Abstract
Background
Sensory nerves innervating the airways play an important role in regulating various cardiopulmonary functions, maintaining homeostasis under healthy conditions and contributing to pathophysiology in disease states. Hypo-osmotic solutions elicit sensory reflexes, including cough, and are a potent stimulus for airway narrowing in asthmatic patients, but the mechanisms involved are not known. Transient receptor potential cation channel, subfamily V, member 4 (TRPV4) is widely expressed in the respiratory tract, but its role as a peripheral nociceptor has not been explored.
Objective
We hypothesized that TRPV4 is expressed on airway afferents and is a key osmosensor initiating reflex events in the lung.
Methods
We used guinea pig primary cells, tissue bioassay, in vivo electrophysiology, and a guinea pig conscious cough model to investigate a role for TRPV4 in mediating sensory nerve activation in vagal afferents and the possible downstream signaling mechanisms. Human vagus nerve was used to confirm key observations in animal tissues.
Results
Here we show TRPV4-induced activation of guinea pig airway–specific primary nodose ganglion cells. TRPV4 ligands and hypo-osmotic solutions caused depolarization of murine, guinea pig, and human vagus and firing of Aδ-fibers (not C-fibers), which was inhibited by TRPV4 and P2X3 receptor antagonists. Both antagonists blocked TRPV4-induced cough.
Conclusion
This study identifies the TRPV4-ATP-P2X3 interaction as a key osmosensing pathway involved in airway sensory nerve reflexes. The absence of TRPV4-ATP–mediated effects on C-fibers indicates a distinct neurobiology for this ion channel and implicates TRPV4 as a novel therapeutic target for neuronal hyperresponsiveness in the airways and symptoms, such as cough.
Key words: Transient receptor potential, sensory nerves, vagus, cough, ion channels, hypotonicity, ATP
Abbreviations used: AUC, Area under the curve; [Ca2+]i, Intracellular free calcium; COPD, Chronic obstructive pulmonary disease; CV, Conduction velocity; DiI, 1,1′-Dioctacetyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate; DMSO, Dimethyl sulfoxide; ECS, Extracellular solution; K50, 50 mmol/L Potassium chloride solution; αβ-MeATP, αβ-Methylene-ATP; NCBI, National Center for Biotechnology Information; 4α-PDD, 4α Phorbol 12,13-didecanoate; Px1, Pannexin 1; RAR, Rapidly adapting stretch receptor; TRP, Transient receptor potential; TRPV4, Transient receptor potential cation channel, subfamily V, member 4
Sensory nerves innervating the airways play an important role in regulating various cardiopulmonary functions, maintaining homeostasis under healthy conditions, and contributing to pathophysiology in disease conditions. Transient receptor potential (TRP) channels are a family of cation channels that are activated by a large number of diverse stimuli, including temperature, pH, and osmolarity.1 TRPA1 and TRPV1 ion channels expressed on peripheral nociceptors act as sensory transducers for chemosensitive stimuli, temperature, and pH. They have also been demonstrated to be involved in initiating the reflex bronchospasm that defines the late asthmatic response2 and cough in human subjects and animal models.3, 4, 5, 6 Hypo-osmotic solutions have been shown to elicit sensory reflexes, including cough,7 and are a potent stimulus for airway narrowing in asthmatic patients,8, 9 and airway surface fluid composition appears to become more hypotonic in asthmatic patients.10 However, the sensory transduction mechanisms involved in regulating reflexes in response to changes in osmolarity are not known.
Transient receptor potential cation channel, subfamily V, member 4 (TRPV4) is a polymodal gated TRP channel that is activated by a diverse range of stimuli, including acidic pH, temperature, mechanical stress, the synthetic phorbol ester 4α phorbol 12,13-didecanoate (4α-PDD), and arachidonic acid metabolites (epoxyeicosatrienoic acids).11 TRPV4 can also be activated in the cellular response to hypotonicity, suggesting it might be an osmosensor, and sensing of osmotic changes by nociceptors is reduced in Trpv4−/− mice and in rats treated with TRPV4 blockers or TRPV4 anti-sense small interfering RNA.12, 13 TRPV4 is widely expressed in the respiratory tract, including the epithelium (human), macrophages (human and murine), and airway smooth muscle (human and guinea pig).14, 15, 16, 17 Furthermore, polymorphisms in the TRPV4 gene are associated with chronic obstructive pulmonary disease (COPD) phenotypes.18 However, limited information is available regarding TRPV4 expression in peripheral nociceptive neurons and in particular those that innervate the lung.
Using calcium imaging techniques, in vivo electrophysiology, an in vivo animal model of cough, and human, guinea pig, and murine bioassays, we have identified a TRPV4-ATP-P2X3 signaling pathway as a key driver of hypotonicity-induced activation of airway afferents. In vivo single-fiber electrophysiologic experiments demonstrated that both a TRPV4 agonist and a hypo-osmotic solution caused a marked and prolonged stimulation of all of the Aδ-fibers examined (both capsaicin-sensitive and insensitive fibers) but had no effect on C-fibers. Unlike the activation of fibers observed with capsaicin and citric acid, which occurred rapidly, activation caused by a TRPV4 ligand was relatively slow, which indicated an indirect mechanism of action. All the TRPV4-mediated effects were inhibited in the presence of a P2X3 antagonist, indicating a role for ATP. It has previously been demonstrated that ATP release from hypotonically or TRPV4-stimulated airway epithelial cells involves Rho-regulated opening of pannexin 1 channels,19 and we have shown this same mechanism to be operative in the TRPV4-induced activation of vagal afferents. The role of ATP in TRPV4 signaling in peripheral Aδ nociceptors is a novel finding, and the absence of TRPV4-ATP–mediated effects on C-fibers provides a distinct neurobiology for this ion channel compared with TRPV1 and TRPA1.
Methods
Additional information can be found in the Methods section in this article's Online Repository at www.jacionline.org.
Animals
Male Dunkin-Hartley guinea pigs (300-500 g; 400-800 g for single-fiber in vivo studies) and C57BL/6 mice (18-20 g) were purchased from Harlan (Bicester, Oxon, United Kingdom) or B&K (Hull, United Kingdom) and housed in temperature-controlled (21°C) rooms with food and water freely available for at least 1 week before commencing experimentation. Homozygous breeding pairs of mice genetically modified to disrupt the TRPV4 gene (Trpv4−/−) or the pannexin 1 gene (Px1−/−) were used. Experiments were performed in accordance with the UK Home Office guidelines for animal welfare based on the Animals (Scientific Procedures) Act of 1986 and the ARRIVE guidelines.20
Isolated primary airway specific vagal neurons
Cell dissociation
Guinea pigs were killed by means of injection of sodium pentobarbitone (200 mg/kg administered intraperitoneally). Nodose and jugular ganglia were dissected free of adhering connective tissue, and neurons were isolated by means of enzymatic digestion, as described previously.21, 22
Calcium imaging
Intracellular free calcium ([Ca2+]i) measurements were performed in dissociated nodose and jugular neurons and neurons projecting fibers specifically to the airways, which were identified as previously described.21, 22 The concentration-response data represent an overview of responding cells only. The criteria for a “responsive cell” was judged as an increase in [Ca2+]i of 10% or greater of the response to 50 mmol/L potassium chloride solution (K50). In each case N is defined as the number of animals, and n is defined as the number of cells tested.
Single-cell RT-PCR
Isolated nodose- and jugular-derived neurons harvested from male Dunkin-Hartley guinea pigs were placed in a Petri dish containing extracellular solution (ECS), and airway terminating (1,1′-dioctacetyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate [DiI]–stained) neurons were identified by using a Widefield Microscope (Olympus IX-71 inverted microscope; Olympus, Center Valley, Pa). Selected individual neurons were carefully harvested by using suction into the end of a custom-made glass micropipette (tip ID, 50-70 μm; OD, 2 mm; FIVEphoton Biochemicals, San Diego, Calif) manipulated into place by using a micromanipulator (Three-axis Water Hydraulic Micromanipulator MHW3; Narishige, Tokyo, Japan). The micropipette tip was then broken into a microreaction tube containing 1 μL of RNaseOUT (Life Technologies, Grand Island, NY) and placed on ice. Protocols for DNA digestion, cDNA synthesis, and PCR of selected targets were carried out based on the methodology described by Kwong et al.23 Single-cell PCR primer information can be found in Table E1 in this article's Online Repository at www.jacionline.org.
Isolated vagus nerve preparation
Guinea pigs and mice (C57BL/6 or Trpv4−/−)24, 25 were killed by means of injection of sodium pentobarbitone (200 mg/kg administered intraperitoneally). The vagus nerves were removed, and experiments were conducted in a fully characterized isolated vagus nerve preparation, as described in previous publications.5, 26 Human vagus nerve sections (n = 6, 38- to 57-year-old male donors and 38- to 68-year-old female donors with no known respiratory disease) were obtained from the International Institute for the Advancement of Medicine (Edison, NJ), and in all cases consent was granted for use in scientific research. Ethics approval was obtained from the Royal Brompton & Harefield Trust.
In vivo single-fiber preparation
Guinea pigs were anesthetized with urethane (1.5 g/kg) intraperitoneally. If required, anesthesia was supplemented with additional urethane. The trachea was cannulated with a short length of Portex tubing (Fisher Scientific UK Ltd, Loughborough, United Kingdom), and blood gases and pH were maintained at physiologic levels by means of artificial ventilation (small-animal ventilator; Ugo Basile, Varese, Italy), with a tidal volume of 10 mL/kg and 50 to 60 breaths/min of laboratory air. The right jugular vein and carotid artery (passed to the ascending aorta/aortic arch) were cannulated for injecting drugs and measuring systemic arterial blood pressure, respectively. Tracheal pressure was measured with an air pressure transducer (SenSym 647, Farnell, United Kingdom) connected to a sidearm of the tracheal cannula. Animals were paralyzed with vecuronium bromide, which was initially administered at a dose of 0.10 mg/kg intravenously, followed every 20 minutes by 0.05 mg/kg administered intravenously to maintain paralysis. Both cervical vagus nerves were located through a cervical incision and dissected free from the carotid artery and sympathetic and aortic nerves, and the nerves were cut at the central end. The left vagus nerve was used for sensory nerve fiber recording, as previously described.27
Single vagus nerve fibers were identified as originating from the major groups of airway sensory nerve endings (ie, slowly adapting stretch receptors, rapidly adapting stretch receptors [RARs], irritant receptors, and Aδ-fibers), which were further subdivided into those that were more acid and/or less capsaicin sensitive and with conduction velocities (CVs) slower than those of conventional RARs, and pulmonary/bronchial C-fiber receptors by using several criteria.27 These include pattern of spontaneous discharge, response to hyperinflation and deflation, adaptation indices, response to capsaicin/citric acid administration, and CVs. As a rule, a receptor that had no obvious pattern to the spontaneous activity (often very sparse) and did not respond to hyperinflation/hyperdeflation but did respond to capsaicin aerosol was pursued as a C-fiber. Alternatively, and in the first instance, a receptor that had a spontaneous discharge with a definite rhythmic respiratory pattern and adapted rapidly/variably to hyperinflation/deflation was pursed as an Aδ-fiber. Finally, verification of fiber type was confirmed at the end of the experiment by determining the CV.
Conscious guinea pig cough model
In all experiments the operator was blind to the treatment group. Conscious unrestrained guinea pigs were placed in individual plastic, transparent, whole-body plethysmograph chambers (Buxco, Wilmington, NC), and cough was detected, as previously described.5, 26
Details on compounds and materials can be found in the Methods section in this article's Online Repository.
Data analysis and statistics
The area under the curve (AUC) of calcium signal (calcium flux: total increase in calcium level to greater than the resting level over time) was used to measure primary neuron responses, which were normalized to calcium flux generated by application of K50. Inhibition of agonist-induced responses was analyzed by using an unpaired t test comparing the K50 percentage (AUC) with and without an antagonist. Data were analyzed for responding cells only, which were defined as a neuron with a response of 10% or greater K50, and are presented as means ± SEMs, where N indicates the number of animals and n indicates the number of cells. Inhibition of agonist-induced responses in the isolated vagus nerve preparation were analyzed by using a 2-tailed paired t test, comparing responses to agonist in the absence and presence of antagonist in the same piece of nerve. Data are presented as means ± SEMs, with statistical significance set at a P value of less than .05.
In the single-fiber experiments data were analyzed by using the paired t test to compare responses (absolute values) after stimulus with baseline values immediately preceding the response. Data are presented as means ± SEMs, with statistical significance set at a P value of less than .05.
Inhibition of fiber firing was analyzed by using a paired t test to compare responses after antagonist with control values before antagonist administration or using an unpaired t test comparing responses to vehicle control as appropriate. Statistical significance was set at a P value of less than .05.
Inhibition of cough by the TRPV4 antagonist or the P2X3 receptor antagonist in vivo was analyzed by using the Mann-Whitney U test to compare responses from the antagonist group with those from the vehicle control group. Data are presented as means ± SEMs, with statistical significance set at a P value of less than .05.
Results
Effect of TRPV4 agonists on airway-stained vagal ganglia neurons
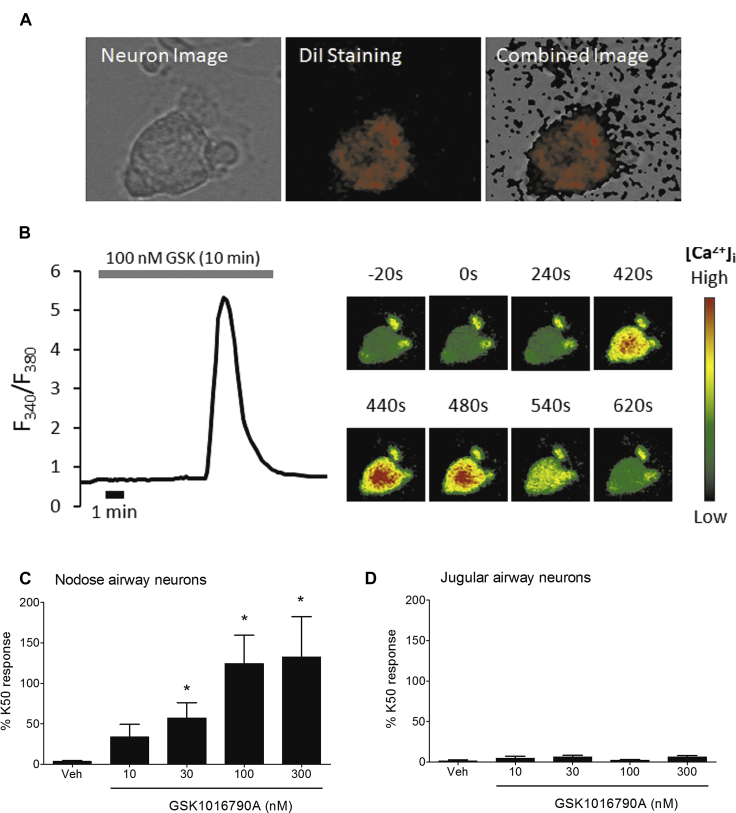
To assess the airway-specific effects of TRPV4 stimulation, we used fluorescent imaging to investigate the effects of GSK1016790A (TRPV4 agonist28) on isolated neurons from the guinea pig vagal ganglia, which were labeled with the retrograde tracer DiI (Fig 1, A). GSK1016790A induced a slow concentration-dependent increase in [Ca2+]i levels in nodose (Fig 1, B and C) but not jugular neurons (Fig 1, D). Maximum [Ca2+]i signals for the responsive nodose neurons (expressed as a percentage of K50 AUC) were 132% ± 50% at 300 nmol/L (Fig 1, C). There were a total of 36 airway nodose cells examined, of which 75% responded to GSK1016790A, and 34 jugular airway cells, of which only 12% were responsive to GSK1016790A stimulation (Fig 1, D). A similar pattern was seen in nonairway neurons: of a total of 58 nonairway nodose neuronal cells, 52% were responsive, and of the 46 nonairway jugular neurons, 20% responded to GSK1016790A stimulation.
