Abstract
Rhizosecretion has many advantages for the production of recombinant pharmaceuticals, notably facile downstream processing from hydroponic medium. The aim of this study was to increase yields of the HIV microbicide candidate, Cyanovirin-N (CV-N), obtained using this production platform and to develop a simplified methodology for its downstream processing from hydroponic medium. Placing hydroponic cultures on an orbital shaker more than doubled the concentration of CV-N in the hydroponic medium compared to plants which remained stationary, reaching a maximum of approximately 20μg/ml in one week, which is more than 3 times higher than previously reported yields. The protein composition of the hydroponic medium, the rhizosecretome, was characterised in plants cultured with or without the plant growth regulator alpha-napthaleneacetic acid by LC-ESI-MS/MS, and CV-N was the most abundant protein. The issue of large volumes in the rhizosecretion system was addressed by using ion exchange chromatography to concentrate CV-N and partially remove impurities. The semi-purified CV-N was demonstrated to bind to HIV gp120 in an ELISA and to neutralise HIVBa-L with an IC50 of 6nM in a cell-based assay. Rhizosecretion is therefore a practicable and inexpensive method for the production of functional CV-N.
Keywords: Cyanovirin-N, HIV microbicide, Hydroponic medium, Molecular pharming, Rhizosecretion
1 INTRODUCTION
Cyanovirin-N (CV-N) is an 11 kDa lectin from the cyanobacterium (blue-green alga) Nostoc ellipsosporum, discovered in a program to screen for molecules with anti-HIV properties [1]. It is a remarkably stable protein, being resistant to continuous freeze-thaw cycles, to dissolution in organic solvents (such as acetonitrile, methanol and dimethyl sulfoxide), high concentrations of salt (8M guanidine hydrochloride), strong detergents (e.g. 0.5% w/v sodium dodecyl sulphate), hydrogen peroxide and high temperatures [1, 2]. CV-N can inactivate many strains of HIV and prevent cell-to-cell transmission by irreversibly binding to high-mannose oligosaccharides of gp120, and interfering with essential interactions for virus fusion and entry into susceptible cells [1, 2]. Consequently, it is a promising molecule for use as a topical microbicide i.e. a compound that can reduce or prevent pathogenic infection of HIV, when applied to the vagina or rectum prior to sexual intercourse [3].
HIV microbicides need to be not only effective, but also stable, safe and inexpensive in order to be widely used [4]. CV-N protective efficacy has been demonstrated when formulated as a gel and applied to the vagina or rectum of macaques prior to challenging with a pseudotyped simian HIV [5, 6]. Additionally, it was shown that CV-N can block the infection of human ectocervical explants by HIV-1BA-L. CV-N has also shown benign behaviour in a rabbit vaginal toxicity model [7]. Potent activity of CV-N in the low nanomolar range against laboratory and primary isolates has been confirmed in ex vivo female genital tissue explants even in the presence of semen or Candida albicans [4]. Moreover, CV-N selective pressure on HIV can result in a virus that is more susceptible to the host immune system [8, 9].
CV-N has already been expressed in several heterologous systems including Escherichia coli [1, 7, 10], Streptococcus gordonii [11], Lactobacillus [12, 13] and Pichia pastoris [7]. It has been suggested that to supply the potential world demand for an HIV microbicide, transgenic plants might be the only option [3]. In this context, the feasibility of the plant system was demonstrated by producing genetically modified tobacco plants expressing recombinant CV-N with a yield in leaves of 0.85% of total soluble protein. The plant-derived CV-N was functional as demonstrated by specific binding to gp120 and protection of T-cells from in vitro HIV infection [14]. Recently, it was reported that functional CV-N was expressed in soya bean seeds. However, CV-N purification from soya seeds was cumbersome, as the protein in the soluble fraction was co-purified with contaminants, and CV-N had to be purified from the insoluble fraction [15].
Sexton et al. also reported the rhizosecretion of CV-N from transgenic tobacco plants in hydroponic medium. CV-N demonstrated high stability accumulating in the medium for up to 24 days [14]. In a subsequent study, addition of the plant-growth regulator alpha-napthaleneacetic acid (NAA) to hydroponic medium was used to increase rhizosecretion yields of CV-N 6-fold reaching a maximum yield of 6μg/ml after 7 days [16].
Rhizosecretion has many advantages for the production of recombinant pharmaceuticals. It is a contained low cost system that uses defined culture medium rather than soil and does not require the use of bioreactors. The principal advantage however, is that downstream processing is simple, as purification is from hydroponic medium rather than from vegetative tissues. For production of recombinant pharmaceutical proteins from plants, several steps are usually required in order to eliminate impurities, and as a consequence, purification can account for up to 80% of the production costs [17].
Recently, a combination of ultrafiltration, centrifugal partition chromatography and aqueous two-phase systems (ATPS) was used to purify recombinant CV-N from other proteins which were co-secreted into a hydroponic plant medium in a rhizosecretion production process. A systematic approach including the use of a Design of Experiment software allowed optimisation of ATPS parameters, but the efficiency of purification could not be optimized sufficiently to establish a robust downstream purification protocol [18].
In the present study, both upstream and downstream elements of the rhizosecretion system for production of CV-N were investigated and optimized. The hydroponic medium was characterized with respect to protein composition by liquid chromatography - electrospray ionisation - tandem mass spectroscopy (LC-ESI-MS/MS) and finally, an efficient first step in the downstream processing of CV-N from hydroponic medium was demonstrated using ion exchange chromatography.
