Abstract
PURPOSE
The purpose of this longitudinal study was to track the symptom experience in a sample of cancer patients, determine the persistence of cancer symptoms and symptom burden, and examine the relationship between symptoms and QOL over time.
METHODS
542 patients provided longitudinal data, completing surveys over a 12 month period. Patients had breast, colorectal, gynecologic, lung or prostate cancer with stage 1, 2 or 3 disease. Surveys included the Memorial Symptom Assessment Scale and the Functional Assessment of Cancer Therapy-General Scale and were administered every three months. Demographic and clinical information and comorbidities were collected from the tumor registry.
RESULTS
The number and type of symptoms experienced by patients varied by cancer type, but about 90% of patient reported one or more symptoms – with prostate cancer patients reporting fewer symptoms and colorectal patients more symptoms. Prostate patients also had the lowest symptom burden at every timepoint. Overall symptom burden decreased over time, as did the Physical subscale for the MSAS. Quality of life was stable over time, except for Physical Well-being, which improved. Quality of life was negatively correlated with symptom burden at every timepoint.
CONCLUSIONS
The differences in symptom experience by cancer type suggest that assessment and management of symptoms must be individually tailored or at least adjusted by cancer type. While symptom burden decreased over time, residual symptom burden was still noteworthy. As quality of life was persistently negatively correlated with symptom burden, the results suggest the need for comprehensive symptom assessment and management.
Keywords: Oncology, Symptoms, Quality of Life, Symptom Persistence
The American Cancer Society estimated that 13.7 million cancer survivors were alive on January 1, 2012, including those cured of disease and those for whom cancer has become a chronic disease [1]. The increased number of survivors is attributable both to earlier diagnosis and advances in treatment. Given the growing number of survivors, it is important for healthcare providers to understand the experience of survivors, especially the long-term effects of cancer and its treatment on their physical and psychological well-being [2].
Most cancer patients are known to experience multiple symptoms [3-6], with symptoms varying by type of treatment, gender, age, and cancer type. Previous studies have established that the average number of symptoms reported by cancer survivors ranges from eight to 13.5, depending on type of cancer and level of care [3,5]. Treatment itself is a contributor to immediate or delayed symptoms [7].
The aforementioned range in number of symptoms is consistent with our previously published paper reporting the baseline symptoms (at six to eight months post-diagnosis) and quality of life (QOL) in 558 patients with breast, colorectal, gynecologic, lung, or prostate cancer [8]. Patients reported an average of 9.2 symptoms, and a negative correlation was found between their symptom burden and QOL. Further, the symptom burden varied among patient groups: lung and colorectal cancer patients reported greater burden, and prostate patients reported less burden.
Most symptom prevalence studies have been based on clinical samples of cancer patients during treatment [9], and many are cross-sectional offering only a snapshot of the cancer experience. Recent longitudinal studies of symptoms during and after treatment have examined symptom persistence and associated symptom burden over time, but were focused on a narrow set of symptoms [10-12]. Some data suggest that fatigue, cognitive limitations, depression, anxiety, sleep problems, pain, and sexual difficulties persist, for up to 10 years after treatment, regardless of cancer type [13]. Fatigue has been reported to be the most common and distressing symptom, with 33% of patients reporting significant fatigue more than 5 years after their last treatment [9,13].
Symptom research is complicated by the fact that symptoms are often underreported [14,15] and that instruments used to measure patients’ symptoms vary widely [16]. Instruments may measure multiple symptoms, specific symptoms, or certain aspects of symptoms. For example, symptom-specific scales provide valuable information on the multiple dimensions of a single symptom or pairs of symptoms, while instruments designed to measure multiple concurrent symptoms provide wider scope but less symptom-specific detail [16]. A comprehensive instrument, such as the one used here which captures both the scope of symptoms and a high level of detail, is essential for developing a more realistic picture of symptom burden for cancer patients.
Symptom burden is “a summative indicator of the severity of the symptoms that are most associated with a disease or a treatment and a summary of the patient's perception of the impact of these symptoms on daily living” [17]. While symptom burden is related to QOL [3,18], QOL is a broader term as it is impacted by such factors as physical function, poor body image, social support, coping strategies, and communication with physicians [19], in addition to symptom burden.
The present article reports on the longitudinal extension of the previously reported baseline study [8], providing information about the persistence of symptoms. A cohort of cancer patients was followed at 3 month intervals over the course of one year in order to assess three aspects of survivorship: 1) the symptom experience in a diverse sample of cancer patients, 2) the persistence of cancer symptoms and symptom burden, and 3) the relationship between symptoms and QOL over time. This study provides detailed information about the symptom experience in survivorship because a comprehensive symptom assessment tool was used.
Methods
This study had a longitudinal, repeated measures design involving outpatients diagnosed with cancer at an NCI-designated Comprehensive Cancer Center. The Protocol Review and Monitoring Committee of the cancer center and the Human Research Protection Office of the affiliated university approved the study and all participants gave informed consent.
Participants
Potential participants were identified through the cancer center's tumor registry. Eligible patients were newly diagnosed with a primary cancer among one of the top five cancers (breast, colorectal, gynecological, lung, or prostate) by volume at the cancer center and with stage 1, 2, or 3 disease. Patients with stage 0 disease were excluded because this stage is unusual outside of breast cancer. Patients with stage 4 disease were excluded to minimize attrition over the course of the longitudinal portion of the study and because of the chronic nature of treatment for patients with this stage. Patients were excluded if they had previously been diagnosed with cancer.
