Abstract
The Bacillus strain (CCUG 66887) has a high capacity to excrete keratinase with the ability to degrade both alpha- and beta keratin. In this study we aimed to show the characteristics of the keratinolytic protease and to identify its gene by using liquid chromatography–electrospray ionization tandem mass spectrometry methods (nanoHPLC–ESI–MS/MS) followed by Mascot data base search. The results showed that the enzyme in fact consists of two different keratinases, both with a molecular mass of 38 kDa. Further, DNA sequencing generated the open reading frame (ORF) of one of the genes (Ker1), and de novo genome sequencing identified the ORF of the second gene (Ker2). The two keratinase genes contain 1153 base pairs each and have a gene similarity of 67 %. In addition, the Bacillus strain was classified as Bacillus pumilus and its genes were annotated in the GeneBank at NCBI (accession: CP011109.1). Amino acid sequences alignment with known B. pumilus proteases indicated that the two keratinases of B. pumilus strain C4 are subtilisin-like serine proteases belonging to the Protease S8 family. Taken together, these result suggest the two keratinases as promising candidates for enzymatic processing of keratinous wastes in waste refinery.
Keywords: Bacillus pumilus, Keratinase, α-Keratin, β-Keratin, NanoHPLC–ESI–MS/MS, DNA sequencing
Introduction
Annually, just the global feather waste from the poultry processing industry reaches 8.5 million tons. At present, the poultry feathers are dumped, buried, used for land filling, or incinerated, resulting in environmental challenges in terms of storage, handling, emission control, and ash disposal (Agrahari and Wadhwa 2010). Poultry feathers are also turned into feather meal used as animal feed because of the high protein content. However, the use of waste for animal feed is becoming tighter (Commission of the European Communities 2000). Additionally, the high treatment costs make the process economically unfeasible. An environmentally and economically promising process to recover the feather waste is to produce renewable energy by e.g. anaerobic digestion. In this process, not only does the valuable methane result as a byproduct, but also digested residues are formed. The latter can safely be used as a fertilizer, since pathogens presented in the feather waste have been eradicated in the process (Salminen and Rintala 2002a, b).
The recalcitrant keratin is the major compound in several biological materials. It is also the waste product in poultry, slaughterhouse, leather- and fur processing industries and consists of feather, hair, horn, hoof, nails, claws, wool, and bristles (Kornillowicz-Kowalska and Bohacs 2011). While some of the materials like hair or wool, to a great extent, are composed of the helix form of α-keratin, other materials such as feather are largely composed of the flat form of β-keratin. Among the two different types of keratin structures, the content of sulfur varies giving the keratin a softer or harder structure and affects the degradation of the keratinous material to a greater extent (Brandelli et al. 2010). A large number of microorganisms have been reported to produce keratinases (Brandelli et al. 2010; Gupta and Ramnani 2006; Onifade et al. 1998), and among bacteria, the best studied are organisms from the genus Bacillus (Gobinath et al. 2014). Keratinases (EC 3.4.99.11) are serine- or metalloproteases (Gupta and Ramnani 2006), and many bacterial keratinases have been sequenced, cloned, and characterized indicating a sequence similarity with the subtilisin family, Family S8, of serine proteases (Rawlings and Barret 1993).
The isolation and characterization of a keratin-degrading bacterium, Bacillus sp C4, has been reported by this lab. The proteolytic activity was broadly specific, and the bacterium could grow and produced a significant level of keratinase when using wool or chicken feather as substrates. A total hydrolysis of the keratinous waste was obtained in less than 3 days (Fellahi et al. 2014). Also this proteolytic enzyme has shown activity and stability over a broad pH range with two distinct optima, one at pH 8.5 and the other at pH 11, indicating that it might be not one but two enzymes. Its activity was completely inhibited by phenylmethylsulfonyl fluoride (PMSF) pointing out that the enzyme is a serine protease (Fellahi 2009).
In an attempt to increase our understanding of the Bacillus strain’s ability to simultaneously hydrolyze both α- and β-keratin, we in this study aimed to show the characteristics of the keratinolytic protease and to identify its gene. So far, the vast majority of the identified keratinase-producing organisms appears to be able to hydrolyze only the β-keratin in the chicken feather (Gupta et al. 2013), which gives the keratinolytic protease from this strain a potential for simultaneous degradation of both types of keratin in waste refinery.
Materials and methods
Bacterial strain and medium
The microorganism used in this study was Bacillus sp. C4; CCUG 66887. It has earlier been isolated from the compost and identified using biochemical tests and 16S rDNA technique (GenBank accession: FJ214667) (Fellahi et al. 2014). Before the strain was used for protease production it was grown at 37 °C for 24 h on peptone yeast extract medium containing Bactopeptone, 10 g/l; Yeast extract, 5 g/l and NaCl, 5 g/l.
