Abstract
A series of porous polymers bearing functional quaternary ammonium salts were solvothermally synthesized through the free radical copolymerization of divinylbenzene (DVB) and functionalized quaternary ammonium salts. The obtained polymers feature highly cross-linked matrices, large surface areas, and abundant halogen anions. These polymers were evaluated as heterogeneous catalysts for the synthesis of cyclic carbonates from epoxides and CO2 in the absence of co-catalysts and solvents. The results revealed that the synergistic effect between the functional hydroxyl groups and the halide anion Br− afforded excellent catalytic activity to cyclic carbonates. In addition, the catalyst can be easily recovered and reused for at least five cycles without significant loss in activity.
Keywords: Ionic polymer, Mesoporous materials, CO2 fixation, Cycloaddition, Solid catalyst
Background
Global climate change and excessive CO2 emission have attracted widespread public concern in recent years. Since CO2 is expected to be a highly abundant, quite cheap, nontoxic, and nonflammable C1 resource in the organic synthesis, the capture and utilization of CO2 to produce higher-value relevant chemicals, such as polycarbonates and cyclic carbonates, are receiving rising attention [1, 2]. In this context, the conversion of CO2 to cyclic carbonates via epoxide substrates is demonstrated to be an atom-economical reaction, and the products can serve as excellent aprotic polar solvents as well as intermediates in the production of pharmaceuticals and fine chemicals [3–5]. In the past few decades, extensive efforts have been devoted to develop efficient catalysts for the synthesis of cyclic carbonates, including salen-metal complexes [6–8], quaternary ammonium/phosphonium salts [9, 10], ionic liquids (ILs) [11, 12], molecular sieves [13], metal-organic frameworks [14, 15], and so on. Among them, ILs have become a class of promising candidates owing to their unique features of high thermal stability, variety of structures available, and easy shaping [16–18]. To simplify the separation process and improve the reusability of ILs, more efforts have been devoted to the supported IL catalysts [19, 20], which usually suffers from tedious preparation process, large mass transfer resistance, and the leaching of IL active sites. Thus, new strategies for the preparation of efficiently heterogeneous IL catalysts for the synthesis of cyclic carbonates are highly desirable.
Nanoporous polymeric materials have attracted increasing attention due to their versatile and tunable structures, high surface area, and tunable surface chemistry, which allow potential applications in gas storage, explosive detection, drug release, and catalysis [21, 22]. Particularly, ionic porous polymers obtained by the polymerization of monomeric ILs or copolymerization of ILs with other monomers have been investigated as innovative solid catalysts or catalyst supports [23, 24]. A wide range of ionic porous polymers have been developed and have shown excellent catalytic performances in numerous organic synthetic reactions, among which, the cycloaddition of CO2 with epoxide is a hot topic [25]. For example, Wang et al. [26] used an ionothermal method to prepare a novel meso-macroporous hierarchical poly(ionic liquid), which was applied as highly efficient heterogeneous catalysts for the conversion of CO2 into cyclic carbonates at ambient pressure. Zhang et al. prepared imidazolium salt-modified porous hypercross-linked polymers and used them as highly efficient solid catalysts for synergistic CO2 capture and conversion [27]. However, the present systems still suffer from long reaction time, high CO2 pressure, or high reaction temperature. Moreover, most of the present ionic porous polymers are based on imidazolium ILs, while the quaternary ammonium salt IL-based ionic porous polymers are scarce, although quaternary ammonium salts have been proved to be one of the most efficient catalysts for CO2 fixation. In addition, it has been reported that the functional groups such as hydroxyl and carboxyl are favorable for the cycloaddition reaction due to the synergistic effect with Br ions [28, 29]. These considerations prompted us to design new hydroxyl-containing quaternary ammonium salt-based ionic porous polymers as “task-specific” catalysts for the cycloaddition of CO2 with epoxides.
Herein, we designed and synthesized a new type of hydroxyl functionalized quaternary ammonium salt-based ionic porous polymer via the radical copolymerization of hydroxyl functionalized triallylamine and divinylbenzene (DVB) (Scheme 1). The resulting porous materials with very high surface area can be sufficiently applied for the production of cyclic carbonates from CO2 and epoxides, showing high conversion and selectivity, easy recovery, and steady reusability.
Scheme 1.

Synthesis of quaternary ammonium salt-based ionic porous polymers
Methods
All chemicals in this work were used as received without further purification. Triallylamine (TAA); divinylbenzene (DVB), 3-bromo-1-propanol, 1-bromobutane, epichlorohydrin (ECH), and propylene oxide (PO) were purchased from Sigma Aldrich Reagent Co., LLC. Styrene oxide (SO), allyl glycidyl ether (AGE), and cyclohexene oxide (CHO) were provided by Aladdin Chemical Reagent Co., Ltd. Other reagents were laboratory-grade reagents from local suppliers.
