Abstract
The present study investigates the anti-proliferative and apoptosis inducing mechanism of CoCl2·6H2O in PC-3 cancer cell line. Preliminary, three different forms of cobalt i.e., cobaltous (CoCl2·6H2O), macro-Co(II,III) oxide and nano-Co(II,III) oxide were screened for antiproliferative activity in PC-3 cell line. The CoCl2·6H2O being the most effective antiproliferative agent, hence it was further tested against lung (A549), prostrate (PC-3) and brain (IMR-32) cell lines. Human embryonic kidney cell line (293T) was used as a normal cell line to compare the toxicity of CoCl2·6H2O. The CoCl2·6H2O induced morphological and anatomical changes in PC-3 cancer cell which were studied using light, confocal and scanning electron microscopy. The lactate dehydrogenase was estimated which showed mild necrotic mode of cell death. The Annexin/PI staining confirmed the apoptotic mode of cell death in PC-3 cells. Further, production of reaction of reactive oxygen species and changes in mitochondrial membrane potential was also assessed spectrofluorimetrically. The cell cycle arrest was also investigated using flow cytometery. Finally, the caspase activity was estimated in CoCl2·6H2O treated PC-3 cancer cell line. Interestingly, it was found that CoCl2·6H2O induces more cell death in cancerous cells as compared to normal non-cancerous cells. These findings presented CoCl2·6H2O as potential antiproliferative agent.
Keywords: Apoptosis, Antiproliferative, CoCl2·6H2O, MTT
Background
The d-block elements confined to 3–12 groups of periodic table represent transition metals. They along with their complexes have found use in drug development since last decade (Gianferrara et al. 2009; Li et al. 2015). Their ability to exist in a number of oxidation states and reacting with other oppositely charged species has been exploited in medicinal chemistry. Some of the transition metals have been reported to possess antimicrobial, antifungal, antidiabetic and anticancer properties (Singh 2014). Different metal complexes viz., Ru(III), Co(II), Cu(II) nitrate, Pd(II) chloride, and coordination complexes of Pt(II), As, Sb, Bi, V, Fe, Rh, Ti and Ga have been investigated for their anticancer activities by various workers (Olivova et al. 2011; Sharma et al. 2008; Soldevila-Barreda et al. 2015; Bandyopadhyay et al. 2014; Chitambar 2013; Geldmacher et al. 2012; Obeid et al. 2012; El-Boraey and El-Din 2014; Novotny and Kombian 2014; Mojic et al. 2014; Via et al. 2014; de Assis et al. 2016; Huang et al. 2016). It has been reported that cytotoxic drugs specifically target frequently dividing cells because of the increased synthesis of nucleic acids in them during cell division (Thind et al. 2008).
Cobalt (Co) is a transition metal which is required as a trace element in both plants and animals. Co exists in numerous inorganic complexes with different oxidation states, but not all the oxidation states are medicinally important. It is a water soluble crystalline complex which gets decomposed on electrolysis. It is used in coloration of paint and glass. Cobalt(III) oxide is an insoluble (Mahey and Thukral 2014) non-conductive complex having optic and ceramic properties. Co plays a major role in the form of cobalamin i.e., vitamin B-12 by maintaining neurological and immune responses, red blood cell formation, synthesis of DNA and in development as well as growth of infants (Andres et al. 2010; Krisch et al. 2013; Hunger et al. 2014; Rush and Yajnik 2014; Chellan and Sadler 2015). It has also been reported as a cardio-, reno- and neuro- protective agent (Salnikow et al. 2000). CoCl2·6H2O has been reported to be used as a doping agent by athletes for improving their performance (Lippi et al. 2005).
The present study was designed to screen the antiproliferative activities of three different forms of cobalt i.e., cobaltous (CoCl2·6H2O), macro-Co(II,III) oxide and nano-Co(II,III) oxide against PC-3 cancer cell line. The most active form of Cobalt was further tested against three cancer cell lines viz., A549 (lung), PC-3 (prostrate), IMR-32 (brain) along with 293T (human kidney embryonic cells) employing MTT assay. Further, the mechanistic studies were carried out on PC-3 cell line using CoCl2·6H2O. Various mechanistic studies gave an insight into process of cellular death, i.e., apoptosis or necrosis in PC-3 cells. Assays employed for this purpose involved microscopic (phase contrast, confocal, scanning electron microscopy) investigations, measurement of reactive oxygen species (ROS), mitochondrial membrane potential (MMP), sub G0 population using flow cytometry, lactate dehydrogenase (LDH), annexin V-FITC/PI and caspase-3 activity.
Methods
Chemicals and reagents
Dulbecco’s modified Eagle’s medium (DMEM), Roswell Park Memorial Institute medium (RPMI), foetal bovine serum (FBS), penicillin, streptomycin, 4′,6-diamidino-2-phenylindole (DAPI), rhodamine123 (RH-123), 5,6-chloromethyl-2′7′-dichlorodihydrofluorescein diacetate (CM-H2DCFDA) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich Corporation (St. Louis, MO, USA). Gentamicin was purchased from, Abbott Healthcare Pvt. Ltd CoCl2·6H2O, Macro-Co(II,III) oxide and Nano-Co(II,III) oxide were procured from Sigma-Aldrich Corporation (St. Louis, MO, USA).
Procurement of cell lines
Cancer cell lines A-549, IMR-32, PC-3 and normal human embryonic cell line 293T used in the present study were procured from National Centre for Cell Sciences (Pune, India). The cell lines were grown in culture flasks containing nutritive medium (DMEM or RPMI) supplemented with 10 % FBS at 37 °C in CO2 incubator (5 % CO2 level + 90 % relative humidity). The cells were trypsanized and seeded in well plates as per the requirement of experiment.
Sample preparation
Different concentrations of CoCl2·6H2O, Macro-Co(II,III) oxide and Nano-Co(II,III) oxide (10, 25, 50, 75, 100 mg/L) were prepared for the evaluation of antiproliferative activity. The sample was dissolved in growth medium (DMEM and RPMI) in which the cancer cell line was grown. The treatments design of the different samples was divided into various groups as follows:
X1: Control group: comprises of medium only (DMEM/RPMI)
X2: comprises of medium + CoCl2·6H2O
X3: composed of medium + CoCl2·6H2O + cell line
X4: composed of medium + cell line
Absorbance of CoCl2·6H2O only was calculated by (X2–X1); % inhibition was calculated by using formula
Although the cancer cells used in the experiment were from single stock, still before each experiment the cells synchronization was done prior to the treatment by serum starvation method.
