombinant human (rh) TGFβ1 for 1 week. Total RNA was purified using an RNeasy kit (Qiagen, Valencia, CA) according to manufacturer's instructions and quantitated by spectrophotometry with NanoDrop 2000c (Thermo Fisher Scientific). RNA was reverse transcribed using a high-capacity cDNA reverse transcriptase kit (Applied Biosystems, Foster City, CA) and subjected to TaqMan Gene Expression Assays (Applied Biosystems) for type I collagen (COL1A1, Hs00164004), α-SMA (ACTA2, Hs00426835), fibronectin (FN1, Hs01549940), and Glyceraldehyde 3-phosphate dehydrogenase (GAPDH, 4352665). PCR reaction was carried out in triplicate using TaqMan Fast Universal PCR Master Mix kit (Applied Biosystems) and 96-well optical plates on the StepOnePlus Real-Time PCR System (Applied Biosystems). The relative expression level of each mRNA transcript was normalized to GAPDH as an internal control as described previously18.
Western blotting
Cells were seeded in 6-well plates and stimulated in triplicate with or without 10 ng/mL rhTGFβ1 for 1 week and lysed with 1X Cell Lysis Buffer (20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM b-glycerophosphate, 1 mM Na3 VO4 and 1 μg/ml leupeptin)(Cell Signaling Technology, Danvers, MA) supplemented with Complete Protease Inhibitor Cocktail (Roche Applied Science, Mannheim, Germany). Protein concentration was determined by BCA assay (Thermo Scientific, Rockford, IL). Twenty ×g of denatured protein was fractionated on a NuPAGE Bis-Tris 4–12% gel (Life Technologies). Following electrotransfer, Immobilon-P membranes (Millipore, Billerica, MA, USA) were incubated with primary antibodies for type I collagen (1:1500; rabbit polyclonal anti-collagen I ab292; Abcam, Cambridge, MA) and β-actin (1:5000 mouse monoclonal anti-β-actin AC-74; Sigma-Aldrich), and then with the appropriate horseradish peroxidase-conjugated secondary antibody (GE Healthcare, Piscataway, NJ, USA). β-Actin served as a loading control. The signal was visualized by an enhanced chemiluminescence solution (ECL Plus; Life Technologies) and exposed to Eastman Kodak Co. (Rochester, NY) X-OMAT LS film. Western blots were quantified by densitometry with ImageJ software (NIH).
Contraction assays
Matrix contraction assays were performed as described previously19. In brief, fibroblasts were mixed with 54% bovine collagen (Organogenesis) and 18% Matrigel (BD Biosciences) in Eagle's Minimum Essential Medium (BioWhittaker, Walkersville, MD) supplemented with 9.4% FBS, 0.8 μM L-glutamine (Cellgro, Manassas, VA), 1.8 mM sodium bicarbonate (Life Technologies) and dispensed into 24-well plates (6×105 cells/0.5 mL gel per well). The gels were allowed to solidify for 90 minutes before detaching them from the surface of the cell culture plate. Floating gels were assessed in duplicate both with and without 10 ng/mL rhTGFβ1-stimulation. The diameter of each gel was measured daily for 4 days.
Fibroblast culture on polyacrylamide gels
To grow fibroblasts in the context of physiologically relevant matrix stiffness, polyacrylamide gel-based platforms with variable stiffness were prepared as described previously17. In brief, polyacrylamide gels with 125 μm thickness were generated on 25 mm-round glass coverslips (Bellco Glass, Vineland, NJ) to the final concentrations of 25.4-38.5% polyacrylamide by varying the amount of 2% bis-acrylamide (Thermo Fisher Scientific) in a mixture of 30% acrylamide (Alfa Aesar, Ward Hill, MA), TEMED (BioRad, Philadelphia, PA), and 10% ammonium persulfate (Sigma) in 250mM HEPES, pH 8 (Thermo Fisher Scientific), resulting in the generation of gels with stiffness ranging from 1 kPa to 12 kPa. The polyacrylamide gel surface was coated with a thin layer of type I collagen (0.1 mg/ml) (Organogenesis, Canton MA) following UV light activation of a cross-linker Sulfo-SANPAH (Pierce, Rockford, IL) to allow cell adherence. Fibroblasts (5×104 cells per gel) were seeded on top of the polyacrylamide gel placed in 6-well dishes and grown for 4 days with full DMEM in the presence or absence of 10 ng/mL rhTGFβ1.Cells were imaged at day 4 on a Nikon Eclipse TS-100 microscope (Tokyo, Japan) using Metavue software. Perimeter measurements were calculated using ImageJ (NIH) by tracing individual cells. At least 3 cells were used for each condition.
Immunofluorescence
Fibroblasts on polyacrylamide gels were fixed in 4% formaldehyde (Sigma-Aldrich) for 15 minutes at room temperature and washed 3 times in phosphate buffered saline (PBS)(Life Technologies). Staining of biopsies or gels was then carried out as previously described18 with primary antibodies: anti-α-SMA antibody (1:400, mouse monoclonal anti-α-smooth muscle actin; Sigma-Aldrich) and phospho-Smad3Ser423/425 (pSMAD3)(1:100, rabbit monoclonal antibody clone C25A9, Cell Signaling Technology). Secondary antibodies, Cy3-conjugated secondary rabbit anti-mouse or donkey anti-rabbit antibody were used respectively (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) at a 1:600 dilution for 30 minutes at room temperature. Counterstaining was done with Vectashield® mounting medium with 4′,6-diamidino-2-phenylindole (DAPI)(Vector Laboratories, Burlingame, CA). Images were captured on an Olympus BX51 microscope and imaged with a digital camera at 200× (biopsies) and 400× (poly acrylamide gels) and relative signal intensity was analyzed using ImageJ software. For α-SMA, relative intensity was measured by multiplying the average intensity by the area stained and dividing by the number of cells. For p-SMAD3, average intensity within individual nuclei was measured.
For human tissue staining, biopsy specimens were collected as above. Specimens were fixed in paraffin, sectioned, and stained with hematoxylin and eosin. Patients were determined to be either normal, active, or inactive by pediatric and adult pathologist in accordance with the 2011 EoE guidelines20. Once this determination was made, two samples with lamina propria were selected for staining against α-SMA for each group. Antibody and concentration used were the same as described above.