Characterizations
1H NMR spectra were collected on a Varian Mercury plus 400-MHz spectrophotometer at ambient temperature using D2O as solvent. CHN elemental analysis was performed on a vario EL cube elemental analyzer. Fourier transform infrared spectroscopy (FT-IR) spectra were recorded on a FT-IR instrument (Nicolet 360, KBr discs) in the 4000–400 cm−1 region. Thermogravimetric (TG) analysis was carried out with a STA409 instrument in nitrogen at a heating rate of 15 °C min−1. The nitrogen adsorption-desorption isotherms were measured on a BELSORP-MINI instrument at liquid nitrogen (77 K) temperature. The samples were evacuated at 423 K for 1.5 h before measuring. The specific surface areas were evaluated using the Brunauer-Emmett-Teller (BET) method, and the pore distribution was calculated by the BJH method from adsorption branches of isotherms. Transmission electron microscopy (TEM) analysis was performed on a JEM-2100 (JEOL) electron microscope operating at 200 kV. Scanning electron microscopy (SEM) images were recorded on a SUPERSCAN SSX-550 electron microscope (Shimadzu, Japan) operating at 20 kV. The morphology and the bromine (Br) element distribution were characterized by a Hitachi S-4800 field emission scanning electron microscope accompanied by energy-dispersive X-ray spectrometry.
Synthesis of catalysts
Synthesis of HTA and BTA
Triallylamine (20 mmol, 2.74 g) and 3-bromo-propanol (20 mmol, 2.78 g) were dissolved in ethanol (15 mL). The mixture was then stirred at 80 °C for 24 h under nitrogen atmosphere. On completion, the solvent was removed by distillation and the solid product was washed with ethyl acetate three times to remove the unreacted substrates. After drying under vacuum, hydroxypropyl functionalized triallylamine (HTA) was obtained. 1H NMR (400 MHz, D2O, TMS) δ(ppm) = 1.93 (t, 2H, CH2), 3.21 (m, 2H, CH2), 3.51 (m, 2H, CH2), 3.62 (d, 6H, CH2), 5.81 (m, 6H, CH2), 5.94 (d, 3H, CH). The butyl functionalized triallylamine (BTA) was prepared accordingly in the same way and then was characterized by 1H NMR (400 MHz, D2O, TMS) δ(ppm) = 1H NMR (400 MHz, D2O, TMS) δ(ppm) = 0.86 (t, 3H, CH3), 1.26 (m, 2H, CH2), 1.69 (m, 2H, CH2), 3.13 (t, 2H, CH2), 3.72 (d, 6H, CH2), 5.59 (d, 6H, CH2), 5.90 (m, 3H, CH).
Synthesis of ionic porous polymers DVB-HTA and DVB-BTA
DVB (1.3 g, 10 mmol) and HTA (0.58 g, 2 mmol) were dissolved in 20 mL ethyl acetate and 4 mL methanol, respectively. These two solutions were mixed, and azodisisobutyronitrile AIBN (0.05 g) was added into it. After stirring at room temperature for 3 h, the mixture was solvothermally treated at 70 °C for 24 h. The yellow solid product DVB-HTA was filtered, washed with methanol three times, and dried under vacuum at 50 °C for 24 h. CHN elemental analysis for DVB-HTA found the following (wt.%): C 86.87, H 8.64, N 1.07. The ionic porous polymer DVB-BTA was prepared in the same way by reacting DVB with BTA. CHN elemental analysis for DVB-BTA found the following (wt.%): C 87.73, H 8.91, N 1.13.
Typical procedure for cycloadditions
As a typical example, ECH (20 mmol) and catalyst DVB-HTA-Br (0.05 g) were added into a 50-mL stainless steel autoclave equipped with a magnetic stirrer. After the reaction mixture was heated to 120 °C, CO2 was then charged into the reactor until the desired pressure of 1.2 MPa was reached. The reactor was cooled to ambient temperature after reacting 6 h, and the resulting mixture was filtered and the filtrate was analyzed by gas chromatography (GC) that was equipped with a FID and a DB-wax capillary column (SE-54 30 m × 0.32 mm × 0.25 μm). Biphenyl was used as an internal standard to calculate the catalytic conversion. GC-MS (SCIONSQ-456-GC) was used to analyze the purity and structure of the products. For the catalyst recycling, the filtered solid catalyst was directly used in the next run after washing with diethyl ether and drying.