Procedures
New cases for the cancer center's tumor registry are entered into a database typically 6-8 months following a patient's initial diagnosis. Each month following study implementation, patients newly entered into the database received a letter informing them of the research study and inviting them to participate through the return of assessment surveys. Those patients agreeing to participate in the study received survey packets every 3 months for one year (baseline, 3, 6, 9, and 12 months) for a total of five surveys.
All survey packets contained two measurement tools: the Memorial Symptom Assessment Scale and the Functional Assessment of Cancer Therapy-General Scale. The initial survey packet also contained a self-report form for demographic information and treatment status. Cancer type, cancer stage and a comorbidity score were captured from the tumor registry database.
Measures
The Memorial Symptom Assessment Survey (MSAS) [20] is a 32 item measure, well-validated in oncology populations. The alpha reliability score for the MSAS has been reported as ranging from 0.83 to 0.88 [20,21], and in this study was 0.90 at baseline [8]. The MSAS takes approximately 10 to 15 minutes to complete. The tool captures the multidimensional nature of symptoms (symptom presence, frequency, intensity, symptom-related distress). We used the Total MSAS score (which combines all of a patient's ratings into one score representing all 32 symptoms) as a measure of overall symptom burden [20,22,23]. The Total MSAS score ranges from 0 to 3.0. In addition, we computed a composite symptom score, which combined the patient's ratings (frequency, intensity, related distress) for each symptom, as a measure of burden associated with a particular symptom (range 0 to 4) [8]. The MSAS has 3 subscales – the Physical Symptom Subscale, the Psychological Symptom Subscale, and the Global Symptom Distress Index (GSDI). The GSDI is a clinically useful measure of global distress based on 10 selected psychological and physical symptoms thought to best represent a patient's clinical status [20].
The Functional Assessment of Cancer Therapy-General Scale (FACT-G) [24] is a 27 item measure of QOL that takes about five minutes to complete. It also is well validated and widely used with oncology patients. The instrument assesses 4 domains of well-being: Physical, Emotional, Social, and Functional, producing 4 subscale scores as well as a Total summary QOL score. The subscales have a range of 0-28, except for the Emotional subscale which has a range of 0-24. The Total score can range from 0 to 108, with higher Total scores or subscale scores reflecting better QOL.
Comorbidity was measured using the Modified Medical Comorbidity Instrument [25] and obtained from the tumor registry database. Developed by Picirillo and colleagues, the MMCI is a modified version of the Kaplan-Feinstein Index [26], a valid comorbidity index that classifies diseases and quantifies the severity of comorbid conditions, resulting in four groups: Grade 0 = none; Grade 1 = mild; Grade 2 = Moderate; and Grade 3 = Severe.
Data Analysis
Descriptive statistics were used to analyze the frequencies and means of demographic and clinical characteristics and scale measures for the study sample. Pearson's correlation coefficients were calculated to examine the correlation between any two continuous variables. To account for correlations among repeated measures from the same patient, the longitudinal data was analyzed using a general linear mixed model to examine the change in symptom burden, symptom scores and QOL over time. All the tests were two-sided and the significance level was set at 0.05. The statistical package SAS 9.3 was used for all the statistical calculations (SAS Institute Inc., Cary, NC).
Results
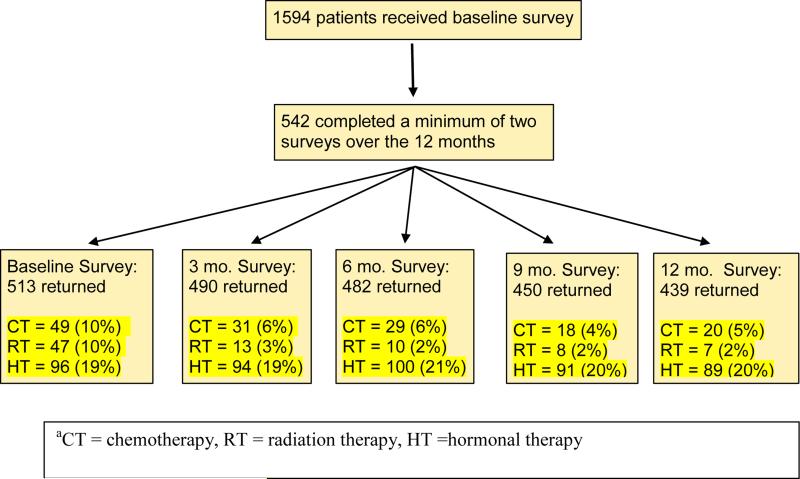
Baseline survey packets were mailed to 1594 patients, with 558 patients returning completed surveys, yielding a participation rate of 35%. For the longitudinal analyses, we excluded 16 survey responders who turned in only one survey; thus the final sample included 542 respondents (34% of the patients receiving the original mailing) who completed a minimum of two surveys over 12 months of follow-up. Figure 1 shows the number of surveys we received from this group of 542 at each time point and the treatment status of those responding. Of this longitudinal sample, the distribution of cancer diagnoses was: prostate = 226, breast = 157, gynecologic = 85, lung = 41, and colorectal = 33 (sample characteristics are presented in Table 1). The sample was fairly evenly balanced in terms of sex; however, most patients reported having stage 2 disease and Grade 1 comorbidities. At the time of baseline assessment, most (65%) had completed treatment. Across the year of follow-up, the percentage of those in treatment dropped, such that 74% had completed treatment by the last assessment timepoint; moreover, at the last timepoint, 20% of participants were receiving hormonal treatment, a more chronic form of treatment.
Figure 1.
Flow Chart of Surveys Returned at Each Time Point for the Longitudinal Sample and Treatment Statusa for the Cohort
Table 1.