Microfabricated-Post-Array- Detectors (mPADs)
mPADs were fabricated as detailed by Yang et al 21. Silicon masters were generously provided by Professor Christopher S. Chen. Fibronectin printed mPADs were seeded with primary esophageal fibroblasts at 2×104 cells per Attofluor chamber (Life Technologies). Cells were imaged 22-24 hours post-seeding on a spinning disk laser confocal Olympus IX71 inverted microscope fitted with a LCI Chamlide stagetop incubation system using a Hamamatsu ImagEM EMCCD camera and Metamorph software. Fluorescent images focused on the plane of post tips were processed via a series of custom MATLAB (The MathWorks, Natick, MA) scripts. These scripts identified fluorescently labeled post centroids, connected centroids in consecutive frames to form trajectories, removed the drift in position from the trajectories, and positioned them relative to their undeflected resting lattice locations. Post spring constants (kspring) were corrected for substrate warping22. An effective substrate Young's modulus (Eeff) was computed using the model of Ladoux and coworkers23. For additional details on the mPAD platform, please see the Supplementary Methods.
Statistical Analysis
Demographic data were analyzed with student's t test (age), Mann-Whitney (among age groups), and Kruskal-Wallis (among age and disease groups) as indicated. Data from qPCR were analyzed using two-way ANOVA. Simple effects testing was done using both Sidak and Tukey analyses of variance at an alpha level of 0.05. Contraction assay data within each phenotype were analyzed at Day 4 using t-tests. Multiple t-tests corrected using the Sidak method were used to analyze contraction across phenotypes. Perimeter and staining intensity on polyacrylamide gels were analyzed for trend using linear regression. mPADs data were analyzed by two-tailed Student's t test and 1-way ANOVA. All results are reported as means ± standard error about the mean. P < 0.05 was considered significant.
Results
Profile of patients from whom primary fibroblasts were derived
Demographic data from each primary cell line was compiled in Table 1. Mean age was significantly different between pediatric and adult patients (p<0.0001). In addition, evidence of rings upon EGD was significantly higher in adults than in children (p=0.045), and specifically higher in adults with disease than in children with disease (p<0.05). There was no statistical difference between pediatric and adult populations in any of the other symptoms, demographics or endoscopy findings.
EoE biopsies show evidence for subepithelial fibrosis and myofibroblast activation
Subepithelial fibrosis is known to occur in EoE, as evidenced by the severe clinical findings of food impaction and stricture12. However, myofibroblast activation in the subepithelial compartment has not been previously documented. We first carried out immunofluorescence for α-SMA, a marker of myofibroblast activation, in endoscopic esophageal biopsies containing sufficient subepithelial stromal components for evaluation. Interestingly, the pediatric and adult active EoE patients (1 B,E) showed upregulation of subepithelial αSMA expression compared to the pediatric and adult non-EoE normal controls (Figure 1A-D). Interestingly, we found that the inactive pediatric patients (Figure 1C) had both resolution of eosinophilia and stromal activation. However, the inactive adult EoE patients had continued myofibroblast activation in the subepithelial compartment (Figure 1F), despite resolved eosinophilia.
Figure 1. Subepithelial activation of fibroblasts in EoE.
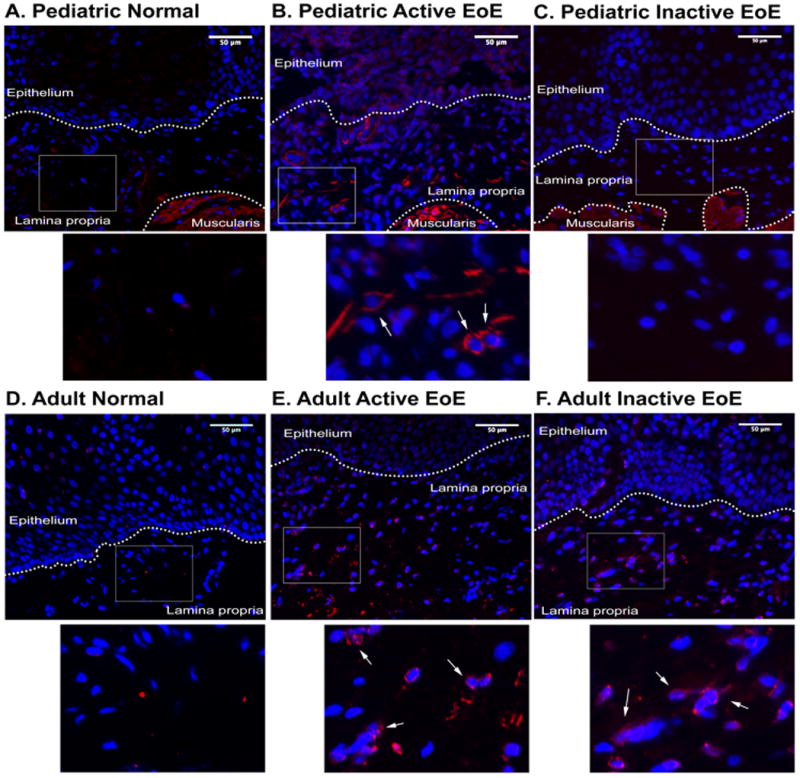
Immunofluorescent staining of human esophageal biopsies from pediatric control (A), active EoE (B), and inactive EoE patients (C). Adult biopsies of control (D), active EoE (E), and inactive EoE patients (F) (N=2 for each group). Biopsy specimens are counterstained with DAPI (blue). α-SMA-positive fibroblasts indicated in insert with white arrows. α-SMA-expressing smooth muscle cells provide a positive control. Scale bar is 50μm. Histogram representing relative intensity per area of lamina propria (G).
