Abstract
Lifestyle behavior changes can prevent progression of prediabetes to diabetes but providers often are not able to effectively counsel about preventive lifestyle changes. We developed and pilot tested the Avoiding Diabetes Thru Action Plan Targeting (ADAPT) program to enhance primary care providers' counseling about behavior change for patients with prediabetes. Primary care providers in two urban academic practices and their patients with prediabetes were recruited to participate in the ADAPT study, an unblinded randomized pragmatic trial to test the effectiveness of the ADAPT program, including a streamlined electronic medical record-based goal setting tool. Providers were randomized to intervention or control arms; eligible patients whose providers were in the intervention arm received the ADAPT program. Physical activity (the primary outcome) was measured using pedometers, and data were gathered about patients' diet, weight and glycemic control. A total of 54 patients were randomized and analyzed as part of the 6-month ADAPT study (2010–2012, New York, NY). Those in the intervention group showed an increase total daily steps compared to those in the control group (+ 1418 vs − 598, p = 0.007) at 6 months. There was also a trend towards weight loss in the intervention compared to the control group (− 1.0 lbs. vs. 3.0 lbs., p = 0.11), although no change in glycemic control. The ADAPT study is among the first to use standard electronic medical record tools to embed goal setting into realistic primary care workflows and to demonstrate a significant improvement in prediabetes patients' physical activity.
Keywords: Applied informatics, Medical informatics, Primary care, Electronic health records, Prediabetes, Behavior change, Goal setting, Clinical decision support
Highlights
-
•
EHRs can be vehicles for embedding prediabetes goal setting into daily primary care practice.
-
•
Technology enhanced goal setting increased daily physical activity among prediabetes patients.
-
•
Using technology to improve primary care goal setting requires careful attention to workflow.
-
•
EHR tools to structure goal setting is a promising tool for prediabetes in primary care.
1. Background
The 2014 prevalence and incidence of type 2 diabetes mellitus (DM2) is increasing worldwide with 29 million Americans (9.3% of the population) and 347 million Europeans (9.5%) diagnosed with diabetes (Danaei et al., 2011, CDC, 2014). The worldwide prevalence rate is estimated to almost double from 2.8% in 2000 to 4.4% in 2030(Danaei et al., 2011, Writing Group Members et al., 2006, Wild et al., 2004). Moreover, in the United States, an additional 86 million adults are estimated to have prediabetes (a condition defined by blood sugar levels greater than normal but below thresholds for diabetes) (CDC, 2014). Several studies have established that DM2 can be prevented through lifestyle behavior changes (ADA, 2008). The landmark Diabetes Prevention Program (DPP) demonstrated that a comprehensive, intensive behavioral change program can reduce progression to DM2 by 58% in people with prediabetes, and this evidence has translated into recommendations that weight control through small increases in physical activity and small reductions in caloric intake can make a significant impact on preventing diabetes (Hill et al., 2003, Craig et al., n.d, Diabetes Prevention Program Research Group, 2002).
For primary care providers (PCPs), counseling patients with prediabetes about lifestyle modification can consume the majority of time during a clinical encounter, often because traditional clinical encounters do not support effective behavior change (Haire-Joshu and Klein, 2011). Providers have limited training on effective behavior change techniques,(Kushner, 2010) and the provider–patient encounter is often brief and consumed by mandatory documentation and reporting requirements. The time remaining to counsel on behavior change is therefore short, unstructured, and ineffective. Consequently, PCPs spend little time discussing physical activity and lifestyle changes (Eakin et al., 2005, Glasgow et al., 2001).
Recent studies have shown that using health technologies including electronic medical records (EMR), the internet or text messaging can help improve behavioral management of diabetes (Holbrook et al., 2011, Hunter et al., 2008, Christian et al., 2008, Welch and Shayne, 2006, Jackson et al., 2005, Dick et al., 2011, Franklin et al., 2006, Richardson et al., 2005). Device technologies such as pedometers have also been shown to improve diabetes related behaviors (Richardson et al., 2005, Yates et al., 2009, Diedrich et al., 2009, Booth et al., 2008). Furthermore, interventions that appear to be most effective in sustaining behavior changes include those that use goal-setting, physical activity prescriptions and reminders via telephone calls (Ammerman et al., 2002, Spink et al., 2008, Eakin et al., 2007).
We developed the Avoiding Diabetes Thru Action Plan Targeting (ADAPT) program to create a streamlined shared goal-setting tool embedded in the EMR to help PCPs more effectively counsel patients with prediabetes to improve lifestyle behaviors. This paper describes the results of a 6-month pilot randomized pragmatic trial to evaluate the effectiveness of the ADAPT program on lifestyle behaviors (physical activity, diet) and clinical outcomes (hemoglobin A1C, weight).
2. Methods
The ADAPT study introduced a novel electronic medical record (EMR)-based tool to embed goal setting into primary care provider counseling for patients with prediabetes. The full details of the study design have been previously published (Mann and Lin, 2012, Lin and Mann, 2012).