MTT [3-(4,5-dimethylthiasol-2-yl)-2,5-diphenyltetrazolium bromide] colorimetric assay
To assess the antiproliferative activity of the samples, MTT assay was performed (Heckenkamp et al. 1999). The cells were seeded in 96 well plate at density of 1 × 104 cells/well. After 24 h, the cells were treated with different concentration of 0.1 mL sample (5–100 mg/L) for 24 h. After treatment, the 0.02 mL of MTT dye (2.5 mg/mL) was added to each well and incubated for 4 h. After this to each well is added 0.1 mL of DMSO to solubilised formazan crystals. These plates were then read on ELISA plate reader at 570 nm. All the treatments were made in triplicates. Camptothecin at a concentration of 10 µM was used as positive standard.
Revival of treated PC-3 cell
The PC-3 and 293T cells (1 × 104) in 96-well plate were treated with IC50 of CoCl2·6H2O for 24 h. The non-adhered cells which come up in the medium and usually regarded as dead cells were harvested by collecting the medium in sterilized 10 mL centrifuge tubes. The medium containing cells were centrifuge at 1500 rpm for 5 min. The medium was discarded and cell pellet was mixed with fresh nutritive medium. The cells along with nutritive medium were transferred in culture flask and incubated in CO2 incubator for 24 h. After incubation, the cells were observed under microscope if they survived the treatment of CoCl2·6H2O and were photographed.
Microscopic studies of CoCl2·H2O treated PC-3 cells
The morphological and anatomical changes induced in PC-3 cells by CoCl2·6H2O were studied with the help of different microscopic techniques.
Light microscopy
The light microscopy of treated PC-3 cells was done using the previously studied method with slight modifications (Ramasamy et al. 2013). The PC-3 cells were incubated in 6 well plate with density of 5 × 105 cells/well. After 24 h, the cells were treated with different concentrations (5, 10, 25, 50, 75 and 100 mg/L) of CoCl2·6H2O for 12–14 h. After treatment, the cells were observed under phase contrast microscope and changes in the morphology of PC-3 cells were photographed.
Confocal microscopy
The confocal microscopy was done using previously followed method with slight modification (Bhushan et al. 2007). The PC-3 cells (5 × 105 cells/well) were seeded in 6-well plate and allowed to adhere for 24 h. The cells were treated with IC50 and IC70 concentration of CoCl2·H2O for 12–14 h. Thereafter, the cells were washed with phosphate buffer saline and incubated with 4 % PFA for half an hour. After fixation, the cells were stained with 4,6-diaminidino-2-phenylindole (DAPI), and slides were prepared for imaging with confocal microscope. The slides were scanned under Nikon eclipse TiE inverted fluorescence microscope equipped with a Nikon AiR laser scanning confocal microscope system (Nikon Corporation, Japan). Fluorescence was observed with long pass 488 emission filters.
Scanning electron microscopy
For scanning electron microscopy, the formerly described method was followed (Ye et al. 2012). The PC-3 cells at concentration of 5 × 105 cells/well in 6 well plate were seeded. After 24 h, the cells were treated with IC50 concentration of CoCl2·6H2O for 12–14 h. Thereafter, the cells were fixed in 4 % osmium tetraoxide. The cells were dehydrated using concentration gradient of ethanol and acetone before freeze drying under vacuum in lyophilizer. The cells were then gold plated using Quarum Q150R ES Rotary-pumped sputter coater. Finally, the cells were observed and photographed under EVO LS 10 scanning electron microscope (Carl Zeiss, Germany).
Cell cycle analysis
Cell cycle analysis was done on PC-3 cells by previously described method (Carnevale et al. 2014). The PC-3 cells at a concentration of 5 × 105 were grown in 6-well plate overnight, and afterwards the cells were treated with different concentrations of CoCl2·6H2O for 24 h. After treatment, the cells were trypsinized and centrifuged at 1500 rpm for 5 min and pellet was washed thrice with PBS. Thereafter, cells were fixed in 1 mL ice cold 70 % ethanol for 3 h at −20 °C. Cells were incubated with DNase free RNase at 37 °C for 1 h and then stained with propidium iodide (5 µg/mL) for 20 min at 4 °C in dark. These fixed and stained cells were then analysed immediately on BD Accuri C6 flow cytometer to study the different phases of cell cycle.
LDH assay
The LDH assay was performed by the method of Linford and Dorsa 2002 with slight modifications. The 96 well plate was seeded with PC-3 cells at density of 1 × 104 cells/well and allowed to adhere for 24 h. Thereafter, the cells were treated with IC50 and IC70 concentrations of CoCl2·6H2O for 12–14 h. After treatment, 50 µL of the culture supernatant was transferred to new 96-well culture plates and mixed with 50 µL of the LDH substrate mixture. The reaction was stopped by adding 100 µL of solution containing 50 % dimethylformamide and 20 % sodium dodecyl sulfate (DMF/SDS, pH 4.7). Absorbance was measured at 570 nm with Biotek synergy HT ELISA reader (Thermo Scientific).
Annexin V-FITC/PI assay
Apoptosis induced by treatments of CoCl2·6H2O in PC-3 cells was analysed using Annexin V-FITC detection kit (Sigma Aldrich Inc., USA.). The procedure was followed as given in the kit. The cells were viewed under Nikon eclipse TiE inverted fluorescence microscope equipped with a Nikon AiR laser scanning confocal microscope system (Nikon Corporation, Japan).
ROS generation
The PC-3 cells were used to examine the ROS generation by method of Shin et al. 2009 with slight modifications (Lippi et al. 2005). The PC cells at concentration of 5 × 105 were seeded in 12 well plate. After 24 h, the cells were treated with IC50 and IC70 concentration of CoCl2·6H2O for 12–14 h. The treated cells were stained with 10 µg/mL of 5,6-chloromethyl-2′-7′dichlorodihydroflurescein diacetate (H2DCFDA). Then, fluorescence was measured using ELISA plate reader (Bio Tek Multi Mode Reader) with excitation and emission wavelengths of 488/20 and 530/20 nm respectively.
