Abstract
Background & Aims
Activating mutations of PIK3CA occur in various tumor types, including human hepatocellular carcinoma. The mechanisms whereby PIK3CA contributes to hepatocarcinogenesis remain poorly understood.
Methods
PIK3CA mutants H1047R or E545K were hydrodynamically transfected, either alone or in combination with NRasV12 or c-Met genes, in the mouse liver.
Results
Overexpression of H1047R or E545K alone was able to induce AKT/mTOR signaling in the mouse liver, leading to hepatic steatosis. However, none of the mice developed liver tumors over long term. In contrast, H1047R or E545K cooperated with NRasV12 or c-Met rapidly induce liver tumor formation in mice. At the molecular level, all the tumor nodules displayed activation of AKT/mTOR and Ras/MAPK cascades. Ablation of AKT2 significantly inhibited hepatic steatosis induced by H1047R or E545K and carcinogenesis induced by H1047R/c-Met or E545K/c-Met. Furthermore, tumorigenesis induced by H1047R/c-Met was abolished in conditional Raptor knockout mice.
Conclusions
In conclusion, both H1047R and E545K are able to activate the AKT/mTOR pathway. An intact AKT2/mTORC1 cascade is required for tumorigenesis induced by H1047R/c-Met or E545K/c-Met in the liver.
Keywords: PIK3CA mutants, NRasV12, c-Met, AKT/mTOR, liver cancer
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common tumor and the third most frequent cause of cancer-related deaths worldwide [1]. When diagnosed at early stage, HCC is amenable to potentially curative treatments such as resection, liver transplantation, and radiofrequency ablation [2]. However, most HCC patients are diagnosed at advanced stage, when effective treatment options are lacking [2, 3]. The only approved drug for the treatment of advanced HCC is Sorafenib, a multi-kinase inhibitor, whose overall efficacy in improving patient’s survival is very limited [4]. Thus, novel therapeutic strategies against advanced HCC are required [5]. For this purpose, a deeper understanding of the molecular mechanisms underlying hepatocarcinogenesis is necessary.
The phosphoinositide 3-kinase (PI3K) cascade is one of the most deregulated pathways along tumorigenesis [6]. PI3Ks catalyze the production of phosphatidylinositol-3,4,5-triphosphate (PIP3) at the cell membrane. PIP3 in turn recruits and activates a wide range of downstream effectors, such as PDK, AKT, and SGK [6, 7]. Among PI3K substrates, AKT is considered the major downstream effector. AKT exerts many biological effects through its key downstream effector, the mTOR complex 1 (mTORC1) [8]. mTORC1 activates two distinct downstream signaling: p70S6K and 4EBP1. p70S6K positively regulates 40S ribosomal protein S6 (RPS6), leading to increased expression of SREBP1 and consequent de novo lipogenesis in cells, whereas 4EBP1 negatively regulates eIF4E, the rate-limiting enzyme for cap-dependent translation [9].
Mutant forms of PIK3CA, the p110α catalytic subunit, have been detected in multiple tumor types, including colon, breast, lung, and gastric cancer [10]. Somatic mutations of PIK3CA cluster around two hotspot regions: helical domain (exon 9, E545K) and kinase domain (exon 20, H1047R). Both E545K and H1047R mutants have been demonstrated to transform cells in vitro [10, 11]. Furthermore, experiments using genetically engineered mouse models (GEMMs) confirmed the oncogenic role of activated PIK3CA mutants in vivo [10]. Interestingly, a recent study reported that while tumor cell lines with PIK3CA H1047R showed high levels of p-AKT, tumor cell lines with PIK3CA E545K tended to show low p-AKT expression. [12]. Subsequent functional analysis suggested that PIK3CA helical domain mutants might drive tumorigenesis predominantly through the PDK1/SKG3 cascade [12]. It is important to note that most GEMMs utilized PIK3CA H1047R, and whether PIK3CA helical domain and kinase domain mutants have similar biological activity in triggering AKT activation and tumorigenesis in vivo, especially in the liver, has not been satisfactorily investigated.
In human HCC, activated mutant forms of PIK3CA occur in approximately 4% of tumor samples (cancer.sanger.ac.uk.cosmic). Similar to other tumor types, PIK3CA mutations in HCC occur around helical and kinases domains. In the present study, we investigated the functional contribution of PIK3CA H1047R and E545K in mouse hepatocarcinogenesis.
Materials and methods
Constructs and reagents
The constructs used for mouse injection, including pT2CAGGS-NRasV12, c-Met and pCMV/sleeping beauty transposase (SB), were described previously [13–15]. pBabe-HA-PIK3CA wild-type (No. 12522), pBabe-HA-PIK3CAH1047R (No. 12524), and pBabe-HA-PIK3CAE545K (No. 12525) constructs were purchased from Addgene. PIK3CA wild-type (PIK3CA WT), H1047R, and E545K were cloned into pT3EF1α vector through the Gateway cloning system (Invitrogen). All the plasmids were purified using the Endotoxin free Maxi prep kit (Sigma, St. Louis, MO).
Hydrodynamic injection and mouse treatment
Wild-type FVB/N mice were obtained from Charles River (Wilmington, MA). AKT2 knockout mice [16] were generated by crossing AKT2+/− mice. Raptorfl/+ mice [17] were purchased from the Jackson Laboratory (Bar Harbor, ME; stock: 013188), and intercrossed to generate Raptorfl/fl mice. Hydrodynamic injections were performed as described [18]. To determine whether overexpression of PIK3CA plasmid alone can induce hepatic steatosis and carcinogenesis, 20μg PIK3CA WT, H1047R or E545K along with 0.8μg SB plasmid were delivered into FVB/N mouse liver by hydrodynamic injection. For the tumorigenesis models, 20μg H1047R or E545K, 20μg NRasV12 or c-Met along with 1.6μg SB plasmid were delivered into FVB/N mouse liver. The same amount and combination of plasmids were delivered into AKT2 wild-type and AKT2 knockout mice. To determine the requirement of mTORC1 in PIK3CA-dependent hepatocarcinogenesis, high dose of Cre (60μg) or pT3EF1α (60μg) was mixed with H1047R (20μg), c-Met (20μg) and SB (4μg), and injected into Raptorfl/fl mice. Mice were housed, fed, and monitored in accordance with protocols approved by the Committee for Animal Research at the University of California, San Francisco.