2 MATERIALS and METHODS
2.1 Establishment of plant cultures
The transgenic tobacco plants expressing CV-N have been described previously [14]. Seeds from a homozygous line (T3 generation) were surface sterilized and established in hydroponic culture in Murashige and Skoog medium (MS) [19] as previously described [20]. Tobacco seedlings were grown in MS medium for a period of 6 weeks. Medium was then replaced with fresh MS containing 1mg/L NAA (30 mL per plant). Medium was harvested (~25 mL per plant) and replaced with fresh MS +NAA (30 mL per plant) at weeks 8, 9 and 10. For one assessment (described later), plants were cultured in an identical manner but without addition of NAA. For all experiments, plants were maintained at 25°C with a 16 h photoperiod.
2.2 Culture vessel agitation
At week 6, eight to ten plants were randomly allocated to either a shaking or stationary control group with the former placed on an orbital shaker (Labotron, Infors HT, Reigate, UK) at 150 rpm with medium replacements as described in section 2.1. Plants in the shaking group remained on the shaker until the end of the experiment. Medium aliquots were taken from each plant at weeks 7, 8, 9 and 10 and refrigerated prior to analysis for CV-N content by enzyme-linked immunosorbent assay (ELISA). The week 8 sample was also analyzed by SDS-PAGE. In all subsequent assessments, medium from plants cultured without shaking were used.
2.3 LC-ESI-MS/MS identification of medium proteins
Hydroponic medium from plants maintained without shaking was collected at week 11. In addition, in order to ascertain the effect of NAA treatment on rhizosecretion of proteins, medium was also collected from plants that had been cultured in MS medium without NAA. Medium was concentrated 20-fold using a Vivaspin 2 column (Sartorius Stedim Biotech, Aubagne, France). The concentrated mixture was then resolved by reducing 1D SDS-PAGE and the gel stained with colloidal Coomassie (Expedeon, Cambridge, UK). Protein bands were cut from the gel, processed and LC-ESI/MS/MS carried out as described previously [21].
2.5 Semi-purification of CV-N by ion exchange (IEX) chromatography
Semi-purification of CV-N from hydroponic medium was undertaken using a 1 ml HiTrap Q HP resin ion-exchange column (GE Healthcare, Little Chalfont, UK) connected to an Akta® FPLC. Medium was initially dialysed in Tris-HCl pH 8.4 (1:100 medium/buffer ratio) and filtered through a 0.22μm syringe filter. The column was washed with 20 ml of Tris-HCl pH 8.4, prior to loading 50 ml of dialyzed medium. The column was again washed with 20 ml of Tris-HCl pH 8.4 and elution was performed in three steps: 1 – gradient from 0 to 200 mM NaCl in Tris-HCl pH 8.4; 2 – gradient from 200mM to 1M NaCl in Tris-HCl pH 8.4 ; 3 – 1M NaCl.
2.6 HIV neutralization assay
A semi-purified CV-N sample was desalted using a PD-10 column (GE Healthcare), and eluted in PBS. The sample was then analyzed in a TZM-bl cell based HIV assay [22]. Samples were sterile-filtered, diluted 1:4 in Dulbecco’s Modified Eagle Medium (DMEM) and loaded onto a 96 well plate (Nunc, Roskilde, Denmark) to give a final volume of 55μl/well. Rhizosecretion-derived CV-N was added at an initial concentration of 1.75 μg/ml, whilst E. coli-derived CV-N was added at an initial concentration of 10 μg/ml. Samples were further titrated 1:5 down the plate in DMEM. Fifty-five microliters of HIVBa-L (of which the appropriate dilution to provide adequate infection had been ascertained by a previous titration) was then added to each sample and incubated at 37°C with 5% v/v CO2 for 1 hour. After incubation, 100μl of the sample and virus mixture was transferred to a 96 well plate (Nunc 96 Microwell flat bottom with lid) previously seeded with 100μl/well of 105 TZM-bl cells/mL (NIH AIDS Research & Reference Reagent Program, # 8129) and incubated at 37°C with 5% v/v CO2 for a maximum of 20 hours to allow formation of a confluent monolayer. TZM-bl cells were incubated with the sample/virus mixture at 37°C with 5% v/v CO2 for 24 hours after which wells were aspirated and rinsed with PBS. Luciferase production by the cells was assessed by a Luciferase Assay Kit (product number E1501, Promega, Fitchburg, USA). Briefly, cells were lysed with 100μl/well of Lysis Buffer diluted 1:5 in water and the plates were frozen at −80°C for a minimum of 2 hours. After thawing, 50μl of lysed cells were transferred to a white 96 well plate (Corning Costar, Sigma-Aldrich) and 50μl/well of Luciferase mixture was added (Luciferase buffer and Luciferase assay substrate mixed according to manufacturer’s instructions). Luciferase production was immediately assessed by a LUMIstar Omega plate reader using a Lum/E filter with a sensitivity threshold of 400nm. The relative infectivity of each sample was measured and the IC50 for each sample determined.
2.7 ELISA
Plates were coated with 50 ng/well of recombinant gp120 IIIB (National Institute for Biological Standards and Control #0607), 50 μl/well, for 2 hours at 37°C. Serial dilutions of samples were added alongside a purified CV-N positive control (produced in recombinant E. coli). Blocking, washing, detection, and measurement of colour development were as previously described [16]. CV-N concentration in samples was calculated by interpolation of values obtained for the positive control titration curve. Calculation was made using the Graphpad Prism™ Software.