Measurement of MMP
The changes in mitochondrial membrane potential in treated PC-3 cells were assessed using the method (Deng et al. 2013). The overnight grown PC-3 (5 × 105) cells were treated with IC50 and IC70 concentrations of CoCl2·6H2O for 12–14 h and followed by staining with rhodamine 123 fluorescence dye (1 µM) for 45 min. Cells were washed thrice with PBS (1×). Thereafter, the rhodamine 123 fluorescence was examined using ELISA plate reader (Bio Tek Multi Mode Reader) with excitation of 488 nm and emission of 530 nm.
Measurement of caspase activity
The caspase activity was measured in PC-3 cells treated with IC50 and IC70 concentrations of CoCl2·6H2O for 12–14 h using the method described in caspase kit purchased from Biovision Inc., USA.
Statistical analysis
The experimental data was analysed in MS-Excel using self-coded software. Two-way analysis of variance (ANOVA) was done to check the significance of differences between and within treatments, and interactions if any. The null hypothesis tested was that there is no significant difference among the means. The alternative hypothesis was at least two means differ from each other. HSD was calculated using Tukey’s multiple comparison test. Chi square test was used to check whether there is a significant difference between the expected frequencies (positive control) and the observed frequencies (different biochemical parameters like LDH, ROS, MMP and caspase). Significance levels of F-ratios and chi- square were checked at P < 0.001. Logarithmic regression analysis was done to determine the significance of correlativity among the variables (Sokal and Rohlf 1981; Bailey 1994). Camptothecin (10 µM) was used as positive standard.
Results
CoCl2·6H2O induces cell death in cancer cell
Three forms of Cobalt i.e., CoCl2·6H2O, Macro-Co(II,III) oxide and Nano-Co(II,III) oxide were tested for antiproliferative activity against PC-3 cancer cell line as shown in Table 1. Among these, CoCl2·6H2O showed maximum antiproliferative activity of 79.56 % at highest tested dose of 100 mg/L followed by 13.70 and 14.12 % of Macro-Co(II,III) oxide and Nano-Co(II,III) oxide respectively. Since, CoCl2·6H2O was found to the most effective antiproliferative agent hence it was further studied against IMR3, A549 and 293T cell lines as well. CoCl2·6H2O inhibited rapid proliferation of cancer cells and induced maximum cell death in IMR-32 followed by PC-3 and A549 with IC50 values of 7.12, 21.91 and 29.81 mg/L respectively. CoCl2·6H2O was found to be more toxic to cancer cell lines as compared to the normal cell line (Table 2).
Table 1.
Percent inhibition of prostrate (PC-3) cancer cell lines treated with different concentration of CoCl2·6H2O, Macro-Co(II,III) oxide and Nano-Co(II,III) oxide
| Conc. (mg/L) | CoCl2·6H2O | Macro-Co(II,III) oxide | Nano-Co(II,III) oxide |
|---|---|---|---|
| % Inhibition | |||
| 10 | 33.13 | 3.94 | −12.31 |
| 25 | 46.86 | 5.95 | −4.47 |
| 50 | 61.45 | 8.81 | 6.29 |
| 75 | 71.12 | 10.56 | 9.93 |
| 100 | 79.56 | 13.70 | 14.12 |
Table 2.
Percent inhibition, IC50 values of various cell lines treated with different concentrations of CoCl2·6H2O (5–100 mg/L) by using best fit regression model
| Concentration of CoCl2·6H2O (mg/L) (x) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cell line | Inhibition (%) (y) | Regression equation | IC50 | |||||
| 5 | 10 | 25 | 50 | 75 | 100 | |||
| 293 T | 7.27 | 16.97 | 27.73 | 47.26 | 62.67 | 69.72 | y = 20.92 ln(x) − 31.03 R = 0.977*** |
48.1 |
| IMR-32 | 31.24 | 66.60 | 77.22 | 78.10 | 79.01 | 79.22 | y = 13.59 ln(x) + 23.33 R = 0.849*** |
7.12 |
| PC-3 | 19.80 | 31.58 | 49.70 | 67.98 | 80.36 | 82.08 | y = 21.86 ln(x) − 17.49 R = 0.995*** |
21.91 |
| A549 | 0.817 | 5.48 | 46.92 | 73.61 | 80.02 | 80.79 | y = 30.61 ln(x) − 53.93 R = 0.980*** |
29.81 |
| Two-way ANOVA summary table | ||||||
|---|---|---|---|---|---|---|
| Source of variation | df | SS | MSS | F-ratio | Tukey’s HSD0.05 | 4.79 |
| Treatment (cell line) | 3 | 8632.36 | 2877.45 | 470.06*** | ||
| Dose (CoCl2·6H2O) | 5 | 39,715.85 | 7943.17 | 1297.59*** | ||
| Treatment × dose (interaction) | 15 | 5669.54 | 377.97 | 61.75*** | ||
| Error | 48 | 293.83 | 6.12 | |||
| Total | 71 | 54,311.57 | ||||
R *** P < 0.001; *** P < 0.001; n = 5
Non revival of treated PC-3 and 293T cells
Figure 1 shows harvested 293T and PC-3 cells with and without treatment with CoCl2·6H2O. It was observed that treated PC-3 as well as 293T cells did not revert back to normalcy even after transferring to fresh growth medium.
Fig. 1.

Subculturing of dead 293T and PC-3 cells after treatment with CoCl2·6H2O after 24 h. a 293T cells without treatment, b 293T cells with CoCl2·6H2O (IC50) treatment, c PC-3 cells without treatment, d PC-3 cells with CoCl2·6H2O (IC50) treatment
CoCl2·6H2O induces morphological and chromatin changes
The microscopic analysis of PC-3 cells treated with various concentrations of CoCl2·6H2O showed the details of the morphological and chromatin changes. Light microscopy showed that with increasing concentration of CoCl2·6H2O, the cell morphology was changed involving cell rounding (Fig. 2). Confocal microscopic studies revealed the nuclear morphological changes induced by CoCl2·6H2O at chromatin level. Changes like chromatin breaks and chromatin condensation were observed in CoCl2·6H2O treated PC-3 cells (Fig. 3). Further, scanning electron microscopy revealed the appearance of apoptotic bodies in PC-3 cells treated with CoCl2·6H2O (Fig. 4).
Fig. 2.