Histology, immunohistochemistry and immunoblotting
Preneoplastic and neoplastic liver lesions were assessed by two board-certified pathologists (M.E. and F.D.) in accordance with the criteria by Frith et al. [19]. Immunohistochemistry and immunoblotting was performed as previously described [20, 21]. Antibodies were described in Supplementary Table 1.
Oil Red O staining
Oil Red O Staining was performed using the Oil Red O Staining Kit (American MasterTech, Lodi, CA).
Additional method is described in Supplementary file.
Results
PIK3CA H1047R and E545K mutants activates the AKT pathway in the mouse liver
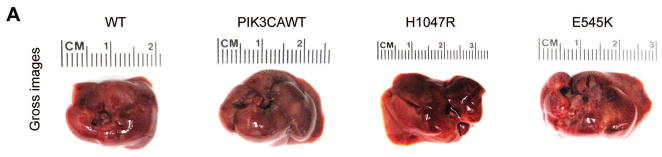
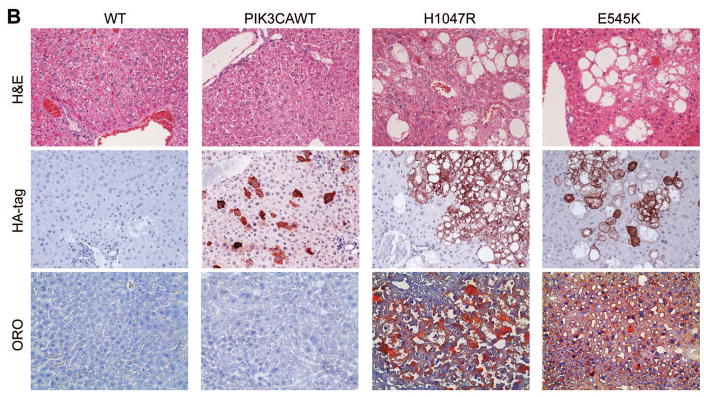
To determine whether PIK3CA helical domain mutant or kinase domain mutant can induce similar or distinct biological processes, we hydrodynamically transfected PIK3CA wild-type (PIK3CAWT), H1047R or E545K constructs into the mouse liver. All mice were harvested 4 weeks post injection. Macroscopically, we found that livers from PIK3CAWT injected mice appeared to be normal, whereas both H1047R and E545K injected mouse livers were pale and spotty (Fig. 1A). At the histological level, PIK3CAWT livers were completely normal, undistinguishable from mouse livers uninjected or injected with empty vector. In contrast, ~30–40% of the liver parenchyma of H1047R or E545K mice was occupied by lipid-rich hepatocytes with an enlarged cytoplasm, leading to hepatic steatosis (Fig. 1B). No signs of inflammation in association with steatosis were detected in H1047R and E545K mouse livers (data not shown). To confirm that the observed changes in hepatocytes were d induced by the ectopically injected oncogene, immunohistochemistry (IHC) was performed in the same livers using an anti-HA antibody, which indicates the expression of the injected PIK3CA wild-type or mutant form. As expected, strong expression of HA-tag was detected in lipid-rich hepatocytes from mice injected with H1047R and E545K mutants (Fig. 1B). In addition, scattered hepatocytes from mice injected with PIK3CAWT exhibited HA immunoreactivity, thus substantiating the successful transfection of the latter gene in mice (Fig. 1B). No HA immunolabeling was detected in wild-type mice, either uninjected mice (Fig. 1B) or mice injected with the empty vector (data not shown). Lipid accumulation in H1047R and E545K mouse livers, but not wild-type and PIK3CAWT livers, was further substantiated by Oil Red O staining (Fig. 1B). Noticeably, this phenotype is highly similar to that observed when an activated form of AKT was overexpressed or when Pten, a cellular inhibitor of AKT, was deleted in the mouse liver [20, 22, 23]. At the molecular level, we found that higher levels of p-AKT (both S473 and T308) and AKT substrates (p-GSK3, p-PRAS40 and p-FoxO1/3a) as well as of p-ERK1/2 could be detected in H1047R or E545K liver tissues (Supplementary Fig. 1B). Higher levels of cell proliferation, as demonstrated by increased Ki67 staining, were detected in liver tissues from H1047R and E545K injected mice, when compared with wild-type and PIK3CAWT-injected mice (Supplementary Fig. 1A). Consistent with the notion that increased lipogenesis is a major metabolic event downstream of activated AKT/mTOR [20], we found high expression of lipogenic enzymes, including Fatty Acid Synthase (FASN) and Stearoyl-CoA desaturase-1 (SCD1), in H1047R or E545K liver tissues (Supplementary Fig. 1A).
Fig. 1. Overexpression of PIK3CA H1047R and E545K mutants induces steatosis in the mouse liver.
(A) Gross images of livers from wild-type mice (WT), and mice injected with WT PIK3CA wild-type (PIK3CAWT), PIK3CA H1047R (H1047R), and PIK3CA E545K (E545K) constructs. PIK3CAWT, PIK3CA H1047R, and PIK3CA E545K mice were sacrificed 6 weeks post hydrodynamic injection. (B) Upper panels: hematoxylin and eosin (H&E) staining of WT, PIK3CAWT, PIK3CA H1047R, and PIK3CA E545K mouse livers. While WT and PIK3CAWT livers were histologically undistinguishable from each other, PIK3CA H1047R and PIK3CA E545K livers displayed the presence of clusters of lipid-rich cells with an enlarged cytoplasm. Middle panels: the successful integration of PIK3CAWT, H1047R, and E545K genes was confirmed by strong immunoreactivity in hepatocytes for the HA-tag present in the transfected construct. Lower panels: Oil Red O (ORO) staining of WT, PIK3CAWT, PIK3CA H1047R, and PIK3CA E545K mouse livers showing intense staining only in PIK3CA H1047R and PIK3CA E545K livers. Magnifications: 200X.
To determine whether these PIK3CA mutants can induce liver tumor formation in mice, we aged H1047R or E545K injected mice up to 40 weeks post injection. No tumor nodules were identified in these mice by macroscopical and microscopical examination (n=5/group, data not shown).
Altogether, our study demonstrates that PIK3CA H1047R and E545K mutants, but not wild-type PIK3CA, can fully activate the AKT signaling, leading to steatosis in the mouse liver.