Fig 1.
A and B, Bright-field and pseudocolor images of an airway-specific neuron stained with the retrograde tracer DiI (Fig 1, A) and the same neuron exposed to GSK1016790A (100 nmol/L) showing a [Ca2+]i level increase, with the right-hand side indicating images taken during real-time recording (Fig 1, B). C and D, Effect of GSK1016790A on [Ca2+]i levels in DiI-stained neurons for responsive-only nodose (Fig 1, C) and jugular neurons (Fig 1, D). Data are presented as means ± SEMs of 4 to 11 observations and 4 to 6 guinea pigs. *Statistical significance (P < .05) compared with relevant control.
Effect of TRPV4 agonists on vagal sensory afferents
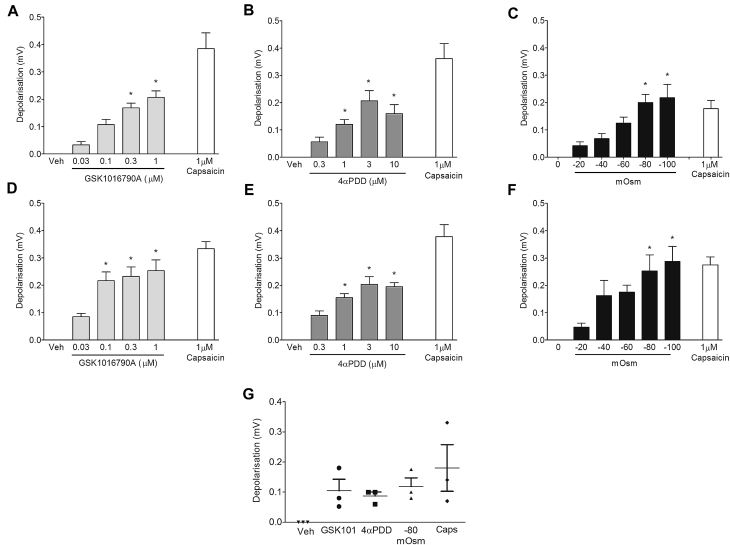
TRPV4 agonists (GSK1016790A and 4α-PDD29) induced concentration-dependent depolarization (a measure of nerve activation) of guinea pig (Fig 2, A and B) and mouse vagal sensory nerves (Fig 2, D and E), reaching a maximum of approximately 50% to 70% of the control capsaicin response. Hypo-osmotic solution also caused an osmotic strength-dependent depolarization in both the guinea pig (Fig 2, C) and mouse (Fig 2, F) vagus nerve. Because of the limited availability of human vagus nerve, only key experiments were performed. Submaximal concentrations of GSK1016790A (300 nmol/L), 4α-PDD (1 μmol/L), and hypo-osmotic solution (−80 mOsm), which were effective in the guinea pig and mouse vagus, were tested on isolated human vagus nerve and induced nerve depolarization (Fig 2, G).
Fig 2.
A-F, Effect of vehicle (0.1% DMSO), the TRPV4 agonists GSK1016790A and 4-αPDD, and hypo-osmotic solution on the activation of guinea pig (Fig 2, A-C) and mouse (Fig 2, D-F) isolated vagus nerve compared with capsaicin as a reference (n = 4-6). G, Activation of human vagus nerve by GSK1016790A (300 nmol/L), 4α-PDD (1 μmol/L), and −80 mOsm (n = 3). Data are presented as means ± SEMs. *Statistical significance (P < .05) compared with relevant control.
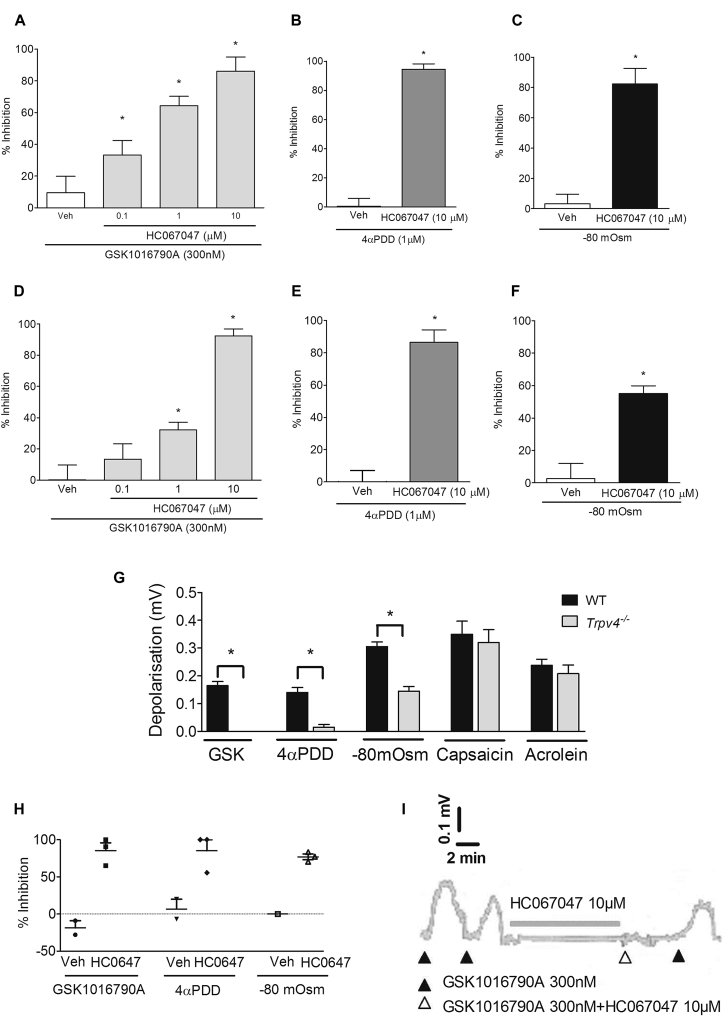
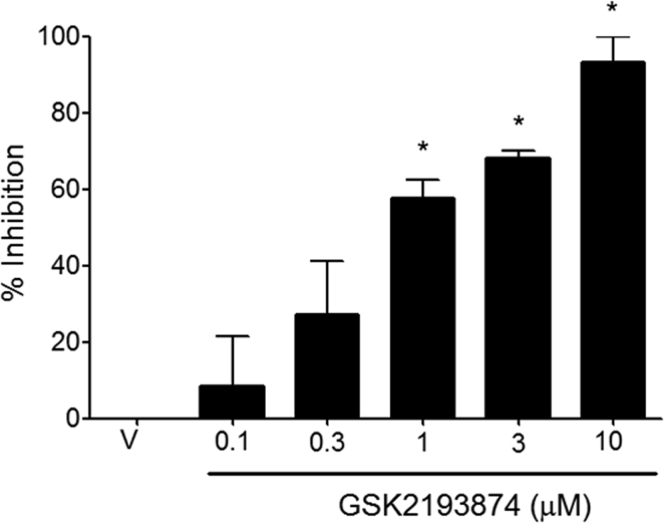
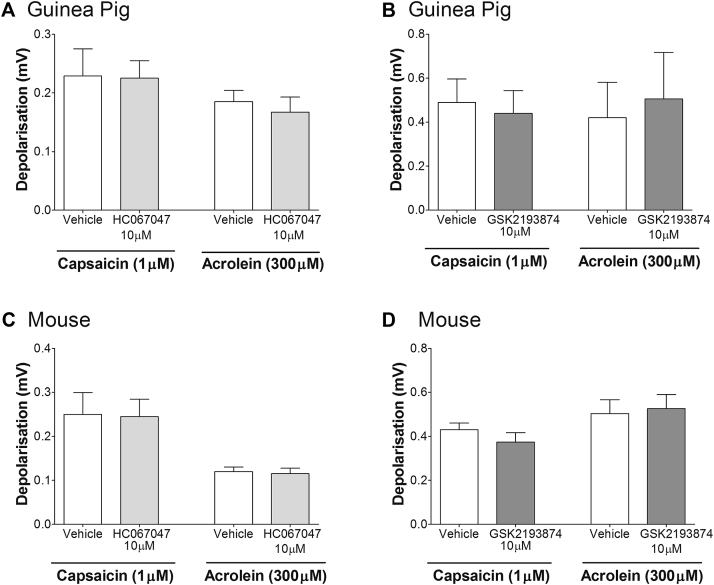
HC067047 (TRPV4 antagonist30) concentration-dependently inhibited GSK1016790A (300 nmol/L)–induced depolarization of guinea pig (Fig 3, A) and mouse (Fig 3, D) vagus. The concentration of HC067047 (10 μmol/L) producing the greatest inhibition of GSK1016790A (Fig 3, A) also inhibited depolarization evoked by 4α-PDD (1 μmol/L) and hypo-osmotic solution (−80 mOsm) in guinea pig (Fig 3, B and C, respectively) and mouse vagus nerve (Fig 3, E and F, respectively). Another TRPV4 antagonist, GSK2193874,31 also inhibited GSK1016790A (300 nmol/L)–induced depolarization in a concentration-dependent manner in guinea pig vagus with a similar potency to HC067047 (see Fig E1 in this article's Online Repository at www.jacionline.org). Neither GSK2193874 (10 μmol/L) nor HC067047 (10 μmol/L) had any effect on capsaicin- or acrolein-induced depolarization in guinea pig or mouse vagus (see Fig E2 in this article's Online Repository at www.jacionline.org). Depolarization of the vagus induced by GSK1016790A, 4α-PDD, and hypo-osmotic solution was reduced in Trpv4−/− mice, whereas responses to TRPV1 (capsaicin) and TRPA1 (acrolein) agonists were unaffected (Fig 3, G). Genetic knockdown of the murine TRPV4 gene in the Trpv4−/− colony was verified by means of genotyping. Finally, the TRPV4 antagonist (HC067047) inhibited depolarization evoked by GSK1016790A, 4α-PDD, and hypotonicity in human vagus (Fig 3, H and I).
Fig 3.
A-F, H, and I, Effect of the TRPV4 antagonist (HC067047) on either the TRPV4 agonists GSK1016790A (300 nmol/L)– or 4α-PDD (1 μmol/L)–induced or hypo-osmotic solution (–80 mOsm)–induced depolarization of guinea pig (Fig 3, A-C; n = 4-6), mouse (Fig 3, D-F; n = 4-6), and human (Fig 3, H) isolated vagus nerve (n = 2-3; example trace is shown in Fig 3, I). G, Depolarization of the vagus from Trpv4−/− and wild-type (WT) control mice to GSK1016790A (300 nmol/L), 4α-PDD (1 μmol/L), hypo-osmotic solution (−80 mOsm), capsaicin (1 μmol/L), and acrolein (300 μmol/L; n = 4-6). Data are presented as means ± SEMs. *Statistical significance (P < .05) compared with relevant control.
Fig E1.
Concentration-response curve of GSK2193874- against GSK1016790A (300 nmol/L)–induced depolarization in the guinea pig (N = 4). *P > .05, t test comparing agonist and antagonist responses in the same piece of nerve. V, Vehicle.
Fig E2.
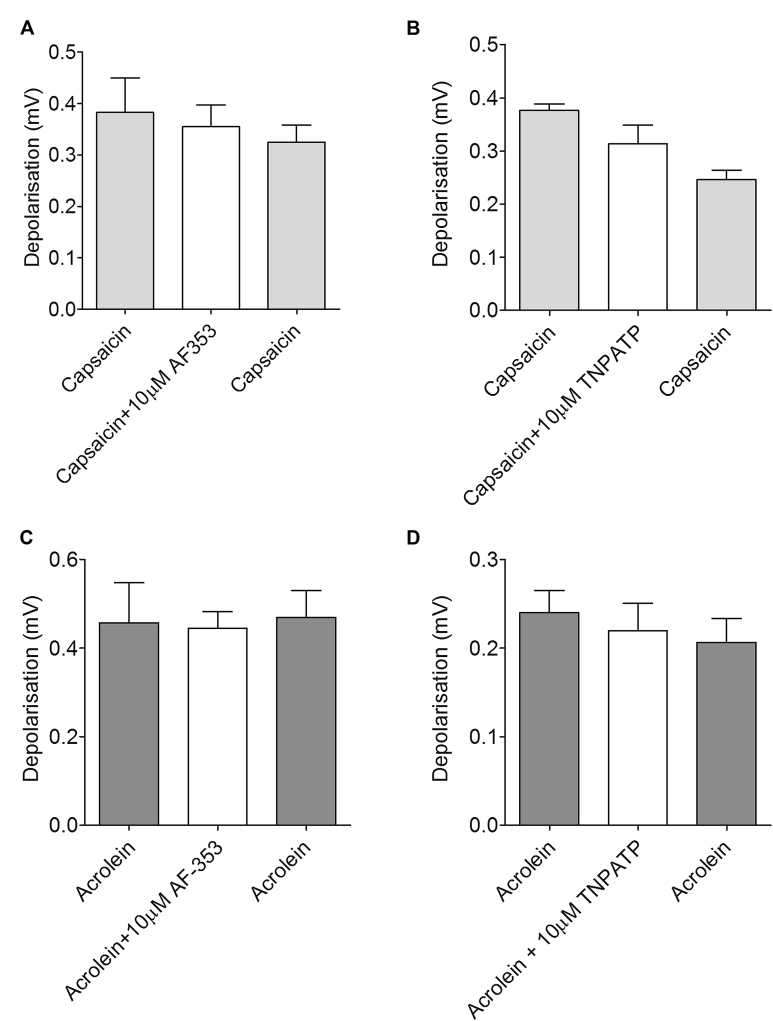
A and B, Neither HC67047 (10 μmol/L; Fig E2, A) nor GSK2193874 (10 μmol/L; Fig E2, B) had any effect on TRPV1 (capsaicin)– or TRPA1 (acrolein)–induced depolarization in the guinea pig. C and D, A similar result was seen in mouse vagus. Data are shown as means ± SEMs of 3 to 6 observations.