Patients were recruited between 2011 and 2012 from two urban, academic primary care practices in New York City. Eligible participants were recruited from practice databases and all study procedures were situated within the context of already scheduled clinical visits (Challenges in Clinical Research, 2010). Eligibility criteria included: age 18 or older, English-speaking, and a diagnosis of prediabetes defined as having a glycosylated hemoglobin A1C (A1C) of 5.7–6.4% or a fasting glucose of 100–125 mg/dL. Patients were excluded if they had a diagnosis of diabetes, had ever been prescribed a diabetic medication, were unable to walk, or did not have access to email. A research assistant obtained informed consent with interested participants and administered a standardized survey at enrollment and at 6 months. All participants were given a pedometer to wear for at least one week upon enrolling in the study. All study activities including baseline and follow-up surveys, laboratory assessments and pedometer disbursements were conducted within the context of ongoing primary care clinical activities. This study was approved by the Institutional Review Board at Mount Sinai Hospital.
2.1. Design
The study was a pragmatic randomized clinical trial whose unit of randomization was at the level of the primary care provider. PCPs were randomly assigned in a 1:1 ratio to intervention or control; their patients were subsequently in the intervention or control arm depending on the group to which their PCP had been randomized. Blinding was infeasible due to the nature of the intervention. Patients were offered a 3 month follow-up with their PCP but it was not mandated since every 3-month visits are not a requirement of routine clinical care for prediabetes follow-up. A 6 month follow-up visit was scheduled for all participants.
2.2. Intervention
Just prior to a routine office visit with their PCP, patients in the intervention arm completed a short survey to identify one diet and one physical activity behavior they were willing to change and would be willing to discuss with their PCP. The survey also assessed their current level of pre-specified lifestyle behaviors. During the office visit, the EMR alerted the PCP about the previously-selected diet and physical activity behaviors that their patient was willing to change and the EMR-embedded action planning tool helped guide PCPs to engage in a conversation about lifestyle behavior change along the SMART goal setting framework (see Appendices for screenshots) (Locke and Latham, 1990). The purpose of the action planning tool was to help PCPs and patients set one concrete diet and one concrete activity goal at the close of the visit (for example, “reduce intake of sweetened beverages to one daily” or “get off one bus stop earlier to walk”). In subsequent visits, the EMR tool would display patient progress on these behaviors to the PCP. A pedometer was given to all patients in the intervention arm to assist them in achieving the physical activity goals set with their PCP.
Patients in the control arm followed the same visit schedule as those in the intervention arm but the EMR tools were not available for their PCPs during their visits and they did not receive a pedometer for the duration of the study. Patients in this group did receive printed information on prediabetes and how to change their lifestyle to treat it.
2.3. Measures
All participants completed a baseline survey that assessed socio-demographics, medical history, family history, self-reported physical activity and attempts to change physical activity, confidence/self-effectiveness to change eating habits and physical activity, and assessed their stage of change regarding diet and physical activity behaviors (Prochaska and Velicer, 1997). Prediabetes knowledge and diabetes risk perception were measured using validated instruments consisting of 8 and 5 item 5-point scales respectively (Weymiller et al., 2007, Walker et al., 2007). Prediabetes knowledge and diabetes risk perception scores were calculated as number of points divided by the maximum number of points in each scale for a maximum value of 1, which would indicate strong knowledge or risk perception.
2.4. Diet
Self-reported diet behavior was assessed using a 13 item 3-point scale subset of the short Rapid Eating and Activity Assessment for Patients (REAP-S) tool, a 16-item instrument to address dietary intake and behavior (Gans et al., 2003, Gans et al., 2006). Diet behavior scores were calculated as a 13 item sum with a maximum value of 39 and higher scores indicating better diets.
2.5. Physical activity
All participants were required to wear a pedometer (portable activity monitor, Omron HJ-720ITC) to measure their daily steps at baseline and after the 6 month study visit. The pedometer was then retained by intervention participants for the duration of the study but collected from control patients and then given back to them at 6 months to collect closeout activity assessment. During the pre and post assessment the LCD display of the pedometer was blinded in both groups. To be considered a valid measure of activity, a participant's pedometer data needed to consist of (1) at least 10 h of non-zero activity per day and (2) at least 2 days of activity (Bodenheimer and Handley, 2009). Hours of activity and days worn could be continuous or interrupted. Steps-per-day were then calculated for each patient for each valid day. At baseline and 6 month primary care office visits, weight, A1C, and fasting lipid panels were measured as part of routine clinical care.
2.6. Statistical analysis
Differences between participants in the control vs. intervention arms were compared using t-test, Wilcoxon Rank Sum, chi-square or Fisher's exact test, as appropriate. Changes between baseline and six months were calculated for average daily steps (measured by pedometer), weight, and A1c levels. Differences between groups with respect to these changes between baseline and 6 months were assessed via t-test or Wilcoxon Rank Sum for normally and non-normally distributed variables, respectively. For all tests, p-values less than 0.05 were considered statistically significant. All analyses were conducted using SAS 9.2 (SAS Institute, Cary, North Carolina).