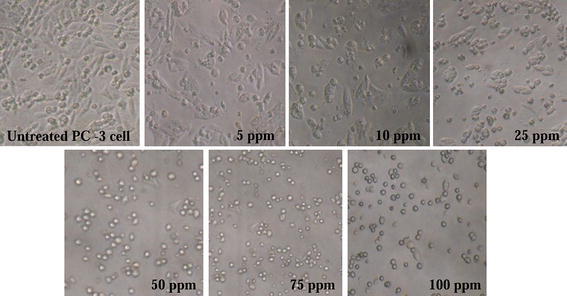
Phase contrast micrographs (40X, Nikon eclipse TS100) of CoCl2·6H2O concentrations effect (5–100 mg/L) on morphology of PC-3 cells observed after 12–14 h of treatment at magnification of ×40
Fig. 3.
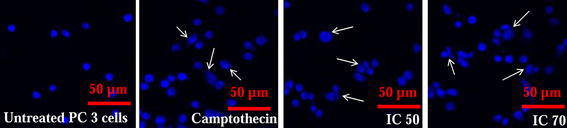
Confocal micrographs (Nikon eclipse TiE inverted fluorescence microscope, Nikon Corp. Japan) of effect of CoCl2·6H2O treatment (IC50 and IC70, 12–14 h treatment) on morphology of PC-3 cells
Fig. 4.

Scanning electron micrographs (EVO LS 10. Carl Zeiss Germany) of effect of CoCl2·6H2O (IC50, 12–14 h treatment) on morphology of PC-3 cells
CoCl2·6H2O arrests PC-3 cells ate sub go phase of cell cycle
The cell cycle arrest induced in PC-3 cells treated with IC50 and IC70 concentrations of CoCl2·6H2O is shown in Fig. 5. The untreated PC-3 showed only 10.8 % of cells in sub Go phase of cell cycle while this number increased to 32.6 and 39.1 % with treatment of IC50 and IC70 concentrations of CoCl2·6H2O respectively. The positive control, camptothecin treatment showed 51.9 % of cells in sub G0 phase.
Fig. 5.
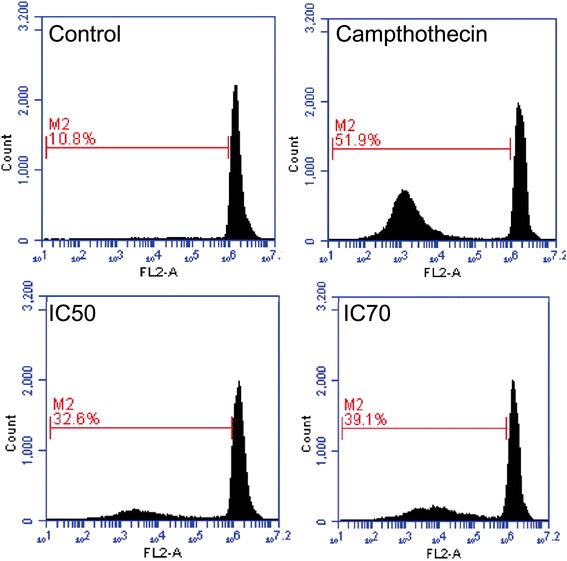
Cell cycle analysis in PC-3 cells of untreated control, positive control (campthothecin), IC50 and IC70 treatment of CoCl2·6H2O. Cells were stained with PI (5 µg/mL for 20 min) and analysed using BD Accuri C6 flow cytometer
CoCl2·6H2O induces LDH release
The LDH assay was employed to study the effect of CoCl2·6H2O on integrity of PC-3 cell membrane. Both the IC50 and IC70 treatments of CoCl2·6H2O induced release of 23.2 and 38.2 % of LDH respectively. The positive control camptothecin resulted in 26.7 % release of LDH over the untreated PC-3 cells (Table 3).
Table 3.
Change in LDH, ROS, MMP and caspase activities at IC50 and IC70 concentrations of CoCl2·6H2O for 12–14 h treatment in PC-3 cancer cell line with respect to untreated PC-3 cells
| LDH | ROS | MMP | Caspase | |
|---|---|---|---|---|
| Untreated PC-3 cells | 100 | 100 | 100 | 100 |
| Camptothecin (10 µM) treated PC-3 cells | 126.7 | 176.5 | 72.7 | 174.1 |
| CoCl2·6H2O (IC50) treated PC-3 cells | 123.2 | 152.9 | 80 | 170.4 |
| CoCl2·6H2O (IC70) treated PC-3 cells | 138.2 | 211.8 | 73.3 | 177.8 |
| χ2 | 27.07*** | 211.42*** | 18.55*** | 164.88*** |
IC50 and IC70 doses calculated using regression equation: y = 21.86 ln(x) − 17.49
*** P < 0.001; n = 3
CoCl2·6H2O induces apoptosis
In order to confirm the apoptotic or necrotic mode of cell death in PC-3 cells, Annexin V/propidium iodide staining dyes were used. Fluorescently labelled annexin gets strongly bound to phosphatidylserine part in plasma membrane of cells upon onset of apoptosis, while propidium iodide was used for conferring necrosis. The cells showed mild necrosis but peculiar features of apoptosis (Fig. 6).
Fig. 6.
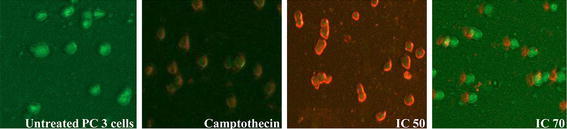
Annexin V/propidium iodide staining for confirmation of apoptosis and necrosis in PC-3 cells with IC50 and IC70 treatments of CoCl2·6H2O in comparison to control and camptothecin (10 µM) as positive standard
CoCl2·6H2O induces ROS generation
The generation of ROS in PC-3 cells treated with IC50 and IC70 concentrations of CoCl2·6H2O was measured spectrofluorimetrically. The results were presented as relative fluorescence as compared to the untreated cells. The CoCl2·6H2O treatments showed dose dependent increase in ROS generation. More than two folds of increase in ROS generation was observed in PC-3 cells treated with IC70 concentration of CoCl2·6H2O while positive standard camptothecin induced 1.76-fold increase (Table 3).