Effect of TRPV4 agonists on action potential firing in guinea pig Aδ-fibers
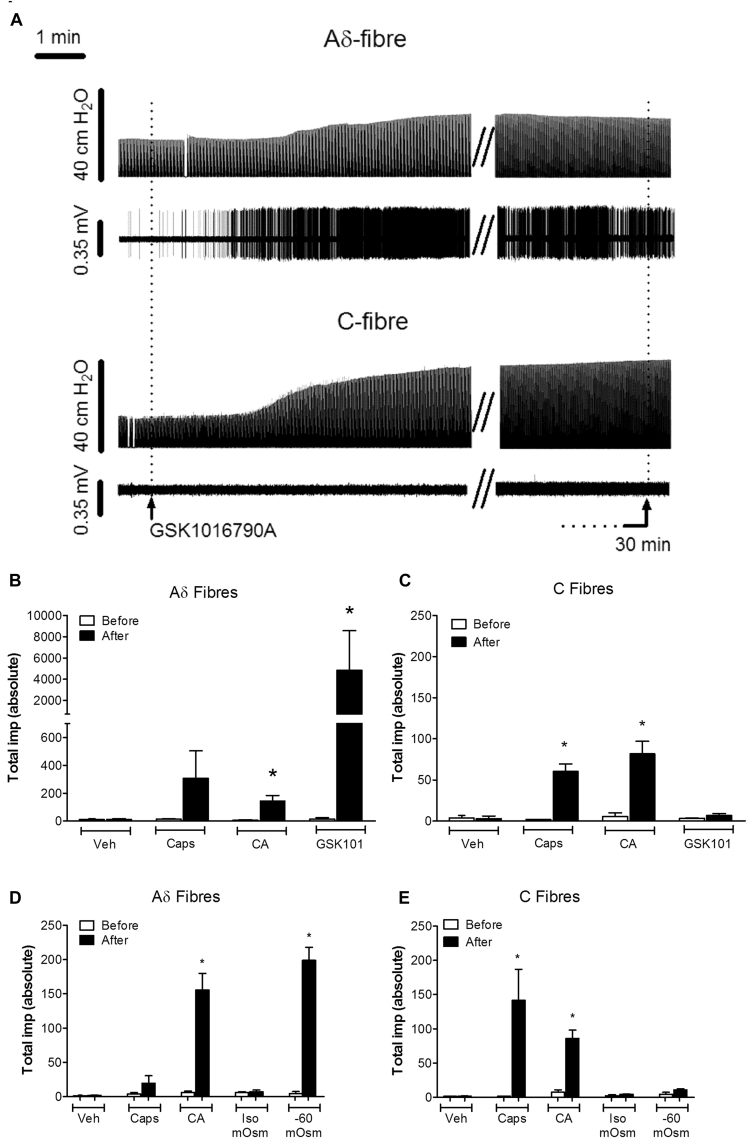
When administered by means of aerosol to anesthetized guinea pigs, capsaicin (100 μmol/L for 15 seconds) activated 2 of the 3 Aδ-fibers (CV range, 2.3-7.14 m/s) and all 5 of the C-fibers (CV range, 0.57-0.92 m/s; could not distinguish between bronchial or pulmonary C-fibers) examined. Capsaicin also evoked bronchoconstriction in all guinea pigs examined, which was preceded by activation of the fiber under examination (except for 1 Aδ fiber). Moreover, citric acid (0.3 mol/L) aerosol activated all of the fibers examined (Fig 4, B and C). In contrast to capsaicin, citric acid did not cause bronchoconstriction. Aerosol administration of GSK1016790A (10 μg/mL) caused a marked prolonged stimulation of all of the Aδ-fibers examined (ie, total impulses increased from 15.3 ± 8.7 to 4854 ± 3708; n = 3) but had no effect on the C-fibers (Fig 4, A-C). Unlike the activation of Aδ-fibers observed with capsaicin and citric acid, which occurred within 35 seconds of administration, that caused by GSK1016790A did not commence until 101.5 ± 21.2 seconds. In addition, although the activation of Aδ-fibers lasted no longer than 3.5 to 4.0 minutes after capsaicin and citric acid, the fibers were still firing vigorously at 30 minutes after GSK1016790A (Fig 4, A). GSK1016790A also caused marked bronchoconstriction with a slow onset and prolonged duration, which ensued after the Aδ-fibers had been activated. Vehicle administration alone had no effects on the firing of either the Aδ- or C-fibers examined.
Fig 4.
A, Trace indicates changes in tracheal pressure (top) and action potential firing (bottom) of Aδ- and C-fibers in response to GSK1016790A (10 μg/mL). B and C, Total impulses were significantly increased after application of citric acid (CA; 0.3 mol/L) and GSK1016790A (10 μg/mL) in Aδ-fibers (Fig 4, B), but only capsaicin (Caps; 100 μmol/L) and citric acid (0.3 mol/L) significantly increased firing in C-fibers (Fig 4, C). D and E, Hypo-osmotic solution (−80 mOsm) significantly increased total impulses in Aδ-fibers but had no effect on C-fibers (Fig 4, D and E, respectively). Data are presented as means ± SEMs of 3 or 4 observations. Veh, Vehicle. *Statistical significance (P < .05), paired t test comparing responses before and after aerosol administration of agonist.
In an independent series of experiments to examine the effects of hypo-osmotic solutions, 4 separate Aδ-fibers (CV range, 4.7-11.3 m/s) and 3 C-fibers (CV range, 0.47-0.85 m/s) were used. Capsaicin activated 2 of the 4 Aδ-fibers and the 3 C-fibers, whereas citric acid activated all fibers. Similar to GSK1016790A, aerosol administration of hypo-osmotic solution (−80 mOsm) caused stimulation of all of the Aδ-fibers examined: total impulses increased from 10.33 ± 2.7 to 203.7 ± 24.5 (n = 3, P < .05) but had no effect on C-fibers (Fig 4, D and E). Control isosmotic solution administration alone had no effect on the firing of either the Aδ- or C-fibers examined.
Mechanism involved in TRPV4-induced activation of airway sensory nerves
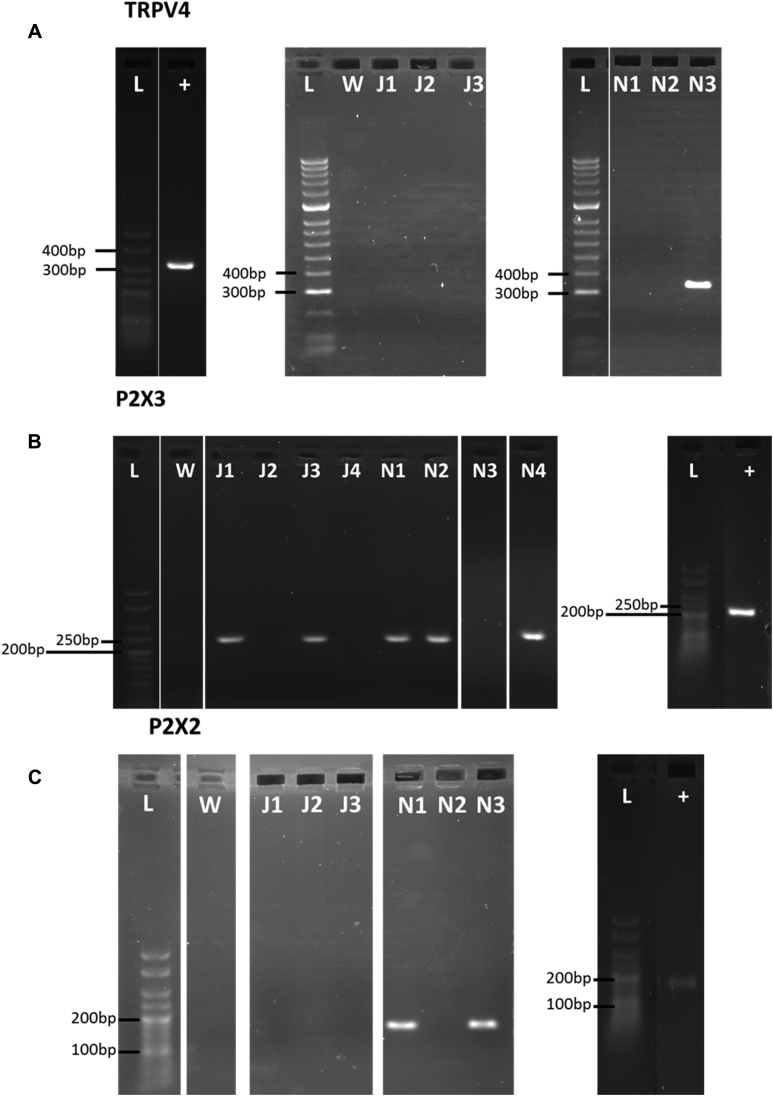
To determine whether TRPV4, P2X2, and P2X3 were expressed in individual neurons, single-cell RT-PCR was carried out on isolated airway terminating nodose and jugular neurons. TRPV4 was not expressed in any of the jugular neurons examined (0/30 cells) but was expressed in 1 of 32 nodose neurons. P2X2 (without P2X3 coexpression) was expressed in 0 of 30 jugular neurons and 2 of 32 nodose neurons. P2X3 (without P2X2 coexpression) was expressed in 22 of 30 jugular neurons and 6 of 32 nodose neurons. P2X2 and P2X3 were coexpressed in 1 of 30 jugular neurons and 11 of 32 of nodose neurons. Of interest, the single nodose TRPV4-positive cell coexpressed P2X2 (but not P2X3). Incidentally, the 1 neuron identified as positive for TRPV4 was confirmed after a second assay using the same primers, indicating the possibility that TRPV4 is expressed on sensory afferents but that its expression is sparse. Both jugular (N = 8, n = 30) and nodose (N = 8, n = 32) neurons examined expressed both β-actin and PGP9.5 (examples are shown in Fig E3 in this article's Online Repository at www.jacionline.org).
Fig E3.
A, Representative examples of electrophoresis gels for PCR products generated by TRPV4 primers in separate jugular- and nodose-derived neurons. TRPV4 was expressed in 0 of 30 jugular neurons and 1 of 32 nodose neurons. The positive control tissue for TRPV4 was the kidney. B, Representative examples of electrophoresis gels for PCR products generated by P2X3 primers in separate jugular- and nodose-derived neurons. P2X3 was expressed in 23 of 30 cells from the jugular and 17 of 32 cells from the nodose ganglia. The positive control tissue for P2X3 was the trigeminal ganglia. C, Representative examples of electrophoresis gels for PCR products generated by P2X3 primers in separate jugular- and nodose-derived neurons. P2X2 was expressed in 1 of 30 jugular neurons and 13 of 32 of nodose neurons. All cells analyzed expressed both PGP9.5 and β-actin. +, Positive control; J, jugular-derived neurons; L, ladder; N, nodose-derived neurons; W, water control.
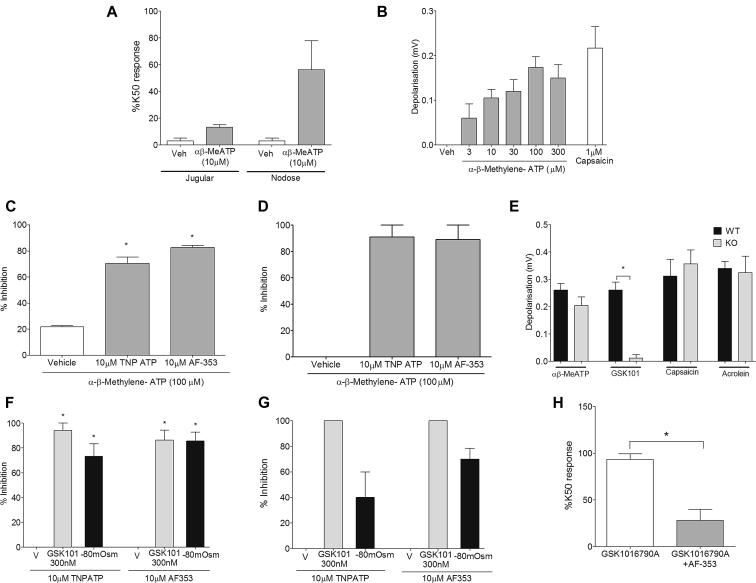
The effect of αβ-methylene-ATP (αβ-MeATP), a more stable agonist at P2X1 and P2X3 receptors, on the activation of vagal sensory nerves was examined to determine whether ATP is able to directly activate airway sensory nerves. αβ-MeATP (10 μmol/L) induced an increase in [Ca2+]i levels predominantly in airway-specific neurons from nodose ganglia in the guinea pig (Fig 5, A) and was also able to cause a concentration-dependent depolarization of the guinea pig vagus nerve (Fig 5, B). The agonist also caused firing of Aδ-fibers but had no effect on bronchospasm (see Fig E4 in this article's Online Repository at www.jacionline.org). This activation was shown to be specific to P2X3 because depolarization was inhibited with the P2X2 and P2X3 inhibitors TNP-ATP32 (10 μmol/L) and AF-35333 (10 μmol/L) in both guinea pig (Fig 5, C) and human (Fig 5, D) vagus, but these inhibitors did not block responses to capsaicin and acrolein (see Fig E5 in this article's Online Repository at www.jacionline.org). Activation of TRPV4 has been hypothesized to cause ATP release and subsequent activation of P2X3.34 Both inhibitors were also shown to inhibit TRPV4-specific depolarization because TNP-ATP (10 μmol/L) and AF-353 (10 μmol/L) inhibited depolarization induced by GSK1016790A and hypo-osmotic (−80 mOsm) solution in guinea pig (Fig 5, F) and human (Fig 5, G) vagus. In addition, depolarization induced by GSK1016790A was abolished in Px1−/− mice, whereas responses to αβ-MeATP (10 μmol/L), capsaicin (1 μmol/L), and acrolein (300 μmol/L) remained unchanged, suggesting that TRPV4 is dependent on pannexin 1 to induce depolarization in the mouse (Fig 5, E). Furthermore, AF-353 (10 μmol/L) significantly inhibited the [Ca2+]i signal induced by GSK1016790A (30 nmol/L) in airway-specific neurons from nodose ganglia. Calcium responses, expressed as a percentage of K50 signal (AUC), induced by GSK1016790A (30 nmol/L) were significantly reduced from 93% ± 6.2% with vehicle control to 28% ± 11.9% after incubation with AF-353 (Fig 5, H).