Patient Characteristics at Baseline
| Responders | Nonresponders | |
|---|---|---|
| N= 542 | N= 1036 | |
| Currently receiving treatment | 189 (35%) | |
| Time since diagnosis | X=9.9 , SD=2.9 | |
| Sexa | ||
| Female | 277 (51%) | 591 (57%) |
| Male | 265 (49%) | 445 (43%) |
| Age | X=60.6, SD=10.4 | X=60.6, SD=12.4 |
| Racec | ||
| Minority | 52 (10%) | 254 (25%) |
| White | 490 (90%) | 782 (75%) |
| Cancer Typec | ||
| Breast | 157 (29%) | 252 (24%) |
| Colorectal | 33 (6%) | 97 (9%) |
| Gynecological | 85 (16%) | 224 (24%) |
| Lung | 41 (8%) | 171 (17%) |
| Prostate | 226 (42%) | 292 (28%) |
| Cancer Stagec | ||
| 1 | 150 (28%) | 382 (37%) |
| 2 | 283 (52%) | 436 (42%) |
| 3 | 109 (20%) | 218 (21%) |
| Comorbidity Scoreb | ||
| 0 | 169 (31%) | 290 (28%) |
| 1 | 246 (45%) | 424 (41%) |
| 2 | 86 (16%) | 181 (17%) |
| 3 | 40 (7%) | 141 (14%) |
| Number of Symptomsd | ||
| Breast | X=11.2, SD=8.1 | |
| Colorectal | X=11.6, SD=8.1 | |
| Gynecologic | X=11.9, SD=8.6 | |
| Lung | X=10.9, SD=6.6 | |
| Prostate | X=5.5, SD=5.7 |
p<.05
p<.01
p<.001
511 patients reported symptoms among 513 patients at the baseline.
We compared longitudinal responders (patients who returned more than one set of surveys, n=542) with non-responders (patients who completed none or one set of surveys, n=1036) on demographic and clinical variables (see Table 1). Our sample was more evenly distributed by sex, but had a greater percentage of white patients (p<.01). Among clinical variables, the groups differed by cancer type (p<.001), with a greater proportion of prostate cancer patients and a smaller proportion of gynecologic and lung patients in our sample. The groups also differed by cancer stage (p<.001), with stage 2 patients overrepresented in our sample and stage 1 patients overrepresented among non-responders. Finally, comorbidity scores also distinguished these groups (p<.01), with a higher percentage of non-responders having comorbidity scores of 3 (14% versus 7%).
A range of 89 to 93% of patients reported at least one symptom at each time period. At baseline the average number of symptoms experienced by patients was 8.9, with a range of 5.5 for patients with prostate cancer and 12.3 for patients with colorectal cancer. Table 3 shows the statistically significant decline in number of symptoms over the 12 month period (p<.01); however, the average number of symptoms for all patients ranged from 8.9 to 8.0 over the 12 months, suggesting that the decline was not clinically significant. Patients with gynecological cancer had the highest number of symptoms among all cancer groups at baseline and 12 months, while patients with colorectal cancer had the highest number of symptoms at 3 months, and lung cancer patients had the greatest number of symptoms at 6 and 9 months.
Table 3.
MSAS and FACT Subscales[1]
| Subscales | Baseline | Month 3 | Month 6 | Month 9 | Month 12 | P value |
|---|---|---|---|---|---|---|
| Physical symptom* | 0.4732 (0.02473) | 0.4291 (0.02322) | 0.4073 (0.02151) | 0.4058 (0.02319) | 0.4021 (0.02199) | 0.0057 |
| Psychological symptom | 0.7567 (0.03654) | 0.7520 (0.03779) | 0.7326 (0.03635) | 0.7050 (0.03720) | 0.6904 (0.03675) | 0.1651 |
| Global distress index | 0.6979 (0.03232) | 0.6650 (0.03235) | 0.6331 (0.03052) | 0.6310 (0.03163) | 0.6331 (0.03085) | 0.0710 |
| Total MSAS* | 0.5346 (0.02239) | 0.4979 (0.02186) | 0.4726 (0.02057) | 0.4724 (0.02168) | 0.4704 (0.02112) | 0.0012 |
| # of symptoms* | 8.9447 (0.3336) | 8.1748 (0.3150) | 8.4362 (0.3234) | 8.0409 (0.3130) | 8.0483 (0.3088) | 0.0066 |
| FACT-G: physical QOL** | 23.5494 (0.2250) | 24.3060 (0.1959) | 24.2046 (0.1975) | 24.3833 (0.1975) | 24.2197 (0.2001) | <0.0001 |
| FACT-G: social QOL | 21.2829 (0.2591) | 20.9131 (0.2771) | 20.6996 (0.2913) | 20.8543 (0.2876) | 20.7523 (0.2854) | 0.0711 |
| FACT-G: emotional QOL | 19.6773 (0.1694) | 19.7248 (0.1732) | 19.9268 (0.1627) | 19.8727 (0.1651) | 19.6612 (0.1809) | 0.1713 |
| FACT-G: functional QOL | 20.9203 (0.2710) | 21.0281 (0.2789) | 20.7534 (0.2979) | 20.9793 (0.2936) | 21.0321 (0.2802) | 0.7641 |
| FACT-G: overall QOL | 85.3746 (0.7197) | 85.7755 (0.7276) | 85.3983 (0.7451) | 85.9918 (0.7435) | 85.5660 (0.7559) | 0.6785 |
p<.01
p<.001
Least square means and standard error
The ten most prevalent symptoms for all patients at baseline varied by cancer diagnosis; however, the most prevalent symptoms for all groups combined at baseline are listed in Table 2. We utilized the composite symptom score to reflect the burden associated with each symptom, and the most burdensome symptoms (versus the most prevalent) in decreasing order of severity were: problems with sexual interest, lack of energy, don't look like myself, numbness/tingling, difficulty sleeping, hair loss, sweats, itching, problems urinating, and lack of appetite. We examined change in the symptom score over time, and determined that, of the most prevalent symptoms, only lack of energy demonstrated significant change over time. An analysis of the composite symptom scores for all symptoms showed a significant decline over the five time points of the study for these symptoms not included among the most prevalent: nausea (p<.01), problems with urination (p<.01), vomiting (p<.01), lack of appetite (p<.01), changes in the way food tastes (p<.001), weight loss (p<.001), don't look like myself (p<.001), and changes in skin (p<.001).