3. Results
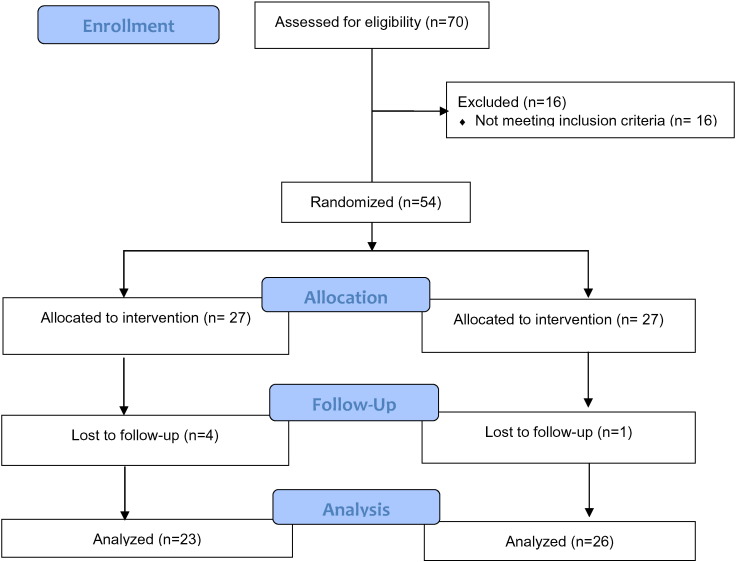
A total of 54 patients participated in the ADAPT study (see Fig. 1 for study flow). Mean age of the participants was 46 (SD 11) and most were female (83.3%); 38.9% were African-American, 40.7% were Hispanic and 11.1% were White; and 76% had a family history of diabetes. Comorbidities such as hypertension and hyperlipidemia were prevalent (44.4% and 29.6%, respectively) and most participants (79.6%) had at least 1 reported comorbidity. There were no significant differences in socio-demographic or medical characteristics between the intervention and control groups (Table 1).
Fig. 1.
Study flow.
Table 1.
Baseline characteristics by randomization group.
| Control (N = 27) | Intervention (N = 27) | p-Value | |
|---|---|---|---|
| Mean age (SD) | 43.67 (9.28) | 47.5 (11.99) | 0.20 |
| Male (%) | 6 (22.22) | 3 (11.11) | 0.47 |
| Race/ethnicity | |||
| White | 1 (3.7) | 5 (18.52) | 0.38 |
| Black | 12 (44.44) | 9 (33.33) | |
| American Indian/Native American | 0 (0) | 1 (3.7) | |
| Asian/Pacific Islander | 2 (7.41) | 2 (7.41) | |
| Hispanic/Latino | 12 (44.44) | 10 (37.04) | |
| Commercial health insurance | 21 (80.77) | 20 (83.33) | 1.00 |
| Some college education | 19 (73.08) | 24 (88.89) | 0.18 |
| Family history of diabetes | 19 (70.37) | 22 (84.62) | 0.22 |
| Comorbidities | |||
| Asthma | 10 (37.04) | 6 (22.22) | 0.23 |
| Hypertension | 13 (48.15) | 11 (40.74) | 0.58 |
| High cholesterol | 8 (29.63) | 8 (29.63) | 1.00 |
| Heart problems | 3 (11.54) | 3 (11.54) | 1.00 |
| Arthritis | 7 (25.93) | 8 (29.63) | 0.76 |
| Sleep apnea | 5 (20) | 5 (19.23) | 1.00 |
| Any comorbidity | 22 (81.48) | 21 (77.78) | 0.74 |
| Diet stage of change | 0.35 | ||
| Contemplation | 2 (7.69) | 1 (4.17) | |
| Preparation | 13 (50) | 7 (29.17) | |
| Action | 9 (34.62) | 11 (45.83) | |
| Maintenance | 2 (7.69) | 5 (20.83) | |
| Physical activity stage of change | 0.09 | ||
| Contemplation | 3 (11.11) | 0 (0) | |
| Preparation | 12 (44.44) | 9 (37.5) | |
| Action | 8 (29.63) | 14 (58.33) | |
| Maintenance | 4 (14.81) | 1 (4.17) | |
| Median REAP-S score (1st quartile, 3rd quartile) | 30 (26, 33) | 31 (28, 34) | 0.41 |
| Median risk knowledge (1st Quartile, 3rd Quartile) | 0.85 (0.78, 0.93) | 0.88 (0.83, 0.9) | 0.31 |
| Median risk perception (1st quartile, 3rd quartile) | 0.43 (0.38, 0.53) | 0.43 (0.3, 0.48) | 0.31 |
(NY, NY 2010–2012).