Results and discussion
Preparation and characterization of catalysts
The hydroxyl functionalized unsaturation quaternary ammonium salt (HTA) was first prepared by reacting 3-bromopropyl alcohol and triallylamine and verified by 1H NMR spectroscopy. Then, quaternary ammonium salt IL-based ionic porous polymer (DVB-HTA) was synthesized through the radical copolymerization of DVB with HTA (Scheme 1). The butyl functionalized quaternary ammonium salt-based ionic polymer DVB-BTA was prepared accordingly. The obtained samples were characterized by FT-IR analysis, and the spectra are shown in Fig. 1. DVB-HTA shows the characteristic bands of –OH (3200~3800 cm−1), C–O (1065 cm−1), and C–N stretching vibrations (1170 cm−1). The peaks approximately between 1448 and 1602 cm−1 can be attributed to the skeletal vibration of aromatic ring in DVB. The results indicate the successful copolymerization of HTA and DVB.
Fig. 1.

FT-IR spectra of (a) DVB-HTA and (b) DVB-BTA
The ionic polymers are white powders and insoluble in water or common organic solvents, such as acetone, ethanol, chloroform, hexanes, N,N-dimethylformamide (DMF), and tetrahydrofuran (THF). The CHN elemental analysis for these polymers reveals that the molar ratio of quaternary ammonium salts to DVB was about 1:9, as illustrated in Scheme 1. The TG profile in Fig. 2 shows that DVB-HTA and DVB-BTA are stable up to 400 °C. For DVB-HTA, the weight loss of nearly 4 % in the range of 200~400 °C may be due to the elimination of hydroxyl functional groups in HTA. The further weight loss in 400~600 °C was attributed to the decomposition of organic polymer framework.
Fig. 2.
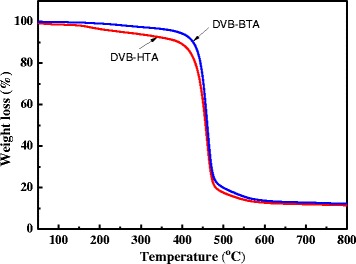
TG curves of DVB-HTA and DVB-BTA
Figure 3 shows the N2 adsorption-desorption isotherms and the corresponding pore size distributions of DVB-HTA and DVB-BTA. They display typical IV-type isotherm with hysteresis loops at relative pressure of 0.8 < P/P0 < 1. Correspondingly, their average pore sizes are distributed at 5–7 nm. The Brunauer-Emmett-Teller (BET) surface areas and pore volumes of DVB-HTA and DVB-BTA are 708 and 655 m2/g and 1.21 and 0.83 cm3/g, respectively.
Fig. 3.
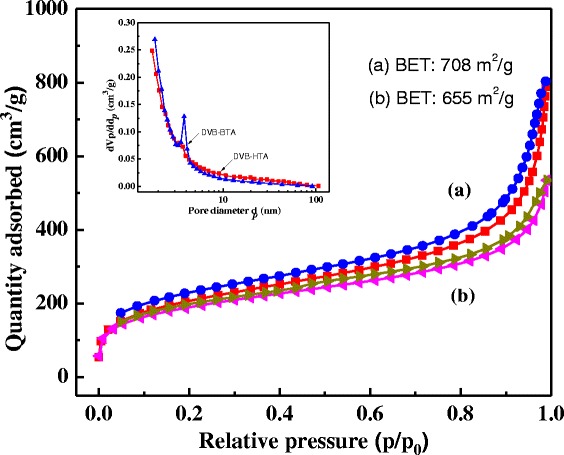
Nitrogen adsorption-desorption isotherms and pore size distributions of a DVB-HTA and b DVB-BTA
The SEM images of DVB-HTA and DVB-BTA in Fig. 4a, b show the abundant wormhole-like morphology with micrometer size. The EDS structural characterization validates the uniform distribution of Br, O, and N on the surface of DVB-HTA, which further confirms the successful combination of HTA and DVB on one catalyst framework. In the TEM images of DVB-HTA and DVB-BTA in Fig. 4g, h, the porosity could be clearly observed. It is proposed that functional quaternary ammonium salt with three vinyl groups acts as the cross-linking node, which intertwines with the DVB to form a highly cross-linked network.
Fig. 4.