Fig 5.
A and B, αβ-MeATP (10 μmol/L) caused an increase in [Ca2+]i levels predominantly from guinea pig airway–specific nodose ganglion neurons (Fig 5, A) and a concentration-dependent depolarization of the guinea pig vagus nerve (Fig 5, B). C and D, This response was inhibited by the P2X3 antagonists AF-353 (10 μmol/L) and TNP-ATP (10 μmol/L) in both guinea pig (Fig 5, C) and donor human (Fig 5, D) vagus. E, Responses to GSK1016790A (300 nmol/L) were virtually abolished in Px1−/− mice. KO, Knockout; WT, wild-type. F and G, Both GSK1016790A- and −80 mOsm–induced depolarization in guinea pig (Fig 5, F) and donor human (Fig 5, G) vagus was inhibited by AF-353 (10 μmol/L) and TNP-ATP (10 μmol/L). H, [Ca2+]i signal induced by GSK1016790A (30 nmol/L) in the guinea pig airway–specific nodose ganglia neurons was also inhibited by AF-353 (10 μmol/L; N = 3; n = 4). Data are presented as means ± SEMs of 4 to 6 observations for guinea pig and 2 to 3 observations for human experiments. *Statistical significance (P < .05), unpaired t test comparing responses in Px1−/− vagus with wild-type control or GSK1016790A-induced [Ca2+]i responses in nodose neurons.
Fig E4.
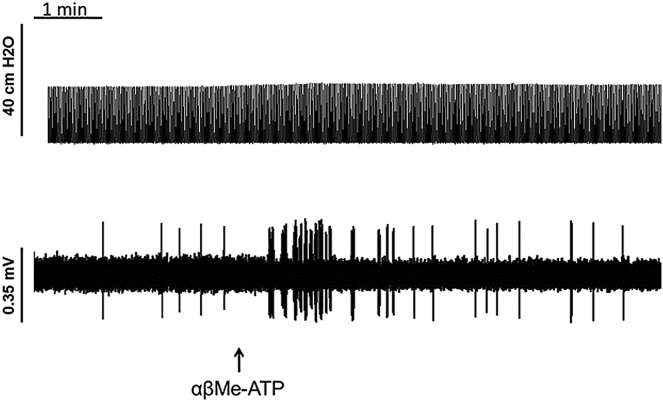
Example trace from an Aδ-fiber where the top panel indicates tracheal pressure (cm H2O) and the bottom panel indicates single-fiber firing. αβ-MeATP (300 μmol/L) caused firing in Aδ-fibers but had no effect on bronchoconstriction.
Fig E5.
Neither AF-353 (10 μmol/L) nor TNPATP (10 μmol/L) had any effect on capsaicin (A and B)– or acrolein (C and D)–induced depolarization in guinea pig vagus. Data are shown as means ± SEMs of n = 3 to 6 observations.
Effect of P2X3 antagonists on activation of airway sensory nerves by TRPV4 in vivo
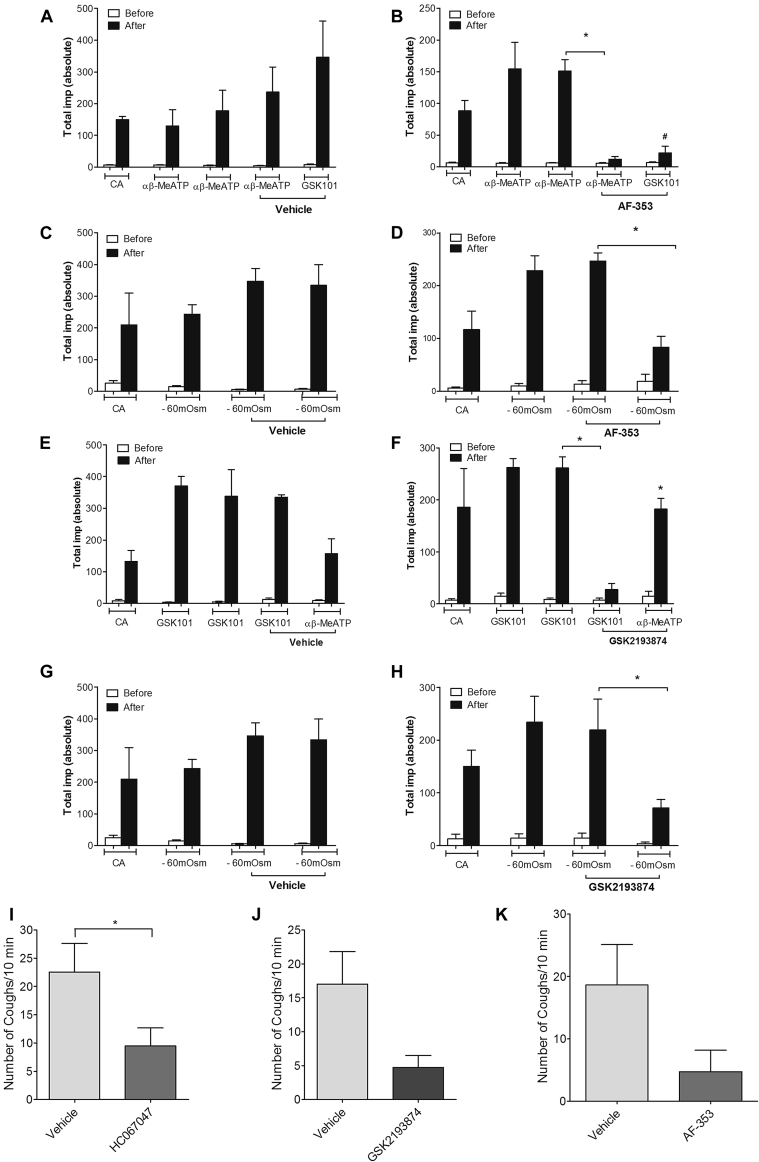
For antagonist studies, only Aδ-fibers were investigated. In all cases citric acid activated the nerves under investigation and was used as an initial test for nerve fiber viability. To investigate the role of the P2X3 antagonist AF-353 on Aδ firing induced by αβMe-ATP, GSK1016790A, and hypo-osmotic solution, Aδ-fibers (n = 12) were investigated, with CVs ranging from 3.8 to 14.1 m/s. After vehicle administration (example trace is available in Fig E6, A, in this article's Online Repository at www.jacionline.org), total impulses induced after aerosol administration of the P2X1/3 agonist αβ-MeATP (300 μmol/L) remained unchanged from 177.0 ± 64.73 impulses before vehicle administration to 236.0 ± 79.38 impulses afterward (Fig 6, A). Total impulses induced by GSK1016790A (100 ng/mL) after vehicle were 346 ± 114.9 (Fig 6, A). After administration of the P2X3 antagonist AF-353 (30 mg/kg; example trace is available in Fig E6, B), total impulses induced by the P2X1/3 agonist αβ-MeATP (300 μmol/L) was significantly reduced from 151.0 ± 18.36 to 12 ± 4.51 impulses, and GSK1016790A firing was also significantly reduced to 22 ± 10.44 impulses compared with vehicle control (Fig 6, B, and see Fig E6, B). Total impulses induced by hypo-osmotic solution were also significantly inhibited after application of the P2X2/3 antagonist AF-353, where total impulses were reduced from 247.0 ± 15.37 to 83.67 ± 20.92 after antagonist administration. Vehicle had no effect on firing (Fig 6, C and D). Treatment with AF-353 (30 mg/kg administered intraperitoneally) had no effect on capsaicin (100 μmol/L)–induced firing (total impulses, 150 ± 58.01 to 209 ± 40.53 after AF-353 administration; n = 2).
Fig E6.
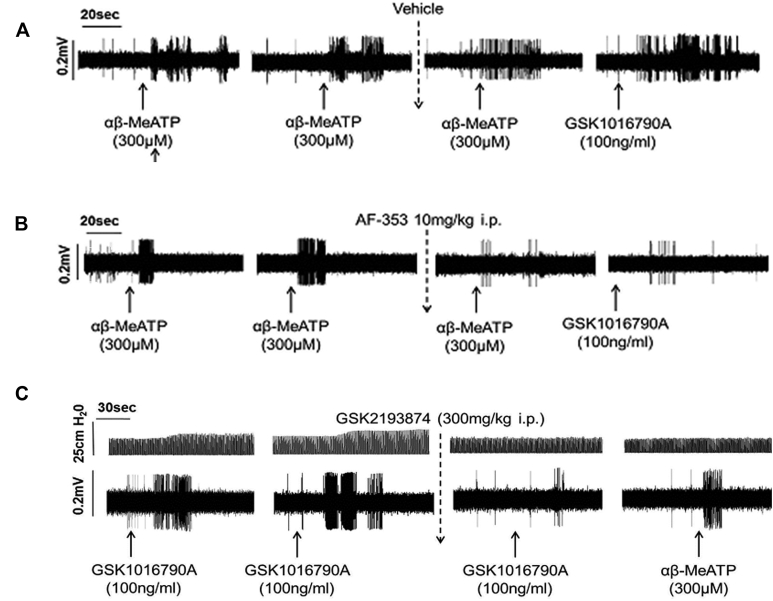
A, Example vehicle trace. After 2 reproducible responses to αβ-MeATP, vehicle (saline in 10% PEG400) was administered, and the following nerve responses to αβ-MeATP or GSK1016790A remained unaffected. B, Conversely, after AF-353 administration, αβ-MeATP response was significantly reduced, as was the response to GSK1016790A compared with vehicle control. C, Example trace after GSK2193874 administration where nerve firing to GSK1016790A was reduced; however, there was no effect on αβ-MeATP–induced firing.
Fig 6.
A-D, Effect of vehicle (10% PEG in saline, 10 mL/kg administered intraperitoneally) or AF-353 (30 mg/kg administered intraperitoneally) on firing of Aδ-fibers induced by αβ-MeATP (aerosolized at 300 μmol/L for 1 minute; shown in Fig 6, A and B, respectively) or hypo-osmotic solution (−80 mOsm aerosolized for 1 minute; Fig 6, C and D). E-H, Effect of vehicle (6% Cavitron in saline, 10 mL/kg administered intraperitoneally) or GSK2193874 (300 mg/kg administered intraperitoneally) on firing of Aδ-fibers induced by GSK1016790A (aerosolized at 100 ng/mL for 1 minute) or αβ-MeATP or hypo-osmotic solution (all n = 3). I-K, Effect of vehicle, TRPV4 (HC067047, 100 mg/kg; GSK2193874, 300 mg/kg administered intraperitoneally), or P2X3 (AF-353, 30 mg/kg administered intraperitoneally) antagonists on cough induced by the TRPV4 agonist GSK1016790A (30 μg/mL, aerosolized for 5 minutes) is shown, and coughs were counted for 10 minutes. Data were expressed as means ± SEMs. *Statistical significance (P < .05) compared with relevant control (n = 8-12). #Statistical significance (P < .05) comparing GSK1016790A to vehicle control.
The TRPV4 antagonist GSK2193874 was also investigated against GSK1016790A-, αβMe-ATP–, and hypo-osmotic solution–induced firing of Aδ-fibers. Aδ-fibers (N = 12) were investigated, with CVs ranging from 3.2 to 14.3 m/s. After vehicle administration, the total number of impulses induced by the TRPV4 agonist GSK1016790A (100 ng/mL) was unchanged from 337.7 ± 84.41 to 335 ± 7.64 (Fig 6, E). The total number of impulses induced by the P2X1/3 agonist αβMe-ATP (300 μmol/L) administered at the end of the experiment was 156.7 ± 46.70 (Fig 6, E). After administration of the antagonist GSK2193874 (300 mg/kg; example trace shown in Fig E6, C), the total number of impulses induced by GSK1016790A (100 ng/mL) was significantly reduced from 261.7 ± 20.92 to 27.67 ± 11.55 impulses (Fig 6, F). Firing induced by αβMe-ATP (300 μmol/L) remained unchanged at 182.3 ± 20.30 impulses compared with vehicle control (Fig 6, F). Similarly, the total number of impulses induced by hypo-osmotic solution was significantly reduced from 219.3 ± 58.46 to 71.67 ± 16.13 (Fig 6, H). Vehicle had no effect on firing (Fig 6, G). Treatment with GSK2193874 (300 mg/kg administered intraperitoneally) had no effect on capsaicin-induced firing (ie, total impulses induced by capsaicin were not significantly changed from 130 ± 35.81 to 177 ± 34.51 after GSK2193874 administration; n = 3).
Aerosolized GSK1016790A, but not vehicle, induced concentration-related coughing in conscious unrestrained guinea pigs (see Fig E7 in this article's Online Repository at www.jacionline.org). The type of coughs induced were a combination of small tussive trains similar to those seen after stimulation with prostaglandin E2 or bradykinin and large single explosive coughs normally induced by agonists, such as acrolein and capsaicin.21 HC067047 (100 mg/kg administered intraperitoneally) significantly inhibited coughing induced by a submaximal concentration of GSK1016790A agonist (3 μg/mL; Fig 6, I). An alternate antagonist, GSK2193874 (300 mg/kg), also inhibited the number of coughs induced by GSK1016790A (30 μg/mL; 6J). The P2X3 antagonist AF-353 (30 mg/kg administered intraperitoneally) inhibited cough induced by the GSK1016790A (30 μg/mL) in the guinea pig to a level similar to that seen with the TRPV4 antagonist (Fig 6, K). Neither AF-353 (30 mg/kg administered intraperitoneally) nor GSK 2193874 (300 mg/kg administered intraperitoneally) were shown to have any effect on capsaicin (60 μmol/L; aerosolized for 5 minutes and coughs counted for 10 minutes)–induced cough. Mean cough counts were not significantly changed from 8.5 ± 3.6 after intraperitoneal vehicle administration versus 6.5 ± 2.8 after GSK2193874 and 7.6 ± 1.9 after AF-353 (n = 8 in each group). Capsaicin-induced cough using the same protocol has previously been shown to be completely inhibited by a TRPV1 receptor antagonist.21
Fig E7.