2.8 SDS-PAGE
Samples were mixed with 4 μl of 4X Tris-Bis loading buffer (Invitrogen, Life Technologies, Paisley, UK) to a final volume of 16 μl, boiled for 3 min and loaded onto 12% Tris-Bis gels (Invitrogen). Samples were electrophoresed at 20 mA/gel and visualisation of the separated proteins was performed by either Coomassie blue or silver staining. Coomassie blue staining of SDS-PAGE gels was with Instant Blue™ (Expedeon). For silver staining, all steps were carried out with gentle shaking. Gels were soaked in fixing solution (30% v/v ethanol, 10% v/v glacial acetic acid in water) for 1h. Solution was replaced with sensitizing solution (30% v/v ethanol, 0.2% w/v sodium thiosulphate, 7% w/v sodium acetate, 0.125% w/v glutaraldehyde, in water) and gels were shaken for another 1h. Solution was removed and the gels were washed four times of 15 min with distilled water. Silver solution (0.25% w/v silver nitrate) was added and gels were gently agitated for 1h and then washed twice with water for 1 min. Development was performed by agitating the gels in developing solution (2.5% w/v sodium carbonate, 0.03% v/v formaldehyde, in water) until bands reached the desired intensity. The reaction was stopped by incubating in 1.5% w/v EDTA-Na2.2H20 in water for at least 1h. Stained gels images were captured by scanning with an Epson XP-102 scanner.
2.9 Western blotting
SDS-PAGE gels were blotted onto nitrocellulose membrane using a semi-dry transfer system (Hoefer, TM TE70, Amersham Biosciences, Pitcataway, USA). Blocking, detection and development were as previously described [16].
3 RESULTS
3.1 Agitation increases CV-N yields in hydroponic medium
CV-N concentrations in the hydroponic medium from plants cultivated with shaking were higher than stationary plants at all time-points (Figure 1A). Yields in the shaking group were at least twice those in the stationary control group across the 5-week period. At week 8, CV-N yield for the shaking group was 21.4 μg/ml (SD=3.33, n=8 plants) and at week 9, yield was 20.1 μg/ml (SD=6.66, n=8 plants). At weeks 10 and 11, CV-N yields in the shaking group dropped slightly but over the 5-week period an average yield of over 16 μg/ml was obtained with approximately 6 μg/ml in the control group. Week 8 samples from all plants in each group were pooled and equal volumes were analyzed by SDS-PAGE stained with Coomassie blue. As demonstrated in Figure 1B, all protein bands were more concentrated for the shaking group. A band at approximately Mr 10 kDa, which was confirmed to be CV-N by LC-ESI-MS/MS analysis, was also much more pronounced in the shaking than in the stationary group.
Figure 1.
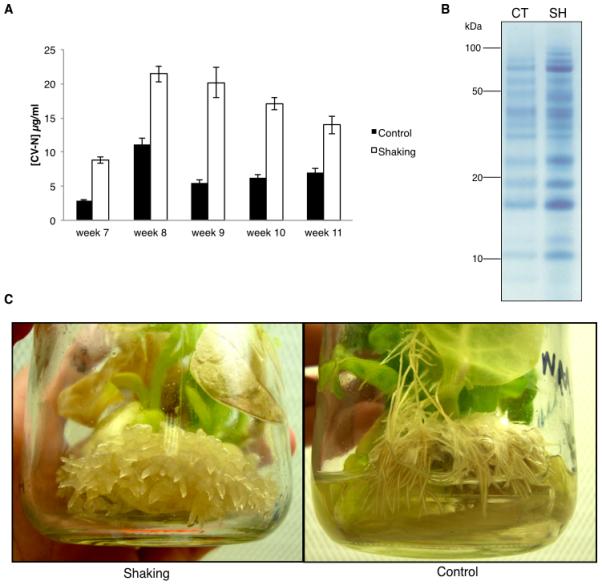
Effect of continuous agitation of cultures on CV-N yields in hydroponic medium. Plants were grown for 6 weeks and then given fresh medium with NAA. Plants in the shaking group were then placed on an orbital shaker at 150 rpm and the control group remained stationary. Medium with NAA was replaced at weeks 8, 9 and 10. (A) Yields at weeks 7-11. Values are means ± SEM for a minimum of 8 plants. (B) Coomassie-stained SDS-PAGE gel of week 8 pooled samples from the stationary control and shaking groups. CT: stationary control, SH: shaking. Molecular weight is indicated on the left in kDa. (C) Root phenotypes of representative plants in the stationary control and shaking groups at week 10.
The phenotype of agitated plants differed to stationary controls (Fig. 1C). At week 8, roots had developed nodular structures. Nodules continued to develop over time, and by week 10, short and thick lateral roots had been formed, resembling callus-like structures (Fig. 1C shaking), which were substantially different from the roots in the stationary plants (Fig. 1C – control).
We investigated sucrose consumption in the stationary and agitated plants: samples from 3 plants in each group were taken immediately after medium was given to the plants at week 7 (day 0) and on days 2, 6, 10 and 14. A sucrose assay (described in Supplementary Figure 1) was then performed to measure sucrose concentration in the samples. From day 0- 2, sucrose consumption for the 2 groups was comparable (Supplementary Figure 1). From day 6 onwards, sucrose became more depleted for the shaking group. On day 14, sucrose concentration in the shaking group was approximately 2 mg/mL - 5 times lower than in the stationary control group - indicating much higher sucrose consumption by the shaking group over the 14-day period.
3.2 The rhizosecretome is a mix of almost 100 proteins, but CV-N is the single most abundant protein
The relative proportions of the protein classes comprising the rhizosecretome for plants cultured with or without NAA are shown in Figure 2. In plants cultured with NAA, a total of 97 proteins were identified in the culture medium (Supplementary table 1). CV-N was the single most abundant protein and accounted for 13% of the proteins by weight. The dominant protein classes were pathogenesis-related proteins (17%), peroxidases (17%) and chitinases (15%). Proteases comprised 9% of protein by weight. In plants cultured without NAA, 74 proteins were identified (Supplementary table 2) with CV-N comprising 6% of the proteins by weight. In this group, peroxidases (33%) were higher and proteases (3%) were lower as a proportion of rhizosecretome protein weight than plants cultured with NAA. In addition, expansins and proteins associated with carbohydrate metabolism and oxidative stress were absent altogether.
