Abstract
Introduction
Diagnosis of peritoneal tuberculosis (pTB) is difficult, even in developed countries, where data are lacking. The aim of the present study was to describe the clinical presentation, diagnosis, and bacterial epidemiology of pTB in France over a 10-year period.
Methods
A retrospective study was conducted on pTB in two university hospitals in France, between January 2004 and December 2014.
Results
Among the 34 patients, 76.5% were migrants from areas of endemic tuberculosis (TB), mainly Africa. The main presentation (85.3%) was a checkup of ascites or suspicion of peritoneal carcinomatosis. On abdominal computed tomography, ascites was found in 90.6% and peritoneal thickening in 75%. Surgery was required for diagnosis in 58.8% of patients. Six of the patients who did not undergo surgery had ultrasound-guided peritoneal biopsy. Bacteriology was positive for ascites in only 58.1% of cases, for peritoneal biopsy in 73.3%, while granuloma was found in 95.5%. TB polymerase chain reaction (PCR) was positive in 25% of peritoneal biopsy. Mycobacterium bovis was isolated in 23.1% of cases and Mycobacterium tuberculosis in 76.9%. Isolates were fully susceptible (except M. bovis naturally resistant to pyrazinamide). Many (38%) belonged to the lineage T (genetic analysis by spoligotyping). Cure rate was high (76.5%), after a 6–9 months of anti-tuberculous therapy.
Conclusion
In developed countries, early diagnosis of pTB is still a challenge. Ultrasound-guided peritoneal biopsy may facilitate diagnosis. TB PCR can be useful on peritoneal biopsy. The lineage T was the most prevalent lineage, but more data are required to directly incriminate this lineage in the pathophysiology of pTB.
Keywords: Ascites, Mycobacterium tuberculosis, Peritoneal biopsy, Peritoneal tuberculosis, Spoligotyping, Tuberculous peritonitis
Introduction
Despite the better control of tuberculosis (TB) in the world, the proportion of extrapulmonary TB has increased in recent years [1]. In this context, peritoneal TB (pTB) represents the most frequent form of abdominal TB [2]. Data are lacking in developed countries since most studies were done in endemic countries of TB [2–4]. The aim of the present study was to describe the clinical presentation, diagnosis, bacterial epidemiology, treatment, and outcome of pTB in two university hospitals in France over a 10-year period.
Methods
All patients diagnosed with pTB in the university hospitals of Lyon (Croix-Rousse Hospital) and Nancy (Brabois Hospital) between January 2004 and December 2014 were included in a retrospective observational study. France is a country with an incidence of 8.7 per 100,000 population (including 27% of extrapulmonary) [5] and the two cities Lyon and Nancy count 1.3 million and 300,000 people, respectively. Patients were identified by using the database of the administrative coding system (patients with diagnosis of pTB in the infectious disease unit) and the database of the mycobacteriological laboratory of Lyon and Nancy (patients with a positive culture or polymerase chain reaction [PCR] of ascites or peritoneum). Children and patients with peritonitis due to non-tuberculous mycobacteria were excluded. Patients without culture or PCR confirmation of TB but with peritonitis consistent with pTB based on clinical, radiographic or histologic evidence were included. Clinical, imaging and bacteriological data were collected. Ascites samples were processed in biosafety level 3 facility as follows: briefly samples were centrifuged for 15 min before inoculation to solid (Löwenstein-Jensen) and liquid (MGIT-Bactec) media. Pellets were heat inactivated and then used for acid fast bacilli smear test with fluorescent microscope. Heat-inactivated samples were further processed for DNA extraction with MagNA Pure Roche System and then used for Mycobacterium tuberculosis specific in house PCR reaction. Cultured strains were characterized by spoligotyping (spacer oligonucleotide typing), a PCR-based method for genotyping strains [6] and categorized by lineage, using the SpolDB4 database [7]. Statistical methods used were medians and percentages.
The study has been approved by the institutional ethics committee. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent waiver was granted as all data were currently in existence.