Table 2.
Top Symptoms by Prevalence from the MSAS for Those Reporting Symptoms N(%)
| Rank and Symptom | Baseline N=513 | Month 3 N=490 | Month 6 N=482 | Month 9 N=450 | Month 12 N=439 |
|---|---|---|---|---|---|
| Lack of Energy | |||||
| Prevalence | 302 (59.1%) | 272 (56.5%) | 266 (55.5%) | 233 (52.5%) | 232 (53.6%) |
| Symptom Scorea | 1.3136 | 1.2007 | 1.1648 | 1.1204 | 1.1797 |
| Difficulty Sleeping | |||||
| Prevalence | 269 (52.7%) | 245 (50.9%) | 240 (50.0%) | 220 (49.4%) | 216 (49.3%) |
| Symptom Score | 1.1293 | 1.1360 | 1.0484 | 1.0480 | 0.9985 |
| Problems with Sexual Interest/Activity | |||||
| Prevalence | 264 (52.6%) | 238 (50.5%) | 244 (52.0%) | 219 (50.2%) | 226 (52.9%) |
| Symptom Score | 1.3987 | 1.3373 | 1.3423 | 1.2796 | 1.3957 |
| Pain | |||||
| Prevalence | 224 (44.0%) | 179 (37.1%) | 183 (38.0%) | 164 (36.8%) | 162 (37.4%) |
| Symptom Score | 0.8932 | 0.7472 | 0.7457 | 0.7749 | 0.7815 |
| Feeling drowsy | |||||
| Prevalence | 206 (42.4%) | 184 (38.4%) | 172 (35.9%) | 162 (36.2%) | 155 (35.6%) |
| Symptom Score | 0.7663 | 0.6704 | 0.6613 | 0.6419 | 0.6693 |
| Worrying | |||||
| Prevalence | 209 (41.1%) | 198 (41.2%) | 194 (40.5%) | 173 (38.9%) | 161 (36.9%) |
| Symptom Score | 0.7832 | 0.8005 | 0.7606 | 0.7225 | 0.7158 |
| Numbness & tingling in hands and feet | |||||
| Prevalence | 202 (39.4%) | 175 (36.3%) | 176 (36.6%) | 163 (36.5%) | 161 (36.8%) |
| Symptom Score | 0.8249 | 0.7980 | 0.7597 | 0.7506 | 0.7609 |
| Feeling sad | |||||
| Prevalence | 198 (38.7%) | 173 (35.8%) | 177 (37.1%) | 157 (35.0%) | 163 (37.4%) |
| Symptom Score | 0.7392 | 0.6966 | 0.6728 | 0.6493 | 0.7068 |
| Difficulty concentrating | |||||
| Prevalence | 197 (38.7%) | 176 (36.5%) | 184 (38.5%) | 154 (34.5%) | 155 (35.5%) |
| Symptom Score | 0.6593 | 0.6350 | 0.6545 | 0.6257 | 0.6526 |
| Feeling irritable | |||||
| Prevalence | 185 (36.2%) | 160 (33.0%) | 155 (32.2%) | 146 (32.6%) | 131 (30.1%) |
| Symptom Score | 0.6527 | 0.6040 | 0.5726 | 0.5720 | 0.5477 |
Prevalence reported as n (%) of those responding “yes” to the presence of this symptom.
p<.01
The average Total MSAS score, representing overall symptom burden, was 0.53 for all patients at baseline (range= 0 - 2.97). Overall symptom burden decreased across the five time periods to 0.47 (p<.001) (Table 3). The Physical Symptom Subscale of the MSAS decreased significantly (p<.01) over the course of the year. The other subscales of the MSAS (Psychological Symptom Subscale and the GDI) were stable over time.
There was a statistically significant difference in change over time in MSAS Total score by diagnosis (p<.01) (see Table 4).The diagnostic group that experienced the highest burden over time was the lung cancer group (MSAS Total score X=.80 at 9 month follow-up), while the prostate cancer group reported the lowest symptom burden (X=.29 at 9 month follow-up). Furthermore, prostate cancer patients reported the lowest symptom burden at every time point. Symptom burden decreased significantly over time for breast cancer patients (X=.66 to X=.60, p<.05) and for colorectal cancer patients (X=.78 to X=.61, p<.001). The only demographic variable that was associated with overall symptom burden was age (p<.05), with patients between 56 and 65 years of age reporting lower average Total MSAS scores (p<.05).
Table 4.