Figure 2.

Relative abundance of protein classes in the rhizosecretome of plants cultured in MS medium with or without NAA. Hydroponic medium was analyzed by LC-ESI-MS/MS followed by a Mascot search with plant taxonomy parameter and a calculation of Exponentially Modified Protein Abundance Index.
3.3 Ion exchange chromatography is suitable for concentration of hydroponic medium and partial purification of CV-N
In order to find optimal conditions for CV-N binding and elution, Vivapure Q Mini H spin columns (Sartorius) were used. The pI of CV-N is 5.2, so the pH values tested for binding to a Q column were 6.4, 7.4, 8.4 and 9.4. Hydroponic medium containing rhizosecreted CV-N was dialyzed against either MES pH 6.4, HEPES pH 7.4, Tris-HCl pH 8.4 or carbonate buffer pH 9.4. Two millilitres of the dialyzed medium were used for each purification using the appropriate buffer, according to the manufacturer’s instructions. Elution was performed with 0.1M, 0.2M, 0.4M, 0.6M, 0.8M and 1M NaCl successively in the appropriate buffer. From this series of experiments, it was concluded that pH 8.4 was the most appropriate for effective CV-N binding to the membrane, and that 20mM followed by 40 mM NaCl efficiently eluted CV-N without eluting many other contaminating proteins (data not shown).
Based on the conditions found with Vivapure Q Mini H columns, a larger volume of hydroponic medium was used for purification of CV-N with a 1 ml HiTrap Q HP resin ion-exchange column (GE) connected to an Akta® FPLC. Samples, before and after ion-exchange chromatography, were analyzed by gp120 binding ELISA (Supplementary figure 2), and most reactive samples were analyzed by SDS-PAGE and silver staining (Figure 3A). Lane ‘CV-N (100 ng)’ shows the E. coli derived purified control CV-N with a faint band at ~11 kDa. A stronger 50 kDa band which may result of the aggregation of the E.coli-derived CV-N can also be observed. This was not observed in subsequent analysis. ‘Loaded sample’ shows the starting hydroponic medium, with the same band at ~11 kDa. Most, but not all of the proteins bound to the ion exchange column (‘flow-through’) and the majority of CV-N was released in the first elution with 200 mM NaCl (‘elution 1’). Subsequent elutions (‘elution 2 and 3 fractions’) showed that the majority of contaminating higher molecular weight proteins were separated from CV-N by the ion exchange protocol, and that the majority of CV-N had been eluted in the first step. A Western blot confirmed the presence of CV-N in the Elution 1 fraction. As shown in Figure 3B, the 11 kDa band observed in the silver stained gel was confirmed as CV-N, but none of the higher molecular weight contaminating bands were immunoreactive.
Figure 3.
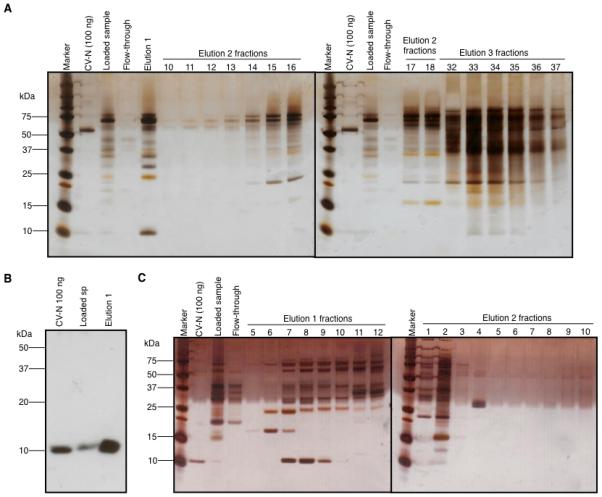
CV-N semi-purification by IEX (A) Silver stained SDS-PAGE gel with samples from the first IEX run for purification of CV-N. (B) Western blot to detect CV-N in IEX Elution 1. (C) Silver-stained SDS-PAGE gel with samples from the second IEX run for purification of CV-N. Marker - Molecular weight protein markers, CV-N (100 ng) - purified E. coli CV-N positive control (100 ng), loaded sample - dialyzed hydroponic medium prior to column loading, flow-through – IEX column flow through.
To allow maximal concentration of CV-N, this method was repeated using the same volume of hydroponic medium, but collecting 1 ml fractions during elution 1 on a 0 to 200 mM NaCl gradient. Elution step 1 was performed with 12×1 ml fractions, elution step 2 with 10×1ml fractions and elution step 3 was individually pooled, with 10 ml of the appropriate elution buffer.
As previously observed, CV-N was eluted early on in the process. It was predominantly concentrated in elution 1, fractions 7, 8 and 9 (Figure 3C), corresponding to 60, 80 and 100 mM [NaCl] respectively. This was confirmed by an antigen-specific binding activity of each fraction in an ELISA (Supplementary Figure 3). Although some CV-N was detected in many fractions in the ELISA, the concentration was below the limit of detection of a silver-stained gel. Most contaminants were eluted separately from CV-N (Fig. 3C, elution 2 fraction 2).