Results
Thirty-four patients were included (29 in Lyon and 5 in Nancy). There was male predominance, with 22 men and 12 women; median age was 40 years. Twenty-six patients (76.5%) were migrants from areas of endemic TB, mainly Africa. One or more underlying disease was found in 13 patients (38.2%): liver cirrhosis (6), diabetes mellitus (6), chronic viral hepatitis (4) or immunodepression as cancer (4), immunosuppressive therapy (3) and human immunodeficiency virus (1, who was already treated and controlled). HIV was tested for 32/34 patients (94.1%). Two patients (5.9%) had history of cured TB; history of TB exposure was reported by seven patients (20.6%). The main presentation that lead to hospitalization (29 patients: 85.3%) was a checkup of ascites or suspicion of peritoneal carcinomatosis; two patients (5.9%) presented peritonitis. The main symptoms and signs are given in Table 1. Four patients (11.8%) presented acute abdominal illness requiring emergency laparotomy (two initially and two secondarily). Median symptom duration before admission was 1 month.
Table 1.
Symptoms and signs at the presentation (N = 34)
| Symptoms | n (%) |
|---|---|
| Fever and/or sweats | 24 (70.6%) |
| Abdominal pain | 24 (70.6%) |
| Ascites or abdominal swelling | 19 (55.9%) |
| Weight loss | 16 (47.1%) |
| Asthenia | 11 (32.4%) |
| Diarrhea | 4 (11.8%) |
| Anorexia | 3 (8.8%) |
| Abdominal mass | 1 (2.9%) |
| Salpingitis | 1 (2.9%) |
Biological findings revealed biological inflammatory syndrome in all patients, with a median C-reactive protein level of 95 mg/L. Lymphopenia (under 1.5 G/L) was found in 23 of the 30 patients investigated (76.7%). Interferon-γ release assay (QuantiFERON-TB®; Cellestis) was positive in only 16/22 patients (72.7%), and 15/24 (62.5%) had positive (at least 10 mm) tuberculin test (Tubertest®; Sanofi Pasteur). Ascitic fluid analysis found exudate in 17/21 patients (80.1%), with lymphocytic predominance in 19/21 cases (90.5%).
On abdominal computed tomography (CT), ascites was found in 29/32 patients (90.6%), and was the only abnormality in 4/32 of these (13.8%). Peritoneal, mesenteric, and/or omentum thickening (Fig. 1) was detected in 24 patients (75%). Only three patients (8.9%) had no ascites on CT scan, corresponding to the “dry plastic” form of pTB, defined by Bhargava [8]. Abdominal ultrasound was equal to CT to detect ascites (25/28, 89.3%) but less efficient in detecting peritoneal thickening (6/28, 21.4%). Six patients (17.8%) had positron emission tomography, showing increased peritoneal or mesenteric and lymph node metabolic activity, which could not be differentiated from carcinomatosis. Thirty-two out of 34 thoracic CT (94.1%) were abnormal with pleural effusion (12), mediastinal lymph node (9), micronodules (8), nodule (4), or excavation (1). Surgery was required for diagnosis in 20 cases (58.8%), including 14 laparoscopies (41.2%) and six laparotomies (17.6%). Intraoperative visual inspection was suggestive of pTB in 13 patients (65%), with typical small white peritoneal granulations or granuloma. Six of the patients who did not undergo surgery (42.9%) had ultrasound-guided peritoneal biopsy. Tuberculous peritonitis was culture-confirmed in 25 patients. Diagnostic performance of the various bacteriological and histological analyses is shown in Table 2. TB PCR, performed on 13/31 ascites samples and on 8/14 peritoneal biopsies, was systematically negative on ascites and positive on only 2 peritoneal biopsies (25%). It should be noted that a large number of peritoneal biopsies were not sent to the bacteriology laboratory (8/22, 36.4%), because only malignant ascites was suspected by the surgeon. Finally, histological and/or bacteriological diagnostic proof was obtained in all patients, except for three (8.8%) who did not have peritoneal biopsy but were cured by the anti-TB treatment.
Fig. 1.
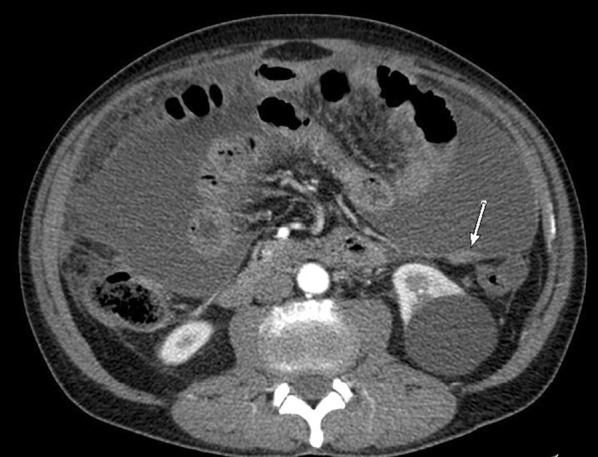
Abdominal computed tomography of a peritoneal tuberculosis, with peritoneal nodular thickening (white arrow) and ascites
Table 2.