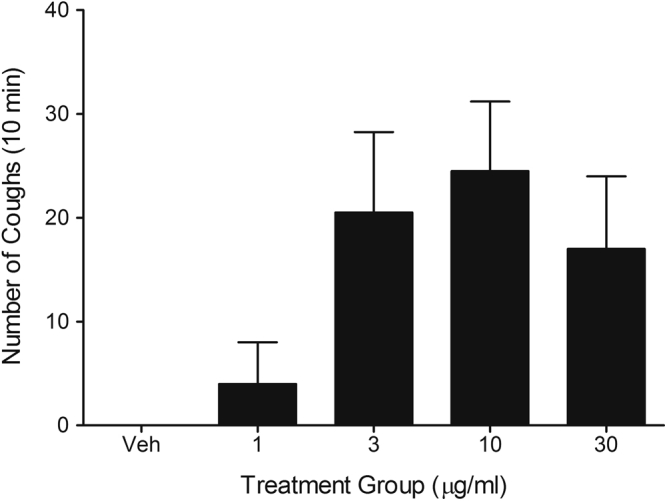
Aerosol of GSK1016790A caused a concentration-dependent increase in the number of coughs in the conscious guinea pig. Vehicle had no effect on coughing. Data are shown as means ± SEMs of 4 observations.
Discussion
The sensory transduction mechanisms involved in regulating reflexes in response to changes in airway osmolarity are not known. TRPV4 has previously been shown to be an osmosensor12, 13 and is widely expressed in the respiratory tract.14, 15, 16, 17, 35 However, not much is known regarding the role of TRPV4 as a peripheral nociceptor in the airway.
To investigate this, we used the guinea pig as a model species because it is widely used for investigations into airway sensory nerve biology and because guinea pigs cough in a similar fashion to human subjects in response to a range of tussive agents.3, 4, 5
In the present study we investigated TRPV4 expression in peripheral nociceptive neurons and in particular those that innervate the lung. Although TRPV4 expression has previously been demonstrated in peripheral sensory nerves with cell bodies located in the trigeminal ganglia36 and dorsal root ganglia,37 it has not been identified in neurons from the vagal ganglia. To assess the airway-specific effects of TRPV4 stimulation, we used fluorescent imaging in isolated guinea pig vagal ganglia neurons, which were labeled with the retrograde tracer DiI. GSK1016790A (TRPV4 agonist) induced concentration-dependent increases in [Ca2+]i levels in nodose but not jugular neurons, and GSK1016790A, 4α-PDD, and hypo-osmotic solution induced depolarization of guinea pig, mouse, and human vagal sensory nerves. These effects were inhibited by the TRPV4 antagonists and reduced in vagus from Trpv4−/− mice (acrolein and capsaicin responses were unaffected). Confirming the activity on airway-specific afferents, we used in vivo single-fiber electrophysiologic experiments. Aerosol administration of GSK1016790A and hypo-osmotic solution caused a marked prolonged stimulation of all of the Aδ-fibers examined (both capsaicin-sensitive and insensitive fibers) but had no effect on the C-fibers. This is in contrast to a previous study in which TRPV4 and hypo-osmolar solutions activated saphenous nerve C-fibers in the rat.13 It is not clear why we did not see a similar profile of C-fiber activation, but perhaps anatomically distinct afferent nerve fibers and different species account for this difference. In addition, although the activation of Aδ-fibers was relatively short lived after capsaicin and citric acid, the fibers were still firing vigorously at 30 minutes after GSK1016790A administration. GSK1016790A also caused marked bronchoconstriction with a slow onset and prolonged duration that ensued after the Aδ-fibers had been activated. Although the bronchospasm occurred after the initiation of fiber firing, we cannot rule out the possibility that firing of Aδ-fibers was secondary to bronchospasm. However, the fact that GSK1016790A evoked compound depolarization of the vagus nerve and increased the [Ca2+]i signal in the vagal ganglia, which are systems free from the influence of bronchospasm, suggests that the firing is independent of bronchospasm. Furthermore, aerosolized GSK1016790A induced cough in conscious unrestrained guinea pigs, which was inhibited by both TRPV4 antagonists (HC067047 and GSK2193874).
It has previously been reported that TRPV4 regulates urothelial ATP release to modulate mechanosensitive bladder afferent nerves.34, 35, 36, 37, 38 Furthermore, TRPV4 has been shown to release ATP from human airway macrophages and epithelial cells.17 In these studies we have established a role for ATP and the P2X3 receptor in TRPV4-mediated activation of airway afferents. We have demonstrated expression of P2X3 and P2X2, and αβ-MeATP caused a [Ca2+]i level increase in guinea pig nodose ganglion neurons, which is in agreement with previous studies.23, 39 αβ-MeATP was also able to depolarize guinea pig and human vagus nerves, and both the increase in calcium levels and the vagal depolarization evoked by αβ-MeATP were inhibited by the P2X1 and P2X3 inhibitor TNP-ATP and the P2X3 inhibitor AF-353. Both inhibitors were also shown to inhibit depolarization induced by TRPV4 ligands and hypo-osmotic solution in guinea pig and human vagus, confirming the hypothesis that TRPV4- and hypotonicity-mediated activation of vagal afferents is mediated by the release of ATP and activation of P2X3 receptors. In vivo single-fiber recording experiments were performed to confirm the role of ATP/P2X3 in the TRPV4- and hypotonicity-induced activation of Aδ airway-specific afferents. Unlike the activation of Aδ-fibers observed with capsaicin and citric acid, which occurred rapidly, activation caused by GSK1016790A was relatively slow, which indicated an indirect mechanism of action. This firing was inhibited after administration of the TRPV4 antagonist GSK2193874. In addition, the P2X3 antagonist AF-353 decreased the firing frequency to αβ-MeATP and GSK1016790A. Furthermore, the P2X3 antagonist AF-353 inhibited cough induced by GSK1016790A in the guinea pig to a similar level to that observed with the TRPV4 antagonist. These studies have identified the TRPV4-ATP-P2X3 axis as a key driver of hypotonicity-induced activation of airway afferents. Although the TRPV4 and P2X3 receptor antagonists inhibited the majority of the response to hypotonicity in most of the systems used, the small residual response might be mediated by an alternative mechanism.
It is not clear what mechanisms are important in TRPV4-induced ATP release. Interestingly, 2 recent studies in human epithelial cells have suggested that TRPV4 plays a role in the release of ATP and that this is associated with opening of the pannexin 1 channel.17, 19 Studies were performed in vagus obtained from pannexin knockout (Px1−/−) and wild-type mice to further investigate the signaling mechanism. The TRPV4 agonist response was absent in Px1−/− mice, whereas responses to αβ-MeATP, capsaicin, and acrolein remained unchanged, suggesting that TRPV4 is dependent on activation of the pannexin 1 pore and subsequent release of ATP to induce depolarization in the mouse vagus. It is also not certain what cell type is important in TRPV4-induced ATP release in vivo. However, in single-cell PCR studies we only demonstrated TRPV4 expression in 1 of 32 nodose ganglion neurons, suggesting that although TRPV4 can release ATP from neuronal cells, it is more likely to be coming from an alternative cell type in close proximity. Furthermore, TRPV4 expression has been demonstrated in neuron-associated cells (eg, glia40), and in our studies, in some cases, an accessory or satellite cell was observed in close proximity to the DiI neuron being imaged (Fig 1, A and B). In addition, TRPV4 can also induce ATP release from several diverse cell types in the airway (eg, epithelial cells and macrophages17), and therefore other sources of ATP cannot be ruled out in the in vivo situation. Interestingly, TRPV4 is expressed on airway smooth muscle, and we and others have shown that it causes bronchospasm,15 which could theoretically lead to indirect activation of Aδ-fibers, action potential generation, and cough. However, these data do not suggest that bronchospasm per se activates Aδ-fibers given that the effect is also inhibited by the P2X3 antagonist and P2X3 is almost exclusively expressed on sensory nerves. However, it is still possible that TRPV4-induced bronchoconstriction could be responsible for ATP release, which then leads to Aδ-fiber activation. However, it is also possible that TRPV4 might elicit ATP release from other cell types (eg, macrophages and epithelial cells) in the appropriate location independent of bronchospasm.
Another interesting finding from this study was the absence of TRPV4-ATP–mediated effects on C-fibers, providing a distinct neurobiology for this target compared with TRPV1/TRPA1, although it is acknowledged that ATP can directly activate both C-fibers and Aδ-fibers independent of TRPV4 activation. This work is of particular interest given recent data suggesting that the P2X3 receptor antagonist AF-219 inhibited cough frequency in patients with refractory chronic cough, an effect for which these studies might provide a potential mechanistic explanation.41 It is not yet clear whether chronic cough in other patient groups involves release of ATP and activation of P2X3 receptors and whether this would happen in a TRPV4-dependent manner.
Chronic cough is associated with many respiratory diseases (eg, asthma, COPD, pulmonary fibrosis, bronchiectasis, and lung cancer) in addition to refractory chronic cough.42 Increased levels of extracellular ATP have been reported in the airways of asthmatic patients and patients with COPD,43, 44 and polymorphisms in the TRPV4 gene have been associated with COPD phenotypes,18 suggesting there might be some rationale for this mechanism being operative in cough associated with asthma and COPD. Current treatment options for chronic cough are limited, and a recent systematic review concluded that over-the-counter remedies lack evidence of effectiveness.45 Furthermore, cough is the most frequent reason for consultation with a family doctor or a general or respiratory physician.46 Despite these facts, cough is a largely ignored research area, and novel antitussive agents are required.
In summary, this study has identified the TRPV4-ATP axis as the key osmosensor involved in initiation of airway sensory nerve reflexes. The absence of TRPV4-ATP–mediated effects on C-fibers provides a distinct neurobiology for this target compared with TRPV1/TRPA1 and as such presents a distinct target for possible antitussive therapies. It is not clear whether TRPV4 antagonists will be more or less effective than TRPV1 or TRPA1 antagonists or whether they will address different cough phenotypes. However, human tussive challenge studies might shed more light on this question if certain patient groups respond more to some tussive agents rather than others (eg, hypo-osmolar solutions indicative of TRPV4-driven cough hypersensitivity vs capsaicin, which is indicative of TRPV1). The effectiveness of the P2X3 antagonist (AF-219) in patients with chronic idiopathic cough might suggest that TRPV4 could be an effective alternative therapy in this patient group, although we await clinical trials to support or reject this hypothesis.
Key messages.
-
•
Inhalation of hypo-osmolar solutions provokes bronchospasm and cough in asthmatic patients, but the mechanisms involved are unknown.
-
•
For the first time, we have identified the TRPV4-ATP-P2X3 axis as a key osmosensor expressed on Aδ nociceptors but not on C-fiber afferents, providing a distinct neurobiology for this channel compared with TRPV1 and TRPA1.
-
•
These observations are of therapeutic importance given data reported from a recent clinical study that showed an unprecedented effect of a P2X3 receptor antagonist on daytime cough rate and cough severity in patients with treatment-resistant chronic cough.
Acknowledgments
Homozygous breeding pairs of mice genetically modified to disrupt the TRPV4 gene (Trpv4−/−; RBRC no. 01939) were obtained from Riken BioResource Center (Tsukuba, Japan). Mice devoid of the pannexin 1 gene (Px1−/−) were a kind gift from Dr Vishva Dixit (Genentech, South San Francisco, Calif). The TRPV4 antagonist GSK2193874 was a kind gift from Almirall (Barcelona, Spain), and the P2X3 inhibitor AF-353 was kindly provided by Afferent Pharmaceuticals (San Mateo, Calif).
Footnotes
Supported by S.A.M., M.S.G., E.D. were funded by project grants from the Medical Research Council (MRC, UK; M.S.G.: G0800195; S.A.M. and E.D.: MR/K020293/1). M.A.W., Y.-M.C., and S.J.B. were supported on an MRC studentship, an MRC/Asthma UK Centre studentship, and the National Heart and Lung Institute, respectively. M.A.W. was also funded by the North West Lung Centre Charity. The human vagus experiments in this study were undertaken with the support of the NIHR Respiratory Disease Biomedical Research Unit at the Royal Brompton and Harefield NHS Foundation Trust and Imperial College London and the Imperial Confidence in Concept Fund.
Disclosure of potential conflict of interest: A. P. Ford is employed by Afferent Pharma. M. Miralpeix and G. Tarrason are employed by Almirall S.A. J. A. Smith has consultant arrangements with Verona Pharma plc, GlaxoSmithKline, Almirall, Reckitt Benckiser, Glenmark, Xention, Patara, Bayer, and Aboca; has received grants from Verona Pharma, the British Lung Foundation, the Medical Research Council Industry Collaboration Agreement research grant with Almirall, MRC AstraZeneca Mechanisms of Disease, GlaxoSmithKline, Xention, Afferent, Medical Research Council Fellowship, Moulton Charitable Trust, and NeRRe; is an inventor on a patent owned by University Hospital of South Manchester for Vitalograph; has received payment for development of educational presentations from GlaxoSmithKline; and has received travel support from GlaxoSmithKline. M. G. Belvisi has received grants from the Medical Research Council, Afferent, the North West Lung Centre Charity, the National Institute for Health Research Respiratory Disease Biomedical Research Unit and the Royal Brompton and Harefield NHS Foundation Trust, and Imperial College London and the Imperial Confidence Concept Fund; is an associate editor for the American Journal of Respiratory and Critical Care Medicine; is an executive editor for Pharmacology and Therapeutics; has consultant arrangements with Ario Pharma, Aboca, Chiesi Pharma, Imperial College Consultants, Patara, Pulmatrix, Sun Pharma, and Skye Pharma; and is Director of IR Pharma, an Imperial College spinout that conducts contract research. The rest of the authors declare that they have no relevant conflicts of interest.