Fractions 7 and 8 and the neat hydroponic medium were analyzed by gp120 ELISA for CV-N quantification and total soluble protein (TSP) quantification by BCA, in order to determine the percentage of active CV-N in each sample. CV-N concentration in crude hydroponic medium was 3 μg/ml, corresponding to 1.3% of the TSP. In fractions 7 and 8, the concentration increased to 53 μg/ml and 81 μg/ml, corresponding to 50% and 45% of the TSP in each fraction, respectively. CV-N recovery from fractions 7 and 8 corresponded to 89% of the amount loaded onto the column.
3.4 Rhizosecreted CV-N has anti-HIV activity comparable to E. coli-derived CV-N
A cell based HIV neutralisation assay was undertaken to demonstrate the functionality of the purified CV-N. CV-N purified from hydroponic medium by IEX (Figure 3C, elution fraction 7) was compared to recombinant CV-N produced in E.coli (Figure 4). The negative control, PBS, had no effect on HIV infectivity. CV-N from both E. coli and plants effectively neutralised HIV with a comparable IC50 (4-6 nM).
Figure 4.
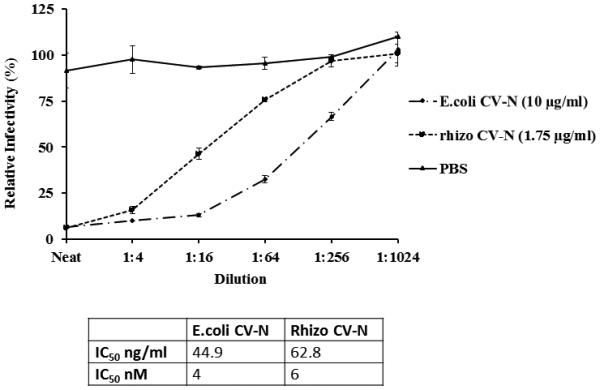
Inhibition of HIV infection by rhizosecreted CV-N following IEX. Luciferase assays were performed by incubating the reporter cell line TZM-bl with HIVBaL and rhizosecreted IEX-purified CV-N or E. coli derived CV-N (initial concentration of 1.75μg/ml and 10 μg/ml, respectively) or PBS. The viral infectivity is plotted against the dilution of the sample, and results are the mean and SEM of three experiments. The table indicates the IC50 of rhizosecreted IEX-purified and E.coli-derived CV-N. Calculations were performed using GraphPad Prism software.
4 DISCUSSION
The development of an effective HIV microbicide is of particular interest because of the global pandemic spread of the virus and the difficulty in developing a successful vaccine [23]. It has been suggested that a successful microbicide would be important to slow transmission, particularly among women, until an effective vaccine is produced [24]. Because microbicides, in contrast to condoms, are potentially not a barrier to intimate contact or conception, it is likely that they will be more readily accepted by men and women. Importantly, women would be in control and responsible of microbicide application [25].
We previously identified CV-N as being a suitable target for production by rhizosecretion [14] as it is a small and stable molecule which should be readily secreted by plant cells and remain intact in the hydroponic medium. Yields were initially around 1 μg/ml [14], but were boosted 6-fold by the addition of NAA reaching a maximum of 6 μg/ml [16]. However, subsequent attempts to increase rhizosecretion yields of CV-N by use of elicitors, cell wall degrading agents and manipulation of medium constituents have been unsuccessful (data not shown).
4.1 Agitation increased sucrose consumption by plants, induced changes in root morphology and resulted in enhanced CV-N yields in hydroponic medium
Agitation of plants increased yields of CV-N in the medium, and this may have been due to several possible mechanisms. Firstly, agitation may have improved the diffusion of proteins from the root cell apoplast to the external hydroponic medium. We also theorised that increase in CV-N yields might be due to greater availability of oxygen to plants in the shaking group leading to increased respiration rates, higher root biomass and greater protein production. If this was the case, we would expect sucrose consumption to be greater in the shaking group and this was indeed observed (Supplementary Figure 1.). Roots of agitated plants were considerably thicker than roots in non-agitated plants and had short lateral roots from which a high density of root hairs proliferated which may have given additional root surface area for secretion of proteins into the medium. Recently, we have reported that the NAA treatment used in our rhizosecretion production system induces extensive proliferation of root lateral primordia [21]. In the present study however, lateral root primordia were not detected in the agitated roots, suggesting that the agitation induced the primordia to elongate into short laterals, possibly by disrupting the gravity sensing mechanism of the roots resulting in decreased auxin transport rate from the root cap to the elongation zone allowing lateral root emergence. Finally, we cannot exclude the possibility that agitation caused damage to the roots and especially the fragile root hairs, resulting in greater secretion of CV-N into the medium.
CV-N concentrations in hydroponic medium were measured in 5 consecutive weekly harvests and in each case were higher in shaken plants than in stationary controls, reaching a maximum of over 20 μg/ml. Whilst this increase in yield is significant – over 3 times higher than previously published data- the increased costs involved in agitation of plants cultures would need to be considered prior to implementation in a large-scale production protocol.
4.2 Downstream processing of recombinant proteins from hydroponic medium is simpler than from vegetative tissues
Perhaps the most important advantage of rhizosecretion as a production system for recombinant pharmaceuticals is the potential relative ease of purification from hydroponic medium compared to vegetative tissues: downstream processing from the latter accounts for up to 80% of the production costs [17]. For extraction from vegetative tissues, downstream processing starts with the harvesting of plant biomass followed by tissue disruption, extraction of the target protein into an aqueous medium, clarification of the crude extract, and finally purification of the product [17]. Because the final phase requires high specificity and is intrinsically expensive, it is important that the first and second phases are efficient and have as few steps as possible in order to reduce overall costs [26]. For plants, this is particular difficult since plant solids, which require early removal after extraction, are generally in higher concentration, wider in size range, and denser than traditional bacterial and mammalian cell culture debris [17].