Primary esophageal fibroblasts from adult subjects have enhanced TGFβ-induced type I collagen expression compared to pediatric fibroblasts
Adult EoE patients are known to have more symptoms suggestive of esophageal fibrosis than pediatric EoE patients 6, which is supported by our clinical data in our patient cohorts (Table 1). Because adult EoE esophageal fibroblasts remained activated despite resolution of inflammation (Figure 1F) we hypothesized that fibroblasts from adult and pediatric subjects would exhibit differential behavior in response to activation ex vivo. TGFβ stimulates production of extracellular matrix proteins in primary human esophageal fibroblasts5. We therefore stimulated primary fibroblasts with rhTGFβ1 for 7 days and quantified mRNA expression for α-SMA (ACTA2), fibronectin (FN1), and type I collagen (COL1A1) using fibroblasts from four different subject cohorts: adult control, adult EoE, pediatric control, and pediatric EoE. Despite increased numbers of activated myofibroblasts in biopsies from EoE patients (Figure 1), primary culture showed no statistically significant difference in the basal expression of α-SMA, fibronectin, or type I collagen genes among the four groups tested (Figure 2A-C). Similarly, stimulation with TGFβ significantly induced expression of α-SMA and fibronectin, to a similar degree in all four phenotypes. Interestingly, however, TGFβ induced significantly more type 1 collagen mRNA expression in adult fibroblasts (both EoE and control adult fibroblasts) than pediatric fibroblasts (Figure 2C). Lastly, TGFβ-induced type 1 collagen mRNA was further corroborated by type I collagen expression at the protein level in whole cell lysates (Figure 2D).
Figure 2. TGFβ enhances expression of relevant markers of fibroblast activation.
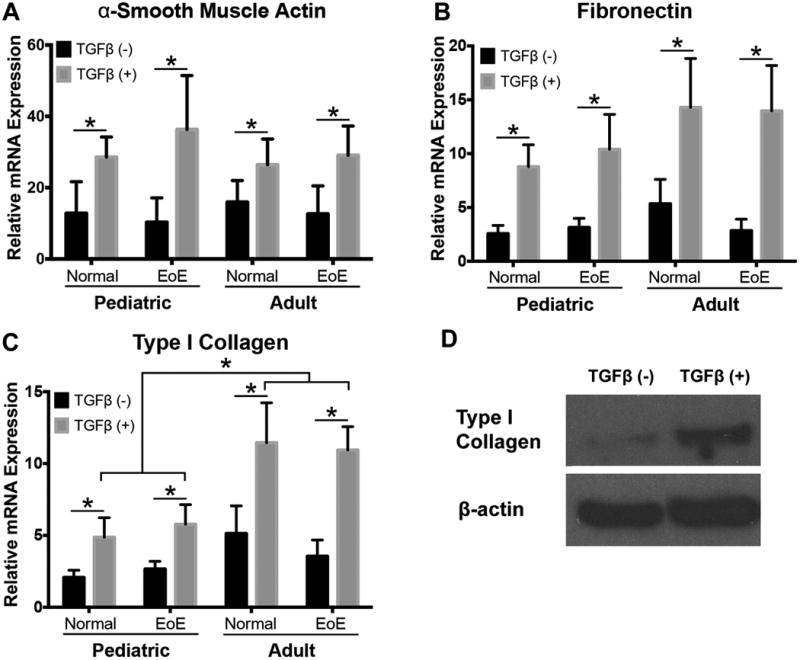
PCR was performed to evaluate markers of fibroblast activation in human esophageal fibroblast cultures. Constitutive expression of α-SMA is similar across phenotypes and increases upon stimulation with TGFβ (A)(N=5, 7, 3, 7 cell cultures respectively) (main effect of TGFβ p=0.0254). Fibronectin expression does not vary at baseline, but is significantly increased with TGFβ stimulation (B) (N=5, 7, 3, 7)(main effect of TGFβ p=0.0001). Constitutive expression of collagen is similar across all phenotypes and TGFβ enhances expression of type 1 collagen in all cell cultures (main effect of TGFβ p<0.0001). Adult derived cell cultures (both EoE and control cell lines) have enhanced response to TGFβ stimulation compared to cells from pediatric patients (C) (n=6, 7, 4, 7) (p<0.05). Increased expression of type I collagen confirmed by Western blot (D), representative picture from normal pediatric primary culture out of total n=4 performed. * p<0.05.
TGFβ enhances esophageal fibroblast contractility in collagen gels
Having characterized TGFβ effects at the mRNA and protein levels, we next sought to determine how fibroblast function changes with exposure to TGFβ. One of the hallmarks of activated myofibroblasts is their ability to contract16. To understand this functional outcome of activation, fibroblasts were seeded in collagen gels and allowed to contract over 4 days in the presence or absence of rhTGFβ1 (Figure 3). Gels without any embedded fibroblasts did not contract (data not shown), while gels containing esophageal fibroblasts demonstrated on average 70.2-78.9% contraction, with no significant differences in basal contraction between phenotypes. Treatment with TGFβ significantly increased contraction to 66.2-69.7% (Figure 3B) with similar results in all cohorts.
Figure 3. TGFβ enhances fibroblast contractility in a soft matrix.
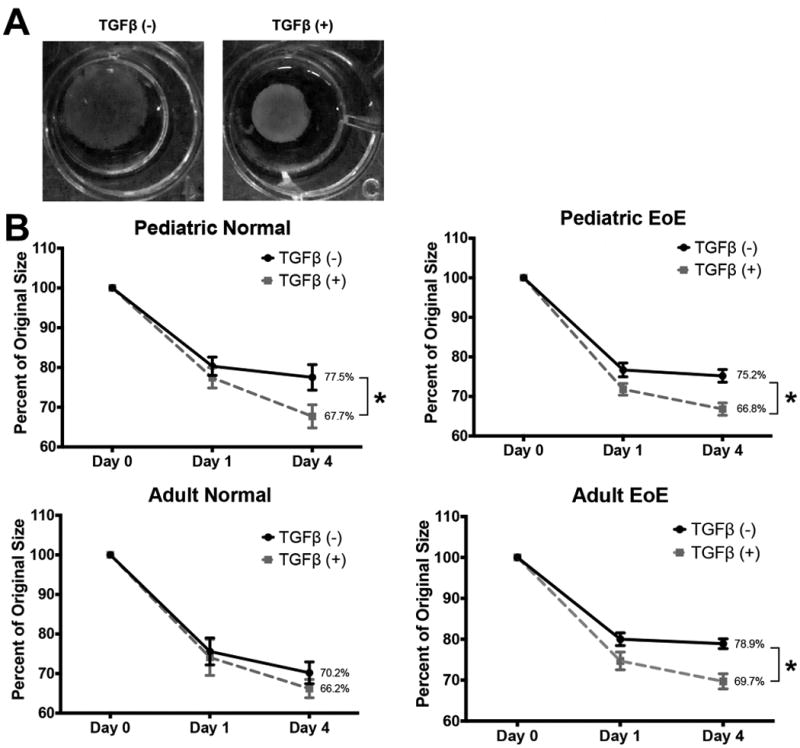
Fibroblasts were embedded into collagen gel matrices and allowed to contract for 4 days. (A) Images of representative gel matrices from Day 0 and Day 4 are shown in panel. (B) Average percent contraction of gels after 4 days of contraction with and without TGFβ in all four patient phenotypes showed significant increases in contraction with TGFβ stimulation of pediatric normal (p=0.01), pediatric EoE (p=0.0015), and adult EoE (p=0.0003) but not adult normal fibroblasts. n=5,7,11, and 4, respectively. *p<0.05 compared to TGFβ(-).