Methods
Animals
Male Dunkin-Hartley guinea pigs (300-500 g; 400-800 g for single-fiber in vivo studies) and C57BL/6 mice (18-20 g) were purchased from Harlan (Bicester, Oxon, United Kingdom) and housed in temperature-controlled (21°C) rooms with food and water freely available for at least 1 week before commencing experimentation. Homozygous breeding pairs of mice genetically modified to disrupt the TRPV4 gene (Trpv4−/−; RBRC no. 01939) were obtained from Riken BioResource Center (Tsukuba, Japan).E1, E2 Mice devoid of the pannexin 1 gene (Px1−/−) were a kind gift from Dr Vishva Dixit (Genentech). Experiments were performed in accordance with the UK Home Office guidelines for animal welfare based on the Animals (Scientific Procedures) Act of 1986 and the ARRIVE guidelines.E3
Isolated primary vagal ganglia
Cell dissociation
Guinea pigs were killed by means of injection of sodium pentobarbitone (200 mg/kg administered intraperitoneally). Nodose and jugular ganglia were dissected free of adhering connective tissue and isolated by means of enzymatic digestion, as described previously.E4, E5 Briefly, nodose and jugular ganglia were separated and incubated with activated papain solution (Sigma, St Louis, Mo; papain type, 200 U/mL in Ca2+- and Mg2+-free HBSS) for 30 minutes at 37°C, followed by incubation for 40 minutes with type 4 collagenase (2 mg/mL; Worthington Biochemical, Lakewood, NJ) and Dispase II (2.4 mg/mL; Roche, Mannheim, Germany). Neurons were dissociated by means of trituration with fire-polished glass Pasteur pipettes and washed by using centrifugation. The supernatant was decanted and the pellet was carefully homogenized in HBSS at room temperature. Cells were separated from the remaining tissue by means of centrifugation in L-15 medium containing 20% Percoll (vol/vol), followed by washing with L-15 medium alone. Cells were resuspended in complete F-12 medium and plated in poly-d-lysine/laminin (22.5 μg/mL)–coated FluoroDishes (World Precision Instruments, Hertfordshire, United Kingdom). Neurons were allowed to adhere for 2 hours and then gently flooded with complete F12 medium (10% FBS, 10,000 U/mL 1% penicillin, and 10 mg/mL streptomycin). Plates were used for experimentation within 24 hours.
[Ca2+]i measurements were performed in dissociated nodose and jugular neurons. Neurons projecting fibers specifically to the airways were identified by using the retrograde tracer DiI (1 mL/kg administered intranasally) dosed 14 to 28 days before neuron isolation to allow time for the dye to travel from the nerve endings to the cell bodies.
Calcium imaging
Fluorodishes were loaded with Fura-2 AM (3 μmol/L; Invitrogen, Carlsbad, Calif) for 40 minutes in the dark at 25°C and allowed to de-esterify for 30 minutes in the dark at 25°C. After washing, a fluorodish was placed in a full incubation chamber mounted on the stage of a Widefield inverted microscope and imaged at excitation and emission fluorescence wavelength λ values of approximately 520 to 550 nm and 570 nm, respectively, to identify DiI-labeled cells. [Ca2+]i signals were recorded, as described previously.E4, E5 Briefly, neurons were constantly superfused with ECS buffer by using an in-house pressurized solution-changing perfusion system, allowing complete bath (600-μL volume) replacement in 3 seconds. Before experiments, the cells were superfused for 10 minutes with ECS only. K50 (50 mmol/L potassium solution as opposed to 5.4 mmol/L used in Krebs solution or ECS) was applied at the start of each experiment for 10 seconds to assess cell viability and normalize responses. The 50 mmol/L solution is made by substituting potassium for sodium (ie, potassium content increased by 43.6 mmol/L and sodium content decreased by the same amount to prevent an osmotic effect). For experiments involving the selective TRPV4 agonist GSK1016790A, after washout of K50, a solution of GSK1016790A agonist (10-300 nmol/L) or vehicle (0.1% dimethyl sulfoxide [DMSO] vol/vol in ECS) was applied for 10 minutes. For experiments involving P2X3 ligands, after K50 washout, a solution of the stable ATP analogue αβ-MeATP (10 μmol/L) or vehicle (0.1% dH20 vol/vol in ECS) was applied for 10 minutes. For antagonist experiments, the P2X3 antagonist AF-353 (10 μmol/L) was incubated with the cells for 10 minutes before application of GSK1016790A (30 nmol/L). One concentration of GSK1016790A or αβ-MeATP was tested per plate. The concentration-response data represent an overview of responding cells only. The criteria for a “responsive cell” were judged to be an increase in [Ca2+]i levels of 10% or greater of the response to K50.E4 In each case N is defined as the number of animals and n is defined as the number of cells tested. Only neurons producing a fast response to K50, which was washable within 5 minutes, and with a diameter of greater than 20 μm were analyzed (N = 4-6 animals, n = 5-27 cells per concentration).
Single-cell RT-PCR
The following protocol for RT-PCR of targets in single neurons was carried out based on the methodology described by Kwong et al.E6 Isolated nodose-derived neurons were placed in a Petri dish containing ECS solution, and airway terminating (DiI-stained) neurons were identified by using a Widefield Microscope (Olympus IX-71 inverted microscope). Selected individual neurons were carefully harvested by using suction into the end of a custom-made pulled-glass micropipette (tip ID, 50-70 μm; OD, 2 mm; FIVEphoton Biochemicals) manipulated into place with a micromanipulator (Three-axis Water Hydraulic Micromanipulator MHW3, Narishige). The micropipette tip was then broken into a microreaction tube containing 1 μL of RNaseOUT (Life Technologies) and placed on ice. Cells were processed by using the Superscript III Direct cDNA Synthesis Kit (Life Technologies) and Random Hexamers (Applied Biosystems) per the manufacturer's instructions and as described by Lieu et al.E7 Some sample was removed immediately before addition of Superscript III reverse transcriptase to be used as −reverse transcriptase (RT) controls. Sample cDNA and −RT controls were stored at −20°C until PCR analysis. The reaction mixture for PCR contained 0.5 U of HotStarTaq DNA Polymerase, 2.5 mmol/L MgCl2, and PCR buffer (HotStarTaq Polymerase; Qiagen, Hilden, Germany), as well as 200 μmol/L dNTP and custom-synthesized intron-spanning primers (0.5 μmol/L each of forward/reverse primer). The PCR reaction conditions were activation at 95°C for 15 minutes and then 50 cycles of denaturation at 94°C for 30 seconds, annealing at 60°C for 30 seconds, and extension at 72°C for 1 minute, followed by a final extension at 72°C for 10 minutes. TRPV4, P2X3, and PGP9.5 primers were designed with the Primer-BLAST Primer Designer Tool from the National Center for Biotechnology Information (NCBI; http://www.ncbi.nlm.nih.gov/tools/primer-blast/), and β-actin and P2X2 primers were from Lieu et alE8 and Kwong et al,E6 respectively. PCR products were separated by means of electrophoresis on 1.5% agarose gels. Data presented here are from samples in which −RT controls produced no product and additionally in which both a specific product was amplified in β-actin and PGP9.5 primer reactions, indicating successful picking of a neuronal cell.
Primer design
TRPV4, P2X3, and PGP9.5 primers were custom designed by using the NCBI's Primer-BLAST Web site and selected, where possible, to span an exon-exon junction (TRPV4 and PGP9.5) or to be positioned on separate exons (intron spanning, P2X3). The NCBI nucleotide database contains 5 predicted TRPV4 mRNA variants: our primers were designed to target all of the potential isoforms to ensure we found any TRPV4 expressed in the single cells. Primer sequences and accession numbers or literature references used for primer design are presented in Table E1. All primers were ordered from Life Technologies. Representative examples of electrophoresis gels for PCR products generated by TRPV4, P2X3, and P2X2 primers in separate jugular- and nodose-derived neurons are shown in Fig E3.
Isolated vagus nerve preparation
Guinea pigs and mice (C57BL/6 or Trpv4−/−)E1, E2 were killed by means of injection of sodium pentobarbitone (200 mg/kg administered intraperitoneally). The vagus nerves were removed, and experiments were conducted in a fully characterized isolated vagal preparation, as described in previous publications.E9, E10 Human vagus nerves (n = 6, 38- to 57-year-old male donors and 38- to 68-year-old female donors with no known respiratory disease) were obtained from the International Institute for the Advancement of Medicine (Edison, NJ). In all cases the tissue was consented for use in scientific research, and ethics approval was obtained from the Royal Brompton & Harefield Trust.
Noncumulative concentrations of the selective TRPV4 agonists GSK1016790A (0.03-1 μmol/L) or 4α-PDD (0.3-10 μmol/L) or the endogenous stimulus hypo-osmotic solution (0 to −100 mOsm below normal airway osmolarity of 297 mOsm) were applied in a random order for 2 minutes, followed by a wash period with Krebs solution to restore baseline membrane potential between stimulations (n = 4-6). From this, submaximal doses of GSK1016790A (300 nmol/L), 1 μmol/L 4α-PDD (1 μmol/L), and hypo-osmotic solution (−80 mOsm) were chosen and subsequently used for inhibitor studies. The ability of all agonists to stimulate human vagus nerve was also determined (n = 3). Concentration responses for the TRPV4-selective inhibitors HC067047 and GSK2193874 or vehicle (0.1% DMSO vol/vol in Krebs solution) were established against both GSK1016790A and 4α-PDD in guinea pig and, for HC067047, in wild-type mouse vagus nerve (n = 4). From this, the dose of antagonist exhibiting maximal inhibition (10 μmol/L) was chosen for further experiments.
We confirmed data generated with pharmacologic tools by using tissues from Trpv4−/− vagal knockout mice (n = 4). Knockdown of the TRPV4 gene was confirmed by using standard genotyping techniques. Subsequently, TRPA1-selective (HC-030031; 10 μmol/L) and TRPV1-selective (JNJ17203212; 100 μmol/L) antagonists were tested against GSK1016790A and 4α-PDD on both guinea pig and mouse vagus (n = 4). In addition, HC067047 (10 μmol/L) was tested against acrolein (300 μmol/L)– and capsaicin (1 μmol/L)–induced vagus nerve depolarization to ensure that the antagonist was not exhibiting off-target effects on the TRPA1 or TRPV1 receptors (n = 4, see Fig E2). Vehicle for all antagonists was 0.1% DMSO vol/vol in Krebs solution. Effective concentrations for the TRPA1 and TRPV1 tools had been previously established in our laboratory.E4
To investigate potential mechanisms of TRPV4-mediated activation of airway sensory nerves, an initial concentration response to αβ-MeATP (0.1-300 μmol/L), a stable agonist at P2X1 and P2X3 receptors, was carried out in guinea pig tissue, and a submaximal concentration was taken forward for inhibitor studies (100 μmol/L). Specificity of the agonist for its receptor was determined by using the P2X2/P2X3 antagonist TNP-ATP (10 μmol/L) and the P2X3 antagonist AF-353 (10 μmol/L). These antagonists were then tested against GSK1016790A-induced depolarization alongside capsaicin- and acrolein-induced depolarization. Vehicle for the antagonists was 0.1% DMSO vol/vol in Krebs solution.
In vivo single-fiber preparation
Guinea pigs were anesthetized with urethane (1.5 g · kg−1) intraperitoneally. If required, anesthesia was supplemented with additional urethane. The trachea was cannulated with a short length of Portex tubing, and blood gases and pH were maintained at physiologic levels by using artificial ventilation (Ugo Basile small-animal ventilator) with a tidal volume of 10 mL · kg−1 and 50 to 60 breaths · min−1 of laboratory air. The right jugular vein and carotid artery (passed to the ascending aorta/aortic arch) were cannulated for injecting drugs and measuring systemic arterial blood pressure, respectively. Systemic arterial blood pressure and heart rate were continuously recorded with a transducer (Gould P23XL). Tracheal pressure was measured with an air pressure transducer (SenSym 647) connected to a side arm of the tracheal cannula. Body temperature was continuously monitored with a rectal thermometer and maintained at 37°C with a heated blanket and control unit (Harvard Apparatus, Holliston, Mass). Animals were paralyzed with vecuronium bromide, which was initially administered at a dose of 0.10 mg · kg−1 administered intravenously, followed every 20 minutes by 0.05 mg · kg−1 administered intravenously to maintain paralysis. The depth of anesthesia was frequently assessed by monitoring the response of heart rate and blood pressure to noxious stimuli. Both cervical vagus nerves were located through a cervical incision and dissected free from the carotid artery and sympathetic and aortic nerves; both vagus nerves were cut at the central end. The left vagus nerve was used for sensory nerve fiber recording and cleared of its surrounding fascia. The skin and muscle in the neck at either side of the incision were lifted and tied to a metal ring to form a well, which was filled with light mineral oil. Bipolar Teflon-coated platinum electrodes (exposed at the tips) were used for recording purposes by using fascia positioned on 1 electrode for a reference. The vagus nerve was placed on a small black Perspex plate to facilitate subsequent dissection. Thin filaments of nerve were teased from the vagus nerve under a binocular microscope and placed on the second electrode until a single active unit or one of not more than 2 or 3 units was obtained. Action potentials were recorded in a conventional manner by using electrodes connected to a pre-amp Headstage (Digitimer NL100K; Digitimer, Welwyn Garden City, United Kingdom). The signal was amplified (×1000-×5000, Digitimer NL104), filtered (in the range of LF30Hz-HF8.5kHz, Digitimer NL125), and passed through a HumBug noise reducer (AutoMate Scientific, San Francisco, Calif) before input sampling and recording. All signals were sampled (50 kHz) and recorded by using the Spike 2 software data acquisition system (Cambridge Electronic Design, Cambridge, United Kingdom) through a CED Micro1401 interface. The software allowed pulse train counting over selected time periods. In addition, monitoring of the input signal to the Spike software was also carried out on a digital storage oscilloscope (Tektronix DPO 2012). The input signal was also fed through an audio amplifier to a loudspeaker. All animals were killed at the end of the experiments with an overdose of pentobarbitone.
CVs were measured to distinguish slow-conducting nonmyelinated C-fibers from fast-conducting myelinated Aδ-fibers by stimulating the vagus nerve close to the thorax with bipolar silver electrodes by using a suprathreshold voltage at 0.5 ms and 1 Hz (Grass stimulator; Grass Technologies, Warwick, RI). The corresponding action potential was recorded in the nerve fiber under observation. The stimulus and the recorded action potential were captured on the Spike software in a single sweep, and the time interval between them was measured to calculate the velocity by using the distance from the cathode-stimulating electrode and the recording electrode. Aerosols were generated by an Aerogen nebulizer (Buxco Nebulizer Control-5) connected to the ventilator and arranged so that the inspired air passed through the medication chamber before entering the lungs of anesthetized animals through the tracheal cannula.
Single vagus nerve fibers were identified as originating from the major groups of airway sensory nerve endings (ie, slowly adapting stretch receptors, RARs, irritant receptors, and Aδ-fibers, which were further subdivided into those that were more acid and/or less capsaicin sensitive and with CVs slower than conventional RARs) and pulmonary/bronchial C-fiber receptors by using several criteria.E11 These include pattern of spontaneous discharge, response to hyperinflation and deflation, adaptation indices, response to capsaicin/citric acid administration, and CVs. As a rule, a receptor that had no obvious pattern to the spontaneous activity (often very sparse), did not respond to hyperinflation/hyperdeflation, but responded to capsaicin aerosol was pursued as a C-fiber. Alternatively, and in the first instance, a receptor that had a spontaneous discharge with a definite rhythmic respiratory pattern and adapted rapidly/variably to hyperinflation/deflation was pursed as an Aδ-fiber. Finally, verification of fiber type was confirmed at the end of the experiment by determining the CV.