H1047R and E545K cooperate with c-Met and NRasV12 to induce liver tumorigenesis in vivo
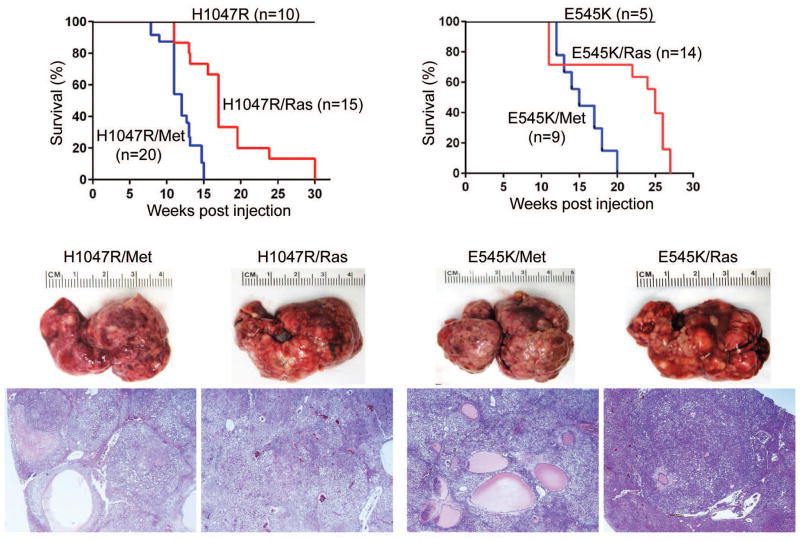
Since overexpression of H1047R or E545K alone failed to induce liver tumorigenesis in vivo, we sought to investigate whether these two activating PIK3CA mutations could cooperate with other oncogenic signals to promote liver malignant transformation in vivo. Previously, we showed that an activated form of AKT cooperates with NRasV12 [21] or c-Met (Hu J, unpublished observation) to induce liver cancer. Thus, we hypothesized that activating PIK3CA mutants could also cooperate with NRasV12 and c-Met to trigger hepatocarcinogenesis in mice. In agreement with previous studies [14], hydrodynamic transfection of NRasV12 alone did not lead to any abnormal liver histology (data not shown). Also, overexpression of c-Met alone resulted in liver dysplasia over long term (data not shown), in accordance with previous findings [24]. To test our hypothesis, we co-expressed the different combinations of the four plasmids (H1047R/NRasV12; H1047R/c-Met; E545K/NRasV12; and E545K/c-Met) into the mouse liver by hydrodynamic transfection. Importantly, we found that all the oncogenic combinations were able to induce liver tumor formation in mice (Fig. 2). In general, H1047R appeared to be slightly more potent than E545K in inducing liver tumor formation, and c-Met promoted tumor development faster than NRasV12.
Fig. 2. PIK3CA H1047R and E545K mutants cooperate with NRasV12 and c-Met to promote HCC development in mice.
Survival curve for PIK3CA H1047R (H1047R), PIK3CA H1047R/c-Met (H1047R/Met) and PIK3CA H1047R/NRasV12 (H1047R/Ras) mice. Survival curve for PIK3CA E545K (E545K), PIK3CA E545K/c-Met (E545K/Met) and PIK3CA E545K/NRasV12 (E545K/Ras) mice. Gross images (upper panels) and hematoxylin and eosin (H&E) staining (lower panels) of tumors developed in the four mouse models. Magnification: 40X.
At the histological level, lesions developed in H1047R/NRasV12, H1047R/c-Met, E545K/NRasV12, and E545K/c-Met were indistinguishable from each other. Indeed, all four mouse models developed preneoplastic and neoplastic lesions in the liver characterized predominantly by a clear cell phenotype, with most of the liver parenchyma being occupied by confluent hepatocellular adenomas and carcinomas (Supplementary Fig. 2). No cholangiocarcinomas were detected in any of the four mouse models. Lesions often displayed areas with basophilic changes and organized necrosis (i.e. pseudocyst formation; Supplementary Fig. 2). Premalignant and malignant hepatocytes consisted of clear cells with enlarged cytoplasm (due to intracellular lipid storage) or small, basophilic cells, showing modest cellular atypia (Supplementary Fig. 2, insets).
At the cellular level, preneoplastic and neoplastic liver lesions from H1047R/NRasV12, H1047R/c-Met, E545K/NRasV12, and E545K/c-Met mice exhibited significantly higher proliferation and apoptosis rates when compared with wild-type mice, without differences among the four mouse models. Both proliferation and apoptosis indices progressively increased from preneoplastic lesions to HCC in the four mouse models (Supplementary Fig. 3,).