Increasing matrix stiffness leads to increases in markers of fibroblast activation
Deposition of extracellular matrix components and their subsequent contraction by activated myofibroblasts leads to stiffening of the cell microenvironment. As such, we investigated the effects of changing the fibroblast mechanical microenvironment on esophageal fibroblast behavior. As the stiffness of the esophagus, either normal or with pathologic changes, has not yet been characterized, we modeled the stiffness of our system loosely on work in Crohn disease in the small intestine, with normal intestine around 2.9 kPa and strictured tissue around 16.7 kPa15. Butcher and colleagues24 estimated normal epithelial cell and fibroblast environments to be around 3-5 kPa, giving us confidence in our selection of soft (1 kPa), normal (3 kPa), and stiffening (9 and 12 kPa) gel models. To determine the effects of stiffening tissue, we cultured fibroblasts on top of polyacrylamide hydrogels (Figure 4A). After culture on the polyacrylamide substrates for 4 days, fibroblasts from pediatric and adult subjects with or without EoE were analyzed for morphological changes and the induction of α-SMA. Representative images of fibroblasts from pediatric EoE patients are shown with similar results found from fibroblasts from the other groups (Figure 4B, D); however, cell morphology was distinct between softer and stiffer matrices, with the latter increasing cell spreading and protrusion of the filopodia as shown with increased perimeter of cells (Figure 4B-C), consistent with accepted morphology of activated myofibroblasts. Additionally, increased matrix stiffness was associated with increased α-SMA expression in the fibroblasts in a dose dependent manner (Figure4D-E).
Figure 4. Fibroblasts respond to increasing matrix stiffness and TGFβ stimulation.
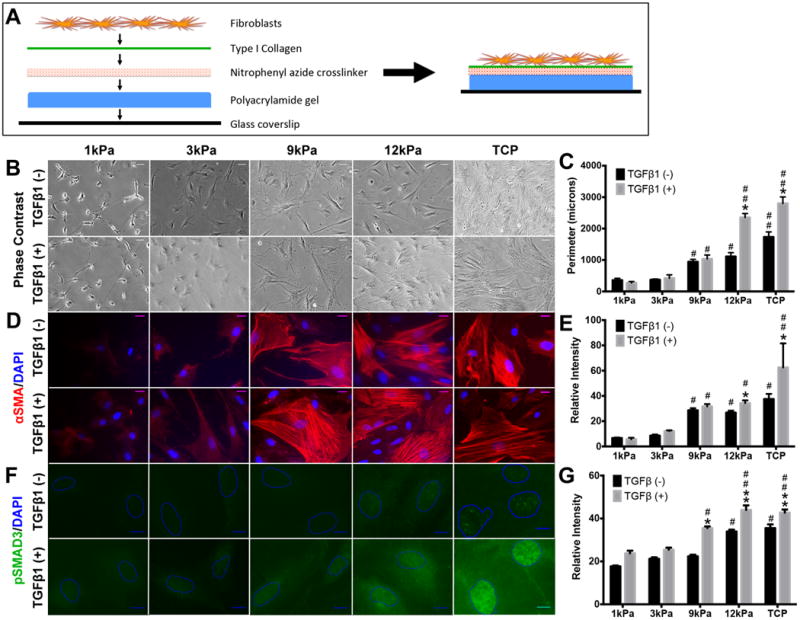
Schematic of experimental design: Fibroblasts were seeded on collagen-coated polyacrylamide gels of varying stiffness (A). Cell morphology differences were observed, with enhanced spindle morphology on stiff matrices (B). Perimeter measurements performed to account for filopodia and cell spreading (C) (significant trend for increasing perimeter from 1kPa to TCP adjusted for TFGβ exposure: p<0.001, with subanalyses demonstrating significant trends for both TGFβ(-) and TGFβ(+) conditions: p<0.001). Immunofluorescent localization (D) and quantification (E) of αSMA (significant trend for increasing relative intensity from 1kPa to TCP adjusted for TFGβ exposure: p<0.001, with subanalyses demonstrating significant trends for both TGFβ(-) and TGFβ(+) conditions: p<0.001 and p=0.001, respectively). Effect of stiffness and TGFβ stimulation upon nuclear co-localization of pSMAD3 (F,G)(significant trend for increasing relative intensity from 1kPa to TCP adjusted for TFGβ exposure: p<0.001, with subanalyses demonstrating significant trends for both TGFβ(-) and TGFβ(+) conditions: p<0.001). * p<0.05 compared to TGFβ(-). # p<0.05 compared to low stiffness (1 and 3kPa(C,E) or 1, 3, and 9kPa (G)). ## p<0.05 compared to 1, 3, and 9kPa (C,G) or 1, 3, 9, and 12kPa (E).
We next determined how TGFβ affects fibroblast activation on substrates with differential stiffness. Interestingly, TGFβ not only stimulated myofibroblast differentiation and α-SMA expression in a dose-dependent manner, but also enhanced such changes more robustly on stiffer matrix (≥ 9kPa)(Figure 4D-E). For example, TGFβ stimulated fibroblasts displayed enhanced organization of the α-SMA-stained filaments on the stiffer matrix (Figure 4D). These data suggest that myofibroblast activation requires matrix stiffness, as myofibroblast differentiation did not occur on soft hydrogels, even in the presence of TGFβ. It should be noted that all cells used for these experiments were grown for at least 2 passages on tissue culture plastic (approx. 1 GPa) where myofibroblast characteristics are fully expressed in the absence of exogenous TGFβ (Figure 4B). However, upon seeding on a soft matrix, fibroblasts adjusted their behavior to reflect their environment (Figure 4B-E) with less spreading and diminished expression of α-SMA, indicating that myofibroblast activation can reverse in the appropriate mechanical setting.