After surgery, animals were allowed to stabilize for at least 30 minutes. For the TRPV4 agonist GSK1016790A, after identification of a lung-afferent fiber, the ensuing protocol was pursued. After a control baseline recording of at least 2 minutes, vehicle (1% ethanol plus 1% Tween 80 in saline) was administered as an aerosol for up to 1 minute, and changes in fiber activity, intratracheal pressure, and blood pressure were continuously recorded until baseline or a steady state was re-established. After an interval of 20 minutes, capsaicin (100 μmol/L) was administered as an aerosol for 15 seconds, and the changes in fiber activity, intratracheal pressure, and blood pressure were continuously recorded until baseline or a steady state was re-established. Citric acid (0.3 mol/L) was administered 20 minutes later as an aerosol for 1 minute, and variables were continuously recorded. After a further 20 minutes, GSK1016790A (10 μg · mL−1) was administered as an aerosol for 1 minute, and the changes in fiber activity, intratracheal pressure, and blood pressure were continuously recorded. When C-fibers were being examined, capsaicin aerosol was repeated at least 30 minutes after GSK1016790A.
In a separate series of experiments for hypo-osmotic solutions, a similar protocol as for GSK1016790A was followed. After establishing responsiveness to capsaicin and citric acid, a 0-mOsm solution was administered for 1 minute as an aerosol, and variables were continuously recorded. After an interval of 20 minutes, a hypo-osmotic solution of −80 mOsm was administered as an aerosol for 1 minute, and the changes in fiber activity, intratracheal pressure, and blood pressure were continuously recorded. A −80-mOsm solution is a solution that is 80 mOsm less than the normal airway osmolarity of −295 mOsm. This was achieved by reducing the chloride concentration and gradual salt compensation with sucrose. The effect of a low-chloride solution on sensory nerves was circumvented by using a modified Krebs solution with a lower chloride concentration and salt compensation with sucrose at the start of every experiment involving hypo-osmotic solution.
A similar protocol was followed for the set of experiments involving αβ-MeATP. After establishing responsiveness to capsaicin and citric acid, αβ-MeATP was administered as an aerosol into the airways for 1 minute, and the changes in fiber activity, intratracheal pressure, and blood pressure were continuously recorded. For antagonist experiments, only Aδ-fibers were investigated. Two control responses to 300 μmol/L αβMe-ATP were carried out 20 minutes apart, after which the antagonist or vehicle was administered intraperitoneally (AF-353: 30 mg/kg, vehicle: 10% polyethylene glycol 400 in 0.9% saline), to investigate the antagonist activity of the P2X3 antagonist AF-353. One hour later, αβ-MeATP was reaerosolized into the airways, and responses were monitored; 20 minutes later, GSK1016790A (100 ng/mL) was readministered to the airways, and responses were monitored. A similar protocol was undertaken for the TRPV4 antagonist GSK2193874, where 2 control responses to GSK1016790A (100 ng/mL) were carried out 20 minutes apart, after which antagonist or vehicle was administered intraperitoneally (GSK2193874: 300 mg/kg, vehicle: 6% Cavitron/2-hydroxypropyl-β-cyclodextrin in saline; Sigma-Aldrich, Poole, United Kingdom). Changes in fiber activity, intratracheal pressure, and blood pressure were continuously recorded throughout the experiment.
Conscious guinea pig cough model
In all experiments the operator was blind to the treatment group. Conscious unrestrained guinea pigs were placed in individual plastic, transparent, whole-body plethysmography chambers (Buxco), and cough was detected, as previously described.E7, E8 A concentration response was initially established for GSK1016790A (1-30 μg/mL) or appropriate vehicle (1% ethanol and 1% Tween 80 in saline, n = 4). The stimulus was aerosolized for 5 minutes, and coughs were counted for 10 minutes by trained observers (2 in the room), who were blind to the treatment group and counted the number of coughs, and a mean of the 2 observer's counts was taken as the final count. For the antagonist studies, guinea pigs were injected intraperitoneally with HC067047 (100 mg/kg) or appropriate vehicle (1% DMSO in 0.9% saline) 1 hour before exposure to a submaximal concentration of aerosolized GSK1016790A (3 μg/mL). A similar protocol was followed for the alternate TRPV4 antagonist GSK2193874 (300 mg/kg; vehicle: 6% Cavitron/2-hydroxypropyl-β-cyclodextrin in saline) and the P2X3 antagonist AF-353 (30 mg/kg; vehicle: 10% polyethylene glycol in saline), which, in separate experiments, were injected intraperitoneally 1 hour before aerosol administration of GSK1016790A (30 μg/mL). GSK1016790A was aerosolized for 5 minutes, and the number of coughs was counted for 10 minutes (n = 12). A further cough study was carried out, investigating the effect of AF-353 and GSK2193874 on capsaicin-induced cough in conscious guinea pigs, to determine the specificity of the antagonists. GSK2193874 (300 mg/kg; vehicle: 6% Cavitron/2-hydroxypropyl-β-cyclodextrin in saline) and the P2X3 antagonist AF-353 (30 mg/kg; vehicle: 10% polyethylene glycol in saline) were injected intraperitoneally 1 hour before aerosol administration of the TRPV1 receptor agonist capsaicin (60 μmol/L). Capsaicin was aerosolized for 5 minutes, and coughs were counted for 10 minutes (n = 8). The dose of AF-353 selected for in vivo experiments is consistent with previous in vivo studies.E12 The alternative TRPV4 antagonist (GSK2193874) described by Thorneloe et alE13 was used at 300 mg/kg, given that some effects of the TRPV4 agonist were not completely inhibited at 30 mg/kg, at least in the rat.
Compounds and materials
The TRPV4 inhibitor HC067047 was purchased from Peakdale Molecular (High Peak, United Kingdom), and GSK2193874 was a kind gift from Almirall (Barcelona, Spain). The TRPA1 inhibitor HC030031 from ChemBridge (San Diego, Calif) and 4α-PDD were purchased from LC Labs (Woburn, Mass). AF-353 was a kind gift from Afferent Pharmaceuticals (San Mateo, Calif), and TNPATP was purchased from Cayman (Ann Arbor, Mich). All other agents were purchased from Sigma-Aldrich (Poole, Dorset, United Kingdom).
Isolated vagal ganglia experiments
DiI was purchased from Invitrogen (Molecular Probes) as solid crystals, which were then diluted to a stock of 25 mg/mL in ethanol. A working solution (500 μg/mL in 0.9% saline) was made fresh from the ethanol stock immediately before use. Fura-2 AM was purchased from Molecular Probes/Invitrogen. L-15 and HBSS were purchased from Gibco/Invitrogen. The K50 solution contained 50 mmol/L KCl, 91.4 mmol/L NaCl, 1 mmol/L MgCl2, 2.5 mmol/L CaCl2, 0.33 mmol/L NaH2PO4, 10 mmol/L glucose, and 10 mmol/L HEPES, with pH adjusted to 7.4 at 37°C by using KOH. ECS buffer was made fresh on a daily basis as follows: 5.4 mmol/L KCl, 136 mmol/L NaCl, 1 mmol/L MgCl2, 2.5 mmol/L CaCl2, 0.33 mmol/L NaH2PO4, 10 mmol/L glucose, and 10 mmol/L HEPES, with pH adjusted to 7.4 at 37°C by using NaOH. All inhibitors were dissolved in 100% DMSO and diluted 1:1000 in ECS buffer to the desired final concentrations.
In vitro vagus experiments
Krebs salts were obtained from BDH (Dorset, United Kingdom), and Krebs-Henseleit solution was made fresh on a daily basis (118 mmol/L NaCl, 5.9 mmol/L KCl, 1.2 mmol/L MgSO4, 2.5 mmol/L CaCl2, 1.2 mmol/L NaH2PO4, 25.5 mmol/L NaHCO3, and 5.6 mmol/L glucose). All inhibitors were dissolved in 100% DMSO and diluted 1:1000 in Krebs buffer to the desired final concentrations. The hypo-osmotic solution was made fresh on the day of the experiment by using a modified Krebs-Henseleit solution with salt reduction, followed by gradual compensation with sucrose. A solution of 197 mOsm (100 mOsm less than normal airway osmolarity) contained 7 mmol/L KCl, 63 mmol/L NaCl, 1.2 mmol/L MgCl2, 1.2 mmol/L NaH2PO4, 5.6 mmol/L D-glucose, 25.5 mmol/L NaHCO3, and 2.5 mmol/L CaCl2. Solutions of increasing osmolarity were then made by addition of sucrose. For solutions of 217 mOsm (−80), 237 mOsm (−60), 257 mOsm (−40), 277 mOsm (−20), and 297 mOsm (0), sucrose was added to the modified Krebs solution (−80 mOsm: 20 mmol/L, −60 mOsm: 40 mmol/L, −40 mOsm: 60 mmol/L, −20 mOsm: 80 mmol/L, and 0 mOsm: 100 mmol/L). Because the chloride content of these solutions is significantly reduced and therefore could have a functional effect, the 0-mOsm solution was used both before and after application of the hypo-osmotic solution. Osmolarity of the solutions was confirmed with a cryo-osmometer (Osmomat030; Gonotec, Berlin, Germany).
In vivo cough and single-fiber experiments
Capsaicin and GSK1016790A (Sigma-Aldrich) were dissolved in 1% ethanol and 1% Tween 80 in 0.9% saline to a working solution. Citric acid was dissolved in 0.9% saline. αβMe-ATP was dissolved in dH20 and 0.9% saline for a working solution. HC067047 (100 mg/mL) was suspended in vehicle (1% DMSO in 0.9% saline), a total dosing volume of 10 mL/kg intraperitoneal GSK2193874 (300 mg/kg) was suspended in vehicle (6% Cavitron/2-gydroxypropyl-β-cyclodextrin in 0.9% saline), a total dosing volume of 30 mL/kg intraperitoneal AF-353 (30 mg/kg) was dissolved in vehicle (polyethylene glycol 400 in 0.9% saline), and a total dosing volume of 1 mL/kg was administered intraperitoneally.
Data analysis and statistics
The AUC of the calcium signal (calcium flux: total increase in calcium levels to greater than resting level over time) was used to measure primary neuron responses, which were normalized to calcium flux generated by application of K50. Inhibition of agonist-induced responses was analyzed by using an unpaired t test comparing the K50 percentage (AUC) with and without antagonist. Data were analyzed for responding cells only, which were defined as neurons with a response of 10% or greater of K50, and are presented as means ± SEMs, where N indicates the number of animals and n indicates the number of cells.
Inhibition of agonist-induced responses in the isolated vagus nerve preparation were analyzed by using the 2-tailed paired t test, comparing responses to agonist in the absence and presence of antagonist in the same piece of nerve. Data are presented as means ± SEMs, with statistical significance set at a P value of less than .05.
In the single-fiber experiments data were analyzed by using the paired t test, comparing responses (absolute values) after stimulus with baseline values immediately preceding the response. Data are presented as means ± SEMs, with statistical significance set at a P value of less than .05.
Inhibition of fiber firing was analyzed by using the paired t test comparing responses after antagonist with control values before antagonist administration or using an unpaired t test comparing responses to vehicle control as appropriate. Statistical significance was set at a P value of less than .05.
Inhibition of cough by the TRPV4 antagonist or the P2X3 receptor antagonist in vivo was analyzed by using the Mann-Whitney test comparing responses from the antagonist group with those from the vehicle control. Data are presented as means ± SEMs, with statistical significance set at a P value of less than .05.
Table E1.