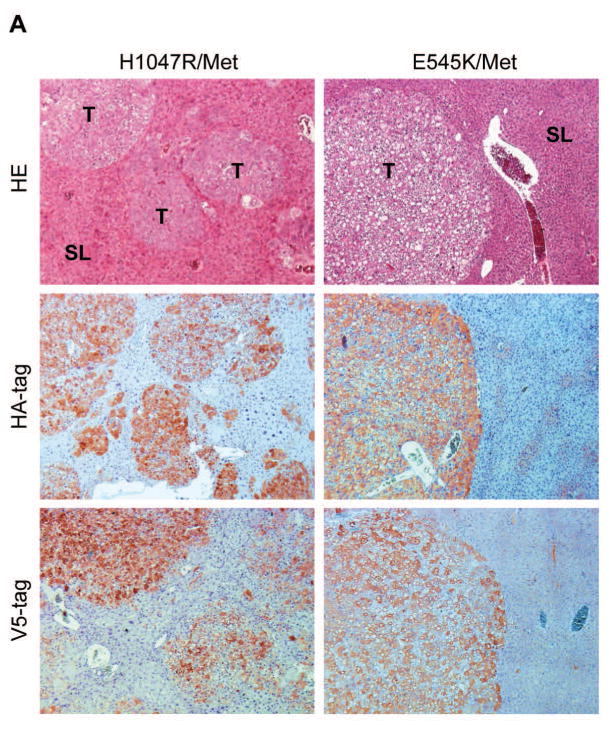
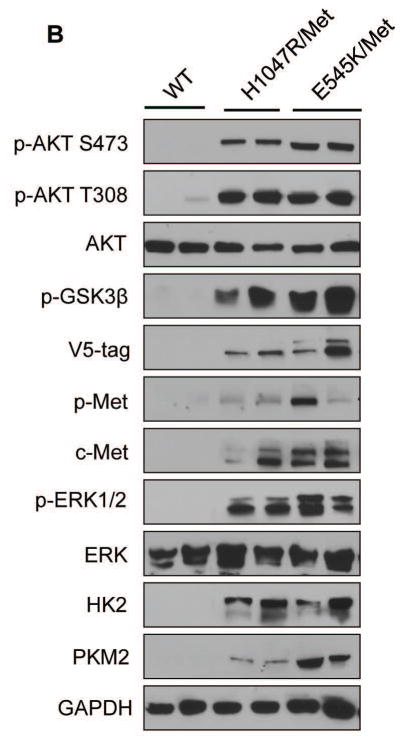
Ras mutations are rare in human HCC, and Ras mutations in the present experiments were used as a tool to enhance the levels of the MAPK cascade. On the other hand, c-Met is a major oncogenic driver along hepatocarcinogenesis [25]. Thus, we chose to focus our genetic and molecular analyses on H1047R/c-Met and E545K/c-Met HCC mice, as we believe those models to be more physiologically relevant to human HCC. By Western blotting and/or immunohistochemistry, we observed that ectopically injected c-Met (with antibodies specific for human c-Met and V5 tag) could be rapidly detected and was activated, as indicated by expression of p-c-Met, in H1047R/c-Met and E545K/c-Met tumor samples (Fig. 3A,B). Furthermore, tumor cells showed high levels of HA-tag of PIK3CA mutants (Fig. 3A) and strong activation of AKT and ERK/MAPK cascades (Fig. 3B). It is well known that increased glycolysis is a hallmark of tumorigenesis. Consistently, we found high expression of PKM2 and HK2, two key enzymes involved in glycolysis, in the liver tumor samples (Fig. 3B, Supplementary Fig. 4). In addition, high levels of FASN (Supplementary Fig. 4), ACC and SCD1 (not shown), key proteins involved in aberrant de novo lipid biosynthesis downstream of mTORC1 [20], were detected in H1047R/c-Met and E545K/c-Met tumor samples (Supplementary Fig. 4).
Fig. 3. Molecular characterization of liver tumors developed in PIK3CA H1047R/c-Met and PIK3CA E545K/c-Met mice.
(A) Upper panels: hematoxylin and eosin (H&E) staining and immunohistochemistry for HA-tagged PIK3CA and V5-tagged c-Met in PIK3CA H1047R/c-Met (H1047R/Met) and PIK3CA E545K/c-Met (E545K/Met) mice. H&E staining shows the presence of multiple hepatocellular tumors (T) in the liver parenchyma of a PIK3CA H1047R mouse and a single, large tumor with a clear-cell phenotype in a PIK3CA E545K/c-Met mouse. Unaffected, surrounding liver (SL) is appreciable in both panels. Middle and lower panel: strong and homogeneous immunoreactivity for HA-tagged PIK3CA (HA-tag) and V5-tagged c-Met (V5-tag) in tumor lesions (but not in surrounding livers) from PIK3CA H1047R/c-Met and PIK3CA/E545K mice, implying the origin of the tumors from doubly transfected cells. Magnification: 100X. (B) Representative Western blotting of total and activated/phosphorylated (p−) AKT, AKT downstream effectors (p-GSK-3β), V-5 tagged c-Met (V5-tag), total and activated c-Met, total and activated ERK1/2, and markers of glycolysis (HK2, PKM2) proteins in WT mice as well as in liver tumors from PIK3CA H1047R/c-Met and PIK3CA E545K/c-Met mice. Of note, only tumors from PIK3CA H1047 and E545K mice exhibited activation of the AKT, c-Met, and ERK/MAPK pathways as well as induction of glycolysis. Five mice per each group were used. GAPDH was used as a loading control.
AKT2 is required for activated PIK3CA mutants driven hepatic steatosis and tumorigenesis
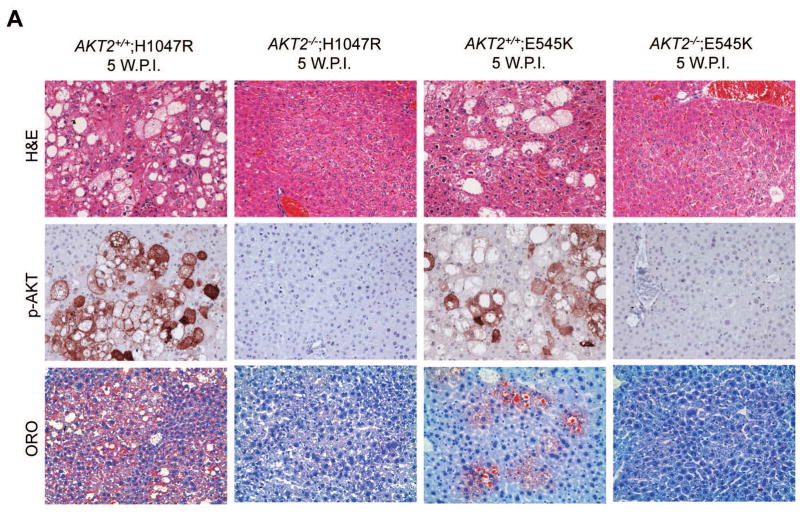
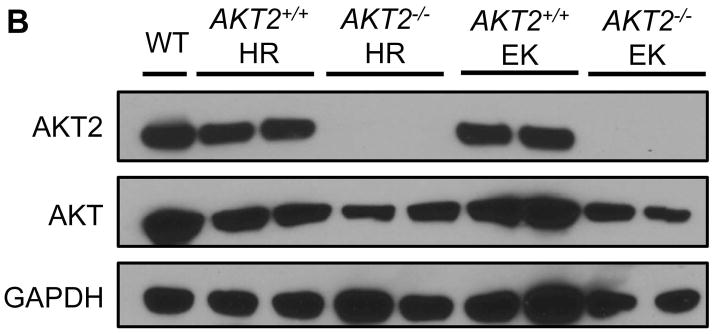
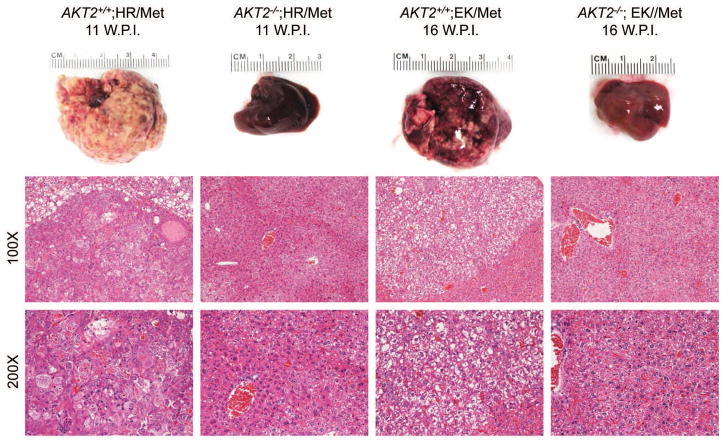
AKT2 is considered the major AKT isoform in the liver [16, 26]. A previous study demonstrated that AKT2 mediates hepatic steatosis induced by PTEN loss in mice [27]. In addition, activated/phosphorylated (p−)AKT2, but not p-AKT1, was detected in H1047R/c-Met and E545K/c-Met preneoplastic and neoplastic lesions (Supplementary Fig. 5). Thus, we investigated whether AKT2 expression is required for the histopathological alterations induced by H1047R or E545K. For this purpose, we generated AKT2+/+ and AKT2−/− mice. In these mice, we hydrodynamically transfected the H1047R or E545K construct. We found that while H1047R or E545K were able to induce hepatic steatosis in AKT2+/+ mice, this phenotype was not observed in AKT2−/− mice (Fig. 4). At the molecular level, loss of AKT2 protein was confirmed in liver tissues of AKT2−/− mice by Western blotting (Fig. 4B), and immunostaining of p-AKT showed that both H1047R and E545K failed to induce p-AKT expression in AKT2−/− mice (Fig. 4A). The