Expression of pSMAD3 increases with substrate stiffness and TGFβ stimulation
We next determined how fibroblast activation is enhanced on stiff matrices, by assessing cytoplasmic and nuclear localization of the phosphorylated form of SMAD3 (pSMAD3), a marker of the activation of canonical TGFβ receptor-mediated signaling. pSMAD3 was not detectable without TGFβ stimulation on softer matrices (<12kPa) while a basal level of pSMAD3 was detected on stiffer matrices (≥12 kPa) (Figure 4). Interestingly, TGFβ stimulation failed to activate pSMAD3 on softer matrices (<3kPa) but TGFβ-mediated pSMAD3 induction was found to occur with matrix stiffness at 9 kPa (Figure 4F-G), suggesting that matrix stiffness influences TGFβ signaling and myofibroblast activation.
Fibroblasts increase their traction forces with substrate stiffness
Because matrix stiffness enhanced fibroblast spreading and α-SMA expression, we next determined how stiffness changed fibroblast function, specifically traction forces. We seeded primary fibroblasts of different age groups and disease states on mPADs of a moderate stiffness (Eeff = 5 kPa, kspring = 6 nN/μm). Similar to our results in the 3-D contraction assay (Figure 3B), neither age nor disease status had any effect on the per-pillar traction forces exerted by individual cells (Figure 5B). Since we observed similar traction forces among all phenotypes, we used a representative primary culture to investigate the role of stiffness on cell traction force. Pediatric EoE fibroblasts plated on stiffer mPADs substrates (Eeff = 20 kPa, kspring = 25 nN/μm) exhibited increased per-pillar traction forces compared to those plated on softer mPADs.
Figure 5. Fibroblast traction force increases with matrix stiffness.
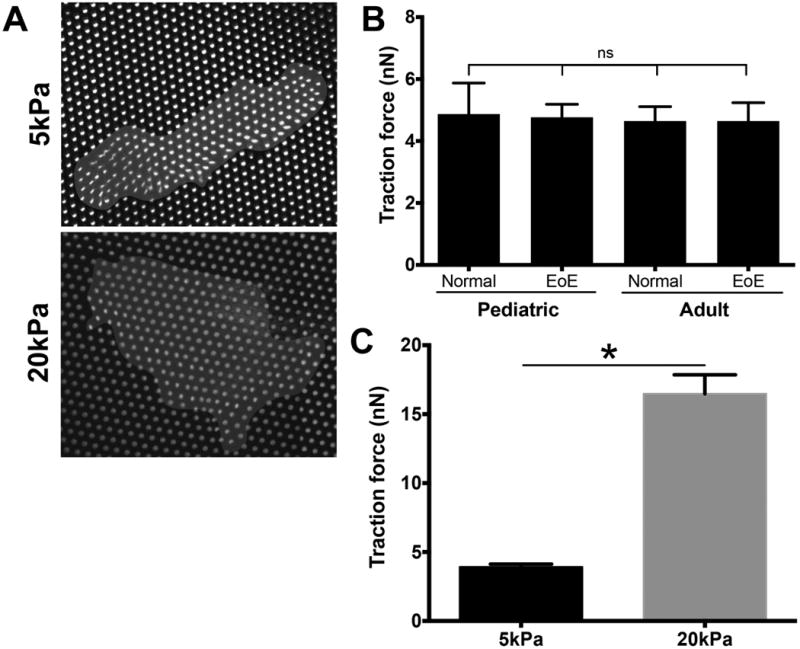
Fibroblasts were seeded on micropost arrays (post diameter = 1.83 μm) with varying post height, thereby varying the spring constant of the post and stiffness of the matrix. Representative images of fibroblasts seeded on 5kPa and 20kPa mPADs (A). Fibroblasts from all phenotypes exerted similar tensile force of engaged microposts of the same stiffness (NS) (B). Traction forces generated by fibroblasts on soft (5kPa) and stiff (20kPa) posts (C). * p<0.0001
In aggregate, our data suggest unique effects of matrix stiffness upon activation of TGFβ signaling and myofibroblast activation as well as age-dependent and independent differential activities of fibroblasts isolated from subjects with or without EoE.
Discussion
We have demonstrated for the first time that the TGFβ-rich tissue microenvironment associated with EoE may facilitate the activation of fibroblasts to myofibroblasts. Our study suggests a potential mechanism by which adults with EoE may have ongoing tissue remodeling despite improvement in tissue eosinophilia. Although TGFβ induces α-SMA in fibroblasts from pediatric and adult subjects with or without EoE to a similar extent, and collagen-matrix contraction upon TGFβ stimulation was comparable in all fibroblasts tested, we show that TGFβ-induced myofibroblastic differentiation with concurrent activation of canonical TGFβ signaling is greatly influenced by matrix stiffness. In addition, increased matrix stiffness resulted in augmentation of cell-generated traction forces. We propose a novel model (Fig. 6) in which the functional interplay between mechanical and biochemical factors enhances myofibroblast activation and ECM deposition, which together may contribute to fibrostenotic pathology and associated clinical manifestations in EoE, suggesting that altering the microenvironment in EoE may have therapeutic potential.
Figure 6. Proposed mechanism of fibroblast activation in EoE.