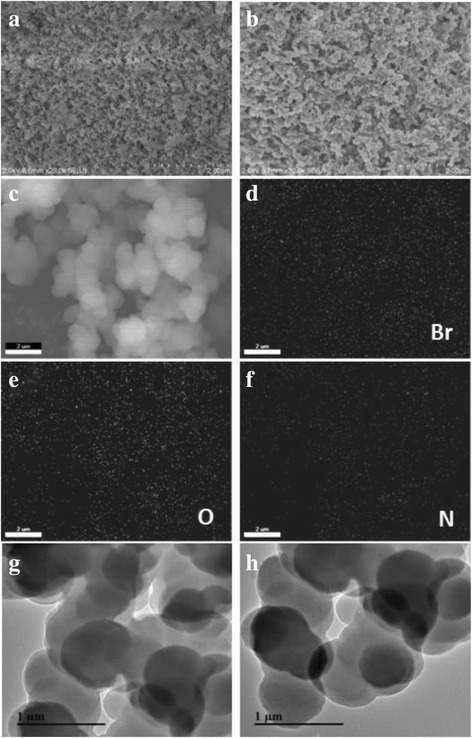
SEM images of a, c DVB-HTA and b DVB-BTA; EDS elemental mapping of d Br element, e O element, and f N element; and TEM images of g DVB-HTA and h DVB-BTA
Catalytic performance
The catalytic activities were first tested on epichlorohydrin (ECH) as the model substrate at 120 °C with 1.2 MPa CO2 under solvent-free conditions, and the results are listed in Table 1. No product was detected in the absence of the catalyst (entry 1). The ionic polymers are insoluble in the reaction system, and DVB-HTA bearing hydroxyl functional groups exhibited 90 % yield with 98 % selectivity (entry 2). It is higher than that of hydroxyl-free ionic polymer DVB-BTA, which gave 84 % yield with a relative low selectivity of 92 % (entry 3). The quaternary ammonium salt monomers HTA offered 84 % yield (entry 4) that is higher than that of hydroxyl-free BTA which showed 77 % yield (entry 5), but it is still lower than that of ionic polymer DVB-HTA. Additionally, HTA and BTA caused homogeneous catalysis, and the catalyst cannot be easily recovered and reused. The results of the above control experiments imply that the highly cross-linked polymer framework endows the catalyst with insolubility and thus result in heterogeneous catalysis. Although the halogen Br− ions act as the active centers that are indispensable for the catalytic efficiency of the catalyst, the mesoporous framework with such high surface area can accelerate the mass transfer and allow the Br− give full play as active centers, thus giving high catalytic activity. Furthermore, the hydroxyl functionalized samples demonstrated higher activity than the hydroxyl-free samples, demonstrating that the –OH groups are more favorable for the cycloaddition reaction mostly due to the synergistic effect with Br− ions. This result is inconsistent with those of the previous reports [30, 31].
Table 1.
Cycloaddition of CO2 and ECH catalyzed by various catalysts
| Entry | Catalyst | Solubility | Yielda (%) | Selb (%) |
|---|---|---|---|---|
| 1 | No catalyst | Homogeneous | – | – |
| 2 | DVB-HTA | Heterogeneous | 90 | 98 |
| 3 | DVB-BTA | Heterogeneous | 84 | 92 |
| 4 | HTA | Homogeneous | 84 | 97 |
| 5 | BTA | Homogeneous | 77 | 95 |
Reaction conditions: ECH (16 mmol), CO2 (1.2 MPa), catalyst (0.035 mmol based on Br), 120 °C, 3 h
aThe yield of cyclic carbonate product
bThe selectivity for the cyclic carbonate product; the byproduct is 3-chloro-1,2-propanediol
The results of the experimental optimization in Fig. 5 show that the reaction conditions, including reaction time, CO2 pressure, the reaction temperature, and the catalyst amount, have remarkable influence on the yield of cyclic carbonate in the presence of DVB-HTA as a catalyst. The best reaction condition is 1.2 MPa CO2 pressure, 120 °C temperature, and 3-h reaction time. Notably, a further increase of the CO2 pressure exceeding 1.5 MPa or reaction temperature exceeding 130 °C led to a slight decrease of the cyclic carbonate yield, which may result from the further oxidation of the cyclic carbonate formed. For the catalyst amount, with the increasing the of the catalyst amount to 0.08 g, a high yield of 94 % with 98 % selectivity could be achieved, demonstrating the high efficiency of this novel catalyst DVB-HTA.
Fig. 5.