latter finding is consistent with a previous study showing that ablation of AKT2 strongly inhibits loss of Pten driven hepatic steatosis [27]. Next, we determined whether liver tumor development driven by PIK3CA mutants and c-Met requires AKT2. Thus, we hydrodynamically transfected H1047R/c-Met or E545K/c-Met into AKT2+/+ or AKT2−/− mice (n=4 to 6 in each cohort). We found that H1047R/c-Met or E545K/c-Met could induce lethal burden of liver tumor in AKT2+/+ mice by 13 weeks or 16 weeks post-injection, respectively (Fig. 5). In striking contrast, none of the H1047R/c-Met/AKT2−/− or E545K/c-Met/AKT2−/− mice showed any sign of abdominal mass at the same stage (Fig. 5). All injected AKT2−/− mice were euthanized 25 weeks post injection. Of note, liver tissues from 4/4 E545K/c-Met/AKT2−/− injected and 4/4 H1047R/c-Met/AKT2−/− mice appeared to be completely normal by histological examination (Fig. 5).
Fig. 4. Hepatic steatosis induced by overexpression of PIK3CA H1047R and E545K mutants requires AKT2.
(A) Upper panels: hematoxylin and eosin staining (H&E) of livers from AKT2 wild-type (AKT2+/+) or AKT2 knockout (AKT2−/−) mice injected with PIK3CA H1047R (H1047R) or PIK3CA E545K (E545K) constructs. While injection of the two PIK3CA mutants induced extensive liver steatosis in AKT2+/+ mice, the lipogenic phenotype driven by the two PIK3CA mutants was completely inhibited in AKT2−/− mice. Magnification: 200X. Middle panels: immunohistochemical staining of activated/phosphorylated AKT (p-AKT) in the same mouse groups. Of note, p-AKT immunolabeling was completely abolished in AKT2−/− livers. Magnification: 200X. Lower panels: Oil Red O (ORO) staining in the same mouse groups confirming the findings of H&E-stained slides. Magnification: 200X. (B) Western blotting for AKT2 and AKT levels in livers from AKT2+/+ or AKT2−/− mice injected with PIK3CA H1047R (HR) or PIK3CA E545K (EK) constructs.
Fig. 5. Loss of AKT2 inhibits liver tumor formation in PIK3CA H1047R/c-Met and PIK3CA E545K/c-Met mice.
Gross images (upper panels) and hematoxylin and eosin (H&E) staining (middle and lower panels) of livers from AKT2 wild-type (AKT2+/+) or AKT2 knockout (AKT2−/−) mice injected with PIK3CA H1047R (HR) or PIK3CA E545K (EK) and c-Met (Met) constructs. Of note, hepatocarcinogenesis driven by the co-expression of PIK3CA mutants and c-Met is completely impaired in AKT2−/− mice. Magnification: 100X in middle panels; 200X in lower panels.
In summary, our results demonstrate that AKT2 is the major AKT isoform downstream of activated PI3K signaling, and both helical and kinase domain PIK3CA mutants require AKT2 to exert their oncogenic potential.
Ablation of Raptor suppresses PIK3CA induced hepatic steatosis and tumorigenesis
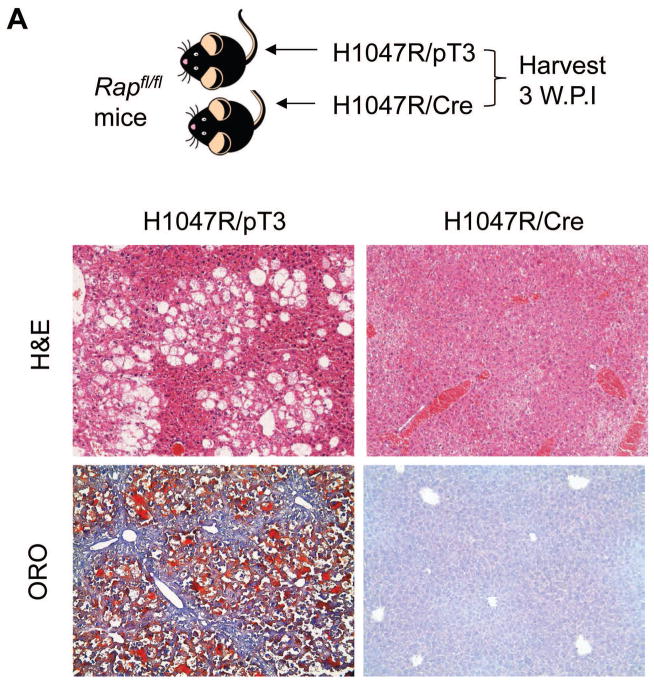
mTORC1 is the major complex downstream of activated AKT [9]. In this complex, Raptor is a critical component [9]. Thus, we investigated whether mTORC1 is required for PIK3CA oncogenic function in vivo. For this purpose, Raptorfl/fl mice were used. Since both H1047R and E545K mutants equally activate the AKT/mTOR signalling pathway, as indicated by the upregulation of mTORC1 targets (FASN, ACC, SCD1, and PKM2; Fig. 3 and Supplementary Fig. 4), only H1047R was used for the investigation. To determine whether mTORC1 is required for H1047R induced hepatic steatosis, we hydrodynamically transfected Raptorfl/fl mice with H1047R and Cre plasmids (H1047R/Cre, n=5). This strategy was successfully used in another previous study from our lab [28], and the co-expression of H1047R and Cre allowed the expression of H1047R in Raptor−/− hepatocytes (Fig. 6A). Indeed, the successfulness of deleting floxed Raptor alleles in H1047R/Cre mouse liver was confirmed by PCR analysis of the Raptor locus using primers described previously [29] (Supplementary Fig. 6). As control, H1047R was co-injected with PT3-EF1α empty vector (H1047R/pT3, n=5). We found that while all H1047R/pT3 mouse livers showed the presence of hepatic steatosis, none of the H1047R/Cre mice exhibited this phenotype (Fig. 6A).