CoCl2·6H2O disrupts mitochondrial membrane potential
The disruption of mitochondrial membrane is a key step in the induction of apoptosis in cancerous cells. The results were presented as percentage rhodamine intensity. The intensity of rhodamine detected in untreated cells was considered as 100 %. The treatment with IC50 and IC70 concentrations of CoCl2·6H2O showed dose dependent decrease in rhodamine intensity as detected by spectrofluorimeter. Positive standard camptothecin showed 72.7 % while IC50 and IC70 showed up to 80 and 73.3 % of rhodamine intensity (Table 3).
CoCl2·6H2O activates caspase 3
Caspase-3 activity was assessed in PC-3 cells treated with IC50 and IC70 concentrations of CoCl2·6H2O (Table 3). A dose dependent increase in caspase activity was observed in PC-3 cells treated with CoCl2·6H2O. The IC50 and IC70 concentrations of CoCl2·6H2O showed significant increase in caspase activity of 170.4 and 177.8 % respectively as compared to untreated PC-3 cells. The positive control camptothecin showed 174.1 % increase in caspase activity as compared to the untreated PC-3 cells.
Discussion
Metals and their complexes have been extensively used for their remedial properties since ages. Recent chaos over their toxic potentials has overlooked their medicinal values, thus warranting a need to delineate the ailment preventive effects of metals substantiated with a scientific data. Recently the mechanism of action of metal ions in cancer cells has been elucidated. It has been reported that metal ions induce hypoxia in cellular entity and resulting in activation of various signalling pathways and hypoxia inducible transcription factors (HIF) (Salnikow et al. 2000; Huang et al. 2013). Cobalt chloride is well known for inducing hypoxia like conditions both in vivo (Alexa et al. 2015) and in vitro (Shweta et al. 2015) conditions. Medicinally, it is also used to treat anaemic patients. Having known its medical uses, it would be pertinent to test its anti-proliferative and anti-cancer activities.
Preliminary, three forms of cobalt were screened for antiproliferative active against PC-3 cancer cell line using MTT assay. MTT is the most widely widely used method to investigate antiproliferative potential of test compound against various cell lines (Dellai et al. 2012; Pieme et al. 2013; Rapisarda et al. 2015). The mechanism behind this assay includes conversion of yellow coloured MTT into blue colored formazon by the action of the enzyme succinate dehydrogenase within mitochondria of living cells. Our results showed CoCl2·6H2O as the most active antiproliferative agent, hence it was further tested against other cancer cell lines. Cell death induced by CoCl2·6H2O inhibited the formation of blue colored formazan crystals. Lower the intensity of blue colour, more will be the antiproliferative potential of the test compound. CoCl2·6H2O was tested at 6 concentrations ranging from 5 to 100 mg/L against four cell lines viz. 293T, PC-3, A549 and IMR-32. It induced maximum cell death in IMR-32 cancer cell line. Similar studies were conducted by (Martinez-Built et al. 2015; Villa-Pérez et al. 2016) using Co(II), Ni(II), Zn(II) and Cu(II) complexes against HeLa, HTC-15, MCF-7 and PC-3 cell lines and they established that ligand binding capability of transition metals is responsible for their antiproliferative activity. Although, the growth of normal non cancerous cells was also inhibited to certain degree but interestingly, this growth inhibition was more in case of cancer cell lines. Further, the LDH assay was also performed to check whether the mode of cell death induced by test compound is apoptosis or necrosis. The results clearly showed a very mild release of LDH from cell culture treated with CoCl2·6H2O. This prompted us to look for other way of cell death i.e., apoptosis. Further studies were conducted on PC-3 cells to eke out the apoptotic signatures from both inside and outside of the cell. All the microscopic studies on CoCl2·6H2O treated PC-3 cells showed the appearance of apoptotic bodies, blebbing, condensation of chromatin and shrinkage of cells. These morphological and cytological changes in PC-3 cells point towards the apoptotic mode of cell death (Brauchle et al. 2014). Similar apoptotic inducing activities of cobalt were studied by Akita et al. (2007) where they found that salts of cobalt reduces expression of Bcl-2 protein at transcriptional as well as translational level in HSG cells and induces apoptosis. The cell cycle analysis using flow cytometry showed an initial PC-3 cell arrest at sub Go phase of cycle. Most of the anticancer drugs act by first arresting the cancer cells at particular phase of cell cycle and then start inducing apoptosis (Pathania et al. 2015). Similar studies were conducted by Van Rijt et al. (2014) and they observed cell arrest in S-phase after treatment with organometallic osmium compounds in A549 cancer cells. The cell surface markers change their positions during apoptosis and to confirm this annexin V-FITC/propidium iodide staining was done and images of stained cells were taken by confocal microscope. The pattern of staining showed the DNA damage and movement of apoptotic markers to cell surface to which annexin was bound. It was further tested that whether apoptosis was operating by intrinsic or extrinsic pathway. The treated and untreated PC-3 cells were checked spectroflurimetrically for ROS and MMP using different dyes. Results showed that CoCl2·6H2O induced generation of ROS which in turn damaged mitochondria as was evident from MMP reading. These results were in accordance with studies by Zhang et al. (2016) where Co(II) complexes were found to alleviate ROS and disrupted mitochondrial membrane in cancer cell lines. The intrinsic pathway requires an induction like overproduction of ROS, radiations that damage mitochondria which in turn activate caspases to bring out apoptotic death (Hajrezaie et al. 2015; Elmore 2007; Tian et al. 2015; Leverson et al. 2015). Thus, caspases being an important class of cysteine proteases play requisite role in apoptosis (Exile et al. 2014). Our results showed a dose dependent increase in caspase activity in PC3 cells with an increase in the concentration of CoCl2·6H2O. Similar results were observed in ruthenium polypyridyl complexes, which lead to increased caspase-3 activity in breast cancer cells (Cao et al. 2015).
Thus, it could be inferred that the hypoxic conditions induced by CoCl2·6H2O deprives the proliferating cancer cells of the oxygen required for their metabolism. This could have increased the expression of HIF transcription factor and the over expression of later is an inducer of apoptosis (Krick et al. 2005). Thus apoptosis was found to execute in PC3 cells by over generation of ROS and concomitant damage to mitochondrial membrane.