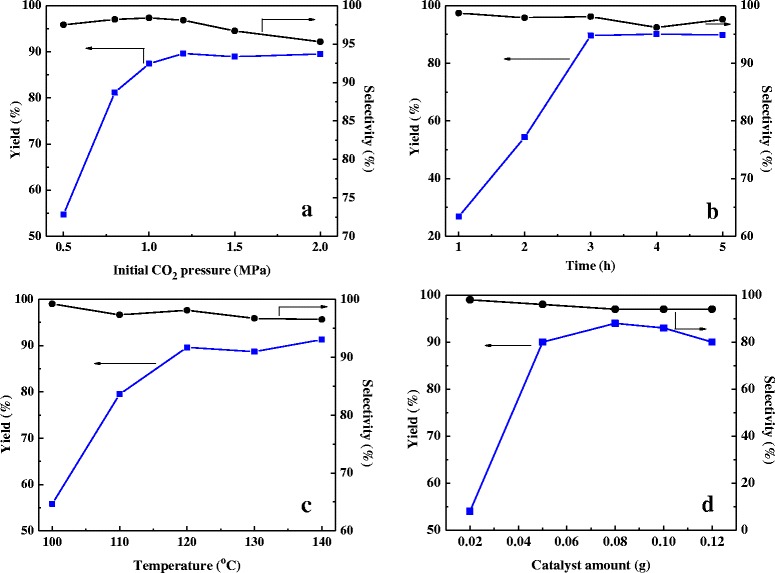
Influences of reaction conditions on the cycloaddition of CO2 and ECH over DVB-HTA. a Influence of initial CO2 pressure. b Influence of reaction time. c Influence of reaction temperature. d Influence of catalyst amount. Reaction conditions: catalyst 0.05 g, ECH 16 mmol, CO2 pressure 1.2 MPa, 120 °C, 3 h; for each figure, there is a specific parameter changed
In order to investigate the scope of the ionic porous polymer DVB-HTA for the fixation of CO2, various functional-group-substituted epoxides, such as propylene oxide, styrene oxide, allyl glycidyl ether, and cyclohexene oxide, were studied as the substrates, and the result are summarized in Table 2. It can be seen that most of the epoxides can be transformed to the corresponding cyclic carbonates in high yield and selectivity at 120 °C with 1.2 MPa CO2 pressure and 0.05 g catalyst under solvent-free condition. In the case of cyclohexene oxide, a moderate yield of 56 % with 63 % selectivity was obtained. Notably, the coupling of CO2 with cyclohexene oxide is a rather difficult reaction, and it could not give a high yield of cyclic carbonate as well over other catalysts [32, 33]. This is possibly ascribed to the limited diffusion of large-sized epoxide substrate molecules into the pore canal of DVB-HTA thus exerting size-selective catalysis.
Table 2.
Cycloaddition of CO2 with different epoxides catalyzed by DVB-HTA
| Entry | Epoxide | Product | Time (h) | Con (%)a | Sel (%)b |
|---|---|---|---|---|---|
| 1 |
|
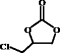
|
3 | 90 | 98 |
| 2 |
|
|
6 | 93 | 99 |
| 3 |

|

|
6 | 89 | 98 |
| 4 |
|

|
4 | 83 | 98 |
| 5 |
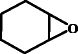
|
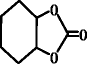
|
12 | 56 | 63 |
Reaction conditions: epoxide (16 mmol), CO2 (1.2 MPa), catalyst DVB-HTA (0.05 g, 0.035 mmol), 120 °C
aThe yield for the cyclic carbonate product
bThe selectivity for the cyclic carbonate product, and the byproduct for entry 5 is mostly 1,2-hexanediol
The reusability studies of the catalyst DVB-HTA in the cycloaddition of CO2 with ECH have been tested. The results of a five-run recycling performance are shown in Fig. 6A. It is obvious that the yield of cyclic carbonate which is 90 % for the first run only marginally decreased down to 82 % for the fifth run. To check if structural deterioration occurred for the recovered catalyst, the recovered DVB-HTA was characterized by FT-IR. The IR spectrum of the reused DVB-HTA in Fig. 6B was very similar to that of the fresh one, demonstrating a durable catalyst structure accounting for the steady catalytic reuse. The weight loss in the operation for recovering the catalyst is usually unavoidable during the recycling tests. Thus, the very slow decrease of yield is mostly probably due to the weight loss in the operation for recovering the catalyst.
Fig. 6.

A Catalytic reusability of DVB-HTA for the cycloaddition of CO2 with ECH and B FT-IR spectra of (a) fresh DVB-HTA and (b) reused DVB-HTA. Reaction conditions: ECH 16 mmol, catalyst 0.05 g (0.035 mmol), 120 °C, CO2 1.2 MPa, 3 h
According to previous reports, the cooperation of nucleophilic attack by a halogen anion and the activation by an onium cation through electron interaction could facilitate the opening of an epoxy ring [34, 35] and the functional groups such as the –OH and –COOH groups can also activate the cycloaddition reaction through hydrogen bonding [28, 29]. In this work, by rationalizing our experimental results and relating them to these numerous literatures, a synergistic catalysis of hydrogen bonding, electronic interaction, and nucleophilic attack for the cycloaddition of epoxides and CO2 to form cyclic carbonate was proposed, as illustrated in Scheme 2. The epoxy ring is first activated by the –OH group via a hydrogen bond interaction and the quaternary ammonium cations via electronic interaction (a). What follows is the opening of the epoxy ring through Br− nucleophilic attack (b) then the insertion of CO2 into the transient species. Finally, the product cyclic carbonate was formed via an intramolecular nucleophilic attack, and the catalyst was regenerated to the original form (d). Notwithstanding, this mechanism is tentative and the detailed mechanistic studies to probe the intermediates during the cycloaddition reaction could be necessary, and research along this line will be conducted in the near future.