MSAS Scores by Clinical and Demographic Variables[1]
| Total MSAS | Baseline | Month 3 | Month 6 | Month 9 | Month 12 | P value | |
|---|---|---|---|---|---|---|---|
| Stage | 1 | 0.6453 (0.04215) | 0.5965 (0.04152) | 0.5781 (0.03895) | 0.5997 (0.04078) | 0.5872 (0.03942) | 0.90 |
| 2 | 0.4497 (0.03050) | 0.4192 (0.02964) | 0.3864 (0.02765) | 0.3693 (0.02915) | 0.3750 (0.02822) | ||
| 3 | 0.6044 (0.04882) | 0.5669 (0.04783) | 0.5557 (0.04539) | 0.5718 (0.04741) | 0.5634 (0.04682) | ||
| Comorbidity Score | 0 | 0.4738 (0.03897) | 0.4155 (0.03787) | 0.4000 (0.03593) | 0.3891 (0.03802) | 0.4142 (0.03670) | 0.43 |
| 1 | 0.5070 (0.03277) | 0.4785 (0.03176) | 0.4756 (0.03012) | 0.4812 (0.03166) | 0.4609 (0.03092) | ||
| 2 | 0.6390 (0.05620) | 0.6048 (0.05476) | 0.5313 (0.05218) | 0.5347 (0.05510) | 0.5253 (0.05418) | ||
| 3 | 0.7546 (0.08281) | 0.7720 (0.08063) | 0.6510 (0.07851) | 0.6405 (0.08166) | 0.6594 (0.07998) | ||
| Gender | Male | 0.3747 (0.03024) | 0.3611 (0.02980) | 0.3465 (0.02835) | 0.3504 (0.02964) | 0.3301 (0.02865) | 0.12 |
| Female | 0.6913 (0.03001) | 0.6316 (0.02976) | 0.5965 (0.02817) | 0.5913 (0.02959) | 0.6080 (0.02858) | ||
| Diagnosisb | Colorectal | 0.7609 (0.08527) | 0.6678 (0.08275) | 0.5407 (0.08122) | 0.4841 (0.08124) | 0.4851 (0.07897) | 0.0034* |
| Breast | 0.6598 (0.03861) | 0.6042 (0.03844) | 0.5794 (0.03619) | 0.5733 (0.03780) | 0.5725 (0.03620) | ||
| Gynecological | 0.7110 (0.05428) | 0.6850 (0.05362) | 0.6245 (0.05035) | 0.6251 (0.05298) | 0.7021 (0.05191) | ||
| Prostate | 0.3184 (0.03203) | 0.3088 (0.03154) | 0.2979 (0.02979) | 0.2900 (0.03111) | 0.2765 (0.03006) | ||
| Lung | 0.7317 (0.07580) | 0.6408 (0.07492) | 0.6919 (0.07149) | 0.7974 (0.07417) | 0.7136 (0.07084) | ||
| Race | Minority | 0.6183 (0.07361) | 0.5945 (0.07223) | 0.5821 (0.06986) | 0.5958 (0.07353) | 0.5507 (0.07029) | 0.87 |
| White | 0.5262 (0.02350) | 0.4881 (0.02292) | 0.4620 (0.02141) | 0.4609 (0.02267) | 0.4619 (0.02211) | ||
| Agea | <=56 | 0.5813 (0.03876) | 0.5153 (0.03796) | 0.4798 (0.03591) | 0.4377 (0.03785) | 0.4796 (0.03679) | 0.0343* |
| >56, <=65 | 0.4893 (0.03739) | 0.4553 (0.03668) | 0.4452 (0.03425) | 0.4472 (0.03586) | 0.4409 (0.03499) | ||
| >65 | 0.5368 (0.03998) | 0.5292 (0.03902) | 0.4977 (0.03698) | 0.5349 (0.03876) | 0.4950 (0.03792) | ||
p<.05
p<.01
c p<.001
Least square means and standard error
Patients’ overall QOL scores were stable over time, as were the QOL subscales except for the Physical subscale, which showed significant improvement (F=7.15, p<.001) (see Table 3). The only clinical variable associated with change in overall QOL over time was cancer diagnosis (p<.05) (see Table 5). Colorectal cancer patients had improvement in overall QOL over time. No demographic variable was associated with change in QOL over time. Overall QOL correlated negatively with MSAS Total scores for each time period: r=−.77 at baseline, r=−.72 at 3 months, r=−.66 at 6 months, r=−.71 at 9 months, and r=−.74 at 12 months – all significant at p<.001. The higher the patient's symptom burden, the poorer their quality of life.
Table 5.