The majority of participants were in the preparation, action or maintenance phases of the Stages of Change for diet and physical activity. Knowledge about prediabetes and diabetes beliefs were similar across groups with most participants holding appropriate concerns about their risks for developing diabetes and ability to prevent it. REAP-S scores were also similar between both groups [median (LCL, UCL): 30 (29, 32) vs. 31 (29, 34), p = 0.41)].
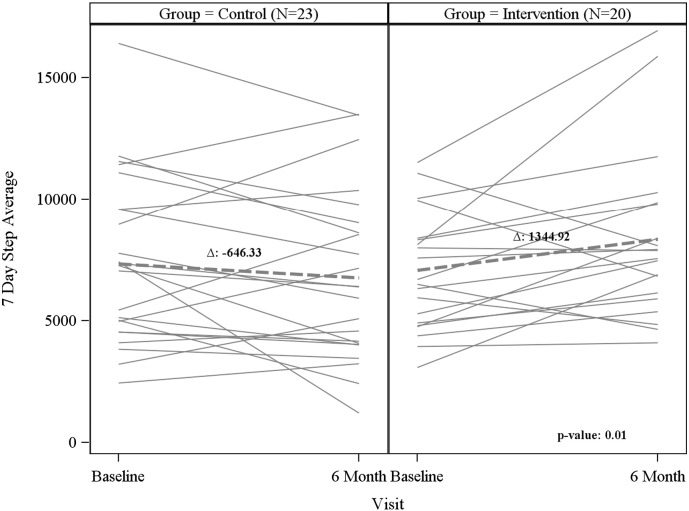
There was a significant increase in total daily steps (mean (LCL, UCL): 1418 (349, 2487)) vs − 598 (− 1600, 405), p = 0.007) and the 7-day average steps (mean (LCL, UCL): 1345 (104, 2586) vs. -646 (− 1648, 356), p = 0.01) in the intervention compared to the control group (Table 2) over the 6 months. Fig. 2 highlights the change in 7-day average steps over the 6 months between groups. There was a trend towards weight loss (median (LCL,UCL): − 1.0 lbs. (− 3.0, 2.0) vs. 3.0 lbs. (− 4.0, 6.0), p = 0.11) in the intervention compared to the control group. There were no differences in A1C change from baseline to 6 months in either group. Additionally, there were no differences between the intervention and control group in diet scores or stage of change for diet or physical activity from baseline to 6 months.
Table 2.
Comparison of change from baseline to 6 months by group.
| Control (N = 27) |
Intervention (N = 27) |
p-Value | |
|---|---|---|---|
| Weight (lbs) | 3.0 (− 4.5, 6.5) | − 1.0 (− 3.0, 2.0) | 0.11 |
| Body mass index (kg/m2) | 0.43 (− 0.79, 1.15) | − 0.18 (− 0.5, 0.37) | 0.11 |
| Hemoglobin A1C (%) | 0.1 (− 0.1, 0.2) | 0 (− 0.1, 0.2) | 0.83 |
| Total cholesterol (mg/dL) | − 4.59 ± 28.59 | 1.38 ± 21.04 | 0.44 |
| Low density lipoprotein (mg/dL) | − 6.23 ± 23.09 | − 0.43 ± 15.87 | 0.35 |
| High density lipoprotein (mg/dL) | − 1.57 ± 8.49 | 1.33 ± 7.45 | 0.25 |
| Triglycerides (mg/dL) | 4 (− 20, 27) | − 1 (− 11, 10) | 0.57 |
| REAP-S score | 1.65 ± 4.48 | 2.81 ± 4.72 | 0.46 |
| Risk knowledge | 0.02 ± 0.14 | − 0.03 ± 0.12 | 0.20 |
| Risk perception | 0.08 (− 0.01, 0.11) | 0.04 (− 0.04, 0.18) | 1.00 |
| Total step average | − 597.5 ± 2317.24 | 1417.99 ± 2284.68 | 0.01 |
| 7 day step average | − 646.33 ± 2317.07 | 1344.92 ± 2652.12 | 0.01 |
Values expressed as mean ± SD or median (1st quartile, 3rd quartile) as appropriate. (NY, NY 2010–2012).
Fig. 2.
Individual and overall 7 day step average by study visit and group Fig. 1: Changes in 7-day step average from baseline to 6-month visit. Solid lines represent individual patient changes in each group; dashed lines represent overall group change. (NY, NY 2010–2012).
4. Discussion
These data give exciting preliminary data demonstrating that brief goal setting by primary care providers, carefully embedded within clinical workflow and enhanced with technology, can significantly promote physical activity among patients with prediabetes. This approach also has a potential to impact weight loss though it does not appear to impact overall glycemic control in the short term.