Keratinase gene sequence determination
Multiple sequence alignment with CLUSTALW2 (http://www.ebi.ac.uk/Tools/msa/clustalw2/) (Chenna et al. 2003) was used to align keratinase genes from different Bacillus pumilus strains to investigate the resemblance among the strains to be able to choose one strain for designing the first sequencing primer set (F: TTAGAAGCCGCTTGAACGTTA, R: ATGTGCGTGAAAAAGAAAAATGTG). Genomic DNA was isolated from strain C4 using MasterPure™ Gram Positive DNA Purification Kit (Epicentre), and the DNA was sent together with the sequence for the first primer set to Eurofins Genomics, Germany where the primers were synthesized and both DNA strands sequenced by Sanger method. From the retrieved two DNA sequences, a new primer set (F: AAGTATTAGATCGTTACGGCGATGGAC, R: CCAAGAACACCAATCGTGTTATCAAGG) was designed and once again sent to Eurofins Genomics together with genomic DNA. This procedure was repeated a third time with primer (F: TTGCCAACGTGAACAGCAAC) to determine the open reading frame (ORF) of the gene.
De novo sequencing and genome annotation
To be able to search the genome of strain C4 for additional putative keratinase genes, de novo sequencing of the whole genome using the instrument MiSeq and the MiSeq Control Software 2.3.0.3 was performed by Eurofins Genomics, Germany. The sequence assembly and scaffolding was done using the Newbler assembler software v2.9. The genome sequence was annotated using the prokaryotic annotation pipeline at the National Center for Biotechnology Information, Bethesda, USA (NCBI).
Partial purification of proteases for nanoHPLC–ESI–MS/MS
The protease production from Bacillus C4 strain was done according to Fellahi and coworkers. (Fellahi et al. 2014). In short: the C4 strain was grown in 50 ml of modified Schaeffer’s medium (Leighton and Doi 1971) containing Beef extract, 3 g/l; Bactopeptone, 5 g/l; KCl, 2 g/l; Yeast extract, 2 g/l; pH 7, and supplemented with 2 mM MgSO4.7H2O; 1 mM CaCl2; 0.1 mM MnCl2; 1 mM FeSO4; and 0.1 % (w/v) glucose. The production was done using a 250-ml E-flask with a 2 % inoculum size in a shaker incubator (Excella 24, New Brunswick Scientific) at 37 °C, 160 rpm. After 24 h, the cell-free supernatant was received by centrifugation at 8000 rpm for 15 min at 4 °C (Optima Max-XP, Beckman Coulter).
For the identification of the enzyme by nanoHPLC–ESI–MS/MS the cell-free supernatant was precipitated by adding NH3SO4 to 65 % saturation at 4 °C and slowly mixing in a shaker incubator (Excella 24, New Brunswick Scientific) for 1 h. The precipitate was collected by centrifugation at 12,000 rpm for 30 min at 4 °C (Optima Max-XP, Beckman Coulter). The pellet was re-suspended in 500 µl of 20 mM Tris–HCl buffer, pH 8, followed by dialysis overnight against the same buffer. Proteolytic activity was measured as described by Cliffe and Law (1982), using Hide Powder Azure (HPA, Sigma) as substrate. Approximately 20 µg of the purified enzyme was run on a 12 % SDS-PAGE-gel, according to Laemmli (1970) along with a molecular weight protein marker (All Blue Protein Precision Standard, Bio-Rad).
Protein identification by nanoHPLC–ESI–MS/MS and data base search
Protein identification of the crude enzyme was performed by Proteome Factory AG, Germany. Two protein spots from a 12 % SDS-PAGE-gel were cut out and digested in-gel by trypsin (Promega, Mannheim, Germany) and analyzed by nanoHPLC–ESI–MS/MS. The LCMS system consisted of an Agilent 1100 nanoHPLC system (Agilent, Waldbronn, Germany), PicoTip electrospray emitter (New Objective, Woburn, MA, USA), and an Orbitrap XL or LTQFT. The retrieved peptides were analyzed using an ultra-mass spectrometer (ThermoFisher Scientific, Bremen, Germany). Peptides were first trapped and desalted on the enrichment column Zorbax 300SB-C18, 0.3 mm × 5 mm (Agilent) for 5 min (solvent: 2.5 % acetonitrile/0.5 % formic acid), then separated on a Zorbax 300SB-C18, 75 μm × 150 mm column (Agilent) using a linear gradient from 10 to 32 % B (Solvent A: 5 % acetonitrile in water, Solvent B: acetonitrile. Both solvents contained 0.1 % formic acid). Ions of interest were data-dependently subjected to MS/MS according to the expected charge state distribution of the peptide ions. MS/MS ion search of the Mascot search engine (Matrix Science, London, England) was performed, and only peptide matches with a score of 20 or above were accepted. Proteins were identified against the B. pumilus entries from the RefSeq protein database available at NCBI, which was appended to an existing bacterial database. The search results were also run against the amino acid sequences retrieved from the Ker1 and Ker2 genes.