4.3 NAA alters the rhizosecretome
In the present study, an investigation of rhizosecreted proteins in tobacco plants was undertaken prior to the establishment of purification protocols for CV-N. LC-ESI-MS/MS allowed the identification of proteins in the hydroponic medium. We routinely include NAA in the culture regime as we have previously demonstrated that it results in an increase in rhizosecretion of recombinant proteins [16]. However, in the present study we also included plants cultured without NAA to ascertain the effect of this plant growth regulator on rhizosecretion of proteins. The proteins found in the rhizosecretome of NAA-treated plants were comparable to what we observed previously for monoclonal antibody-expressing plants [21]. Comparison of the samples by category abundance revealed differences between the CV-N and CV-N NAA samples. One such difference was the much lower abundance of peroxidases in the NAA sample (17% vs. 33%). Peroxidases are related to either lignification of cell wall or response to wounding [27] and it has been demonstrated that the auxins IAA and NAA strongly suppress the expression of these enzymes in tobacco protoplasts [28]. Another difference elicited by NAA treatment was the presence of additional protein categories in the CV-N NAA sample. One category was expansins, proteins that can degrade the polysaccharides in the cell wall and have been described as responsible for the acid growth triggered by auxins [29]. Another category was proteins involved in oxidative stress, which included cytochrome-C and reactive oxygen species (ROS) scavenging proteins, such as thioredoxin h. The fact that these proteins were found in the extracellular medium of plants treated with NAA suggests that treatment induced apoptosis or senescence of the root cells. Another indication that NAA triggered early senescence, was the presence of carbohydrate metabolism proteins in CV-N NAA samples. Proteins such as fructose-biphosphate aldolase or malate dehydrogenase detected here are not extracellular, and are normally resident inside the mitochondria: their presence in the medium suggests that intracellular compounds were released, possibly as a result of cell death.
Previously, we observed that a monoclonal antibody (mAb) was less degraded when produced by rhizosecretion than when extracted with leaf tissue. In addition, the proteolytic activity of leaf intercellular fluid was demonstrated to be greater than the hydroponic medium in which plants were cultured [16]. We hypothesized that a mAb produced in the leaf would remain in the apoplast, a protease-rich environment (17 peptidases have been identified in tobacco intercellular fluid [30]), whilst mAbs produced by rhizosecretion could diffuse from the root cell into the hydroponic medium which had lower proteolytic activity.
In the present study, proteases accounted for 9% of protein content in the CV-N NAA hydroponic medium. A subtilisin-like (serine) protease was identified which has previously been demonstrated to be present in the extracellular medium of BY-2 cells [31]. In addition, another protein with protease activity, CND41, was also present in the medium. CND41 is a chloroplast nucleoid DNA-binding protein, containing an aspartic protease domain, which has strong proteolytic activity at acidic pH [32].
4.4 IEX is a simple and inexpensive methodology to reduce large medium volumes and partially purify functional CV-N
The purification of mAb from hydroponic medium has already been demonstrated to be extremely simple: following adjustment of pH, there is a single filtration step prior to affinity chromatography using an established ligand such as protein A [16, 21]. However, for CV-N there is no inexpensive, readily sourced, affinity ligand which can be incorporated into a column or resin. Several alternative options are available however. A combination of ultrafiltration, centrifugal partition chromatography and ATPS has previously been investigated to purify CV-N from MS-based hydroponic medium [18], but in the present study IEX was employed as it is a relatively inexpensive method and the membrane or resin can be recycled. The protocols comprised 3 simple steps – dialysis, sterile-filtration and chromatography. Sterile-filtration was used as a standard method prior to chromatography, but in large scale it might be unnecessary – hydroponic medium derived from the rhizosecretion system is a clear solution with a very small amount of root debris, and filtration through a wider membrane is presumably a possibility. Similarly, dialysis may not be regarded as a scalable process, and an alternative would be the use of salt-tolerant anion exchangers, such as the HyperCel STAR AX, which have been demonstrated to provide significant operations and cost benefits in downstream purification [33].
Several conditions were tested and optimized for CV-N binding and elution in order to maximize CV-N concentration and the elimination of impurities. Optimal purification was obtained at pH 8.4 using resin IEX and eluting at 60, 80 and 100 mM salt. The CV-N collected in elutions 7 and 8 was substantially concentrated compared to hydroponic medium, with impurities in this fraction above Mr 25 kDa. The majority of contaminating proteins were eluted in subsequent fractions. The IEX semi-purified product bound to gp120 in an ELISA which also confirmed the increase in concentration of CV-N compared to the amount in hydroponic medium. Total CV-N recovery from fractions 7 and 8 was 89% with approximately 50% purity. The principal contaminating protein was 75 kDa in size, and by reference to the rhizosecretome data (supplementary table 2, hit number 5) may well be a thaumatin-like pathogenesis-related protein (Genbank accession #: 131015). Ultrafiltration has been previously demonstrated to be suitable for elimination of high molecular weight proteins from hydroponic medium [18], and this technique could be applied in tandem to IEX for a complete purification of CV-N.
In a cell-based HIV neutralisation assay, the IEX semi-purified CV-N had an IC50 of 6nM which was of comparable to that of the purified E. coli control (4nM). Taken together, these results confirm that IEX chromatography is suitable for CV-N purification from plant-derived hydroponic medium.