Scheme 2.

The proposed mechanism of the DVB-HTA-catalyzed fixation of CO2 with epoxides
Conclusions
In summary, we have developed a new type of quaternary ammonium salt-based ionic porous polymer, DVB-HTA, by the copolymerization of DVB and hydroxyl functionalized quaternary ammonium salts. The DVB-HTA was a highly cross-linked porous material with a large surface area of 708 m2/g and abundant Br− anions and acted as efficient heterogeneous catalysts for the transformation of CO2 and epoxides into cyclic carbonates under metal-solvent-free conditions. The excellent catalytic performance of DVB-HTA results from the synergistic effect between the Br− active centers and functional –OH groups. Moreover, the DVB-HTA had good recyclability, attributed to the durable highly cross-linked framework structure. This catalyst is potentially useful in industrial applications due to its low cost, excellent catalytic efficiency, and good stability.
Acknowledgements
Financial support was provided by the Doctoral Fund of Ministry of Education of China, the Guangdong Government (S20120011226), and the MOST of China (2014AA020512).
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
JZ and SC designed the experiment. SC and YZ carried out the experiment, as well as wrote the manuscript. DZ and YZ were involved in the characterization and analysis of the data, as well as helped to prepare the manuscript. All authors read and approved the final manuscript.
Contributor Information
Dongliang Zhu, Email: zhudl1980@163.com.
Jing Zhao, Email: jingzhao@nju.edu.cn.
References
- 1.Fiorani G, Guoa WS, Kleij AW. Sustainable conversion of carbon dioxide: the advent of organocatalysis. Green Chem. 2015;17:1375–1389. doi: 10.1039/C4GC01959H. [DOI] [Google Scholar]
- 2.Adhikari D, Nguyen ST, Baik MH. A computational study of the mechanism of the [(salen)Cr + DMAP]-catalyzed formation of cyclic carbonates from CO2 and epoxide. Chem Commun. 2014;50:2676–2678. doi: 10.1039/c3cc48769e. [DOI] [PubMed] [Google Scholar]
- 3.Luo RC, Zhou XT, Chen SY, Li Y, Zhou L, Ji HB. Highly efficient synthesis of cyclic carbonates from epoxides catalyzed by salen aluminum complexes with built-in “CO2 capture” capability under mild conditions. Green Chem. 2014;16:1496–1506. doi: 10.1039/c3gc42388c. [DOI] [Google Scholar]
- 4.He Q, O’Brien JW, Kitselman KA, Tompkins LE, Curtisa GCT, Kerton FM. Synthesis of cyclic carbonates from CO2 and epoxides using ionic liquids and related catalysts including choline chloride–metal halide mixtures. Catal Sci Technol. 2014;4:1513–1528. doi: 10.1039/c3cy00998j. [DOI] [Google Scholar]
- 5.Xu BH, Wang JQ, Sun J, Huang Y, Zhang JP, Zhang XP, Zhang SJ. Fixation of CO2 into cyclic carbonates catalyzed by ionic liquids: a multi-scale approach. Green Chem. 2015;17:108–122. doi: 10.1039/C4GC01754D. [DOI] [Google Scholar]
- 6.Jiseul C, Kang S, Kang N, Lee SM, Kim HJ, Son SUK. Microporous organic networks bearing metal-salen species for mild CO2 fixation to cyclic carbonates. J Mater Chem A. 2013;1:5517–5523. doi: 10.1039/c3ta10477j. [DOI] [Google Scholar]
- 7.North M, Wang BD, Young C. Influence of flue gas on the catalytic activity of an immobilized aluminium (salen) complex for cyclic carbonate synthesis. Energy Environ Sci. 2011;4:4163–4170. doi: 10.1039/c1ee01074c. [DOI] [Google Scholar]