Amino acid sequence alignment
The amino acid sequences of the retrieved enzymes from the ultra-mass spectrometer analysis were compared to other proteases produced by the B. pumilus strains using the Basic Local Alignment Search Tool Blastp 2.2.3.1 available at NCBI.
Results
Keratinase gene sequence determination
The multiple sequence alignment followed by synthesis of primers and Sanger sequencing resulted in an ORF of a keratinase gene, named Ker1, which contains 1153 base pairs. The gene was subsequently submitted to the NCBI GenBank (Accession Number: KX184831).
De novo sequencing and genome annotation
The de novo sequencing of the whole genome indicated that the organism is a B. pumilus and that it possesses a genome of 3.6 million base pairs. The organism was annotated to NCBI (Accession Number: CP011109.1) using the prokaryotic annotation pipeline, and the result indicated that this B. pumilus strain has around 4000 genes. One of these genes is a homolog to Ker1 and was named Ker2. The gene was subsequently submitted to NCBI GenBank (Accession Number: KX184832).
Partial purification of proteases from strain C4 for nano-HPLC-ESI-MS/MS
The crude protease fraction showed after SDS-PAGE and staining with Coomassie brilliant blue R-250 one distinct band and one weaker band with molecular weights of about 28 and 36 kDa, respectively (Fig. 1).
Fig. 1.
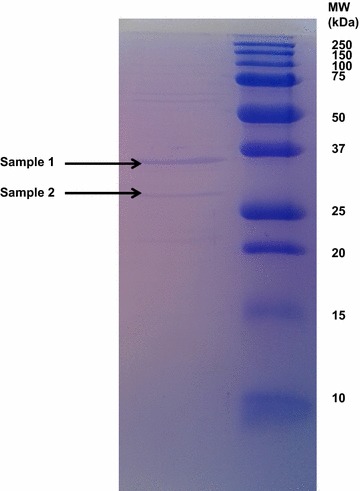
The dialyzed proteolytic enzyme investigated on SDS-PAGE using a 12 % gel. Two bands at 36 and 28 kDa, respectively, were excised for protein identification
Protein identification by nanoHPLC–ESI–MS/MS and data base search
The Mascot Search Result using the B. pumilus entries from the RefSeq protein data base and the amino sequences retrieved from the Ker1 and Ker2 genes can be seen in Table 1. The band corresponding to 28 kDa only contained keratinase 1, while the band corresponding to 38 kDa contained keratinase 1 and keratinase 2. These results indicated that the partial purified keratinolytic protease in fact consists of two enzymes, both with a molecular weight of 38 kDa, and corresponding to the identified genes Ker1 and Ker2 of the B. pumilus strain C4. When using the B. pumilus entries from the RefSeq protein data the peptides from MS/MS identified Peptidase S8. The result also showed a resemblance between keratinase 2 and peptidase S8.
Table 1.
Protein identification by MASCOT Search on the purified enzyme from Bacillus strain C4 using RefSeq protein data base and the two amino acid sequences retrieved from Ker1 and Ker2
| SDS-Page lane | Identified protein | Sequence identity | MW (kDa) | MASCOT peptides identified | MASCOT ion scorea |
|---|---|---|---|---|---|
| 1 and 2 | Keratinase 1 | WP_008348814.1 | 38.8 | QRLENTATPLGNSFYYGK | 81 |
| GVVVVAAAGNSGSTGSTSTVGYPAK | 146 | ||||
| YDSTIAVANVNSNNVR | 122 | ||||
| LENTATPLGNSFYYGK | 102 | ||||
| GLINVQAASN | 85 | ||||
| 1 | Keratinase 2 | WP_017357922.1 | 38.3 | VGVVGVAPK | 61 |
| VADENGDGYYSWIIK | 80 | ||||
| SGTSMASPHVAGAAAVILSK | 107 | ||||
| HPNLTNDELR | 53 | ||||
| HPNLTNDELRER | 34 | ||||
| 1 | Peptidase S8 | gi|648268958 | 38.8 | VGVVGVAPK | 61 |
| VADENGDGYYSWIIK | 80 | ||||
| LGEPFYYGAGLVNVQK | 106 | ||||
| SGTSMASPHVAGAAAVILSK | 107 |
aIndividual ion scores >26 indicates identity or extensive homology at 95 % level of confidence
Figure 2 illustrates the peptides from Ms/Ms identified in keratinase 1, keratinase 2 as well as peptidase S8 and their distribution and coverage in the respective enzymes. As can be seen keratinase 2 shares three peptides with peptidase S8 but also has unique one while keratinase 1 does not have any peptides in common with the identified Peptidase S8.