Single-cell PCR primer information: list of primer sequences, product sizes, and coding DNA sequence accession ID/references
| Target | Accession ID/reference | Forward primer sequence (5′-3′) | Reverse primer sequence (5′-3′) | Size |
|---|---|---|---|---|
| β-Actin | Lieu et alE8 | TGGCTACAGTTTCACCACCA | GGAAGGAGGGCTGGAAGA | 212 |
| TRPV4 | XM_003477912.2 | TGGGCAAGAACTCAGATGGC | TCCACAGTCCTAGAGGGGAG | 335 |
| P2X2 | Kwong et alE6 | GCTGCTCATCCTGCTCTACTTT | GGGCTTCACATACTCCTCCAC | 157 |
| P2X3 | XM_003464224.1 | AGGGAGGCTGAGAACTTCAC | CAGCCAATCTTGATGCCCAG | 239 |
| PGP9.5 | XM_003471552.2 | GCCAGTGTCGGGTAGATGAC | CGTGTGTGCAGAACCAAAGG | 349 |
References
- 1.Wu L.J., Sweet T.B., Clapham D.E. International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol Rev. 2010;62:381–404. doi: 10.1124/pr.110.002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raemdonck K., de Alba J., Birrell M.A., Grace M., Maher S.A., Irvin C.G. A role for sensory nerves in the late asthmatic response. Thorax. 2012;67:19–25. doi: 10.1136/thoraxjnl-2011-200365. [DOI] [PubMed] [Google Scholar]
- 3.Laude E.A., Higgins K.S., Morice A.H. A comparative study of the effects of citric acid, capsaicin and resiniferatoxin on the cough challenge in guinea-pig and man. Pulm Pharmacol. 1993;6:171–175. doi: 10.1006/pulp.1993.1023. [DOI] [PubMed] [Google Scholar]
- 4.Lalloo U.G., Fox A.J., Belvisi M.G., Chung K.F., Barnes P.J. Capsazepine inhibits cough induced by capsaicin and citric acid but not by hypertonic saline in guinea pigs. J Appl Physiol. 1995;79:1082–1087. doi: 10.1152/jappl.1995.79.4.1082. [DOI] [PubMed] [Google Scholar]
- 5.Birrell M.A., Belvisi M.G., Grace M., Sadofsky L., Faruqi S., Hele D.J. TRPA1 agonists evoke coughing in guinea pig and human volunteers. Am J Respir Crit Care Med. 2009;180:1042–1047. doi: 10.1164/rccm.200905-0665OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andrè E., Gatti R., Trevisani M., Preti D., Baraldi P.G., Patacchini R. Transient receptor potential ankyrin receptor 1 is a novel target for pro-tussive agents. Br J Pharmacol. 2009;158:1621–1628. doi: 10.1111/j.1476-5381.2009.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuller R.W., Collier J.G. Sodium cromoglycate and atropine block the fall in FEV1 but not the cough induced by hypotonic mist. Thorax. 1984;39:766–770. doi: 10.1136/thx.39.10.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allegra L., Bianco S. Nonspecific bronchoreactivity obtained with an ultrasonic aerosol of distilled water. Eur J Respir Dis. 1980;61:41–49. [PubMed] [Google Scholar]
- 9.Anderson S.D., Schoeffel R.E., Finney M. Evaluation of ultrasonically nebulized solutions for provocation testing in patients with asthma. Thorax. 1983;38:284–291. doi: 10.1136/thx.38.4.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joris L., Dab I., Quinton P.M. Elemental composition of human airway surface fluid in healthy and diseased airways. Am Rev Respir Dis. 1993;148:1633–1637. doi: 10.1164/ajrccm/148.6_Pt_1.1633. [DOI] [PubMed] [Google Scholar]
- 11.Nilius B., Vriens J., Prenen J., Droogmans G., Voets T. TRPV4 calcium entry channel: a paradigm for gating diversity. Am J Physiol Cell Physiol. 2004;286:C195–C205. doi: 10.1152/ajpcell.00365.2003. [DOI] [PubMed] [Google Scholar]
- 12.Liedtke W. Transient receptor potential vanilloid channels functioning in transduction of osmotic stimuli. J Endocrinol. 2006;191:515–523. doi: 10.1677/joe.1.07000. [DOI] [PubMed] [Google Scholar]
- 13.Alessandri-Haber N., Yeh J.J., Boyd A.E., Parada C.A., Chen X., Reichling D.B. Hypotonicity induces TRPV4-mediated nociception in rat. Neuron. 2003;39:497–511. doi: 10.1016/s0896-6273(03)00462-8. [DOI] [PubMed] [Google Scholar]
- 14.Jia Y., Wang X., Varty L., Rizzo C.A., Yang R., Correll C.C. Functional TRPV4 channels are expressed in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2004;287:L272–L278. doi: 10.1152/ajplung.00393.2003. [DOI] [PubMed] [Google Scholar]
- 15.McAlexander M.A., Luttmann M.A., Hunsberger G.E., Undem B.J. Transient receptor potential vanilloid 4 activation constricts the human bronchus via the release of cysteinyl leukotrienes. J Pharmacol Exp Ther. 2014;349:118–125. doi: 10.1124/jpet.113.210203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamanaka K., Jian M.Y., Townsley M.I., King J.A., Liedtke W., Weber D.S. TRPV4 channels augment macrophage activation and ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2010;299:L353–L362. doi: 10.1152/ajplung.00315.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baxter M., Eltom S., Dekkak B., Yew-Booth L., Dubuis E.D., Maher S.A. Role of transient receptor potential and pannexin channels in cigarette smoke triggered ATP release in the lung. Thorax. 2014;69:1080–1089. doi: 10.1136/thoraxjnl-2014-205467. [DOI] [PubMed] [Google Scholar]
- 18.Zhu G., ICGN Investigators. Gulsvik A., Bakke P., Ghatta S., Anderson W. Association of TRPV4 gene polymorphisms with chronic obstructive pulmonary disease. Hum Mol Genet. 2009;18:2053–2062. doi: 10.1093/hmg/ddp111. [DOI] [PubMed] [Google Scholar]
- 19.Seminario-Vidal L., Okada S.F., Sesma J.I., Kreda S.M., van Heusden C.A., Zhu Y. Rho signaling regulates pannexin 1-mediated ATP release from airway epithelia. J Biol Chem. 2011;286:26277–26286. doi: 10.1074/jbc.M111.260562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kilkenny C., Browne W.J., Cuthill I.C., Emerson M., Altman D.G. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8:e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grace M.G., Birrell M.A., Dubuis E., Maher S.A., Belvisi M.G. Transient receptor potential channels mediate the tussive response to prostaglandin E2 and bradykinin. Thorax. 2012;67:891–900. doi: 10.1136/thoraxjnl-2011-201443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dubuis E., Grace M., Wortley M.A., Birrell M.A., Belvisi M.G. Harvesting, isolation, and functional assessment of primary vagal ganglia cells. Curr Protoc Pharmacol. 2013;62 doi: 10.1002/0471141755.ph1215s62. Unit 12.15. [DOI] [PubMed] [Google Scholar]
- 23.Kwong K., Kollarik M., Nassenstein C., Ru F., Undem B.J. P2X2 receptors differentiate placodal vs. neural crest C-fiber phenotypes innervating guinea pig lungs and esophagus. Am J Physiol Lung Cell Mol Physiol. 2008;295:L858–L865. doi: 10.1152/ajplung.90360.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suzuki M., Mizuno A., Kodaira K., Imai M. Impaired pressure sensation in mice lacking TRPV4. J Biol Chem. 2003;278:22664–22668. doi: 10.1074/jbc.M302561200. [DOI] [PubMed] [Google Scholar]
- 25.Mizuno A., Matsumoto N., Imai M., Suzuki M. Impaired osmotic sensation in mice lacking TRPV4. Am J Physiol Cell Physiol. 2003;285:C96–C101. doi: 10.1152/ajpcell.00559.2002. [DOI] [PubMed] [Google Scholar]
- 26.Maher S.A., Birrell M.A., Belvisi M.G. Prostaglandin E2 mediates cough via the EP3 receptor: implications for future disease therapy. Am J Respir Crit Care Med. 2009;180:923–928. doi: 10.1164/rccm.200903-0388OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adcock J.J., Douglas G.J., Garabette M., Gascoigne M., Beatch G., Walker M. RSD931, a novel anti-tussive agent acting on airway sensory nerves. Br J Pharmacol. 2003;138:407–416. doi: 10.1038/sj.bjp.0705056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thorneloe K.S., Sulpizio A.C., Lin Z., Figueroa D.J., Clouse A.K., McCafferty G.P. N-((1S)-1-{[4-((2S)-2-{[(2,4-dichlorophenyl)sulfonyl]amino}-3-hydroxypropanoyl)-1-piperazinyl] carbonyl} -3-methylbutyl)-1-benzothiophene-2-carboxamide (GSK1016790A), a novel and potent transient receptor potential vanilloid 4 channel agonist induces urinary bladder contraction and hyperactivity: Part I. J Pharmacol Exp Ther. 2008;326:432–442. doi: 10.1124/jpet.108.139295. [DOI] [PubMed] [Google Scholar]
- 29.Watanabe H., Davis J.B., Smart D., Jerman J.C., Smith G.D., Hayes P. Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J Biol Chem. 2002;277:13569–13577. doi: 10.1074/jbc.M200062200. [DOI] [PubMed] [Google Scholar]
- 30.Everaerts W., Zhen X., Ghosh D., Vriens J., Gevaert T., Gilbert J.P. Inhibition of the cation channel TRPV4 improves bladder function in mice and rats with cyclophosphamide-induced cystitis. Proc Natl Acad Sci U S A. 2010;107:19084–19089. doi: 10.1073/pnas.1005333107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thorneloe K.S., Cheung M., Bao W., Alsaid H., Lenhard S., Jian M.Y. An orally active TRPV4 channel blocker prevents and resolves pulmonary edema induced by heart failure. Sci Transl Med. 2012;4:159ra148. doi: 10.1126/scitranslmed.3004276. [DOI] [PubMed] [Google Scholar]
- 32.Virginio C., Robertson G., Surprenant A., North R.A. Trinitrophenyl-substituted nucleotides are potent antagonists selective for P2X1, P2X3, and heteromeric P2X2/3 receptors. Mol Pharmacol. 1998;53:969–973. [PubMed] [Google Scholar]
- 33.Gever J.R., Soto R., Henningsen R.A., Martin R.S., Hackos D.H., Panicker S. AF-353, a novel, potent and orally bioavailable P2X3/P2X2/3 receptor antagonist. Br J Pharmacol. 2010;160:1387–1398. doi: 10.1111/j.1476-5381.2010.00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aizawa N., Wyndaele J.J., Homma Y., Igawa Y. Effects of TRPV4 cation channel activation on the primary bladder afferent activities of the rat. Neurourol Urodyn. 2012;31:148–155. doi: 10.1002/nau.21212. [DOI] [PubMed] [Google Scholar]
- 35.Grace M.S., Baxter M., Dubuis E., Birrell M.A., Belvisi M.G. Transient receptor potential (TRP) channels in the airway: role in airway disease. Br J Pharmacol. 2014;171:2593–2607. doi: 10.1111/bph.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liedtke W., Choe Y., Martí-Renom M.A., Bell A.M., Denis C.S., Sali A. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000;103:525–535. doi: 10.1016/s0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guler A.D., Lee H., Iida T., Shimizu I., Tominaga M., Caterina M. Heat-evoked activation of the ion channel, TRPV4. J Neurosci. 2002;22:6408–6414. doi: 10.1523/JNEUROSCI.22-15-06408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mochizuki T., Sokabe T., Araki I., Fujishita K., Shibasaki K., Uchida K. The TRPV4 cation channel mediates stretch-evoked Ca2+ influx and ATP release in primary urothelial cell cultures. J Biol Chem. 2009;284:21257–21264. doi: 10.1074/jbc.M109.020206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sato D., Sato T., Urata Y., Okajima T., Kawamura S., Kurita M. Distribution of TRPVs, P2X3, and Parvalbumin in the human nodose ganglion. Cell Mol Neurobiol. 2014;34:851–858. doi: 10.1007/s10571-014-0062-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi M., Du F., Liu Y., Li L., Cai J., Zhang G.F. Glial cell-expressed mechanosensitive channel TRPV4 mediates infrasound-induced neuronal impairment. Acta Neuropathol. 2013;126:725–739. doi: 10.1007/s00401-013-1166-x. [DOI] [PubMed] [Google Scholar]
- 41.Abdulqawi R., Dockry R., Holt K., Layton G., McCarthy B.G., Ford A.F. A randomised, double-blind, placebo-controlled study of AF-219, an inhibitor of ATP-gated P2X3 channels, in refractory chronic cough. Lancet. 2015;385:1198–1205. doi: 10.1016/S0140-6736(14)61255-1. [DOI] [PubMed] [Google Scholar]
- 42.Morice A.H., Fontana G.A., Sovijarvi A.R., Pistolesi M., Chung K.F., Widdicombe J. The diagnosis and management of chronic cough. Eur Respir J. 2004;24:481–492. doi: 10.1183/09031936.04.00027804. [DOI] [PubMed] [Google Scholar]
- 43.Idzko M., Hammad H., van Nimwegen M., Kool M., Willart M.A., Muskens F. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. 2007;13:913–919. doi: 10.1038/nm1617. [DOI] [PubMed] [Google Scholar]
- 44.Lommatzsch M., Cicko S., Müller T., Lucattelli M., Bratke K., Stoll P. Extracellular adenosine triphosphate and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181:928–934. doi: 10.1164/rccm.200910-1506OC. [DOI] [PubMed] [Google Scholar]
- 45.Schroeder K., Fahey T. Systematic review of randomised controlled trials of over the counter cough medicines for acute cough in adults. BMJ. 2002;324:329–331. doi: 10.1136/bmj.324.7333.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schappert S.M., Burt C.W. Ambulatory care visits to physician offices, hospital outpatient departments, and emergency departments: United States, 2001-02. Vital Health Stat 13. 2006;159:1–66. [PubMed] [Google Scholar]
References
- Suzuki M., Mizuno A., Kodaira K., Imai M. Impaired pressure sensation in mice lacking TRPV4. J Biol Chem. 2003;278:22664–22668. doi: 10.1074/jbc.M302561200. [DOI] [PubMed] [Google Scholar]
- Mizuno A., Matsumoton N., Imai M., Suzuki M. Impaired osmotic sensation in mice lacking TRPV4. Am J Physiol Cell Physiol. 2003;285:C96–C101. doi: 10.1152/ajpcell.00559.2002. [DOI] [PubMed] [Google Scholar]
- Kilkenny C., Browne W.J., Cuthill I.C., Emerson M., Altman D.G. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8:e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace M.S., Birrell M.A., Dubuis E., Maher S.A., Belvisi M.G. Transient receptor potential channels mediate the tussive response to prostaglandin E2 and bradykinin. Thorax. 2012;67:891–900. doi: 10.1136/thoraxjnl-2011-201443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubuis E., Grace M., Wortley M.A., Birrell M.A., Belvisi M.G. Harvesting, isolation, and functional assessment of primary vagal ganglia cells. Cur Protoc Pharmacol. 2013;62 doi: 10.1002/0471141755.ph1215s62. Unit 12.15. [DOI] [PubMed] [Google Scholar]
- Kwong K., Kollarik M., Nassenstein C., Ru F., Undem B.J. P2X2 receptors differentiate placodal vs. neural crest C-fiber phenotypes innervating guinea pig lungs and esophagus. Am J Physiol Lung Cell Mol Physiol. 2008;295:L858–L865. doi: 10.1152/ajplung.90360.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieu T.M., Myers A.C., Meeker S., Undem B.J. TRPV1 induction in airway vagal low-threshold mechanosensory neurons by allergen challenge and neurotrophic factors. Am J Physiol Lung Cell Mol Physiol. 2012;302:L941–L948. doi: 10.1152/ajplung.00366.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieu T., Kollarik M., Myers A.C., Undem B.J. Neurotrophin and GDNF family ligand receptor expression in vagal sensory nerve subtypes innervating the adult guinea pig respiratory tract. Am J Physiol Lung Cell Mol Physiol. 2011;300:L790–L798. doi: 10.1152/ajplung.00449.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher S.A., Birrell M.A., Belvisi M.G. Prostaglandin E2 mediates cough via the EP3 receptor: implications for future disease therapy. Am J Respir Crit Care Med. 2009;180:923–928. doi: 10.1164/rccm.200903-0388OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birrell M.A., Belvisi M.G., Grace M., Sadofsky L., Faruqi S., Hele D.J. TRPA1 agonists evoke coughing in guinea pig and human volunteers. Am J Respir Crit Care Med. 2009;180:1042–1047. doi: 10.1164/rccm.200905-0665OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adcock J.J., Douglas G.J., Garabette M., Gascoigne M., Beatch G., Walker M. RSD931, a novel anti-tussive agent acting on airway sensory nerves. Br J Pharmacol. 2003;138:407–416. doi: 10.1038/sj.bjp.0705056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz A., Somogyi G.T., Boone T.B., Ford A.P., Smith C.P. Modulation of bladder afferent signals in normal and spinal cord-injured rats by purinergic P2X3 and P2X2/3 receptors. BJU Int. 2012;110(8 Pt B):E409–E414. doi: 10.1111/j.1464-410X.2012.11189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorneloe K.S., Cheung M., Bao W., Alsaid H., Lenhard S., Jian M.Y. An orally active TRPV4 channel blocker prevents and resolves pulmonary edema induced by heart failure. Sci Transl Med. 2012;4:159ra148. doi: 10.1126/scitranslmed.3004276. [DOI] [PubMed] [Google Scholar]