- 8.Rulev YA, Gugkaeva Z, Maleev VI, North M, Belokon YN. Robust bifunctional aluminium–salen catalysts for the preparation of cyclic carbonates from carbon dioxide and epoxides. Beilstein J Org Chem. 2015;11:1614–1623. doi: 10.3762/bjoc.11.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chatelet B, Joucla L, Dutasta JP, Martinez A, Szeto KC, Dufaud Vé R. Azaphosphatranes as structurally tunable organocatalysts for carbonate synthesis from CO2 and epoxides. J Am Chem Soc. 2013;135:5348–5351. doi: 10.1021/ja402053d. [DOI] [PubMed] [Google Scholar]
- 10.Wang JQ, Gan J, Yang W, Yi GS, Zhang YG. Phosphonium salt incorporated hypercrosslinked porous polymers for CO2 capture and conversion. Chem Commun. 2015;51:15708–15711. doi: 10.1039/C5CC06295K. [DOI] [PubMed] [Google Scholar]
- 11.Wang JQ, Leong JY, Zhang YG. Efficient fixation of CO2 into cyclic carbonates catalysed by silicon-based main chain poly-imidazolium salts. Green Chem. 2014;16:4515–4519. doi: 10.1039/C4GC01060D. [DOI] [Google Scholar]
- 12.North M, Quek Sophie CZ, Pridmore NE, Whitwood AC, Wu X. Aluminum (salen) complexes as catalysts for the kinetic resolution of terminal epoxides via CO2 coupling. ACS Catal. 2015;5:3398–3402. doi: 10.1021/acscatal.5b00235. [DOI] [Google Scholar]
- 13.Ravi S, Kang DH, Roshan R, Tharun J, Kathalikkattil AC, Park DW. Organic sulphonate salts tethered to mesoporous silicas as catalysts for CO2 fixation into cyclic carbonates. Catal Sci Technol. 2015;5:1580–1587. doi: 10.1039/C4CY01321B. [DOI] [Google Scholar]
- 14.Beyzavi MH, Klet RC, Tussupbayev S, Borycz J, Vermeulen NA, Cramer CJ, Stoddart JF, Hupp JT, Farha OK. A hafnium-based metal−organic framework as an efficient and multifunctional catalyst for facile CO2 fixation and regioselective and enantioretentive epoxide activation. J Am Chem Soc. 2014;136:15861–15864. doi: 10.1021/ja508626n. [DOI] [PubMed] [Google Scholar]
- 15.Babu R, Kathalikkattil AC, Roshan R, Tharun J, Kim DW, Park DW. Dual-porous metal organic framework for room temperature CO2 fixation via cyclic carbonate synthesis. Green Chem. 2016;18:232–242. doi: 10.1039/C5GC01763G. [DOI] [Google Scholar]
- 16.Chen GJ, Zhou Y, Wang XC, Li J, Xue S, Liu YQ, Wang QN, Wang J. Construction of porous cationic frameworks by crosslinking polyhedral oligomeric silsesquioxane units with N-heterocyclic linkers. Sci Rep. 2015;5:1–14. doi: 10.1038/srep11236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu MS, Liu B, Zhong SF, Shi L, Liang L, Sun JM. Kinetics and mechanistic insight into efficient fixation of CO2 to epoxides over N-heterocyclic compound/ZnBr2 catalysts. Ind Eng Chem Res. 2015;54:633–640. doi: 10.1021/ie5042879. [DOI] [Google Scholar]
- 18.Roy T, Kureshy RI, Khan NH, Abdi SHR, Bajaj HC. Asymmetric cycloaddition of CO2 and an epoxide using recyclable bifunctional polymeric Co(III) salen complexes under mild conditions. Catal Sci Technol. 2013;3:2661–2667. doi: 10.1039/c3cy00325f. [DOI] [Google Scholar]
- 19.Adam F, Appaturi JN, Ng EP. Halide aided synergistic ring opening mechanism of epoxides and their cycloaddition to CO2 using MCM-41-imidazolium bromide catalyst. J Mol Catal A. 2014;386:42–48. doi: 10.1016/j.molcata.2014.02.008. [DOI] [Google Scholar]
- 20.Pourjavadi A, Hosseini SH, Doulabi M, Fakoorpoor SM, Seidi F. Multi-layer functionalized poly(ionic liquid) coated magnetic nanoparticles: highly recoverable and magnetically separable Brønsted acid catalyst. ACS Catal. 2012;2:1259–1266. doi: 10.1021/cs300140j. [DOI] [Google Scholar]
- 21.Gao CJ, Chen GJ, Wang XC, Li J, Zhou Y, Wang J. A hierarchical meso-macroporous poly(ionic liquid) monolith derived from a single soft template. Chem Commun. 2015;51:4969–4972. doi: 10.1039/C4CC09091H. [DOI] [PubMed] [Google Scholar]