Fig. 2.
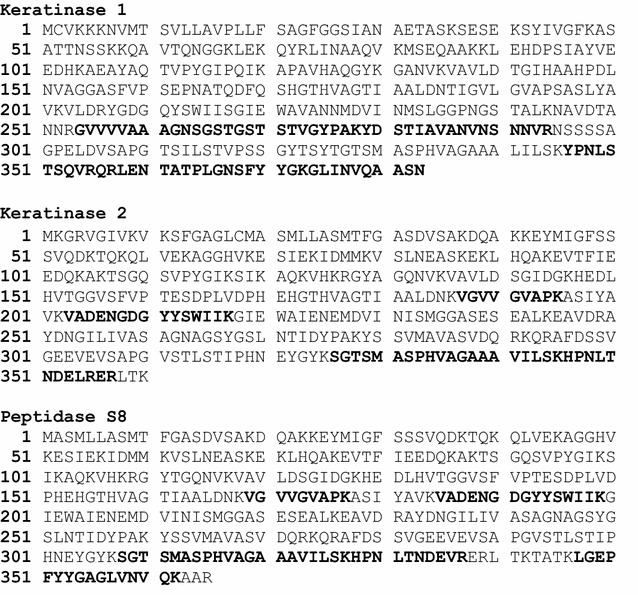
The distribution of peptides from MS/MS in keratinase 1, keratinase 2 and peptidase S8. Matched peptides are shown in bold black
Amino acid sequence alignment
By comparing the amino acid sequences of the two retrieved keratinase enzymes with known proteases of other B. pumilus strains the results indicated that these enzymes are subtilisin-like serine proteases belonging to the Protease S8 family (Tables 2, 3).
Table 2.
Amino acid sequence alignment of keratinase 1 with proteases of Bacillus strains using Blastp 2.2.3.1
| Description | Organism | Amino acid sequence identity (%) | Accession number |
|---|---|---|---|
| Alkaline serine proteinase | B. pumilus | 100 | ACO94164.1 |
| Lehensis serine protease | B. lehensis | 100 | AFP23380.1 |
| Alkaline serine protease | B. pumilus | 99 | BAE79641.1 |
| Dehairing protease precursor | B. pumilus | 99 | AAR19220.1 |
| Peptidase S8 | B. pumilus | 99 | WP_026050071.1 |
| Alkaline serin proteinase | B. pumilus | 99 | BAA93474.1 |
| Protease | B. pumilus | 99 | ADK63096.1 |
| Peptidase S8 | MULTISPECIES: Bacillus | 99 | WP_008348814.1 |
| Serine alkaline protease (subtilisin) | B. stratosphericus LAMA585 | 99 | EMI14709.1 |
| Subtilisin Carlsberg | Bacillus pumilus ATCC 7061 | 99 | EDW22774.1 |
| Peptidase S8 | B. pumilus | 99 | WP_034620013.1 |
| Peptidase S8 | B. pumilus | 99 | WP_041093123.1 |
| Peptidase S8 | B. stratosphericus | 99 | WP_039962807.1 |
| Peptidase S8 | B. invictae | 99 | WP_045034875.1 |
| Peptidase S8 | B. pumilus | 99 | WP_044140726.1 |
| Peptidase S8 | B. aerophilus | 99 | WP_041507592.1 |
| Peptidase S8 | B. altudinis | 99 | WP_039167642.1 |
| Peptidase S8 | B. pumilus | 98 | WP_012009474.1 |
| Serine alkaline protease | B. circulans | 98 | ADN04910.1 |
| Serine alkaline protease (subtilisin E) | B. pumilus | 98 | KIL22204.1 |
| Serine alkaline protease, preproprotein | B. pumilus | 98 | CAO03040.1 |
| Peptidase S8 | B. sp. DW5-4 | 98 | WP_034323660.1 |
| Peptidase S8 | B. pumilus | 98 | WP_041117216.1 |
| Peptidase S8 | B. safensis | 98 | WP_034282323.1 |
| Peptidase S8 | B. pumilus | 98 | WP_034663897.1 |
| Serine alkaline protease (subtilisin E) | B. pumilus | 97 | KIL10386.1 |
| Peptidase S8 | B. pumilus | 97 | WP_041110188.1 |
| Peptidase S8 | B. sp. WP8 | 96 | WP_039183048.1 |
| Keratinase precursor | B. pumilus | 96 | ACM47735.1 |
| Serine alkaline protease (subtilisin E) | Bacillus sp. HYC-10 | 96 | KIL09959.1 |
| Peptidase S8 | B. xiamenensis | 96 | WP_008359041.1 |
| Organic solvent tolerant protease | B. pumilus | 96 | AAU88064.1 |
| Keratinase | B. pumilus | 96 | ADK11996.1 |
| Peptidase S8 | B. safensis | 96 | WP_029706931.1 |
| Peptidase S8 | B. pumilus | 96 | WP_041089929.1 |
| Peptidase S8 | B. safensis | 96 | WP_044335827.1 |
| MULTISPECIES: peptidase S8 | Bacillus | 96 | WP_025093353.1 |
| Peptidase S8 | B. pumilus | 95 | WP_024426548.1 |