Conclusions
The present work unequivocally demonstrates the antiproliferative potential of CoCl2·6H2O against brain (IMR-32), lung (A549) and prostrates (PC-3) cancer cell lines. CoCl2·6H2O increased ROS production, decreased mitochondrial membrane potential, increased the activity of casapse-3 and sub G0 population of cells. The microscopic studies further confirmed the cellular and nuclear morphological changes following CoCl2·6H2O treatment. The mechanism of cellular death in PC-3 is apoptosis. Further, in vivo studies are required to investigate the anticancerous properties of cobaltous chloride hexahydrate.
Authors’ contributions
SM performed MTT, Annexin-V FITC/PI assay, phase contrast microscopy, biochemical studies and wrote the manuscript. RK performed confocal and SEM microscopy. RA performed flow cytometery. JM standardised caspase assay. SA interpreted images and edited manuscript. RB designed the experiment. AKT conceived the idea, supervised experiment and analysed data. All authors read and approved the final manuscript.
Acknowledgements
Research fellowship granted (vide Grant No. F.14-2(SC)/2010(SA-III) by the University Grants Commission, New Delhi to the first author Sonia is duly acknowledged for this work. Thanks are also due to the Head, Department of Botanical and Environmental Sciences for research facilities. For this work the instrumentation facilities at the UGC-UPE centre for emerging life sciences were used.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Sonia Mahey, Email: sonia.gndu11@gmail.com.
Rakesh Kumar, Email: raakysh@gmail.com.
Rohit Arora, Email: rtarora23@gmail.com.
Jyoti Mahajan, Email: mahajanjyoti9@gmail.com.
Saroj Arora, Email: sarojarora.gndu@gmail.com.
Renu Bhardwaj, Email: renubhardwaj82@gmail.com.
Ashwani Kumar Thukral, Email: akthukral.gndu@gmail.com.
References
- Akita K, Okamura H, Yoshida K, Morimoto H, Ogawa-Iyehara H, Haneji T. Cobalt chloride induces apoptosis and zinc chloride suppresses cobalt-induced apoptosis by Bcl-2 expression in human submandibular gland HSG cells. Int J Oncol. 2007;31:923–929. [PubMed] [Google Scholar]
- Alexa T, Luca A, Dondas A, Bohotin CR. Preconditioning with cobalt chloride modifies pain perception in mice. Exp Ther Med. 2015;9:1465–1469. doi: 10.3892/etm.2015.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres E, Fothergill H, Mecili M. Efficacy of oral cobalamin (vitamin B12) therapy. Opin Pharmacother. 2010;11:249–256. doi: 10.1517/14656560903456053. [DOI] [PubMed] [Google Scholar]
- Bailey NTJ. Statistical methods in biology. Cambridge: Cambridge University Press; 1994. [Google Scholar]
- Bandyopadhyay D, Rivera G, Sanchez JL, Rivera J, Granados JC, Guerrero AM, Chang F-M, Dearth RK, Short JD, Banik BK. Bismuth nitrate-induced novel nitration of estradiol: an entry to new anticancer agents. Eur J Med Chem. 2014;82:574–583. doi: 10.1016/j.ejmech.2014.06.010. [DOI] [PubMed] [Google Scholar]
- Bhushan S, Kumar A, Malik F, Andotra SS, Sethi VK, Kaur IP, Taneja SC, Qazi GN, Singh J. A triterpenediol from Boswellia serrata induces apoptosis through both the intrinsic and extrinsic apoptotic pathways in huma leukemia HL-60 cells. Apoptosis. 2007;12:1911–1926. doi: 10.1007/s10495-007-0105-5. [DOI] [PubMed] [Google Scholar]
- Brauchle E, Thude S, Brucker SY, Schenke-Layland K. Cell death stages in single apoptotic and necrotic cells monitored by Raman microspectroscopy. Sci Rep. 2014;4:1–9. doi: 10.1038/srep04698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W, Zheng W, Chen T. Ruthenium polypyridyl complex inhibits growth and metastasis of breast cancer cells by suppressing FAK signaling with enhancement of TRAIL-induced apoptosis. Sci Rep. 2015;5:1–11. doi: 10.1038/srep09157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnevale R, Pignatelli P, Nocella C, Loffredo L, Pastori D, Vicario T, Petruccioli A, Bartimoccia S, Violi F. Extra virgin olive oil blint post-prandial oxidative stress via NOX2 down-regulation. Artherosclerosis. 2014;235:649–658. doi: 10.1016/j.atherosclerosis.2014.05.954. [DOI] [PubMed] [Google Scholar]
- Chellan P, Sadler PJ. The elements of life and medicine. Philos Trans A. 2015 doi: 10.1098/rsta.2014.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitambar CR. Gallium-containing anticancer compounds. Future Med Chem. 2013;4:1257–1272. doi: 10.4155/fmc.12.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Assis FL, Visentin LC, de Souza FS, DaMatta RA, Horn A, Jr, Fernandes C. Synthesis, crystal structure and relevant antiproliferative activity against Toxoplasma gondii of a new binuclear Co(II) complex. Inorg Chem Commun. 2016;67:47–50. doi: 10.1016/j.inoche.2016.02.017. [DOI] [Google Scholar]
- Dellai A, Deghrigue M, Laroche- Clary A, Masour HB, Chouchane N, Robert J, Bouraoui A. Evaluatiom of antiproliferative and anti-inflammatory activities of methanol extract and its fractions from the Mediterranean sponge. Cancer Cell Int. 2012;12:1–6. doi: 10.1186/1475-2867-12-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng S, Yuan H, Yi J, Lu Y, Wei Q, Guo C, Wu J, Yuan L, He Z. Gossypol acetic acid induces apoptosis in RAW264. 7 cells via a caspase dependent mitochondrial signaling pathway. J Vet Sci. 2013;14:281–289. doi: 10.4142/jvs.2013.14.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Boraey HA, El-Din AAS. Transition metal complexes of a new 15-membered [N5] penta-azamacrocyclic ligand with their spectral and anticancer studies. Spectrochim Acta A Mol Biomol Spectrosc. 2014;132:663–671. doi: 10.1016/j.saa.2014.05.018. [DOI] [PubMed] [Google Scholar]
- Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exile MC, Justiniano S, Hollyfield JL, Berhe F, Besecker BY, Das S, Wewers MD, Sarkar A. Microvesicular caspase-1 mediates lymphocyte apoptosis in sepsis. PLoS ONE. 2014;9:1–8. doi: 10.1371/journal.pone.0090968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldmacher Y, Oleszak M, Sheldrick WS. Rhodium(III) and iridium(III) complexes as anticancer agents. Inorg Chim Acta. 2012;393:84–102. doi: 10.1016/j.ica.2012.06.046. [DOI] [Google Scholar]
- Gianferrara T, Bratsos I, Alessio E. A categorization of metal anticancer compound based on their mode of action. Dalton Trans. 2009 doi: 10.1039/b905798f. [DOI] [PubMed] [Google Scholar]
- Hajrezaie M, Paydar M, Looi CY, Moghadamtousi SZ, Hassandarvish P, Salga MS, Karimian H, Shams K, Zahedifard M, Majid NA, Ali HM, Abdulla MA. Apoptotic effect of novel schiff based CdCl2(C14H21N3O2) complex is mediated via activation of the mitochondrial pathway in colon cancer cells. Sci Rep. 2015;5:1–11. doi: 10.1038/srep09097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckenkamp J, Leszczynski D, Schiereck J, Kung J, Lamuraglia GM. Different effects of photodynamic therapy and γ-irradiation on vascular smooth muscle cells and matrix: implications for inhibiting restenosis. Arterioscler Thromb Vasc Biol. 1999;19:2154–2161. doi: 10.1161/01.ATV.19.9.2154. [DOI] [PubMed] [Google Scholar]
- Huang Y, Zitta K, Bein B, Steinfath M, Albrecht M. An insert based enzymatic cell culture system to rapidly and reversibly induce hypoxia investigations of hypoxia cell damage, protein expression and phosphorylation in neuronal IMR-32 cells. Dis Models Mech. 2013;6:1507–1514. doi: 10.1242/dmm.013078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang QW, Wang SX, Liu SG, Su WY, Li GB, He YM. Crystal structure and antitumor activities of a dinuclear cobalt(II) complex based on meso-1,2,3,4-tetra (1H-benzo[d]imidazol-2-yl) butane. J Struct Chem. 2016;57:188–193. doi: 10.1134/S0022476616010236. [DOI] [Google Scholar]
- Hunger M, Mutti E, Rieder A, Enders B, Nexo E, Krautler B. Organometallic B12–DNA conjugate: synthesis, structure analysis, and studies of binding to human B12-transporter proteins. Chem Eur J. 2014;20:13103–13107. doi: 10.1002/chem.201404359. [DOI] [PubMed] [Google Scholar]
- Krick S, Eul BG, Hanze J, Savai R, Grimminger F, Seeger W, Rose F. Role of hypoxia inducible factor-1α in hypoxia-induced apoptosis of primary alveolar epithelial type-II cells. Am J Respir Cell Mol Biol. 2005;32:395–403. doi: 10.1165/rcmb.2004-0314OC. [DOI] [PubMed] [Google Scholar]
- Krisch SH, Herrmann W, Obeid R. Genetic defects in folate and cobalamin pathways affecting the brain. Clin Chem Lab Med. 2013;51:139–155. doi: 10.1515/cclm-2012-0673. [DOI] [PubMed] [Google Scholar]
- Leverson JD, Zhang H, Chen J, Tahir SK, Philips DC, Xue J, Nimmer P, Jin S, Smith M, Xiao Y, Kovar P, Tanaka A, Bruncko M, Sheppard GS, Wang L, Gierke S, Kategaya L, Anderson DJ, Wong C, Eastham-Anderson J, Ludlam MJ, Sampath D, Fairbrother WJ, Wertz I, Rosenberg SH, Tse C, Elmore SW, Souers AJ. Potent and selective small-molecule MCL-1 inhibitors demonstrate on-target cancer cell killing activity as single agents and in combination with ABT-263 (navitoclax) Cell Death Dis. 2015 doi: 10.1038/cddis.2014.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Collins JG, Keene FR. Ruthenium complexes as antimicrobial agents. Chem Soc Rev. 2015 doi: 10.1039/c4cs00343h. [DOI] [PubMed] [Google Scholar]
- Linford NJ, Dorsa DM. 17β-Estradiol and the phytoestrogen genistein attenuate neuronal apoptosis induced by the endoplasmic reticulum calcium-ATPase inhibitor thapsigargin. Steroids. 2002;67:1029–1040. doi: 10.1016/S0039-128X(02)00062-4. [DOI] [PubMed] [Google Scholar]
- Lippi G, Franchini M, Guidi GC. Cobalt chloride administration in athletes: a new perspective in blood doping. Br J Sports Med. 2005;39:872–873. doi: 10.1136/bjsm.2005.019232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahey S, Thukral AK. Effects of macro- and nano-cobalt oxide particles on barley seedlings and remediation of cobalt chloride toxicity using sodium hypochlorite. Int J Plant Soil Sci. 2014;3:751–762. doi: 10.9734/IJPSS/2014/8206. [DOI] [Google Scholar]
- Martinez-Built P, Garza- Ortiz A, Mijangos E, Barron-Sosa L, Sanchez-Bartez F, Gracia-Mora I, Flores-Parra A, Contreras R, Reedijk J, Barba-Behrens N. 2,6-Bis(2,6-diethylphenyliminomethyl) pyridine coordination compounds with cobalt(II), nickel(II), copper(II), and zinc(II): synthesis, spectroscopic characterization, X-ray study and in vitro cytotoxicity. J Inorg Biochem. 2015;142:1–7. doi: 10.1016/j.jinorgbio.2014.09.007. [DOI] [PubMed] [Google Scholar]
- Mojic M, Pristov JB, Maksimovic- Ivanic D, Jones DR, Stanic M, Mijatovic S, Spasojevic I. Extracellular iron diminishes anticancer effects of vitamin C: an in vitro study. Sci Rep. 2014;4:1–8. doi: 10.1038/srep05955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny L, Kombian SB. Vanadium: possible use in cancer chemoprevention and therapy. J Cancer Res Updates. 2014;3:97–102. doi: 10.6000/1929-2279.2014.03.02.3. [DOI] [Google Scholar]