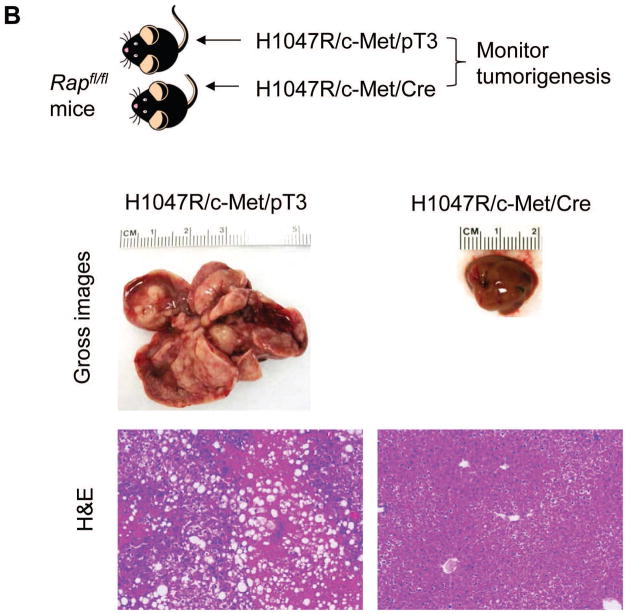
Fig. 6. Ablation of Raptor blunts H1047R driven hepatic steatosis and H1047R/c-Met induced HCC development.
(A) Upper panel: study design of H1047R experiment; lower panels: H&E and ORO staining of H1047R/pT3 and H1047R/Cre injected Raptorfl/fl mice. (B) Upper panel: study design of H1047R/c-Met experiment; lower panels: gross images and H&E staining of H1047R/c-Met/pT3 and H1047R/c-Met/Cre injected Raptorfl/fl mice. Original magnification: 100X in B and D. Abbreviations: H&E, hematoxylin and eosin staining; ORO, Oil Red O staining.
Finally, to determine the role of mTORC1 in PIK3CA/c-Met hepatocarcinogenesis, we transfected Raptorfl/fl mice with H1047R/c-Met plus Cre (H1047R/c-Met/Cre, n=5); or H1047R/c-Met plus PT3-EF1α (H1047R/c-Met/pT3, n=5). Consistent with our previous findings, all H1047R/c-Met/pT3 injected mice developed lethal burden of liver tumor by 15 weeks post injection. Remarkably, none of the H1047R/c-Met/Cre injected mice developed instead liver tumors by 15 weeks post injection (Fig. 6B).
Altogether, our data suggest that an intact mTORC1 is required for activated PIK3CA to induce liver steatosis and carcinogenesis.
Discussion
PI3K mutations have been detected in many tumors, including HCC [6, 30, 31]. Although PIK3CA H1047R alone has been shown to be oncogenic in vitro and in vivo in some tumor types [11, 32, 33], whether PIK3CA mutations are sufficient to promote liver tumorigenesis remained unknown. In the current study, we show that activating mutant forms of PIK3CA, including kinase domain mutant H1047R and helical domain mutant E545K, can induce liver steatosis, but not tumorigenesis, in mice. We also demonstrated that H1047R or E545K mutants can cooperate with NRasV12 and c-Met to rapidly induce hepatocarcinogenesis in vivo. Thus, our findings provide strong evidence that activated mutant forms of PIK3CA are capable of inducing tumor formation in cooperation with an additional oncogenic stimulus. Of note, an equivalent tumorigenic potential was observed for H1047R or E545K oncogenes in the mouse models developed for this study. Also, histopathological lesions developed in H1047R- and E545K-injected mice were undistinguishable. To the best of our knowledge, the present study is the first to directly compare the oncogenic potential of different PIK3CA mutants in liver tumorigenesis using in vivo modelling.
One of the major findings from the current study is that both PIK3CA mutants are capable to trigger AKT activation. Furthermore, using AKT2 KO mice, we convincingly demonstrated that their oncogenic activity requires an activated AKT cascade. Our data are in disagreement with a previous report suggesting that only kinase domain PIK3CA mutant, but not helical domain mutant PIK3CA, is able to activate the AKT pathway [12]. The latter study also showed that inhibiting AKT1 has limited growth inhibitory activity against cell lines harbouring E545K mutant. Further biochemical studies conducted by these authors suggested that helical domain mutant PIK3CA regulates tumor cell growth via PDK1/SGK3 cascade. The molecular mechanisms underlying the different observation between the study by Vasudevan et al. and our current data are unknown. One obvious possibility is that mutant PIK3CA regulates different downstream effectors depending on the cell/tissue context. On the other hand, we cannot exclude that tumor cells may behave differently in culture than in vivo.