| Serine alkaline keratinase | Brevibacillus brevis | 95 | AGO58466.1 |
| Alkaline serine protease precursor | B. pumilus | 95 | ACM07731.1 |
| Serine alkaline keratinase | B. circulans | 94 | AGN91700.1 |
Table 3.
Amino acid sequence alignment of keratinase 2 with proteases of Bacillus strains using Blastp 2.2.3.1
| Description | Organism | Amino acid sequence identity (%) | Accession number |
|---|---|---|---|
| Serine alkaline protease (subtilisin E) | B. stratosphericus LAMA 585 | 100 | EMI12150.1 |
| Peptidase S8 | MULTISPECIES: Bacillus | 100 | WP_035390997.1 |
| Subtilisin Carlsberg | B. sp. M 2-6 | 99 | EIL84986.1 |
| Peptidase S8 | MULTISPECIES: Bacillus | 99 | WP_034647494.1 |
| Peptidase S8 | B. pumilus | 99 | WP_029575389.1 |
| Subtilisin Carlsberg | B. pumilus | 99 | KIL26870.1 |
| Peptidase S8 | B. altitudinis | 99 | WP_035702958.1 |
| Peptidase S8 | B. pumilus | 99 | WP_026050107.1 |
| Pubtilisin | B. altitudinis 41KF2b | 99 | KDE30915.1 |
| Peptidase S8 | B. sp. DW5-4 | 95 | WP_034319985.1 |
| Peptidase S8 | B. pumilus | 95 | WP_044141213.1 |
| Peptidase S8 | B. pumilus | 94 | WP_034620505.1 |
| Peptidase S8 | B. pumilus | 94 | WP_034661158.1 |
| Subtilisin Carlsberg | B. pumilus | 94 | KIL17080.1 |
| Subtilisin Carlsberg | B. pumilus ATCC7061 | 94 | EDW21217.1 |
| Peptidase S8 | B. pumilus | 93 | KDE52880.1 |
| Peptidase S8 | B. safensis | 93 | WP_034622393.1 |
| Peptidase S8 | MULTISPECIES: Bacillus | 93 | WP_029708034.1 |
| Peptidase S8 | B. pumilus | 93 | WP_041117873.1 |
| Peptidase S8 | B. safensis | 93 | KEP30825.1 |
| Peptidase S8 | B. safensis | 93 | WP_029706051.1 |
| Peptidase S8 | B. pumilus | 93 | WP_041109684.1 |
| Pubtilisin Carlsberg | B. pumilus | 93 | KIL21504.1 |
| Pubtilisin Carlsberg | B. pumilus | 92 | KIL11523.1 |
| Peptidase S8 | B. sp. HYC-10 | 92 | WP_008361817.1 |
| Peptidase S8 | B. safensis | 92 | WP_034280781.1 |
| Peptidase S8 | B. sp. WP8 | 92 | WP_039183179.1 |
| Subtilisin | B. safensis FO-36b | 92 | KDE29455.1 |
| Peptidase S8 | B. safensis | 91 | WP_046312283.1 |
| Peptidase S8 | B.pumilus | 91 | WP_041086842.1 |
| Subtilisin Carlsberg | B. pumilus | 90 | KIL15277.1 |
Discussion
The fast growth rate of the microorganisms, the accessibility for genetic engineering and the short time for the production and purification steps make them the ideal source for production of proteases (Rao et al. 1998). The far most popular source of commercial alkaline proteases is from the Bacillus species. The main reason for this is their ability to produce large amounts of alkaline proteases having significant proteolytic activity and stability at high pH as well as high temperature (Jacobs 1995; Yang et al. 2000). So far, the vast majority of the identified keratinase producing organisms appear to be able to hydrolyze only β-keratin in chicken feather and few are known to hydrolyze both α- and β-keratin. (Gupta et al. 2013).