The observed increase in physical activity, trends towards weight loss but stable A1C is in line with other pedometer-based physical activity interventions (Yates et al., 2009, Dorough et al., 2012, De Greef et al., 2010). In a study of a pragmatic structured education program with a pedometer, prediabetes patients randomized to the intervention group increased their pedometer activity by a mean of 1902 steps and decreased their fasting glucose only 6 mg/dl at 1 year (Yates et al., 2009). In a study of pre-hypertensive patients, a 10 week comprehensive technology-enhanced lifestyle intervention using a pedometer led to statistically significant increases in activity (2900 steps, p < 0.01) and weight loss (4.8 kg, p < .05) (Dorough et al., 2012). The ADAPT study extends these data by demonstrating an approach towards increasing physical activity that uses the primary care encounter and the EMR to deliver a brief intervention in a focused, clinically pragmatic and scalable manner.
Despite a clear need, there has been little use of the EMR to promote goal setting within primary care in a feasible manner. The ADAPT intervention leveraged EMR based goal setting to promote behavior change among prediabetes patients in primary care. Goal setting using the SMART (Specific, Measurable, Attainable, Relevant, Time-bound) criteria is a well-established technique for promoting lifestyle behavior change in diabetes and other lifestyle sensitive conditions (Mann and Lin, 2012, DeWalt et al., 2009, Brown et al., 2012, Shilts et al., 2004). Primary care is the most common touch point with the healthcare system for prediabetes patients so it is a logical focus for promoting lifestyle change with goal setting (Bodenheimer and Handley, 2009, Ma et al., 2012). This natural pairing of goal setting within primary care is highlighted by the inclusion of goal setting into the NCQA (National Committee for Quality Assurance) patient centered medical home recognition program (NCQA).
ADAPT combined a pre-visit brief lifestyle behavior screening survey with EMR-embedded alerts and shared goal setting documentation tools to tightly package goal setting and embed goal setting into the primary care provider workflow. The study used the EMR tools to constrain goal setting activities to more closely adhere to the SMART goal setting guidelines to encourage providers and patients to focus on realistic achievable concrete goals for lifestyle behavior change. This approach helped focus PCP counseling towards the most evidence-based approaches and used the pedometer to ensure clear goal setting.
Each of the components of the ADAPT intervention may have contributed to its success. However, the lack of change in A1C highlights that multifactorial interventions are required to significantly impact outcomes for lifestyle sensitive conditions like prediabetes. Goal setting with primary care providers, even when optimized using EMR and pedometer tools, likely has a ceiling effect among most patients. Increasing physical activity, as was observed in ADAPT, does not ensure reduced A1C. The lack of significant changes in dietary behavior further limited the potential for A1C reductions. The relative resistance of dietary behavior change may reflect the relative complexity of changing diet compared to physical activity and the lack of an objective measure of diet which hinders the development of accurate behavioral feedback loops to drive goal setting based behavior change. Moreover, the lack of substantial changes in dietary behavior likely contributed to the relatively small observed changes in weight compared to the DPP (Diabetes Prevention Program Research Group, 2002).
Our study findings may be used as a guide for operational and research leaders to demonstrate how to embed goal setting into the clinical workflow, how to leverage rapid usability studies to iteratively refine clinically integrated tools, how to use pre- and post- visit tools to complement office visit counseling, and how to use technology to assist goal setting in primary care. The study demonstrates that these tools can be used to effectively support a complex behavioral technique like goal setting into routine clinical care. These new workflows can then be incorporated into more comprehensive population health programs that harness the full resources of all members of the healthcare team (including the patient).
These study findings should be viewed in the context of several limitations. First, this was a pilot study so the small sample size limits its ability to find significant differences in several outcome variables and should not be interpreted as an inability for the intervention to affect these outcomes more generally. The study recruited mostly women, a common phenomenon in behavior change trials that limits generalizability to male populations. The participants also frequently had comorbid conditions limiting the relevance of the results to healthier populations. The study was designed as a pragmatic trial, embedding the research methods within real clinical workflows. This choice enhances the study generalizability but limits the control of the data collection and measurement battery. In particular, we cannot fully define what parts of the intervention worked and how they mediated and/or moderated the outcomes. The pragmatic study design also allowed for more missing data points than a typical controlled clinical trial further reducing the power of the sample size. The study used EMR tools but these tools are in constant evolution as is provider comfort with them. This affects our ability to apply the study findings to current clinical workflows though the guiding principles still hold.
Despite these limitations, the successful uptake of the study tools by providers and patients and the changes in physical activity support continued experimentation with EMR tools to support behavior change efforts in primary care. Future studies should leverage advances in EMR decision support technologies that facilitate seamless workflow integration and more powerful, context sensitive behavioral interactions. Mobile and other new telehealth technologies can also be used to extend the interactions between primary care provider recommendations and patients well beyond the clinic. In addition, advances in self-monitoring technologies for diet and physical activity should be integrated into EMR based goal setting tools to make them more robust. Finally, as healthcare transforms to a value based system that targets populations and leverages team based care to promote health — behavior change decision support tools like ADAPT should be modified to support the involvement of all members of the team (including the patient's social network) in helping patients achieve their behavior change goals.