5 Concluding remarks
Despite the advantages of the rhizosecretion system, large volumes and product dilution are still greater than in the whole leaf systems. However, CV-N production by rhizosecretion has now been substantially improved since it was first reported [14], and this has facilitated the options for downstream processing, including IEX. Overcoming the first step in downstream purification was important as it addressed the critical issue of volume reductions and target product concentration. The next stage will be to refine polishing steps to achieve greater product purity, for clinical administrations. Future investigations will focus on further purifying CV-N using a combination of ultrafiltration, IEX and flash chromatography.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Dr Helen Byers from the St. George’s Biomics Center for the LC/MS analysis, to Dr. Barry O’Keefe from the Nation Cancer Institute, NIH, for kindly providing purified CV-N and anti-CV-N antibody, and to Dr. Viviana Buffa from St. George’s, University of London, for assistance with the HIV assays. This project was jointly funded by the NIH Microbicide Innovation Program (III), the Comopharm European Union FP7 project grant, EU COST ACTION FA0804, the Sir Joseph Hotung Endowment and the Dr Hadwen Trust.
Abbreviations
- ACN
acetonitrile
- ATPS
aqueous two-phase systems
- BSA
bovine serum albumin
- CV-N
cyanovirin-N
- DMEM
Dulbecco’s modified eagle medium
- IEX
ion exchange
- LC-ESI-MS/MS
liquid chromatography-electrospray ionisation tandem mass spectroscopy
- mAb
monoclonal antibody
- MS
Murashige and Skoog
- NAA
alpha napthaleneacetic acid
- ROS
reactive oxygen species
Footnotes
The authors declare no financial or commercial conflict of interest
References
- [1].Boyd MR, Gustafson KR, McMahon JB, Shoemaker RH, et al. Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: potential applications to microbicide development. Antimicrob Agents Chemother. 1997;41:1521–1530. doi: 10.1128/aac.41.7.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Shenoy SR, O'Keefe BR, Bolmstedt AJ, Cartner LK, Boyd MR. Selective interactions of the human immunodeficiency virus-inactivating protein cyanovirin-N with high-mannose oligosaccharides on gp120 and other glycoproteins. J Pharmacol Exp Ther. 2001;297:704–710. [PubMed] [Google Scholar]
- [3].Shattock RJ, Moore JP. Inhibiting sexual transmission of HIV-1 infection. Nat Rev Microbiol. 2003;1:25–34. doi: 10.1038/nrmicro729. [DOI] [PubMed] [Google Scholar]
- [4].Buffa V, Stieh D, Mamhood N, Hu Q, et al. Cyanovirin-N potently inhibits human immunodeficiency virus type 1 infection in cellular and cervical explant models. J Gen Virol. 2009;90:234–243. doi: 10.1099/vir.0.004358-0. [DOI] [PubMed] [Google Scholar]
- [5].Tsai CC, Emau P, Jiang Y, Agy MB, et al. Cyanovirin-N inhibits AIDS virus infections in vaginal transmission models. AIDS Res Hum Retroviruses. 2004;20:11–18. doi: 10.1089/088922204322749459. [DOI] [PubMed] [Google Scholar]
- [6].Tsai CC, Emau P, Jiang Y, Tian B, et al. Cyanovirin-N gel as a topical microbicide prevents rectal transmission of SHIV89.6P in macaques. AIDS Res Hum Retroviruses. 2003;19:535–541. doi: 10.1089/088922203322230897. [DOI] [PubMed] [Google Scholar]
- [7].Mori T, Barrientos LG, Han Z, Gronenborn AM, et al. Functional homologs of cyanovirin-N amenable to mass production in prokaryotic and eukaryotic hosts. Protein Expr Purif. 2002;26:42–49. doi: 10.1016/s1046-5928(02)00513-2. [DOI] [PubMed] [Google Scholar]
- [8].Hu Q, Mahmood N, Shattock RJ. High-mannose-specific deglycosylation of HIV-1 gp120 induced by resistance to cyanovirin-N and the impact on antibody neutralization. Virology. 2007;368:145–154. doi: 10.1016/j.virol.2007.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Witvrouw M, Fikkert V, Hantson A, Pannecouque C, et al. Resistance of human immunodeficiency virus type 1 to the high-mannose binding agents cyanovirin N and concanavalin A. J Virol. 2005;79:7777–7784. doi: 10.1128/JVI.79.12.7777-7784.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Colleluori DM, Tien D, Kang F, Pagliei T, et al. Expression, purification, and characterization of recombinant cyanovirin-N for vaginal anti-HIV microbicide development. Protein Expr Purif. 2005;39:229–236. doi: 10.1016/j.pep.2004.10.009. [DOI] [PubMed] [Google Scholar]
- [11].Giomarelli B, Provvedi R, Meacci F, Maggi T, et al. The microbicide cyanovirin-N expressed on the surface of commensal bacterium Streptococcus gordonii captures HIV-1. Aids. 2002;16:1351–1356. doi: 10.1097/00002030-200207050-00006. [DOI] [PubMed] [Google Scholar]
- [12].Liu X, Lagenaur LA, Simpson DA, Essenmacher KP, et al. Engineered vaginal lactobacillus strain for mucosal delivery of the human immunodeficiency virus inhibitor cyanovirin-N. Antimicrob Agents Chemother. 2006;50:3250–3259. doi: 10.1128/AAC.00493-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pusch O, Boden D, Hannify S, Lee F, et al. Bioengineering lactic acid bacteria to secrete the HIV-1 virucide cyanovirin. J Acquir Immune Defic Syndr. 2005;40:512–520. doi: 10.1097/01.qai.0000187446.76579.d3. [DOI] [PubMed] [Google Scholar]