Diagnostic performance of bacteriological and histological analyses in patients with peritoneal tuberculosis
| Ascites | Peritoneal biopsy | |
|---|---|---|
| Total | 32 | 22 |
| Positive culture | 58.1% (18/31) | 71.4% (10/14) |
| Positive PCR | 0.0% (0/13) | 25.0% (2/8) |
| Total bacteriological performance | 58.1% (18/31) | 73.3% (11/15) |
| Histological performance: epithelioid giant-cell granuloma | 95.5% (21/22) | |
| Including with caseous necrosis | 36.4% (8/22) | |
PCR polymerase chain reaction
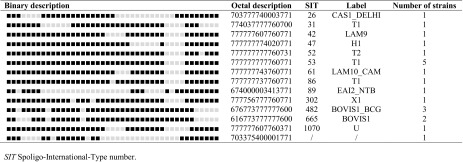
Twenty-six infections were documented. Mycobacterium bovis was isolated in 6 patients (23.1%) and M. tuberculosis in the others 20 (76.9%). All isolates were susceptible to classical anti-mycobacterial drugs, except one case of isoniazid resistance (M. bovis is naturally resistant to pyrazinamide). Genetic analysis by spoligotyping, performed in 21 cases, revealed that 8 (38%) belonged to lineage T (Table 3). T family lineage is characterized by the absence of spacers 33–36 among the 43 spacers targeted by spoligotyping; the prototypic shared spoligotype is SIT53. There was no specific clinical characteristic for the spoligotype T.
Table 3.
Spoligotyping of the 21 strains responsible for peritoneal tuberculosis
Sixteen patients (47.1%) showed one or more other TB location, including lung (five patients), and digestive (four patients), gynecological (four patients) and urological (two patients) tract. Sputum smear examination or bronchoalveolar lavage was performed in 32 patients (94.1%) and cultures were positive for 5 patients (14.7%). Extensive surgery with intestinal resection was required in two patients because of digestive perforation; corticosteroids were prescribed for subocclusive syndrome in two patients; and three malnourished patients required artificial nutrition. Median admission-to-treatment time was 18 days. Antimycobacterial therapy comprised standard regimen with daily rifampicin, isoniazid, ethambutol and pyrazinamide during 2 months followed by at least 4 months of rifampicin and isoniazid for 24 patients (70.6%); the others had therapy without pyrazinamide or without isoniazid and/or with quinolone because of toxicity or hepatic disorder before treatment. Side-effects led to dose decrease or a change of treatment in 13 patients (38.2%). Twenty-six patients (76.5%) completed treatment and were clinically and radiologically cured; four were lost to follow-up; one was still under treatment at the time of writing; and three died during treatment (only one because of disseminated TB). Median treatment duration was 8.5 months, and follow-up without relapse after end of treatment was 12 months.
Discussion
Early diagnosis of pTB is a challenge as the main presentation of our cohort (85.3%) was a checkup of ascites or suspicion of peritoneal carcinomatosis. Many different clinical presentations of pTB have been previously described [3]. Due to non-specific and insidious symptoms, there is a significant delay before diagnosis and treatment [4], even in developed countries. Notably, most cases of pTB mimic peritoneal carcinomatosis, especially in women with elevated blood Ca 125 [9]. In cirrhotic patients, pTB can mimic ascitic decompensation. In addition, imaging findings may point to carcinomatosis [2]. Thus, TB should be systematically considered in case of peritonitis.
Bacteriological performance is imperfect since culture of ascites was positive in only 58.1% and peritoneal biopsy in 73.3% of cases. Biopsy for bacterial culture and histology is essential for diagnosis [10]. Histology allows to correct the diagnosis of TB in a large number of cases where bacterial culture was forgotten: it is essential to routinely do mycobacterial culture of peritoneal biopsy during an exploration of ascites or peritoneal thickening, unless there is a known intra-abdominal malignancy. Laparoscopic peritoneal biopsy is the reference method for diagnosis of pTB but, as described here, ultrasound-guided biopsy may facilitate diagnosis, with a lower risk of complications [11]. Culture of ascites fluid must be systematic, even if the positivity rate is lower than for peritoneal biopsy, probably because of the low number of bacilli in the processed samples. In the present study, TB PCR seemed to be insensitive when performed on ascites, but can be useful on peritoneal biopsy [12].