The ADAPT study is one of the first to use standard EMR tools to embed goal-setting into realistic primary care workflows and to demonstrate a significant improvement in physical activity. This pragmatic trial leveraged usability studies as an agile design guide, ultimately leading to an intervention that was tightly embedded into clinical workflow and successfully promoting evidence-based counseling in routine office visits. Our experience can be incorporated into future studies and clinical improvement programs seeking to leverage primary care for changing behaviors contributing to prediabetes and other lifestyle sensitive health conditions.
Conflicts of interest
The authors have no conflicts of interest to disclose. They have no financial or personal relationships with other people or organizations that could inappropriately influence their work.
Funding
This study was supported by a grant from the National Institute for Diabetes, Digestive and Kidney Diseases (5K23DK081665).
Acknowledgments
We wish to acknowledge Lucas Romero for his help with conducting patient and provider interviews, Diego Chiluisa for his help with patient recruitment and study management, Daniel Edonyabo for his work with the electronic medical record tool development and Michael O′Leary for his work with the patient website development.
Footnotes
Clinicaltrials.gov.: NCT01473654.
Appendix A.
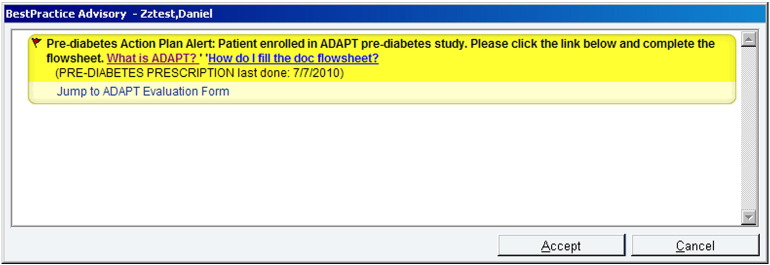
Fig. A.
Screenshot of the ADAPT alert.
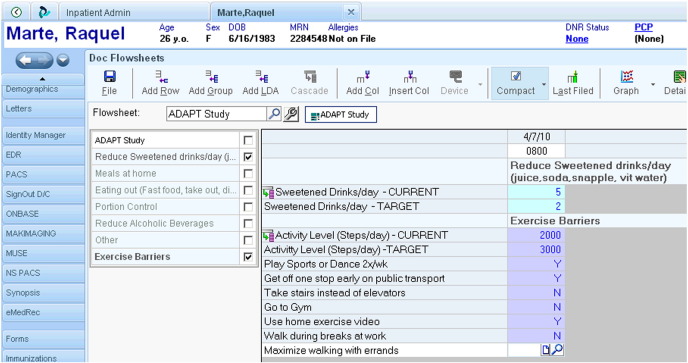
Fig. B.
Screenshot of the ADAPT EMR goal setting tool.
Fig. C.
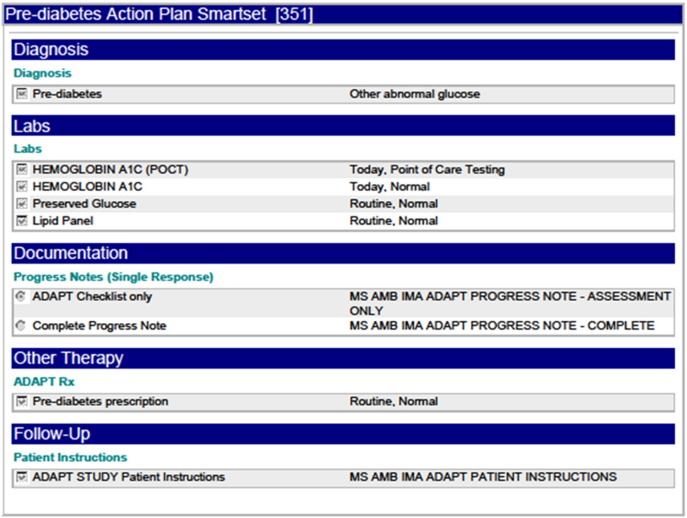
Screenshot of ADAPT order set.