- [14].Sexton A, Drake PM, Mahmood N, Harman SJ, et al. Transgenic plant production of Cyanovirin-N, an HIV microbicide. Faseb J. 2006;20:356–358. doi: 10.1096/fj.05-4742fje. [DOI] [PubMed] [Google Scholar]
- [15].O'Keefe BR, Murad AM, Vianna GR, Ramessar K, et al. Engineering soya bean seeds as a scalable platform to produce cyanovirin-N, a non-ARV microbicide against HIV. Plant Biotechnol J. 2015 doi: 10.1111/pbi.12309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Drake PMW, Barbi T, Sexton A, McGowan E, et al. Development of rhizosecretion as a production system for recombinant proteins from hydroponic cultivated tobacco. Faseb Journal. 2009;23:3581–3589. doi: 10.1096/fj.09-131771. [DOI] [PubMed] [Google Scholar]
- [17].Drossard J. Downstream Processing of Plant-Derived Recombinant Therapeutic Proteins. In: Fischer R, Schillberg S, editors. Molecular Farming: Plant-Made Pharmaceutical and technical Problems. Wiley-VCH Veriag GmbH & Co.; Weinheim, Germany: 2004. pp. 217–232. [Google Scholar]
- [18].Grudzień Ł, Madeira L, Fisher D, Ma J, Garrard I. Phase system selection with fractional factorial design for purification of recombinant cyanovirin-N from a hydroponic culture medium using centrifugal partition chromatography. Journal of Chromatography A. 2013;1285:57–68. doi: 10.1016/j.chroma.2013.02.011. [DOI] [PubMed] [Google Scholar]
- [19].Murashige T, Skoog F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiologia Plantarum. 1962;15:473–497. [Google Scholar]
- [20].Drake PMW, Chargelegue DM, Vine ND, van Dolleweerd CJ, et al. Rhizosecretion of a monoclonal antibody protein complex from transgenic tobacco roots. Plant Molecular Biology. 2003;52:233–241. doi: 10.1023/a:1023909331482. [DOI] [PubMed] [Google Scholar]
- [21].Madeira LM, Szeto TH, Henquet M, Raven N, et al. High-yield production of a human monoclonal IgG by rhizosecretion in hydroponic tobacco cultures. Plant Biotechnol J. 2016;14:615–624. doi: 10.1111/pbi.12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Montefiori DC. Current Protocols in Immunology. John Wiley & Sons, Inc.; 2001. Evaluating Neutralizing Antibodies Against HIV, SIV, and SHIV in Luciferase Reporter Gene Assays. [DOI] [PubMed] [Google Scholar]
- [23].Balzarini J, Van Damme L. Microbicide drug candidates to prevent HIV infection. Lancet. 2007;369:787–797. doi: 10.1016/S0140-6736(07)60202-5. [DOI] [PubMed] [Google Scholar]
- [24].Lederman MM, Offord RE, Hartley O. Microbicides and other topical strategies to prevent vaginal transmission of HIV. Nat Rev Immunol. 2006;6:371–382. doi: 10.1038/nri1848. [DOI] [PubMed] [Google Scholar]
- [25].Stone A. Microbicides: a new approach to preventing HIV and other sexually transmitted infections. Nat Rev Drug Discov. 2002;1:977–985. doi: 10.1038/nrd959. [DOI] [PubMed] [Google Scholar]
- [26].Lahtinen T, Linder MB, Nakari-Setala T, Oker-Blom C. ydrophobin (HFBI): A potential fusion partner for one-step purification of recombinant proteins from insect cells. Protein Expr Purif. 2008;59:18–24. doi: 10.1016/j.pep.2007.12.014. [DOI] [PubMed] [Google Scholar]
- [27].Lagrimini LM, Rothstein S. Tissue Specificity of Tobacco Peroxidase Isozymes and Their Induction by Wounding and Tobacco Mosaic Virus Infection. Plant Physiology. 1987;84:438–442. doi: 10.1104/pp.84.2.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sitbon F, Hennion S, Little CHA, Sundberg B. Enhanced ethylene production and peroxidase activity in IAA-overproducing transgenic tobacco plants is associated with increased lignin content and altered lignin composition. Plant Sci. 1999;141:165–173. [Google Scholar]
- [29].Cosgrove DJ, Li ZC. Role of Expansin in Cell Enlargement of Oat Coleoptiles (Analysis of Developmental Gradients and Photocontrol) Plant Physiol. 1993;103:1321–1328. doi: 10.1104/pp.103.4.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Delannoy M, Alves G, Vertommen D, Ma J, et al. Identification of peptidases in Nicotiana tabacum leaf intercellular fluid. Proteomics. 2008;8:2285–2298. doi: 10.1002/pmic.200700507. [DOI] [PubMed] [Google Scholar]
- [31].Navarre C, De Muynck B, Alves G, Vertommen D, et al. Identification, gene cloning and expression of serine proteases in the extracellular medium of Nicotiana tabacum cells. Plant Cell Reports. 2012;31:1959–1968. doi: 10.1007/s00299-012-1308-y. [DOI] [PubMed] [Google Scholar]
- [32].Murakami S, Kondo Y, Nakano T, Sato F. Protease activity of CND41, a chloroplast nucleoid DNA-binding protein, isolated from cultured tobacco cells. FEBS Lett. 2000;468:15–18. doi: 10.1016/s0014-5793(00)01186-8. [DOI] [PubMed] [Google Scholar]
- [33].Champagne J, Balluet G, Gantier R, Toueille M. “Salt tolerant” anion exchange chromatography for direct capture of an acidic protein from CHO cell culture. Protein Expression and Purification. 2013;89:117–123. doi: 10.1016/j.pep.2013.03.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