It is important to underline that despite overexpression of activated mutant forms of PIK3CA alone was found to be capable to activate the AKT/mTOR and Ras/MAPK cascades in the mouse liver, PIK3CA H1047R and E545K overexpressing mice did not develop HCC. However, mutant forms of PIK3CA were able to trigger hepatocarcinogenesis in cooperation with activated Ras or overexpression of c-Met. The latter findings suggest that activation of AKT/mTOR and Ras/MAPK cascades is not sufficient to induce HCC development in vivo, and additional pathways are required for liver malignant transformation. The nature of these signalling cascades remains to be determined. One of such candidates is the Hippo pathway [34]. Accordingly, we found that Yap, the transcriptional activator downstream of the Hippo cascade, is activated in H1047R/c-Met and E545K/c-Met tumor samples, and its inhibition strongly inhibited H1047R/c-Met driven hepatocarcinogenesis (Tao J, unpublished observation). Further studies are needed to determine the mechanisms whereby the Hippo cascade contributes to PIK3CA dependent hepatocarcinogenesis.
There are three major isoforms of AKT in mammals (AKT1, AKT2 and AKT3). AKT2 is the major isoform expressed in the liver and mediates insulin response in mice [16, 26]. Importantly, our study shows that loss of AKT2 completely blocks hepatic steatosis induced by H1047R and E545K oncogenes, providing strong in vivo evidence that AKT2 is a major downstream effector of PIK3CA mutant induced lipogenesis. This notion is also consistent with a prior study showing that AKT2 mediates hepatic steatosis induced by PTEN loss in mouse model [27]. Similarly, we found that ablation of AKT2 strongly inhibits PIK3CA and c-Met driven liver tumor development. The results are again consistent with the report that loss of AKT2 impairs hepatocarcinogenesis driven by the loss of Pten [35].
Similar to the other tumor types, HCC development depends on the cellular balance between apoptosis and proliferation. We found that preneoplastic and neoplastic liver lesions from H1047R/NRasV12, H1047R/c-Met, E545K/NRasV12, and E545K/c-Met mice exhibited significantly higher proliferation and apoptosis rates when compared with wild-type mice, without differences among the four mouse models (Supplementary Fig. 3). Therefore, it is conceivable that increased cell proliferation outpaced the augmented cell apoptosis in preneoplastic lesions from the four mouse models, finally leading to tumor formation. The precise mechanisms underlying the increased cell proliferation in preneoplastic and neoplastic lesions from H1047R/NRasV12, H1047R/c-Met, E545K/NRasV12, and E545K/c-Met mice need further investigation. Intriguingly, we have detected a strong upregulation of PAK1 (p21-activated kinase), whose role as a major downstream effector of c-Met has been recently envisaged [36], in H1047R/c-Met lesions (Wang C, data not shown). We are currently investigating the functional contribution of PAK1 in H1047R/c-Met driven proliferation.
HCC is a deadly malignancy with limited treatment options. Thus, novel targeted therapeutic strategies are needed for a better treatment of HCC. Because the PI3K/AKT/mTOR pathway is critical for tumorigenesis and activation of this cascade has been established in human HCC, targeting PI3K/AKT/mTOR is an attractive therapeutic option for HCC [37, 38]. Unexpectedly, a recent phase III trial with Everolimus, a Rapalog, failed to show any overall survival benefit for advanced HCC patients. Currently, multiple drugs targeting PI3K/mTOR or mTORC1/mTORC2 are tested for HCC treatment [37, 38], with some of them showing promising results in early stage phase I trials. Our present findings, showing the requirement of AKT/mTORC1 activity in PIK3CA mutant tumors, suggest that HCC patients with PIK3CA mutations might benefit from treatments based on AKT/mTORC1 inhibition. Recently, we performed a preliminary study of treating H1047R/c-Met tumor bearing mice with Rapamycin or NVP/BEZ-235, with the latter being a dual PI3K/mTOR inhibitor [39]. Noticeably, Rapamycin treatment was ineffective in reducing tumor burden in H1047R/c-Met HCC mice. The results are consistent with the limited therapeutic potential of Rapalogs for the treatment of patients with advanced HCC [39]. In contrast, NVP/BEZ-235 treatment led to a ~30% reduction of tumor burden in H1047R/c-Met tumor bearing mice when compared with vehicle-treated mice (Wang C, unpublished observation). Although our preliminary data show encouraging results, additional experiments using the PIK3CA mouse models that we have developed are required to further validate the therapeutic potential of second-generation PI3K/AKT/mTOR inhibitors for HCC treatment.
Supplementary Material
Key points.
Overexpression of PIK3CA H1047R and E545K induces hepatic steatosis in mice.
PIK3CA H1047R and E545K cooperate with NRasV12 or c-Met to induce liver tumor development in mice.
All tumor nodules displayed activation of AKT/mTOR and Ras/MAPK cascades.
An intact AKT2/mTORC1 axis is required for hepatocarcinogenesis induced by PIK3CA mutants and c-Met in mice.
Acknowledgments
This work was supported by grant R01CA136606 and R03CA165122 from NIH, and P30DK026743 from UCSF Liver Center to XC; and by grant CRP-26489 from Region Sardinia L.R.7 2007 to DFC. We would like to thank Dr. Morris J. Birnbaum (University of Pennsylvania, USA) for providing the AKT2 KO mice.