In this study two keratinases from the Bacillus sp C4 strain were identified. The project started by trying to find a keratinase gene in the genome of the strain by attaching primers designed for the Ker A gene of Bacillus licheniformis PWD-1 identified by Lin and coworkers (1995). After conventional PCR followed by agarose gel electrophoresis we found many different gene products due to unspecific binding of the primers but no gene product comparable in size with a keratinase gen. Still we decided to DNA sequence three fragments by the Sanger method which resulted in three DNA sequences with the lengths of 236–396 base pairs. By using NCBI blast network service (http://www.blast.ncbi.nlm.nih.gov/Blast.cgi), we found that these gene sequences might belong to the genome of a B. pumilus strain. With this information at hand we aligned genes from twelve different B. pumilus strains (Table 4) using CLUSTAL W2 (http://www.ebi.ac.uk/Tools/msa/clustalw2/). The outcome of the process indicated high resemblance between many sequences, mostly in the beginning and the end of the different aligned genes. One of the strains, B. pumilus strain A1, produces a keratinase (Fakhfakh-Zouari et al. 2010) which has comparable qualities with the keratinolytic enzyme produced by Bacillus sp C4 (Fellahi 2009). Both enzymes are active and stabile over a broad pH range and also their activity is completely inhibited by PMSF suggesting that they both are serine proteases. The keratinase precursor gene from the B. pumilus strain A1 was for this reason chosen for primer design of the first pair of sequencing primers for the keratinase gene. After Sanger sequencing the complete ORF of the keratinase gene (Ker1) was identified. To find resemblance with other genes from B. pumilus strains, nucleotide BLAST was used. The gene showed a high similarity to several serine protease genes from different B. pumilus strains. As much as 99 % similarity was found with the peptidase S8 gene of the B. pumilus strain W3, GenBank accession: CP011150.1 (Zheng-Bing et al. 2015). This result confirmed that the isolated fragment of the genome encodes one of the serine protease from the Bacillus sp. C4 strain. By de novo sequencing and annotation of the genome, we could identify a homolog to the peptidase S8 gene, the Ker2 gene with equal amount of base pairs as Ker1. The two genes had a gene sequence similarity of 67 %.
Table 4.
Bacillus pumilus strains used for gene alignment in CLUSTAL W2
| B. pumilus strain | GI number and accession version | Gene |
|---|---|---|
| NJM4 | gi|226938414|gb|FJ869878.1| | Alkaline serine proteinase |
| N/A | gi|38373993|gb|AY458140.1| | Dehairing protease precursor |
| N/A | gi|7415641|dbj|AB029082.1| | Alkaline serine proteinase |
| SG2 | gi|301131525|gb|GQ398415.1| | Protease gene |
| SGMM8 | gi|290472378|gb|GU143024.1| | Protease (Alp) gene |
| bppA | gi|87886606|dbj|AB211527.1| | Alkaline serine |
| A1 | gi|222353759|gb|FJ619651.1| | Keratinase precursor |
| 115b | gi|52843271|gb|AY743586.1 | Organic solvent protease gene |
| KS12 | gi|300429855|gb|HM219183.1| | Keratinase gene |
| TMS55 | gi|221193393|gb|FJ584420.1| | Alkaline serine protease precursor |
| 3–19 | gi|297342830|gb|AY754946.2| | Subtilisin like serine |
| sapB | gi|186928863|emb|AM748727.1| | Serine alkaline protease preprotein |
The crude protease showed after partial purification followed by SDS-PAGE-gel and staining with Coomassie brilliant blue R-250 one distinct band and one weaker band. Their molecular weights were estimated to about 28 and 36 kDa, respectively, when comparing with the molecular weight marker. When the bands on the gel were analyzed by a nanoHPLC–ESI–MS/MS system the result indicated two proteins with a molecular weight of 38. 8 and 38.3 kDa, respectively (Table 1). The molecular size difference between the excised gel bands and the molecular size of the identified enzymes may be due to a degradation prior to the SDS-PAGE. When the proteins were identified by Mascot Search the result indicated that the two proteins in fact are corresponding to the two genes Ker1 and Ker2. That the crude enzyme in fact contains two different keratinases is in agreement with earlier conclusions (Yamamura et al. 2002). Yamamura and his group found that for the bacterium Stenotrophomonas sp D-1 it was not sufficient with one protease for effective keratin degradation but two different keratinases were needed. This suggests that degradation requires the cooperative action of multiple enzymes. We are well aware that Bacillus sp C4 may need more than two keratinases for optimal degradation although we have not found any additional.