- Obeid A, El-Shekeil A, Al-Aghbari S, Al-Shabi J. Anticancer, DNA cleavage, and antimicrobial activity studies of some new Schiff-base titanium (IV) complexes. J Coord Chem. 2012;65:2762–2770. doi: 10.1080/00958972.2012.703780. [DOI] [Google Scholar]
- Olivova R, Kasparkova J, Vrana O, Vojtiskova M, Suchankova T, Novakova O, He W, Guo Z, Brabec V. Unique DNA binding mode of antitumor trinuclear tridentate platinum(II) compound. Mol Pharm. 2011;8:2368–2378. doi: 10.1021/mp200298g. [DOI] [PubMed] [Google Scholar]
- Pathania AS, Wani ZA, Guru SK, Kumar S, Bhushan S, Korkaya H, Seals DF, Kumar A, Mondhe DM, Ahmed Z, Chandan BK, Malik F. The anti angiogenic and cytotoxic effects of the boswellic acid analog BA145 are potentiated by authophagy inhibitors. Mol Cancer. 2015;14:2–15. doi: 10.1186/1476-4598-14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieme CA, Guru SK, Ambassa P, Kumar S, Ngameni B, Ngogang JY, Bhushan S, Saxena AK. Induction of mitochondrial dependent apoptosis and cell arrest in human promyelocytic leukemia HL-60 cells by an extract from Dorstenia psilurus: a spice from Cameroon. BMC Complement Altern Med. 2013;13:1–9. doi: 10.1186/1472-6882-13-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy S, Abdul WN, Zainal AN, Manickam S. Effect of extracts from Phyllanthus watsonii Airy Shaw on cell apoptosis in cultured human breast cancer MCF-7 cells. Exp Toxicol Pathol. 2013;65:341–349. doi: 10.1016/j.etp.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Rapisarda V, Loreto C, Musumeci G, Bracci M, Santarelli L, Renis M, Ferrante M, Cardile V. Cytotoxicity, oxidative stress and genotoxicity induced by glass fibers on human alveolar epithelial cell line A549. Toxicol Vitro. 2015;29:551–557. doi: 10.1016/j.tiv.2014.12.010. [DOI] [PubMed] [Google Scholar]
- Rush EC, Yajnik CS. Vitamin B12: one carbon metabolism, fetal growth and programming chronic disease. Eur J Clin Nutr. 2014;6:2–7. doi: 10.1038/ejcn.2013.232. [DOI] [PubMed] [Google Scholar]
- Salnikow K, Blagosklonny MV, Ryan H, Johnson R, Costa M. Carcinogenic nickel induces genes involved in hypoxic stress. Cancer Res. 2000;60:38–41. [PubMed] [Google Scholar]
- Sharma P, Perez D, Cabrera A, Rosas N, Arias JL. Perspectives of antimony compounds in oncology. Acta Pharmacol Sin. 2008;29:881–890. doi: 10.1111/j.1745-7254.2008.00818.x. [DOI] [PubMed] [Google Scholar]
- Shin YJ, Kim JH, Seo JM, Lee SM, Hyon JY, Yu YS, Wee WR. Protective effect of clusterin on oxidative stress-induced cell death of human corneal endothelial cells. Mol Vis. 2009;15:2789–2795. [PMC free article] [PubMed] [Google Scholar]
- Shweta, Mishra KP, Chanda S, Singh SB, Ganju L. A comparative immunological analysis of CoCl2 treated cells with in vitro hypoxic exposure. Biometals. 2015;28:175–185. doi: 10.1007/s10534-014-9813-9. [DOI] [PubMed] [Google Scholar]
- Singh VP. A review: development of new anticancer drugs complementary to transition metal complexes such as copper(II), zinc(II), nickel(II) and cobalt(II) J Chem Chem Sci. 2014;4:70–117. [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry: the principles and practice of statistics in biological research. San Francisco: WH Freeman and Co.; 1981. [Google Scholar]
- Soldevila-Barreda JJ, Romero-Canelon I, Habtemariam A, Sadler PJ. Transfer hydrogenation catalysis in cells as a new approach to anticancer design. Nat Commun. 2015;6:1–9. doi: 10.1038/ncomms7582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thind TS, Agrawal SK, Saxena AK, Arora S. Studies on cytotoxic, hydroxyl radical scavenging and topoisomerase inhibitory activities of extracts of Tabernaemontana divaricate (L.) R.Br. ex Roem. and Schult. Food Chem Toxicol. 2008;46:2922–2927. doi: 10.1016/j.fct.2008.05.036. [DOI] [PubMed] [Google Scholar]
- Tian H, Gao Z, Li H, Zhang B, Wang G, Zhang Q, Pei D, Zheng J. DNA damage response–a double-edged sword in cancer prevention and cancer therapy. Cancer Lett. 2015;358:8–16. doi: 10.1016/j.canlet.2014.12.038. [DOI] [PubMed] [Google Scholar]
- Van Rijt SH, Romero-Canelon I, Fu Y, Shnyder SD, Sadler PJ. Potent organometallic osmium compounds induce mitochondria- mediated apoptosis and S-phase cell cycle arrest in A549 non-small cell lung cancer cells. Metallomics. 2014;6:1014–1022. doi: 10.1039/c4mt00034j. [DOI] [PubMed] [Google Scholar]
- Via LD, Garcia-Argaez AN, Martinez-Vazquez M, Grancara S, Martinis P, Toninello A. Mitochondrial permeability transition as target of anticancer drugs. Curr Pharm Des. 2014;20:223–244. doi: 10.2174/13816128113199990033. [DOI] [PubMed] [Google Scholar]
- Villa-Pérez C, Cadavid-Vargas JF, Camí GE, Giannini F, Villalba MC, Echeverria G, Ortega IC, Valencia-Uribe GC, Etcheverry SB, Soria DB. Synthesis, Physicochemical and Biological Studies of a ternary Co(II) complex with Sulfaquinoxaline and 2,2′-Bipyrimidine as ligands. Inorg Chim Acta. 2016;447:127–133. doi: 10.1016/j.ica.2016.03.043. [DOI] [Google Scholar]
- Ye LH, Li WJ, Jiang XQ, Chen YL, Tao SX, Qian WL, He JS. Study on the autophagy of prostate cancer PC-3 cells induced by oridonin. Anat Rec. 2012;295:417–422. doi: 10.1002/ar.21528. [DOI] [PubMed] [Google Scholar]
- Zhang HR, Huang KB, Chen ZF, Liu YC, Liu YN, Meng T, Ping QQ, Zou BQ, Liang H. Cobalt(II) 8-hydroxyquinoline complexes: structure, cytotoxicity, and action mechanism. Med Chem Commun. 2016 [Google Scholar]