FACT-G Scores by Clinical and Demographic Variables[1]
| FACT-G: overall QOL | Baseline | Month 3 | Month 6 | Month 9 | Month 12 | P value | |
|---|---|---|---|---|---|---|---|
| Stage | 1 | 83.2582 (1.3547) | 84.7900 (1.3758) | 84.2029 (1.4295) | 84.0752 (1.4042) | 83.3988 (1.4163) | 0.41 |
| 2 | 87.4568 (0.9892) | 87.5483 (0.9980) | 87.0040 (1.0223) | 87.9343 (1.0148) | 88.1986 (1.0218) | ||
| 3 | 82.9254 (1.5886) | 82.5187 (1.6180) | 82.8841 (1.6590) | 83.6525 (1.6586) | 81.4817 (1.6996) | ||
| Comorbidity Score | 0 | 87.0647 (1.2689) | 88.2854 (1.2852) | 88.4076 (1.3050) | 89.4751 (1.3127) | 88.6606 (1.3102) | 0.21 |
| 1 | 86.5571 (1.0492) | 86.1626 (1.0665) | 85.9807 (1.0788) | 85.5074 (1.0743) | 86.1021 (1.0967) | ||
| 2 | 81.8246 (1.7813) | 83.4348 (1.8188) | 81.5932 (1.8748) | 84.0539 (1.8881) | 82.0103 (1.9300) | ||
| 3 | 78.3535 (2.6064) | 77.7807 (2.6124) | 76.9813 (2.7201) | 77.9414 (2.7075) | 76.7204 (2.7096) | ||
| Gender | Male | 89.3331 (1.0061) | 88.3911 (1.0263) | 88.2646 (1.0577) | 89.1622 (1.0422) | 89.0948 (1.0604) | 0.0587 |
| Female | 81.6105 (0.9820) | 83.2737 (1.0059) | 82.6631 (1.0365) | 82.9808 (1.0264) | 82.1735 (1.0439) | ||
| Diagnosis* | Colorectal | 82.3528 (2.7647) | 81.8076 (2.8401) | 81.1064 (2.9227) | 85.4101 (2.8633) | 86.7956 (2.9004) | 0.0110* |
| Breast | 84.2722 (1.2615) | 85.1097 (1.3123) | 84.7817 (1.3537) | 84.2902 (1.3264) | 84.3870 (1.3406) | ||
| Gynecological | 76.5443 (1.7319) | 79.05219 (1.7900) | 78.7133 (1.8460) | 79.2939 (1.8444) | 76.4219 (1.8705) | ||
| Prostate | 91.0397 (1.0570) | 89.7052 (1.0920) | 89.7167 (1.1213) | 90.8148 (1.1037) | 90.7510 (1.1156) | ||
| Lung | 78.9794 (2.4882) | 82.7560 (2.5716) | 81.1693 (2.6728) | 80.2319 (2.5740) | 78.9025 (2.6053) | ||
| Race | Minority | 81.2724 (2.3360) | 82.5551 (2.3704) | 81.4045 (2.4654) | 79.1041 (2.4379) | 81.5529 (2.4853) | 0.24 |
| White | 85.8115 (0.7540) | 86.1190 (0.7616) | 85.8163 (0.7790) | 86.6803 (0.7765) | 85.9934 (0.7912) | ||
| Age | <=56 | 83.0813 (1.2427) | 84.0151 (1.2613) | 83.8896 (1.2915) | 85.1994 (1.3016) | 84.5906 (1.3166) | 0.51 |
| >56, <=65 | 86.9144 (1.1983) | 87.2904 (1.2106) | 86.3620 (1.2464) | 87.4025 (1.2316) | 86.4137 (1.2519) | ||
| >65 | 86.0559 (1.2833) | 85.9187 (1.3024) | 85.8952 (1.3400) | 85.3210 (1.3199) | 85.6715 (1.3593) | ||
p<.05
Least square means and standard error
Discussion
The findings from this study provide a broad view of the cancer symptom experience for five cancer groups. The sample was generally representative, although the proportions for early stage disease were different in this sample in comparison to nonresponders. Also, our sample was slightly less likely to have level 3 comorbidities than those who chose not to participate, suggesting that these results may minimize the symptom experience of some cancer patients. Nonetheless, these findings reveal a significant level of symptom burden over the course of time.
In this study, symptom burden varied by cancer diagnosis, with lung cancer patients reporting the highest average symptom burden across the five time periods, and prostate patients reporting the lowest average burden. This finding suggests that supportive care for cancer patients must be tailored, with some groups of patients having greater need for symptom management.
Age was the only demographic variable associated with symptom burden. While this study was not designed to specifically examine the association of age with symptom burden, and cancer treatment may be confounded with age, our preliminary results suggest that both older and younger cancer patients may carry more symptom burden than middle-aged patients. While some have found no relationship between age and symptom burden [32, 33], these have tended to be studies focused on a single cancer type. Other studies have documented a relationship between symptom experience and age as found here, with most indicating higher symptom burden in younger patients [34-36]. There are methodological differences in terms of how age is stratified; therefore the age range for “younger” and “older” patients can vary by study. Moreover, in studies that divide patients into two age groups [e.g. 35], there is a loss of detail regarding the effect of age.
Notably, overall symptom burden (Total MSAS scores) diminished significantly over time; however, there was little change in the burden associated with the most prevalent symptoms over time (except for lack of energy). Also, over 82% of the study sample experienced at least one symptom throughout the 12 months. From a different perspective, the average number of symptoms was 8 at the 12 month follow up, and it is concerning that symptom experience was minimally changed over time. For example, even though patients in this study reported their lack of energy to be significantly improved over time, this symptom, nevertheless, yielded the highest symptom scores across all time periods. Furthermore, this is a symptom which has been somewhat amenable to treatment, such as exercise [27,28]. Similarly, although the degree of difficulty sleeping also declined over time, it remained the second most burdensome symptom over the 12 month period. Savard and colleagues also reported the prevalence of insomnia in breast cancer patients remaining consistent during an 18 month period [29]. Despite the number of medications available to treat sleep problems and the array of nonpharmacologic therapies also shown to be effective [29], these results suggest that sleep problems persist for many patients.
Patients’ QOL scores were predictably lower as their symptom burden increased. With growing attention to survivorship issues [19], the impact of symptoms on survivors’ quality of life and general well-being will likely become the focus of more research. It is interesting to note that of the top ten symptoms by prevalence in our sample, three are psychological symptoms (feeling sad, worrying, and feeling irritable) with others having a psychological component (difficulty concentrating, difficulty sleeping, and problems with sexual interest or activity). Other researchers have identified persistence of psychosocial distress in breast cancer patients longitudinally [30]. These results suggest the need for psychosocial support for cancer survivors, not just those receiving treatment, as advocated in previous IOM reports [19,31].