When comparing the amino acid sequences of the two retrieved keratinases with known proteases of other B. pumilus strains using Blastp we found that keratinase 1 and 2 are subtilisin-like serine proteases belonging to the Protease S8 family (Tables 2, 3). These proteases show broad substrate specificity, have usually a molecular mass in the range 18–90 kDa. They are generally active at neutral and alkaline pH, with optima at pH 7–11 (Rao et al. 1998) and irreversibly inhibited by PMSF (Powers et al. 2002). This is in agreement with our earlier findings (Fellahi 2009).
As a conclusion, the present study confirmed that the keratinolytic protease produced by the non-genetically modified B. pumilus strain C4 consists of two different enzymes belonging to the Protease S8 family. This may explain why the strain is able to simultaneously hydrolyze both α- and β-keratin in less than three days. It also makes the bacterium a potent candidate in a cost effective pretreatment step of keratinase rich waste in waste refinery as the two waste fractions, avian feather and sheep wool, do not have to be separated before hydrolyzation of the protein into valuable feedstuff for the biogas production.
Authors’ contributions
SF has contributed with planning and implementation of the research work as well as interpretation of data and article preparation. AC has contributed with planning and design of research work as well as interpretation of data. EFL has contributed with planning and implementation of the research work as well as interpretation of data and article preparation. MJT has contributed with planning and implementation of research work as well as revising the article critically. All authors read and approved the final manuscript.
Acknowledgements
The authors thank Dr. Johan O. Westman for his advice relating to the molecular biology investigations.
Competing interests
The authors declare that they have no competing interests.
Fundings
This study was supported by the University of Borås and Sparbanksstiftelsen Sjuhärad in Sweden, as well as Mostaganem University in Algeria.
Contributor Information
Soltana Fellahi, Email: soltana.fellahi@yahoo.com.
Abdelwaheb Chibani, Email: abdel2001mohamed@yahoo.com.
Elisabeth Feuk-Lagerstedt, Phone: +46 334354710, Email: elisabeth.feuk@hb.se.
Mohammad J. Taherzadeh, Email: mohammad.taherzadeh@hb.se
References
- Agrahari S, Wadhwa N. Degradation of chicken feather a poultry waste product by keratinolytic bacteria isolated from dumping site at Ghazipur poultry processing plant. Int J Poult Sci. 2010;9(5):482–489. doi: 10.3923/ijps.2010.482.489. [DOI] [Google Scholar]
- Brandelli A, Daroit DJ, Riffel A. Biochemical features of microbial keratinases and their production and applications. Appl Microbiol Biot. 2010;85(6):1735–1750. doi: 10.1007/s00253-009-2398-5. [DOI] [PubMed] [Google Scholar]
- Chenna R, Sugawara H, Koike T, Lopez R, Gibson TJ, Higgins DG, Thompson JD. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 2003;31(13):3497–3500. doi: 10.1093/nar/gkg500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliffe AJ, Law BA. A new method for the detection of microbial proteolytic enzymes in milk. J Dairy Res. 1982;49(02):209–219. doi: 10.1017/S0022029900022317. [DOI] [Google Scholar]
- Commission of the European Communities White paper on food safety. Off J. 2000;C076:1–4. [Google Scholar]
- Fakhfakh-Zouari N, Hmidet N, Haddar A, Kanoun S, Nasri M. A novel serine metallokeratinase from a newly iIsolated Bacillus pumilus A1 grown on chicken feather meal: biochemical and molecular characterization. Appl Ciochem Biotechnol. 2010;162:329–344. doi: 10.1007/s12010-009-8774-x. [DOI] [PubMed] [Google Scholar]
- Fellahi S. Isolation, identification, and proteolytic activity of some protease-producing bacteria. M.Sc. Dissertation. Alexandria: Alexandria University; 2009.