Interestingly, we found that a high percentage of isolates responsible for pTB belonged to lineage T. Extrapulmonary TB is usually more frequently associated with the East African-Indian or Central-Asian lineages, whereas lineage T, like the Beijing lineage, seems to be more frequently linked to pulmonary forms [13, 14]. The T lineage is the predominant lineage in Europe, but not in Africa, where most of our patients have lived before migrating in France [7]. Finally, it is not known if particular lineages are more frequently responsible for pTB and more data are required to directly incriminate the T lineage in the pathophysiology of pTB.
In case of suspected pTB, especially with concordant histology, physicians should not wait for culture before initiating treatment, since treatment delay is a very important factor of poor prognosis [4]. The present cure rate was high, and there was no relapse during follow-up.
Conclusions
In developed countries, early diagnosis of pTB is still a challenge. Ultrasound-guided peritoneal biopsy may facilitate diagnosis. TB PCR can be useful on peritoneal biopsy. When histology is concordant, prompt treatment initiation may improve prognosis. Data are required to know if particular lineages are responsible for pTB.
Acknowledgments
The study was founded by Hospices Civils de Lyon (data collection and performance of spoligotyping). The article processing charges for this publication were funded by EZUS Lyon 1. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published. We have received substantial contributions from Catherine Laurain, Badis Menassel and the Lyon TB Study group: L. Adelaïde, F. Ader, F. Biron, A. Boibieux, A. Bouaziz, K. Bouledrak, E. Braun, C. Broussolle, G. Carret, G. Catho, N. Charhon, C. Chidiac, W. Chumbi-Flores, B. Coppere, S. Couraud, M. Demontclos, G. Devouassoux, O. Dumitrescu, S. Ernesto, T. Ferry, D. Floret, I. Fredenucci, N. Freymond, S. Gardes, S. Gerbier-Colomban, Y. Gillet, M.H. Girard-Madoux, S. Goutelle, J. Grando, R. Grima, L. Hees, E. Hodille, A. Hot, J. Karsenty, L. Kiakouama-Maleka, G. Lina, J. M. Maury, P. Miailhes, L. Moreau, P. Nesme, J. Ninet, L. Perard, T. Perpoint, E. Perrot, A. G. Ranc, JP. Rasigade, R. Reix, A. S. Renaud-Baron, J. Saison, A. Senechal, P. Sève, P. J. Souquet, H. van Thai, F. Tronc, F. Valour, P. Vanhems.
Disclosures
Zoé Cavalli, Florence Ader, Florent Valour, Julien Saison, Loïc Boussel, Oana Dumitrescu, Thomas Perpoint, Christian Chidiac, Thierry May, and Tristan Ferry declare that they have no conflicts of interest.
Compliance with Ethics Guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent waiver was granted as all data were currently in existence.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
On behalf of the Lyon TB Study group.
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/C6D4F0603D2C3510.
Contributor Information
Zoé Cavalli, Email: zoe.cavalli@chu-lyon.fr.