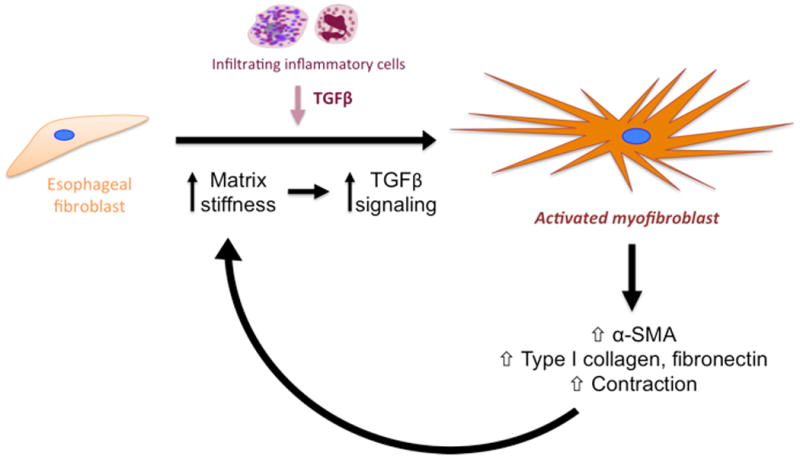
Together, our model suggests that esophageal fibroblasts are stimulated by the EoE inflammatory milieu to produce extracellular matrix proteins and begin remodeling the ECM. This increases stiffness of the environment, leading to enhanced TGFβ signaling in fibroblasts. The increasing stiffness and cytokine response fully activates fibroblasts, which continue to produce α-SMA and extracellular matrix components, such as collagen and fibronectin, and contract their environment to further increase stiffness, giving rise to a positive feedback loop of fibroblast stimulation and activation.
This is the first study to evaluate a large number of primary esophageal fibroblast cultures from both pediatric and adult patients with or without EoE. In vitro fibroblast behavior varies with both anatomical origin of the fibroblasts and disease state25. Fibroblasts isolated from lung tissue produce less IL-8 compared to bone marrow, breast, and spleen derived fibroblasts. Furthermore, disease state alters ex vivo fibroblast behavior. Specifically, pulmonary fibroblasts derived from mice sensitized to Schistosoma mansoni produced more monocyte chemotactic protein-1 compared to healthy mouse fibroblasts and the fibroblasts of mice sensitized to Mycoplasma tuberculosis 26. For the first time, we report that esophageal fibroblasts from adult EoE subjects have increased TGFβ-mediated type 1 collagen mRNA expression compared to pediatric fibroblasts (Fig. 2C), suggesting age-related changes in esophageal fibroblast function that are independent of an EoE disease condition. Aging may affect gene expression in fibroblasts via altered epigenetic landscape of DNA27,28. Despite this difference in collagen gene expression, we found that ex vivo fibroblast transdifferentiation does not change with age or disease state- it changes with alterations of the chemical and mechanical microenvironment. Chronic inflammation and increased matrix stiffness leads to enhanced fibroblast activation, tensile strength, and ECM deposition independent of patient age and disease state.
Canonical TGFβ signaling is closely associated with fibrosis. Mice deficient in SMAD3 after skin injury have been shown to have decreased accumulation of extracellular matrix, decreased myofibroblasts, and decreased recruitment of inflammatory cells29,30. In the context of EoE, human esophageal fibroblasts stimulated with TGFβ and eosinophil sonicates demonstrate increased phospho-SMAD2/3 production 5. In a murine model of EoE, SMAD3 deficient mice had decreased lamina propria thickness and angiogenesis when compared to wild type EoE mice13. In the current study, our results now suggest that stiffness of the esophageal microenvironment could potentiate TGFβ signaling and subsequent fibrosis.
We demonstrate that esophageal fibroblasts, after being cultured and passaged on tissue-culture plastic (TCP) (approx. 1GPa) and returned to a soft matrix (1-3kPa) revert back to a quiescent phenotype with decreased spreading, decreased tensile forces, and decreased α-SMA production. These results suggest that fibroblast activation may be reversible once inflammation and stiffness cease, although one study suggests that fibroblasts in culture have a ‘mechanical memory’ and remain at least partially activated even if returned to soft environment 31.
Increased traction forces may exacerbate fibrosis by causing the release of latent TGFβ from the matrix. The ECM is a major reservoir of TGFβ. This TGFβ is bound to both a latency-associated protein (LAP) and a latent-TGFβ-binding protein, known collectively as the latent complex32,33. Interestingly, intracellular tension, which is increased in cells in a stiff environment, may be one mechanism for the activation of latent TGFβ34. We have shown that in a stiff microenvironment, esophageal fibroblasts generate increased traction force. We speculate that in the setting of a fibrotic stricture, increased traction forces lead to increased latent TGFβ activation and further fibroblast activation and fibrosis.
Our finding that matrix stiffness enhances the myofibroblastic differentiation of esophageal fibroblasts has broad clinical implications. Because the median delay in diagnosis of EoE is 6 years35, fibrosis, matrix remodeling, and increased stiffness may already be present at the initiation of therapy. In fact, patients with a history of food impactions have decreased distensibility and are at increased risk for future food impaction14. Our results suggest that once mechanical changes have occurred, there may be continued fibroblast activation and subepithelial remodeling despite therapeutic interventions. Nevertheless, no link between restricted esophageal distensibility and esophageal fibrosis in EoE patients has been fully established. The functional assessment of esophageal distensibility by impedance palimetry (EndoFLIP) coupled with other novel imaging modalities such as endoscopic ultrasound and manometry may lead to earlier detection of fibrosis and allow for prevention of further esophageal dysfunction36,37. Such studies are currently underway.
In order to avoid continued complications, therapeutic strategies may be more effective if they simultaneously target the matrix and inflammatory cells. Possible targets include matrix metalloproteinases and collagen fibrils. Our results suggest that softening the microenvironment will not only act to prevent further fibroblast activation, but will also decrease the downstream effects of TGFβ stimulation.
Though clinical symptoms of dysphagia can be improved by treatment with topical steroids38, studies suggest that complete histologic reversal of esophageal fibrosis is difficult to achieve. Lucendo et al showed that EoE therapy (400 μg of fluticasone) led to decreases of TGFβ, eosinophils, and IL-5 in adult EoE biopsies, but the decrease in fibrosis was not statistically significant 39. Similarly, Straumann et al showed that there was a trend toward improvement in the thickness and collagen content of the lamina propria but not complete resolution of pathology after 50 weeks of low-dose budesonide (0.25 mg twice daily) therapy40. Both of these studies were performed in adult patients, presumably after years of unchecked inflammation. As corroborated by our data, there is continued fibroblast activation in the lamina propria of adults with inactive disease (Figure 1). However, in a similar pediatric study, there was a significant improvement in histologic fibrosis scores in addition to the decrease in pro-inflammatory cytokines after steroid therapy41. Early detection of disease and aggressive therapy during childhood may prevent tissue stiffening and make future therapy more effective.