This study has several limitations, most particularly the use of a convenience sample. Only 34% of patients invited chose to participate in the project, a relatively low response rate. Comparing responders to nonresponders highlighted some differences that may limit the generalizability of our results, most notably, the greater number of Caucasian patients among the responders. Furthermore, the participants were unevenly distributed among cancer types, with greater numbers in the diagnostic groups with less symptom burden, suggesting that these data may underrepresent the experience of patients who are more symptomatic. The response rate could likely have been improved with a different methodology (e.g. phone surveys) or use of reminders to participants to encourage return of study data. Finally, we did not establish receipt of treatment as an exclusion factor, as our recruitment was based on time since diagnosis. That some patients were receiving treatment likely contributed to symptom burden; however, most patients had completed treatment by the last assessment timepoint, except for more chronic treatment (hormonal therapy) which often impacts survivors for years.
There is no standard for “acceptable” symptom burden; however, there is likely consensus that patient-reported symptom burden suggests the need for symptom management or supportive care. Understanding the experience of survivors is essential for implementing successful palliative and supportive care, decreasing the morbidity associated with uncontrolled symptoms,and, thus, improving patients’ QOL and long term outcomes. The persistence of symptoms documented here poses challenges for long-term medical management of survivors, particularly when follow-up care is less frequent and more likely to be provided by primary care physicians. The persistence of symptoms also raises numerous questions regarding communication about symptoms in the oncology clinic, particularly the thoroughness of clinicians’ symptom assessment and the extent of patient disclosure about symptoms. Communication about symptoms between health care providers and patients is an area ripe for future research.
Acknowledgments
Conflict of Interest: This research project was funded by Barnes-Jewish Hospital which employs Dr. Deshields, Dr. Potter, and Ms. Olsen. The authors acknowledge the support of the Biostatistics Core of the Siteman Cancer Center, which is funded by the NCI Cancer Center Support Grant P30 CA091842. The authors have full control of all primary data and agree to allow the journal to review the data if requested.
Footnotes
There was no external funding for this research project.
Contributor Information
Teresa L. Deshields, Barnes-Jewish Hospital Siteman Cancer Center
Patricia Potter, Barnes-Jewish Hospital.
Sarah Olsen, Barnes-Jewish Hospital.
Jingxia Liu, Washington University School of Medicine, Division of Biostatistics
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA: Ca J for Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute US cancer survivors grow to nearly 12 million. http://www.cancer.gov/newscenter/pressreleases/2011/survivorshipMMWR2011.
- 3.Portenoy RK, Thaler HT, Kornblith AB, et al. Symptom prevalence, characteristics and distress in a cancer population. Qual Life Res. 1994;3:183–189. doi: 10.1007/BF00435383. [DOI] [PubMed] [Google Scholar]
- 4.Baggott C, Dodd M, Kennedy N, et al. Multiple symptoms in pediatric oncology patients: A systematic review. J Pediatr Oncol Nurs. 2009;26:325–339. doi: 10.1177/1043454209340324. [DOI] [PubMed] [Google Scholar]
- 5.Chang VT, Hwang SS, Feuerman M, et al. Symptom and quality of life survey of medical oncology patients at a veterans affairs medical center: A role for symptom assessment. Cancer. 2000;88:1175–83. doi: 10.1002/(sici)1097-0142(20000301)88:5<1175::aid-cncr30>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 6.Kroenke K, Zhong X, Theobald D, et al. Somatic symptoms in patients with cancer experiencing pain or depression. Arch Intern Med. 2010;170(18):1686–1694. doi: 10.1001/archinternmed.2010.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooley ME. Symptoms in adults with lung cancer: A systematic research review. J Pain Symptom Manage. 2000;19:137–153. doi: 10.1016/s0885-3924(99)00150-5. [DOI] [PubMed] [Google Scholar]
- 8.Deshields TL, Potter P, Olsen S, et al. Documenting the symptom experience of cancer patients. J Support Oncol. 2011;9(6):216–223. doi: 10.1016/j.suponc.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Cella D, Davis K, Breitbart W, et al. Cancer-related fatigue: Prevalence of proposed diagnostic criteria in a United States sample of cancer survivors. J Clin Onc. 2001;19(14):3385–3391. doi: 10.1200/JCO.2001.19.14.3385. [DOI] [PubMed] [Google Scholar]
- 10.Savard J, Ivers H, Villa J, et al. Natural course of insomnia comorbid with cancer: An 18-month longitudinal study. J Clin Oncol. 2011;29(26):3580–3586. doi: 10.1200/JCO.2010.33.2247. [DOI] [PubMed] [Google Scholar]
- 11.Andreu Y, Galdon MJ, Dura E, et al. A longitudinal study of psychosocial distress in breast cancer: Prevalence and risk factors. Psychol Health. 2012;27(1):72–87. doi: 10.1080/08870446.2010.542814. [DOI] [PubMed] [Google Scholar]
- 12.Burakgazi AZ, Messersmith W, Vaidya D, et al. Longitudinal assessment of oxaliplatin-induced neuropathy. Neurology. 2011;77(10):980–986. doi: 10.1212/WNL.0b013e31822cfc59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrington CB, Hansen JA, Moskowitz M, et al. It's not over when it's over: Long-term symptoms in cancer survivors- a systematic review. Int J Psych Med. 2010;40(2):163–181. doi: 10.2190/PM.40.2.c. [DOI] [PubMed] [Google Scholar]