- Fellahi S, Zaghloul T, Feuk-Lagerstedt E, Taherzadeh M. A Bacillus strain able to Hydrolyze alpha- and beta-keratin. J Bioprocess Biotechnol. 2014;4:1000181–1000187. doi: 10.4172/2155-9821.1000181. [DOI] [Google Scholar]
- Gobinath SCB, Anbu P, Lakshmipriya T, Tang T-H, Chen Y, Hashim U, Ruslinda AR, Arshad MK. Biotechnological aspects and perspective of microbial keratinase production. Biomed Res Int. 2014;2015:1–10. doi: 10.1155/2015/140726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R, Ramnani P. Microbial keratinases and their prospective applications: an overview. Appl Microbiol Biotechnol. 2006;70(1):21–33. doi: 10.1007/s00253-005-0239-8. [DOI] [PubMed] [Google Scholar]
- Gupta R, Sharma R, Qasim KB. Revisiting microbial keratinases: next generation proteases for sustainable biotechnology. Crit Rev Biotechnol. 2013;33(2):216–228. doi: 10.3109/07388551.2012.685051. [DOI] [PubMed] [Google Scholar]
- Jacobs MF. Expression of the subtilis in Carlsberg-encoding gene in Bacillus licheniformis and Bacillus subtilis. Gene. 1995;152:67–74. doi: 10.1016/0378-1119(94)00655-C. [DOI] [PubMed] [Google Scholar]
- Kornillowicz-Kowalska T, Bohacs J. Biodegradation of keratin waste: theory and practical aspects. Waste Manag. 2011;31(8):1689–1701. doi: 10.1016/j.wasman.2011.03.024. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of the structural proteins during the assembly of the head of the bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leighton TJ, Doi RH. The stability of messenger ribonucleic acid during sporulation in Bacillus subtilis. J Biol Chem. 1971;246:3189–3195. [PubMed] [Google Scholar]
- Lin X, Kelemen DW, Miller ES, Shih JC. Nucleotide sequence and expression of kerA, the gene encoding a keratinolytic protease of Bacillus licheniformis PWD-1. Appl Environ Microbiol. 1995;61(4):1469–1474. doi: 10.1128/aem.61.4.1469-1474.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onifade A, Al-Sane N, Al-Musallam A, Al-Zarban S. A review: potentials for biotechnological applications of keratin-degrading microorganisms and their enzymes for nutritional improvement of feathers and other keratins as livestock feed resources. Bioresour Technol. 1998;66(1):1–11. doi: 10.1016/S0960-8524(98)00033-9. [DOI] [Google Scholar]
- Powers JC, Asgian JL, Ekici ÖD, James KE. Irreversible inhibitors of serine, cysteine, and threonine proteases. Chem Rev. 2002;102(12):4639–4750. doi: 10.1021/cr010182v. [DOI] [PubMed] [Google Scholar]
- Rao MB, Tanksale AM, Ghatge MS, Deshpande VV. Molecular and biotechnological aspects of microbial proteases. Microbiol Mol Biol Rev. 1998;62(3):597–635. doi: 10.1128/mmbr.62.3.597-635.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings ND, Barret AJ. Evolutionary families of peptidases. Biochem J. 1993;290:205–218. doi: 10.1042/bj2900205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen EA, Rintala JA. Anaerobic digestion of organic solid poultry waste-a review. Bioresour Technol. 2002;83(1):13–26. doi: 10.1016/S0960-8524(01)00199-7. [DOI] [PubMed] [Google Scholar]
- Salminen EA, Rintala JA. Semi-continuous anaerobic digestion of solid poultry slaughterhouse waste: effect of hydraulic retention time and loading. Water Res. 2002;36(13):3175–3182. doi: 10.1016/S0043-1354(02)00010-6. [DOI] [PubMed] [Google Scholar]
- Yamamura S, Morita Y, Hasan Q, Yokoyama K, Tamiya E. Keratin degradation: a cooperative action of two enzymes from Stenotrophomonas sp. Biochem Biophys Res Commun. 2002;294:1138–1143. doi: 10.1016/S0006-291X(02)00580-6. [DOI] [PubMed] [Google Scholar]
- Yang JK, Shih IL, Tzeng YM, Wang SL. Production and purification of protease from a Bacillus subtilis that can deproteinize crustacean wastes. Enzyme Microb Technol. 2000;26:406–413. doi: 10.1016/S0141-0229(99)00164-7. [DOI] [PubMed] [Google Scholar]
- Zheng-Bing G, Yu-Jie C, Yan-Zhou Z, Zxiang-Ru L. Complete genome sequence of Bacillus pumilus W3: a strain exhibiting high laccase activity. J Biotechnol. 2015;207:8–9. doi: 10.1016/j.jbiotec.2015.04.019. [DOI] [PubMed] [Google Scholar]