In summary, we show that tissue stiffness and cytokine stimulation are important factors in esophageal fibroblast activation and subsequent contraction and collagen deposition in culture. Our work suggests that profibrotic behaviors are enhanced in a stiff environment, and may explain why presenting symptoms and pathology change with the duration of disease. Prevention of tissue stiffness with an early EoE diagnosis and aggressive therapy could prevent life long swallowing dysfunction and improve quality of life.
What is known about this subject?
Eosinophilic esophagitis is a chronic inflammatory disease that leads to food impaction and stricture.
There is increased collagen deposition and TGFβ in the histologic specimens of patients with EoE, yet little is known about the pathophysiology of remodeling.
Nothing is known about how the microenvironment shapes esophageal fibroblast behavior.
What are the new findings?
Primary esophageal fibroblasts display increased activation, and fibrogenesis and contraction in the setting of TGFβ stimulation.
The stiffness of the fibroblast environment perpetuates fibroblast activation and contractility.
Downstream TGFβ signaling is largely dependent on environment stiffness in esophageal fibroblast.
Acknowledgments
Grant Support: This study was supported by the following NIH Grants: R01DK087789 (MLW, JMS), NIH/NIDDK P30-DK050306 Center of Molecular Studies in Digestive and Liver Diseases, The Molecular Pathology and Imaging, Molecular Biology/Gene Expression and Cell Culture Core Facilities, K01DK103953 (KAW), K08DK106444 (ABM), F32DK100088 (ABM), NIH GM104287 (SJH, DAH). Additional support was provided by American Partnership For Eosinophilic Disorders Hope Award (ABM), Grosvenor Fund Management (to MLW, ABM), Abbott Nutrition (MLW), Joint Penn-CHOP Center in Digestive, Liver and Pancreatic Medicine at The Perelman School of Medicine (ABM, HN), and the CHOP Eosinophilic Esophagitis Allergy Fund. We also wish to thank the endoscopy suite staff, faculty, and fellows in the CHOP Division of GI, Hepatology, and Nutrition for their time, support, and technical assistance with this study.
Footnotes
Disclosures: none
Author Contributions: Experimental design: HN KAW ABM MLW JMS GF SJH RGW DAH KD MD
Experimental execution: ABM KD AJB SJH DL MD
Data analysis and interpretation: ABM DL KAW RGW GF HN JMS MLW AJB AJB
Reagents/materials/analysis tool contributions: ABM RGW DAH SJH HN MLW AJB
Manuscript writing/editing: ABM KD JMS MLW HN GF
References
- 1.Aceves SS, Chen D, Newbury RO, et al. Mast cells infiltrate the esophageal smooth muscle in patients with eosinophilic esophagitis, express TGF-β1, and increase esophageal smooth muscle contraction. J Allergy Clin Immunol. 2010;126:1198–204. doi: 10.1016/j.jaci.2010.08.050. e4. [DOI] [PubMed] [Google Scholar]
- 2.Noti M, Wojno EDT, Kim BS, et al. Thymic stromal lymphopoietin–elicited basophil responses promote eosinophilic esophagitis. Nature Medicine. 2013;19:1005–1013. doi: 10.1038/nm.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alain M, Schoepfer MD, Ekaterina Safroneeva P, Christian Bussmann MD, et al. Accepted Manuscript. YGAST. 2013:1–31. [Google Scholar]
- 4.Falk GW. Clinical presentation of eosinophilic esophagitis in adults. Gastroenterol. Clin. North Am. 2014;43:231–242. doi: 10.1016/j.gtc.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 5.Rieder F, Nonevski I, Ma J, et al. T-Helper 2 Cytokines, Transforming Growth Factor β1, and Eosinophil Products Induce Fibrogenesis and Alter Muscle Motility in Patients With Eosinophilic Esophagitis. Gastroenterology. 2014 doi: 10.1053/j.gastro.2014.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noel RJ, Putnam PE, Rothenberg ME. Eosinophilic esophagitis. N Engl J Med. 2004;351:940–941. doi: 10.1056/NEJM200408263510924. [DOI] [PubMed] [Google Scholar]
- 7.Alain M, Schoepfer MD, Ekaterina Safroneeva P, Christian Bussmann MD, et al. Accepted Manuscript. YGAST. 2013:1–31. [Google Scholar]
- 8.Kishi K, Okabe K, Shimizu R, et al. Fetal skin possesses the ability to regenerate completely: complete regeneration of skin. Keio J Med. 2012;61:101–108. doi: 10.2302/kjm.2011-0002-ir. [DOI] [PubMed] [Google Scholar]
- 9.HESS A. Reactions of mammalian fetal tissues to injury. II Skin Anat Rec. 1954;119:435–447. doi: 10.1002/ar.1091190404. [DOI] [PubMed] [Google Scholar]
- 10.Whitby DJ, Ferguson MW. The extracellular matrix of lip wounds in fetal, neonatal and adult mice. Development. 1991;112:651–668. doi: 10.1242/dev.112.2.651. [DOI] [PubMed] [Google Scholar]
- 11.Whitby DJ, Ferguson MW. Immunohistochemical localization of growth factors in fetal wound healing. Dev Biol. 1991;147:207–215. doi: 10.1016/s0012-1606(05)80018-1. [DOI] [PubMed] [Google Scholar]
- 12.Aceves SS, Newbury RO, Dohil R, et al. Esophageal remodeling in pediatric eosinophilic esophagitis. Journal of Allergy and Clinical Immunology. 2007;119:206–212. doi: 10.1016/j.jaci.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 13.Cho JY, Doshi A, Rosenthal P, et al. Smad3 Deficient Mice Have Reduced Esophageal Fibrosis and Angiogenesis in a Mouse Model of Egg Induced Eosinophilic Esophagitis. Journal of Pediatric Gastroenterology and Nutrition. 2014;59:10–16. doi: 10.1097/MPG.0000000000000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicodème F, Hirano I, Chen J, et al. Esophageal Distensibility as a Measure of Disease Severity in Patients With Eosinophilic Esophagitis. Clinical Gastroenterology and Hepatology. 2013;11:1101–1107. doi: 10.1016/j.cgh.2013.03.020. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson LA, Rodansky ES, Sauder KL, et al. Matrix stiffness corresponding to strictured bowel induces a fibrogenic response in human colonic fibroblasts. Inflamm Bowel Dis. 2013;19:891–903. doi: 10.1097/MIB.0b013e3182813297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hinz B, Phan SH, Thannickal VJ, et al. The Myofibroblast. The American Journal of Pathology. 