Lyon TB Study group:
L. Adelaïde, F. Ader, F. Biron, A. Boibieux, A. Bouaziz, K. Bouledrak, E. Braun, C. Broussolle, G. Carret, G. Catho, N. Charhon, C. Chidiac, W. Chumbi-Flores, B. Coppere, S. Couraud, M. Demontclos, G. Devouassoux, O. Dumitrescu, S. Ernesto, T. Ferry, D. Floret, I. Fredenucci, N. Freymond, S. Gardes, S. Gerbier-Colomban, Y. Gillet, M. H. Girard-Madoux, S. Goutelle, J. Grando, R. Grima, L. Hees, E. Hodille, A. Hot, J. Karsenty, L. Kiakouama-Maleka, G. Lina, J. M. Maury, P. Miailhes, L. Moreau, P. Nesme, J. Ninet, L. Perard, T. Perpoint, E. Perrot, A. G. Ranc, J. P. Rasigade, R. Reix, A. S. Renaud-Baron, J. Saison, A. Senechal, P. Sève, P. J. Souquet, H. van Thai, F. Tronc, F. Valour, and P. Vanhems
References
- 1.Peto HM, Pratt RH, Harrington TA, LoBue PA, Armstrong LR. Epidemiology of extrapulmonary tuberculosis in the United States, 1993–2006. Clin Infect Dis Off Publ Infect Dis Soc Am. 2009;49(9):1350–1357. doi: 10.1086/605559. [DOI] [PubMed] [Google Scholar]
- 2.Guirat A, Koubaa M, Mzali R, Abid B, Ellouz S, Affes N, et al. Peritoneal tuberculosis. Clin Res Hepatol Gastroenterol. 2011;35(1):60–69. doi: 10.1016/j.gcb.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 3.Sanai FM, Bzeizi KI. Systematic review: tuberculous peritonitis–presenting features, diagnostic strategies and treatment. Aliment Pharmacol Ther. 2005;22(8):685–700. doi: 10.1111/j.1365-2036.2005.02645.x. [DOI] [PubMed] [Google Scholar]
- 4.Chow KM, Chow VCY, Hung LCT, Wong SM, Szeto CC. Tuberculous peritonitis-associated mortality is high among patients waiting for the results of mycobacterial cultures of ascitic fluid samples. Clin Infect Dis Off Publ Infect Dis Soc Am. 2002;35(4):409–413. doi: 10.1086/341898. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Global tuberculosis report 2015. 2015. Disponible sur: http://apps.who.int/iris/bitstream/10665/191102/1/9789241565059_eng.pdf?ua=1.
- 6.Kamerbeek J, Schouls L, Kolk A, van Agterveld M, van Soolingen D, Kuijper S, et al. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol. 1997;35(4):907–914. doi: 10.1128/jcm.35.4.907-914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brudey K, Driscoll JR, Rigouts L, Prodinger WM, Gori A, Al-Hajoj SA, et al. Mycobacterium tuberculosis complex genetic diversity: mining the fourth international spoligotyping database (SpolDB4) for classification, population genetics and epidemiology. BMC Microbiol. 2006;6:23. doi: 10.1186/1471-2180-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhargava DK, Shriniwas, Chopra P, Nijhawan S, Dasarathy S, Kushwaha AK. Peritoneal tuberculosis: laparoscopic patterns and its diagnostic accuracy. Am J Gastroenterol. 1992;87(1):109–112. [PubMed] [Google Scholar]
- 9.Oge T, Ozalp SS, Yalcin OT, Kabukcuoglu S, Kebapci M, Arik D, et al. Peritoneal tuberculosis mimicking ovarian cancer. Eur J Obstet Gynecol Reprod Biol. 2012;162(1):105–108. doi: 10.1016/j.ejogrb.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Chow KM, Chow VC-Y, Szeto CC. Indication for peritoneal biopsy in tuberculous peritonitis. Am J Surg. 2003;185(6):567–573. doi: 10.1016/S0002-9610(03)00079-5. [DOI] [PubMed] [Google Scholar]
- 11.Vardareli E, Kebapci M, Saricam T, Pasaoglu O, Açikalin M. Tuberculous peritonitis of the wet ascitic type: clinical features and diagnostic value of image-guided peritoneal biopsy. Dig Liver Dis Off J Italy Soc Gastroenterol Italy Assoc Study Liver. 2004;36(3):199–204. doi: 10.1016/j.dld.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 12.Hong KD, Lee SI, Moon HY. Comparison between laparoscopy and noninvasive tests for the diagnosis of tuberculous peritonitis. World J Surg. 2011;35(11):2369–2375. doi: 10.1007/s00268-011-1224-2. [DOI] [PubMed] [Google Scholar]
- 13.Kong Y, Cave MD, Zhang L, Foxman B, Marrs CF, Bates JH, et al. Association between Mycobacterium tuberculosis Beijing/W lineage strain infection and extrathoracic tuberculosis: insights from epidemiologic and clinical characterization of the three principal genetic groups of M. tuberculosis clinical isolates. J Clin Microbiol. 2007;45(2):409–414. doi: 10.1128/JCM.01459-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Svensson E, Millet J, Lindqvist A, Olsson M, Ridell M, Rastogi N, et al. Impact of immigration on tuberculosis epidemiology in a low-incidence country. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2011;17(6):881–887. doi: 10.1111/j.1469-0691.2010.03358.x. [DOI] [PubMed] [Google Scholar]