References
- ADA Executive summary: Standards of Medical Care in Diabetes 2008. Diabetes Care. 2008;31(Supplement_1):S5–11. [Google Scholar]
- Ammerman A.S., Lindquist C.H., Lohr K.N., Hersey J. The efficacy of behavioral interventions to modify dietary fat and fruit and vegetable intake: a review of the evidence. Prev. Med. 2002;35(1):25–41. doi: 10.1006/pmed.2002.1028. [DOI] [PubMed] [Google Scholar]
- Bodenheimer T., Handley M.A. Goal-setting for behavior change in primary care: an exploration and status report. Patient Educ. Couns. 2009;76(2):174–180. doi: 10.1016/j.pec.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Booth A.O., Nowson C.A., Matters H. Evaluation of an interactive, Internet-based weight loss program: a pilot study. Health Educ. Res. 2008;23(3):371–381. doi: 10.1093/her/cyn007. [DOI] [PubMed] [Google Scholar]
- Brown M.J., Sinclair M., Liddle D., Hill A.J., Madden E., Stockdale J. A systematic review investigating healthy lifestyle interventions incorporating goal setting strategies for preventing excess gestational weight gain. PLoS ONE. 2012;7(7):e39503. doi: 10.1371/journal.pone.0039503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC . In: National Diabetes Statistics Report: Estimates of Diabetes and its Burden in the United States, 2014. Services HaH, editor. 2014. (Atlanta) [Google Scholar]
- Christian J.G., Bessesen D.H., Byers T.E., Christian K.K., Goldstein M.G., Bock B.C. Clinic-based support to help overweight patients with type 2 diabetes increase physical activity and lose weight. Arch. Intern. Med. 2008;168(2):141–146. doi: 10.1001/archinternmed.2007.13. [DOI] [PubMed] [Google Scholar]
- Craig CL, Tudor-Locke C, Bauman A. Twelve-Month Effects of Canada on the Move: A Population-Wide Campaign to Promote Pedometer Use and Walking. vol. 222007:406–413. [DOI] [PubMed]
- Danaei G., Finucane M.M., Lu Y. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378(9785):31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- De Greef K., Deforche B., Tudor-Locke C., De Bourdeaudhuij I. A cognitive–behavioural pedometer-based group intervention on physical activity and sedentary behaviour in individuals with type 2 diabetes. Health Educ. Res. 2010 doi: 10.1093/her/cyq017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWalt D.A., Davis T.C., Wallace A.S. Goal setting in diabetes self-management: taking the baby steps to success. Patient Educ. Couns. 2009;77(2):218–223. doi: 10.1016/j.pec.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick J.J., Nundy S., Solomon M.C., Bishop K.N., Chin M.H., Peek M.E. Feasibility and usability of a text message-based program for diabetes self-management in an urban African-American population. J. Diabetes Sci. Technol. 2011;5(5):1246–1254. doi: 10.1177/193229681100500534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrich A., Munroe D.J., Romano M. Promoting physical activity for persons with diabetes. Diabetes Educ. 2009;0145721709352382 doi: 10.1177/0145721709352382. [DOI] [PubMed] [Google Scholar]
- Dorough A.E., Winett R.A., Anderson E.S., Davy B.M., Martin E.C., Hedrick V. DASH to wellness: emphasizing self-regulation through E-health in adults with prehypertension. Health Psychol. 2012 doi: 10.1037/a0030483. [DOI] [PubMed] [Google Scholar]
- Eakin E., Smith B., Bauman A. Evaluating the population health impact of physical activity interventions in primary care — are we asking the right questions? J. Phys. Act. Health. 2005;2:197–215. [Google Scholar]
- Eakin E.G., Lawler S.P., Vandelanotte C., Owen N. Telephone interventions for physical activity and dietary behavior change: a systematic review. Am. J. Prev. Med. 2007;32(5):419–434. doi: 10.1016/j.amepre.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Franklin V.L., Waller A., Pagliari C., Greene S.A. A randomized controlled trial of Sweet Talk, a text-messaging system to support young people with diabetes. Diabet. Med. 2006;23(12):1332–1338. doi: 10.1111/j.1464-5491.2006.01989.x. [DOI] [PubMed] [Google Scholar]
- Gans K.M., Ross E., Barner C.W., Wylie-Rosett J., McMurray J., Eaton C. REAP and WAVE: new tools to rapidly assess/discuss nutrition with patients. J. Nutr. 2003;133(2):556S–562S. doi: 10.1093/jn/133.2.556S. [DOI] [PubMed] [Google Scholar]
- Gans K.M., Risica P.M., Wylie-Rosett J. Development and evaluation of the nutrition component of the Rapid Eating and Activity Assessment for Patients (REAP): a new tool for primary care providers. J. Nutr. Educ. Behav. 2006;38(5):286–292. doi: 10.1016/j.jneb.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Glasgow R.E., Eakin E.G., Fisher E.B., Bacak S.J., Brownson R.C. Physician advice and support for physical activity: results from a national survey. Am. J. Prev. Med. 2001;21(3):189–196. doi: 10.1016/s0749-3797(01)00350-6. [DOI] [PubMed] [Google Scholar]