Abbreviations
- PI3KCA
p110α catalytic subunit of phosphatidylinositol 3-kinase
Footnotes
Conflict of interest: None.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 3.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 5.Villanueva A, Llovet JM. Targeted therapies for hepatocellular carcinoma. Gastroenterology. 2011;140:1410–1426. doi: 10.1053/j.gastro.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 7.Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discov. 2014;13:140–156. doi: 10.1038/nrd4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thorpe LM, Yuzugullu H, Zhao JJ. PI3K in cancer: divergent roles of isoforms, modes of activation and therapeutic targeting. Nat Rev Cancer. 2015;15:7–24. doi: 10.1038/nrc3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao L, Vogt PK. Class I PI3K in oncogenic cellular transformation. Oncogene. 2008;27:5486–5496. doi: 10.1038/onc.2008.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vasudevan KM, Barbie DA, Davies MA, Rabinovsky R, McNear CJ, Kim JJ, et al. AKT-independent signaling downstream of oncogenic PIK3CA mutations in human cancer. Cancer Cell. 2009;16:21–32. doi: 10.1016/j.ccr.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patil MA, Lee SA, Macias E, Lam ET, Xu C, Jones KD, et al. Role of cyclin D1 as a mediator of c-Met- and beta-catenin-induced hepatocarcinogenesis. Cancer Res. 2009;69:253–261. doi: 10.1158/0008-5472.CAN-08-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu CR, Lee S, Ho C, Bommi P, Huang SA, Cheung ST, et al. Bmi1 functions as an oncogene independent of Ink4A/Arf repression in hepatic carcinogenesis. Mol Cancer Res. 2009;7:1937–1945. doi: 10.1158/1541-7786.MCR-09-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carlson CM, Frandsen JL, Kirchhof N, McIvor RS, Largaespada DA. Somatic integration of an oncogene-harboring Sleeping Beauty transposon models liver tumor development in the mouse. Proc Natl Acad Sci U S A. 2005;102:17059–17064. doi: 10.1073/pnas.0502974102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB, 3rd, et al. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta) Science. 2001;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 17.Sengupta S, Peterson TR, Laplante M, Oh S, Sabatini DM. mTORC1 controls fasting-induced ketogenesis and its modulation by ageing. Nature. 2010;468:1100–1104. doi: 10.1038/nature09584. [DOI] [PubMed] [Google Scholar]
- 18.Chen X, Calvisi DF. Hydrodynamic transfection for generation of novel mouse models for liver cancer research. Am J Pathol. 2014;184:912–923. doi: 10.1016/j.ajpath.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frith CH, Ward JM, Turusov VS. Tumours of the liver. IARC Sci Publ. 1994:223–269. [PubMed] [Google Scholar]
- 20.Calvisi DF, Wang C, Ho C, Ladu S, Lee SA, Mattu S, et al. Increased lipogenesis, induced by AKT-mTORC1-RPS6 signaling, promotes development of human hepatocellular carcinoma. Gastroenterology. 2011;140:1071–1083. doi: 10.1053/j.gastro.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho C, Wang C, Mattu S, Destefanis G, Ladu S, Delogu S, et al. AKT and N-Ras coactivation in the mouse liver promotes rapid carcinogenesis by way of mTOR, FOXM1/SKP2, and c-Myc pathways. Hepatology. 2012;55:833–845. doi: 10.1002/hep.24736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horie Y, Suzuki A, Kataoka E, Sasaki T, Hamada K, Sasaki J, et al. Hepatocyte-specific Pten deficiency results in steatohepatitis and hepatocellular carcinomas. J Clin Invest. 2004;113:1774–1783. doi: 10.1172/JCI20513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stiles B, Wang Y, Stahl A, Bassilian S, Lee WP, Kim YJ, et al. Liver-specific deletion of negative regulator Pten results in fatty liver and insulin hypersensitivity [corrected] Proc Natl Acad Sci U S A. 2004;101:2082–2087. doi: 10.1073/pnas.0308617100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang R, Ferrell LD, Faouzi S, Maher JJ, Bishop JM. Activation of the Met receptor by cell attachment induces and sustains hepatocellular carcinomas in transgenic mice. J Cell Biol. 2001;153:1023–1034. doi: 10.1083/jcb.153.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giordano S, Columbano A. Met as a therapeutic target in HCC: facts and hopes. J Hepatol. 2014;60:442–452. doi: 10.1016/j.jhep.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 26.Whiteman EL, Cho H, Birnbaum MJ. Role of Akt/protein kinase B in metabolism. Trends Endocrinol Metab. 2002;13:444–451. doi: 10.1016/s1043-2760(02)00662-8. [DOI] [PubMed] [Google Scholar]
- 27.He L, Hou X, Kanel G, Zeng N, Galicia V, Wang Y, et al. The critical role of AKT2 in hepatic steatosis induced by PTEN loss. Am J Pathol. 2010;176:2302–2308. doi: 10.2353/ajpath.2010.090931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang C, Cigliano A, Jiang L, Li X, Fan B, Pilo MG, et al. 4EBP1/eIF4E and p70S6K/RPS6 axes play critical and distinct roles in hepatocarcinogenesis driven by AKT and N-Ras proto-oncogenes in mice. Hepatology. 2015;61:200–213. doi: 10.1002/hep.27396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalaitzidis D, Sykes SM, Wang Z, Punt N, Tang Y, Ragu C, et al. mTOR complex 1 plays critical roles in hematopoiesis and Pten-loss-evoked leukemogenesis. Cell Stem Cell. 2012;11:429–439. doi: 10.1016/j.stem.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 31.Lee JW, Soung YH, Kim SY, Lee HW, Park WS, Nam SW, et al. PIK3CA gene is frequently mutated in breast carcinomas and hepatocellular carcinomas. Oncogene. 2005;24:1477–1480. doi: 10.1038/sj.onc.1208304. [DOI] [PubMed] [Google Scholar]
- 32.Samuels Y, Diaz LA, Jr, Schmidt-Kittler O, Cummins JM, Delong L, Cheong I, et al. Mutant PIK3CA promotes cell growth and invasion of human cancer cells. Cancer Cell. 2005;7:561–573. doi: 10.1016/j.ccr.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 33.Ikenoue T, Kanai F, Hikiba Y, Obata T, Tanaka Y, Imamura J, et al. Functional analysis of PIK3CA gene mutations in human colorectal cancer. Cancer Res. 2005;65:4562–4567. doi: 10.1158/0008-5472.CAN-04-4114. [DOI] [PubMed] [Google Scholar]
- 34.Yimlamai D, Fowl BH, Camargo FD. Emerging evidence on the role of the Hippo/YAP pathway in liver physiology and cancer. J Hepatol. 2015;63:1491–1501. doi: 10.1016/j.jhep.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galicia VA, He L, Dang H, Kanel G, Vendryes C, French BA, et al. Expansion of hepatic tumor progenitor cells in Pten-null mice requires liver injury and is reversed by loss of AKT2. Gastroenterology. 2010;139:2170–2182. doi: 10.1053/j.gastro.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cao HH, Cheng CY, Su T, Fu XQ, Guo H, Li T, et al. Quercetin inhibits HGF/c-Met signaling and HGF-stimulated melanoma cell migration and invasion. Mol Cancer. 2015;14:103. doi: 10.1186/s12943-015-0367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ashworth RE, Wu J. Mammalian target of rapamycin inhibition in hepatocellular carcinoma. World J Hepatol. 2014;6:776–782. doi: 10.4254/wjh.v6.i11.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matter MS, Decaens T, Andersen JB, Thorgeirsson SS. Targeting the mTOR pathway in hepatocellular carcinoma: current state and future trends. J Hepatol. 2014;60:855–865. doi: 10.1016/j.jhep.2013.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maira SM, Stauffer F, Brueggen J, Furet P, Schnell C, Fritsch C, et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther. 2008;7:1851–1863. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.