2007;170:1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olsen AL, Bloomer SA, Chan EP, et al. Hepatic stellate cells require a stiff environment for myofibroblastic differentiation. AJP: Gastrointestinal and Liver Physiology. 2011;301:G110–8. doi: 10.1152/ajpgi.00412.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muir AB, Lim DM, Benitez AJ, et al. Esophageal epithelial and mesenchymal cross-talk leads to features of epithelial to mesenchymal transition in vitro. Exp Cell Res. 2013;319:850–859. doi: 10.1016/j.yexcr.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muir AB, Dods K, Noah Y, et al. Esophageal epithelial cells acquire functional characteristics, of activated myofibroblasts after undergoing an epithelial to mesenchymal transition. Exp Cell Res. 2014 doi: 10.1016/j.yexcr.2014.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MD CAL, MD GTF, MD IH, et al. Eosinophilic esophagitis: Updated consensus recommendations for children and adults. Journal of Allergy and Clinical Immunology. 2011;128:3–20. doi: 10.1016/j.jaci.2011.02.040. e6. [DOI] [PubMed] [Google Scholar]
- 21.Desai R, Yang M, Sniadecki N, et al. Microfabricated post-array-detectors (mPADs): an approach to isolate mechanical forces. J Vis Exp. 2007;311 doi: 10.3791/311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schoen DT, Schoen AP, Hu L, et al. High speed water sterilization using one-dimensional nanostructures. Nano Lett. 2010;10:3628–3632. doi: 10.1021/nl101944e. [DOI] [PubMed] [Google Scholar]
- 23.Ghibaudo M, Trichet L, Xayaphounmmine A, et al. Traction forces and rigidity sensing regulate cell functions. Small Matter. 2008;4:1836–1843. [Google Scholar]
- 24.Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer. 2009;9:108–122. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brouty-Boyé D, Pottin-Clémenceau C, Doucet C, et al. Chemokines and CD40 expression in human fibroblasts. Eur J Immunol. 2000;30:914–919. doi: 10.1002/1521-4141(200003)30:3<914::AID-IMMU914>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 26.Hogaboam CM, Steinhauser ML, Chensue SW, et al. Novel roles for chemokines and fibroblasts in interstitial fibrosis. Kidney International. 1998;54:2152–2159. doi: 10.1046/j.1523-1755.1998.00176.x. [DOI] [PubMed] [Google Scholar]
- 27.Sanders YY, Liu H, Liu G, et al. Epigenetic mechanisms regulate NADPH oxidase-4 expression in cellular senescence. Free Radic Biol Med. 2015;79:197–205. doi: 10.1016/j.freeradbiomed.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 28.Thijssen PE, Tobi EW, Balog J, et al. Chromatin remodeling of human subtelomeres and TERRA promoters upon cellular senescence: commonalities and differences between chromosomes. Epigenetics. 2013;8:512–521. doi: 10.4161/epi.24450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ashcroft GS, Yang X, Glick AB, et al. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat Cell Biol. 1999;1:260–266. doi: 10.1038/12971. [DOI] [PubMed] [Google Scholar]
- 30.Flanders KC, Sullivan CD, Fujii M, et al. Mice lacking Smad3 are protected against cutaneous injury induced by ionizing radiation. AJPA. 2002;160:1057–1068. doi: 10.1016/S0002-9440(10)64926-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balestrini JL, Chaudhry S, Sarrazy V, et al. The mechanical memory of lung myofibroblasts. Integr Biol (Camb) 2012;4:410–421. doi: 10.1039/c2ib00149g. [DOI] [PubMed] [Google Scholar]
- 32.Cheng E, Souza RF, Spechler SJ. Tissue remodeling in eosinophilic esophagitis. AJP: Gastrointestinal and Liver Physiology. 2012;303:G1175–G1187. doi: 10.1152/ajpgi.00313.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyazono K, Olofsson A, Colosetti P, et al. A role of the latent TGF-beta 1-binding protein in the assembly and secretion of TGF-beta 1. EMBO J. 1991;10:1091–1101. doi: 10.1002/j.1460-2075.1991.tb08049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wipff P-J, Rifkin DB, Meister J-J, et al. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol. 2007;179:1311–1323. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schoepfer AM, Safroneeva E, Bussmann C, et al. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology. 2013;145:1230–6. doi: 10.1053/j.gastro.2013.08.015. e1–2. [DOI] [PubMed] [Google Scholar]
- 36.Roman S, Hirano I, Kwiatek MA, et al. Manometric features of eosinophilic esophagitis in esophageal pressure topography. 2011;23:208–14. doi: 10.1111/j.1365-2982.2010.01633.x. e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fox VL, Nurko S, Teitelbaum JE, et al. High-resolution EUS in children with eosinophilic “allergic” esophagitis. YMGE. 2003;57:30–36. doi: 10.1067/mge.2003.33. [DOI] [PubMed] [Google Scholar]
- 38.Sawas T, Dhalla S, Sayyar M, et al. Systematic review with meta-analysis: pharmacological interventions for eosinophilic oesophagitis. Aliment Pharmacol Ther. 2015 doi: 10.1111/apt.13147. n/a–n/a. [DOI] [PubMed] [Google Scholar]
- 39.Lucendo AJ, Arias A, De Rezende LC, et al. Subepithelial collagen deposition, profibrogenic cytokine gene expression, and changes after prolonged fluticasone propionate treatment in adult eosinophilic esophagitis: a prospective study. J Allergy Clin Immunol. 2011;128:1037–1046. doi: 10.1016/j.jaci.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 40.Straumann A, Conus S, Degen L, et al. Long-term budesonide maintenance treatment is partially effective for patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2011;9:400–9. doi: 10.1016/j.cgh.2011.01.017. e1. [DOI] [PubMed] [Google Scholar]
- 41.Aceves SS, Newbury RO, Chen D, et al. Resolution of remodeling in eosinophilic esophagitis correlates with epithelial response to topical corticosteroids. Allergy. 2010;65:109–116. doi: 10.1111/j.1398-9995.2009.02142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