- Haire-Joshu D., Klein S. Is primary care practice equipped to deal with obesity?: comment on “preventing weight gain by lifestyle intervention in a general practice setting”. Arch. Intern. Med. 2011;171(4):313–315. doi: 10.1001/archinternmed.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J.O., Wyatt H.R., Reed G.W., Peters J.C. Obesity and the environment: where do we go from here? Science. 2003;299(5608):853–855. doi: 10.1126/science.1079857. [DOI] [PubMed] [Google Scholar]
- Holbrook A., Pullenayegum E., Thabane L. Shared electronic vascular risk decision support in primary care: computerization of medical practices for the enhancement of therapeutic effectiveness (COMPETE III) randomized trial. Arch. Intern. Med. 2011;171(19):1736–1744. doi: 10.1001/archinternmed.2011.471. [DOI] [PubMed] [Google Scholar]
- Challenges in clinical research. 2010. http://www.ncbi.nlm.nih.gov/books/NBK50888
- Hunter C.M., Peterson A.L., Alvarez L.M. Weight management using the internet a randomized controlled trial. Am. J. Prev. Med. 2008;34(2):119–126. doi: 10.1016/j.amepre.2007.09.026. [DOI] [PubMed] [Google Scholar]
- Jackson C.L., Batts-Turner M.L., Falb M.D., Yeh H.C., Brancati F.L., Gary T.L. Computer and internet use among urban African Americans with type 2 diabetes. J. Urban Health. 2005;82(4):575–583. doi: 10.1093/jurban/jti126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner R.F. Tackling obesity: is primary care up to the challenge? Arch. Intern. Med. 2010;170(2):121–123. doi: 10.1001/archinternmed.2009.479. [DOI] [PubMed] [Google Scholar]
- Lin J.J., Mann D.M. Application of persuasion and health behavior theories for behavior change counseling: design of the ADAPT (avoiding diabetes thru action plan targeting) program. Patient Educ. Couns. 2012;88(3):460–466. doi: 10.1016/j.pec.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke E., Latham G. Englewood Cliffs, NJ; Prentice Hall: 1990. A Theory of Goal Setting and Task Performance. [Google Scholar]
- Ma J., Yank V., Xiao L. Translating the diabetes prevention program lifestyle intervention for weight loss into primary care: a randomized trial. Arch. Intern. Med. 2012;1-9 doi: 10.1001/2013.jamainternmed.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann D.M., Lin J.J. Increasing efficacy of primary care-based counseling for diabetes prevention: rationale and design of the ADAPT (avoiding diabetes thru action plan targeting) trial. Implement. Sci. 2012;7:6. doi: 10.1186/1748-5908-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCQA Patient-centered medical home recognition. http://www.ncqa.org/Programs/Recognition/Practices/PatientCenteredMedicalHomePCMH.aspx (Accessed August 7, 2015)
- Prochaska J.O., Velicer W.F. The transtheoretical model of health behavior change. Am. J. Health Promot. 1997;12(1):38–48. doi: 10.4278/0890-1171-12.1.38. [DOI] [PubMed] [Google Scholar]
- Richardson C.R., Brown B.B., Foley S., Dial K.S., Lowery J.C. Feasibility of adding enhanced pedometer feedback to nutritional counseling for weight loss. J. Med. Internet Res. 2005;7(5):e56. doi: 10.2196/jmir.7.5.e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilts M.K., Horowitz M., Townsend M.S. Goal setting as a strategy for dietary and physical activity behavior change: a review of the literature. Am. J. Health Promot. 2004;19(2):81–93. doi: 10.4278/0890-1171-19.2.81. [DOI] [PubMed] [Google Scholar]
- Spink K.S., Reeder B., Chad K., Wilson K., Nickel D. Examining physician counselling to promote the adoption of physical activity. Can. J. Public Health. 2008;99(1):26–30. doi: 10.1007/BF03403736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker E.A., Caban A., Schechter C.B. Measuring comparative risk perceptions in an urban minority population: the risk perception survey for diabetes. Diabetes Educ. 2007;33(1):103–110. doi: 10.1177/0145721706298198. [DOI] [PubMed] [Google Scholar]
- Welch G., Shayne R. Interactive behavioral technologies and diabetes self-management support: recent research findings from clinical trials. Curr. Diab. Rep. 2006;6(2):130–136. doi: 10.1007/s11892-006-0024-9. [DOI] [PubMed] [Google Scholar]
- Weymiller A.J., Montori V.M., Jones L.A. Helping patients with type 2 diabetes mellitus make treatment decisions: statin choice randomized trial. Arch. Intern. Med. 2007;167(10):1076–1082. doi: 10.1001/archinte.167.10.1076. [DOI] [PubMed] [Google Scholar]
- Wild S., Roglic G., Green A., Sicree R., King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- Writing Group Members, Rosamond W., Flegal K. A Report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Vol. 115. 2007. Heart disease and stroke statistics—2007 update; pp. e69–e171. (Circulation). [DOI] [PubMed] [Google Scholar]
- Yates T., Davies M., Gorely T., Bull F., Khunti K. Effectiveness of a pragmatic education program designed to promote walking activity in individuals with impaired glucose tolerance. Diabetes Care. 2009;32(8):1404–1410. doi: 10.2337/dc09-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]