- 14.Cleeland CS. The measurement of pain from metastatic bone disease: capturing the patient's experience. Clinical Cancer Research. 2006;12(20Pt2):6236s–6242s. doi: 10.1158/1078-0432.CCR-06-0988. [DOI] [PubMed] [Google Scholar]
- 15.Donovan HS, Hartenbach EM, Method MW. Patient-provider communication and perceived control for women experiencing multiple symptoms associated with ovarian cancer. Gynecol Oncol. 2005;99(2):404–411. doi: 10.1016/j.ygyno.2005.06.062. [DOI] [PubMed] [Google Scholar]
- 16.Kim JE, Dodd MJ, Aouizerat BE, et al. A review of the prevalence and impact of multiple symptoms in oncology patients. J Pain Symptom Manage. 2009;37(4):715–736. doi: 10.1016/j.jpainsymman.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burkett VS, Cleeland CS. Symptom burden in cancer survivorship. J. Cancer Surviv. 2007;1(2):167–175. doi: 10.1007/s11764-007-0017-y. [DOI] [PubMed] [Google Scholar]
- 18.Kirkova J, Davis MP, Walsh D, et al. Cancer symptom assessment instruments: A systematic review. J Clin Oncol. 2006;24(9):1459–1473. doi: 10.1200/JCO.2005.02.8332. [DOI] [PubMed] [Google Scholar]
- 19.Committee on Cancer Survivorship: Improving Care and Quality of Life, Institute of Medicine and National Research Council . From cancer patient to cancer survivor: Lost in transition. The National Academy Press; Washington, DC: 2005. [Google Scholar]
- 20.Portenoy RK, Thaler HT, Kornblith AB, et al. The Memorial Symptom Assessment Scale: An instrument for the evaluation of symptom prevalence, characteristics and distress. Eur. J. Cancer. 1994;30A:1326–36. doi: 10.1016/0959-8049(94)90182-1. [DOI] [PubMed] [Google Scholar]
- 21.Molassiotis A, Wengstrom Y, Kearney N. Symptom cluster patterns during the first year after diagnosis with cancer. J Pain Symptom Manage. 2010;39:847–858. doi: 10.1016/j.jpainsymman.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 22.Akin S, Can G, Aydiner A, et al. Quality of life, symptom experience and distress of lung cancer patients undergoing chemotherapy. Eur J Oncol Nurs. 2010;14:400–409. doi: 10.1016/j.ejon.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Sun CC, Bodurka DC, Weaver CB, et al. Rankings and symptom assessments of side effects from chemotherapy: Insights from experienced patients with ovarian cancer. Support Care Cancer. 2005;13:219–227. doi: 10.1007/s00520-004-0710-6. [DOI] [PubMed] [Google Scholar]
- 24.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy Scale: Development and validation of the general measure. J Clin Oncol. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 25.Piccirillo J, Creech C, Zequeira R, et al. Inclusion of comorbidity into oncology data registries. Journal of Registry Management. 1999;26(2):66–70. [Google Scholar]
- 26.Kaplan MH, Feinstein AR. The importance of classifying initial comorbidity in evaluating the outcome of diabetes mellitus. J Chron Disease. 1974;27:387–404. doi: 10.1016/0021-9681(74)90017-4. [DOI] [PubMed] [Google Scholar]
- 27.Mock V, Dow KH, Meares CJ, et al. Effects of exercise on fatigue, physical functioning, and emotional distress during radiation therapy for breast cancer. Oncol Nurs Forum. 1997;24(6):991–1000. [PubMed] [Google Scholar]
- 28.Cramp F, Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database of Systematic Reviews. 2008 doi: 10.1002/14651858.CD006145.pub2. 10.1002/14651858. [DOI] [PubMed] [Google Scholar]
- 29.Savard J, Villa J, Simard S, et al. Feasibility of a self-help treatment for insomnia comorbid with cancer. Psychooncology. 2011;20(9):1013–1019. doi: 10.1002/pon.1818. [DOI] [PubMed] [Google Scholar]
- 30.Andreu Y, Galdon MJ, Dura E, et al. A longitudinal study of psychosocial distress in breast cancer: Prevalence and risk factors. Psychol Health. 2012;27(1):72–87. doi: 10.1080/08870446.2010.542814. [DOI] [PubMed] [Google Scholar]
- 31.National Research Council . Cancer Care for the Whole Patient: Meeting Psychosocial Health Needs. The National Academies Press; Washington, DC: 2008. [PubMed] [Google Scholar]
- 32.Prigozin A, Uziely B, Musgrave CF. The relationship between symptom severity and symptom interference, education, age, marital status, and type of chemotherapy treatment in Israeli women with early-stage breast cancer. Oncol Nurs Forum. 2010;37(6):E411–E418. doi: 10.1188/10.ONF.E411-E418. [DOI] [PubMed] [Google Scholar]
- 33.Oksholm T, Miaskowski C, Kongerud JS, et al. Does age influence the symptom experience of lung cancer patients prior to surgery? Lung Cancer. 2013;82:156–161. doi: 10.1016/j.lungcan.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 34.Mao JJ, Armstrong K, Bowman MA, et al. Symptom burden among cancer survivors: Impact of age and comorbidity. J Am Board Fam Med. 2007;20(5):434–443. doi: 10.3122/jabfm.2007.05.060225. [DOI] [PubMed] [Google Scholar]
- 35.Cheung WY, Le LW, Gagliese L, Zimmermann C. Age and gender differences in symptom intensity and symptom clusters among patients with metastatic disease. Support Care Cancer. 2011;19:417–423. doi: 10.1007/s00520-010-0865-2. [DOI] [PubMed] [Google Scholar]
- 36.Kirkova J, Rybicki L, Walsh D, Aktas A. Symptom prevalence in advanced cancer: Age, gender, and performance status interactions. Am J Hosp Palliat Med. 2012;29(2):139–145. doi: 10.1177/1049909111410965. [DOI] [PubMed] [Google Scholar]