- 22.Soll S, Zhang PF, Zhao Q, Wang Y, Yuan JY. Mesoporous zwitterionic poly(ionic liquid)s: intrinsic complexation and efficient catalytic fixation of CO2. Polym Chem. 2013;4:5048–5051. doi: 10.1039/c3py00823a. [DOI] [Google Scholar]
- 23.Yuan JY, Giordano C, Antonietti M. Ionic liquid monomers and polymers as precursors of highly conductive, mesoporous, graphitic carbon nanostructures. Chem Mater. 2010;22:5003–5012. doi: 10.1021/cm1012729. [DOI] [Google Scholar]
- 24.Soll S, Zhao Q, Weber J, Yuan JY. Activated CO2 sorption in mesoporous imidazolium-type poly(ionic liquid)-based polyampholytes. Chem Mater. 2013;25:3003–3010. doi: 10.1021/cm4009128. [DOI] [Google Scholar]
- 25.Xie Y, Zhang ZF, Jiang T, He JL, Han BX, Wu TB, Ding KL. CO2 cycloaddition reactions catalyzed by an ionic liquid grafted onto a highly cross-linked polymer matrix. Angew Chem Int Ed. 2007;46:7255–7258. doi: 10.1002/anie.200701467. [DOI] [PubMed] [Google Scholar]
- 26.Wang XC, Zhou Y, Guo ZJ, Chen GJ, Li J, Shi YM, Liu YQ, Wang J. Heterogeneous conversion of CO2 into cyclic carbonates at ambient pressure catalyzed by ionothermal-derived meso-macroporous hierarchical poly(ionic liquid)s. Chem Sci. 2015;6:6916–6924. doi: 10.1039/C5SC02050F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang JQ, Sng WH, Yi GS, Zhang YG. Imidazolium salt-modified porous hypercrosslinked polymers for synergistic CO2 capture and conversion. Chem Commun. 2015;51:12076–12079. doi: 10.1039/C5CC04702A. [DOI] [PubMed] [Google Scholar]
- 28.Zhang YY, Yin SF, Luo SL, Au CT. Cycloaddition of CO2 to epoxides catalyzed by carboxyl functionalized imidazolium-based ionic liquid grafted onto cross-linked polymer. Ind Eng Chem Res. 2012;51:3951–3957. doi: 10.1021/ie203001u. [DOI] [Google Scholar]
- 29.Wang JQ, Cheng WG, Sun J, Shi TY, Zhang XP, Zhang SJ. Efficient fixation of CO2 into organic carbonates catalyzed by 2-hydroxymethyl-functionalized ionic liquids. RSC Adv. 2014;4:2360–2367. doi: 10.1039/C3RA45918G. [DOI] [Google Scholar]
- 30.Chen X, Sun J, Wang JQ, Cheng WG. Polystyrene-bound diethanolamine based ionic liquids for chemical fixation of CO2. Tetrahedron Lett. 2012;53:2684–2688. doi: 10.1016/j.tetlet.2012.03.058. [DOI] [Google Scholar]
- 31.Liu MS, Gao KQ, Liang L, Wang FX, Shi L, Li S, Sun JM. Insights into hydrogen bond donor promoted fixation of carbon dioxide with epoxides catalyzed by ionic liquids. Phys Chem Chem Phys. 2015;17:5959–5965. doi: 10.1039/C4CP05464D. [DOI] [PubMed] [Google Scholar]
- 32.Gao WY, Wojtas L, Ma SQ. A porous metal–metalloporphyrin framework featuring high-density active sites for chemical fixation of CO2 under ambient conditions. Chem Commun. 2014;50:5316–5318. doi: 10.1039/C3CC47542E. [DOI] [PubMed] [Google Scholar]
- 33.Talapaneni SN, Buyukcakir O, Je SH, Srinivasan S, Seo Y, Polychronopoulou K, Coskun A. Nanoporous polymers incorporating sterically confined N-heterocyclic carbenes for simultaneous CO2 capture and conversion at ambient pressure. Chem Mater. 2015;27:6818–6826. doi: 10.1021/acs.chemmater.5b03104. [DOI] [Google Scholar]
- 34.Zhang W, Liu TY, Wu HH, Wu P, He MY. Direct synthesis of ordered imidazolyl-functionalized mesoporous polymers for efficient chemical fixation of CO2. Chem Commun. 2015;51:682–684. doi: 10.1039/C4CC08062A. [DOI] [PubMed] [Google Scholar]
- 35.Dai WL, Jin B, Luo SL, Luo XB, Tu XM, Au CT. Polymers anchored with carboxyl-functionalized di-cation ionic liquids as efficient catalysts for the fixation of CO2 into cyclic carbonates. Catal Sci Technol. 2014;4:556–562. doi: 10.1039/C3CY00730H. [DOI] [Google Scholar]


